Abstract
DNA preparation is indispensable for genotyping by DNA polymorphism analysis, and that for a large number of plants is laborious. In the present study, a small leaf disk of rice, 1–2 mm in diameter, punched by a mini cork borer was found to be directly usable as a PCR template. DNA fragments <300 bp were amplified efficiently. Leaf disks of 1–1.5 mm in diameter were better than those of 2 mm for a small volume of reaction mixture. Multiplex PCR was possible with four or eight primer pairs using the small leaf disk as a template. Leaf disks of Arabidopsis, Lotus, wheat, soybean, tomato, Chinese cabbage, and melon were also good PCR templates. This method for preparation of PCR templates, named the leaf-punch method, was applicable to SNP analysis of a large number of plants by dot-blot-SNP analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent development of techniques for DNA polymorphism analysis have enabled genotyping of a large number of plants, which is important for fine mapping of genes for map-based cloning (Konishi et al. 2006; Murakami et al. 2006; Zhang et al. 2006), population genetic analysis of gene frequencies (Maruyama et al. 2004; Bittencourt and Sebbenn 2007; Hanson et al. 2008), and plant breeding by direct selection of desired genotypes (Shirasawa et al. 2006). For these studies, techniques for single nucleotide polymorphism (SNP) analysis, which can analyze any change of nucleotide sequence and therefore can identify the genotype of each plant, are highly powerful tools. Among various SNP techniques, the dot-blot-SNP technique is considered to be a highly laborsaving and cost-effective method (Shirasawa et al. 2006). Although this SNP technique enables genotyping of a large number of DNA samples without much labor, preparation of DNA samples from a large number of plants still requires much labor.
Various techniques for DNA preparation from plants have been developed. Among them, the CTAB method (Murray and Thompson 1980) is commonly used because of the high DNA purity possible with this method. As a simple technique without use of hazardous organic solvents, the method of Edwards et al. (1991) is also widely used. These methods require pulverization of plant tissues using a mortar with a pestle or a plastic tube containing a rod is used with liquid nitrogen, and handling of a large number of samples requires much labor. Special instruments for grinding tissues in tubes by shaking with glass beads or metal blocks have been developed for handling a large number of samples (MM300 Mixer Mill, Retsch, Germany), and are used for plant DNA preparation (Xu et al. 2005), but the centrifugation still requires much labor and time for preparation of many DNA samples.
Hammer blotting has been used for blotting of viruses from plant tissues onto filter paper (Romero-Durbán et al. 1995; Olmos et al. 1996). FTA™ cards (Flinder Technology Associates, Moscoso et al. 2005) are used for fixing DNA squeezed from plant tissues, and can be used as a PCR template (Roy and Nassuth 2005). In these methods, only crushing plant tissues by a hammer and washing to remove substances interfering with PCR are required. In our preliminary investigation, DNA amplifications from templates fixed on FTA Cards, nylon membrane, and filter paper were successful, but the efficiency of amplification and reproducibility were not high.
In the present study, we found that a small leaf disk of rice punched by a mini cork borer can be used directly as a PCR template for amplification of short DNA fragments. The efficiency of DNA amplification from rice leaf disks for dot-blot-SNP analysis was examined. This method for preparation of PCR templates, termed leaf-punch method, and the dot-blot-SNP technique were applied to fine mapping of a mutant gene and genotyping of B1F4 plants in a conventional crossbreeding program of rice. Applicability of the leaf-punch method to other plant species was also investigated.
Materials and methods
Plant materials
Seven cultivars in rice (Oryza sativa L.), i.e., ‘Nipponbare’, ‘Koshihikari’, ‘Itadaki’, ‘Kihou’, ‘Akihikari’, ‘Kirara 397’, and ‘Hitomebore’, were used for studying the efficiency of DNA amplification by PCR. Wheat (Triticum aestivum cv. ‘Shirasagikomugi’), soybean (Glycine max cv. ‘Natsunoyosooi’), melon (Cucumis melo cv. ‘Andes’), tomato (Solanum lycopersicum cv. ‘Ailsa craig’), Chinese cabbage (Brassica rapa L. cv. ‘Harusakari’), Arabidopsis thaliana landrace Columbia-O, and Lotus japonicus ecotype Miyakojima MG20 were also used for comparing DNA amplification efficiency. For fine mapping of a mutated gene, F2 plants between a genic male sterile mutant, C204, and a rice line having chromosome nine from ‘Kasalath’ with ‘Nipponbare’ background, CSSL228 (Ebitani et al. 2005), were used. B1F4 plants developed by crossing between ‘Yumemusubi’ and ‘Hitomebore’ (Yumemusubi/Hitomebore//Hitomebore) in a conventional rice crossbreeding program were genotyped using the leaf-punch method and dot-blot-SNP analysis.
Preparation of DNA
Leaves were punched by mini cork borers 1, 1.5, or 2 mm in diameter, and the resulting leaf disks were used directly as PCR templates. Genomic DNA (20 ng for each reaction mixture) prepared by the CTAB method (Murray and Thompson 1980) was used as a control of the PCR template.
DNA amplification
Primers for amplification of 100-, 200-, 300-, 500-, and 1000-bp DNA fragments of plant genomic DNA by PCR were designed (Supplementary Table 1). The reaction mixture of 10 μl consisted of the PCR template, 20 pmol of each primer, 1× PCR buffer, and 2 nmol dNTPs, and 0.5 units of DNA polymerase, i.e., rTaq, ExTaq (TaKaRa Biomedicals, Japan), or KOD-Plus (TOYOBO, Japan). ExTaq and rTaq yielded comparable results. PCR in a 20- or 50-μl reaction mixture was also carried out at the same concentration of each component. The thermal cycle of PCR was set as follows: 1-min denaturation at 94°C, 40 cycles of 30-s denaturation at 94°C, 30-s annealing at 55°C, and 30-s extension at 72°C, and a 1-min final extension at 72°C.
Dot-blot-SNP analysis
Dot-blot-SNP analysis was performed according to Shirasawa et al. (2006). A nucleotide sequence having an SNP was amplified by PCR using leaf disks or genomic DNA as the template. The PCR products were mixed with an equal volume of 0.4 N NaOH containing 10 mM EDTA for denaturation, and dot-blotted onto a nylon membrane by a Multi-pin blotter (ATTO, Japan). The membrane was hybridized with an oligonucleotide probe labeled by digoxigenin or biotin at the 5′ end at an appropriate temperature for each marker (Supplementary Table 1) and washed with washing buffer (0.1× or 1× SSC and 0.1% SDS). Signals of digoxigenin and biotin were detected by Dig-detection Kit (Roche Diagnostics, Switzerland) and streptavidin-alkaline phosphatase conjugate (Promega, USA), respectively.
Results
Efficiency of DNA amplification from a rice leaf disk as a template
Efficiencies of DNA amplification of different-sized DNA fragments by PCR with a 2-mm leaf disk as a template in a 50-μl reaction mixture were compared using young leaves of seven rice cultivars. Nine loci of three different sizes, i.e., 100, 200, and 300 bp, were amplified using 27 primer pairs with two repetitions, and amplified DNAs were detected by the probes for dot-blot-SNP analysis. With most of the primer pairs, the efficiency of amplification and allele-specific signal detection was 100%, 14/14 (Table 1). In the primer pair in which a 200-bp fragment of marker No. 8 was amplified, the efficiency of amplification was lowest, 11/14. Total amplification efficiencies of 100-, 200-, and 300-bp DNA fragments were 98.4, 96.8, and 100%, respectively. There was no tendency for shorter DNA fragments to be amplified more efficiently than longer DNA fragments among 100-, 200-, and 300-bp fragments. However, the efficiencies of amplification of nine 500-bp DNA fragments and nine 1-kb DNA fragments were only 49.2 and 24.6%, respectively. DNA fragments of 1.5 kb could not be amplified with this method.
Leaf disks obtained from young plants at the three-leaf stage and mature ‘Nipponbare’ plants with open flowers were used as PCR templates. Three loci of three different sizes were amplified with six repetitions and detected by the probes for dot-blot-SNP analysis (Table 2). The same analysis was repeated twice. DNAs of 100, 200, and 300 bp were amplified with 100% efficiency, 108/108, from the leaves of both young and mature plants. Leaf disks of immature leaves just after germination were also found to be good PCR templates (data not shown). The efficiency of amplification from leaves frozen in liquid nitrogen was 100%, while that of leaves dried at 50°C was 68.5%.
Since 1 μl of PCR mixture is sufficient for dot-blot-SNP analysis, a smaller volume of the PCR mixture is desirable to economize when genotyping a large number of plants. Different-sized leaf disks, i.e., 1.0, 1.5, and 2.0 mm in diameter, were used as templates in different volumes of the PCR mixture, i.e., 10, 20, and 50 μl (Table 3). DNAs were amplified with high efficiencies from the 1.0- and 1.5-mm leaf disks in all the volumes tested, while no DNA was amplified from the 2.0-mm leaf disks in the 10-μl reaction mixture, suggesting that high concentration of impurities exuded from a large leaf disk inhibited PCR. Thus smaller disks were better as PCR templates.
Multiplex PCR was performed with mixtures of four and eight primer pairs using the leaf disks as templates, and DNA amplification was tested by dot-blot-SNP analysis (Table 4). In multiplex PCR with eight primer pairs, allele-specific signals were detected by the probes of six SNP markers, but not by the other two SNP markers. Signals of these two markers were not detected in multiplex PCR with four primer pairs, either. Furthermore, multiplex PCR using genomic DNA prepared by the CTAB method as a template did not amplify DNA fragments with these two markers, suggesting that the two primer pairs are not suitable for multiplex PCR. Although there were some unsuitable markers, multiplex PCR was found to be useful in terms of labor and cost-efficiency in PCR using leaf disks as templates.
Application of the leaf-punch method to other plant species
The leaf punch method developed using rice was applied to other plant species including model plants and important crops. Three primer pairs amplifying ca. 300-bp fragments were used in PCR for each plant species, and DNA amplification was detected by agarose gel electrophoresis with ethidium bromide staining. DNA fragments were efficiently amplified from 1-mm leaf disks of Arabidopsis thaliana, Lotus japonicus, wheat, Chinese cabbage, and melon, while no DNA could be amplified from soybean and tomato (Table 5).
DNA polymerase was changed to KOD-Plus polymerase for amplification of DNA fragments from leaf disks of soybean and tomato. Although DNA was not amplified by KOD-Plus polymerase with a standard buffer solution for KOD-Plus, the addition of DMSO (5%) and Triton X-100 (0.02%) into the reaction mixture enabled amplification of DNA from the leaf disks of these plants.
Selection of recombinants for fine mapping of a mutated gene in rice
A mutated gene of a genic male-sterile (GMS) mutant C204 in rice was mapped between SCAR9 and RM7343 on chromosome nine in our preliminary study. For fine mapping of the MS gene, a search for SNPs near these markers was carried out using published SNP data (Nasu et al. 2002; Monna et al. 2006), and two dot-blot-SNP markers, i.e., dsG9-1 and dsG9-2, were designed (Supplementary Table 1). Genotypes of 1,056 F2 progeny between C204 and a chromosome substitution line having ‘Kasalath’ genome in chromosome nine with ‘Nipponbare’ background, CSSL228 (Ebitani et al. 2005), were analyzed using the leaf-punch method with these two dot-blot-SNP markers. The efficiency of DNA amplification of dsG9-1 was 80%, and that of dsG9-2 was 88%. Eighty-three recombinants between these two SNP markers were obtained, and we are presently growing these recombinants to investigate their pollen fertility for further localization of the GMS gene.
Genotyping of rice plants in a crossbreeding program
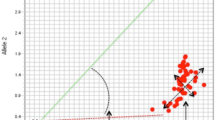
Leaves of B1F4 plants of Yumemusubi/Hitomebore//Hitomebore were punched by 1-mm mini cork borers and used as PCR templates. Genotypes of 381 B1F4 plants at ten loci were analyzed by PCR and dot-blot-SNP analysis using the leaf disks as the templates (Fig. 1). Homozygotes of the ‘Yumemusubi’ allele, homozygotes of the ‘Hitomebore’ allele, and their heterozygotes at the locus of E20943 were 89, 248, and 11, respectively. Signals of 33 plants were not detected. By the probes for the locus of C30021, signals were detected in 357 plants, but not in 24 plants. Total efficiency of signal detection of SNP markers at the ten loci was 90.5%.
Genotyping of rice breeding lines using dot-blot-SNP analysis of DNAs amplified from leaf disks by PCR. The PCR products were dot-blotted onto three nylon membranes (a–c) and analyzed by the marker E20943. Signals were detected by a ‘Hitomebore’-type probe (a), a ‘Yumemusubi’-type probe (b), and a mixture of both the probes (c). H ‘Hitomebore’ DNA; Y ‘Yumemusubi’ DNA; and HY a DNA mixture of ‘Hitomebore’ and ‘Yumemusubi’
Discussion
A bacterial colony can be used directly as a PCR template for testing DNA insertion into plasmids possessed by a bacterial clone (Sandhu et al. 1989). In the present study, we found that a small piece, 1–1.5 mm in diameter, cut out from a rice leaf by a minicork borer can also be directly used as a template of PCR. A similar observation has been reported by Berthomieu and Meyer (1991), but efficiencies of amplification have not been shown. They used pieces of leaves and roots of transgenic plants obtained by co-cultivation with Agrobacterium as the templates of PCR, and detected transgenes. However, PCR using DNAs of T0 plants as templates sometimes amplifies sequences in Agrobacterium attached to the surface of T0 plants. This technique has long been ignored. We found that small leaf disks of not only rice but also of other plant species, e.g., Arabidopsis, Lotus, wheat, soybean, tomato, Chinese cabbage, and melon, are good PCR templates for amplification of single copy DNAs in their genomes. Mature and immature leaves and even frozen or dried leaves can be used as materials. These results suggest a wide applicability of the leaf-punch method to DNA polymorphism analysis of plants. The leaf-punch method is considered to be the simplest and the most cost-effective technique for preparation of PCR templates from plants.
Although the efficiency of amplification of long DNA fragments, more than 500 bp, was low, DNA fragments from 100 to 300 bp, which are sufficient for analysis of SNPs by the dot-blot-SNP technique, were efficiently amplified, more than 95% in the tests using a small number of plants and 80–90% in those using a large number of plants. This difference of the efficiencies between the tests using a small number and a large number of plants is inferred to be due to the difference of time required for sampling. In our experience, rapid handling of leaf samples was important for successful amplification. Since DNA amplification from leaf disks is not always successful, genotyping by random amplified polymorphic DNA (RAPD) or dominant sequence characterized amplified region (SCAR) markers relying on the presence or absence of amplified DNA fragments is less reliable. In dot-blot-SNP analysis, PCR products without amplified DNA are not detected by either probe for the wild-type allele nor for the mutant allele, and therefore the plants whose DNA was not amplified can be genotyped by retrial without error. Furthermore, carrying over of a trace of DNA does not interfere with genotyping in dot-blot-SNP analysis because the relative amounts of DNA fragments of two genotypes are compared by this method. At first we washed the minicork borer carefully with detergent and water after punching a leaf of each plant, but we later omitted such washing and yet achieved successful genotyping of every plant. Dot-blot-SNP analysis is one of the most appropriate techniques for genotyping of plants using the leaf-punch method.
A combination of the leaf-punch method and the dot-blot-SNP technique can be used as a method for fine mapping of genes with SNP markers. For fine mapping of genes, DNA polymorphism analysis of closely linked DNA markers in a large number, more than 1,000, of plants is necessary (Konishi et al. 2006; Murakami et al. 2006; Zhang et al. 2006). In the studies of Konishi et al. (2006) using rice and Murakami et al. (2006) using Lotus japonicus, more than 10,000 plants were genotyped for gene mapping. We have presented an example of genotyping of 1,056 plants using linked SNP markers for the selection of recombinants to perform fine mapping of a mutated gene in this paper. The obtained recombinants between the two SNP markers are important materials for further localization of the mutated gene. Selection of recombinants between two closely linked SNPs may help elucidation of the nucleotide change responsible for the difference of a phenotype among several nucleotide changes in a sequenced region containing the gene controlling the phenotype. Combining different point mutations in a gene by intragenic recombination can produce a new allele, and such new alleles may contribute to the understanding of important domains of proteins encoded by the gene and may be used for production of plants with a new genetic trait.
Investigation of the frequency of an allele and its dynamics in a population is important for understanding of adaptability of each allele and the evolution of genetic traits controlled by this gene. The frequencies of a cytoplasmic male-sterility gene and its nuclear restorer gene in natural populations of Raphanus sativus have been investigated to understand how gynodioecy is maintained (Maruyama et al. 2004). The frequency of a mutated allele of a gene responsible for domestication in old and new cultivars in many cultivated areas and their wild ancestors may give insight into the process of domestication by humans. Selective sweep of a single mutation in the Rc gene determining red pericarp in rice seeds has been observed (Sweeney et al. 2007). Such studies will be accelerated by using the leaf-punch method and dot-blot-SNP technique.
The leaf-punch method together with the dot-blot-SNP technique enables efficient and reliable plant breeding by marker-assisted selection (MAS). In MAS, DNA markers closely linked to the genes controlling important traits as breeding objectives, such as disease resistance and tolerance to environmental stresses, can be used as selection markers (Tuberosa et al. 2002; Liu et al. 2006; Steele et al. 2006). However, use of the linked DNA markers may bring about error in selection, which is caused by recombination between the DNA marker and the target gene for selection. The target gene located between two closely linked DNA markers can be selected without error, but other genes linked between these two markers, some of which may have undesirable functions, will always accompany the target gene. Direct selection of a target gene by SNP analysis, termed DNA-selection breeding (Shirasawa et al. 2004), will be a more promising breeding technique than MAS using linked DNA markers. The dot-blot-SNP technique has been applied to the selection of genotypes of wx and sd1 genes in rice (Shirasawa et al. 2006), but DNA has been prepared with the method of Wang et al. (1993), in which DNA is extracted with NaOH solution and the DNA extract is diluted with buffer solution. In the present study, the leaf-punch method and the dot-blot-SNP technique were successfully used for genotyping of plants in a conventional rice breeding program.
The combination of these two techniques is also useful for seed purity testing. In a genebank maintaining plant germplasms, propagation and renewal of seeds are performed by growing many different lines of plants in the same season. Cross-contamination of seeds or pollen grains sometimes occurs. Therefore, seed purity testing is indispensable in the plant germplasm project. For marketing F1 hybrid seeds, seed purity testing is necessary to check contamination of self-pollinated seeds in the F1 seeds (Nishio et al. 1994). Evaluation of seed contamination requires analysis of genotypes of a large number of seeds. Immature leaves of young seedlings obtained in germination tests can be used as materials.
References
Berthomieu P, Meyer C (1991) Direct amplification of plant genomic DNA from leaf and root pieces using PCR. Plant Mol Biol 17:55–557. doi:10.1007/BF00040656
Bittencourt JVM, Sebbenn AM (2007) Patterns of pollen and seed dispersal in a small, fragmented population of the wind-pollinated tree Araucaria angustifolia in southern Brazil. Heredity 99:580–591. doi:10.1038/sj.hdy.6801019
Ebitani T, Takeuchi Y, Nonoue Y, Yamamoto T, Takeuchi K, Yano M (2005) Construction and evaluation of chromosome segment substitution lines carrying overlapping chromosome segments of indica rice cultivar ‘Kasalath’ in a genetic background of japonica elite cultivar ‘Koshihikari’. Breed Sci 55:65–73. doi:10.1270/jsbbs.55.65
Edwards K, Johnstone C, Thompson C (1991) A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res 19:1349. doi:10.1093/nar/19.6.1349
Hanson TR, Brunsfeld SJ, Finegan B, Waits LP (2008) Pollen dispersal and genetic structure of the tropical tree Dipteryx panamensis in a fragmented Costa Rican landscape. Mol Ecol 17:2060–2073. doi:10.1111/j.1365-294X.2008.03726.x
Konishi S, Izawa T, Lin SY, Ebana K, Fukuta Y, Sasaki T, Yano M (2006) An SNP caused loss of seed shattering during rice domestication. Science 312:1392–1396. doi:10.1126/science.1126410
Liu S, Zhang X, Pumphrey MO, Stack RO, Gill BS, Anderson JA (2006) Complex microcolinearity among wheat, rice, and barley revealed by fine mapping of the genomic region harboring a major QTL for resistance to Fusarium head blight in wheat. Funct Integr Genomics 6:83–89. doi:10.1007/s10142-005-0007-y
Maruyama K, Yahara T, Terachi T (2004) Variation of female frequency and cytoplasmic male sterility gene frequency among natural gynodioecious populations of wild radish (Raphanus sativus L.). Mol Ecol 13:2459–2464. doi:10.1111/j.1365-294X.2004.02231.x
Monna L, Ohta R, Masuda H, Koike A, Minobe Y (2006) Genome-wide searching of single-nucleotide polymorphisms (Oryza sativa L.) and a wild accession (Oryza rufipogon Griff.). DNA Res 13:43–51. doi:10.1093/dnares/dsi030
Moscoso H, Raybon EO, Thayer SG, Hofacre CL (2005) Molecular detection and serotyping of infectious bronchitis virus from FTA filter paper. Avian Dis 49:24–29. doi:10.1637/7220
Murakami Y, Miwa H, Imaizumi-Anraku H, Kouchi H, Downie JA, Kawaguchi M, Kawasaki S (2006) Positional cloning identifies Lotus japonius NSP2, a putative transcription factor of the GRAS family, required for NIN and ENOD40 gene expression in nodule initiation. DNA Res 13:255–265. doi:10.1093/dnares/dsl017
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4325. doi:10.1093/nar/8.19.4321
Nasu S, Suzuki J, Ohta R, Hasegawa K, Yui R, Kitazawa N, Monna L, Minobe Y (2002) Search for and analysis of single nucleotide polymorphisms (SNPs) in rice (Oryza sativa, Oryza rufipogon) and establishment of SNP markers. DNA Res 9:163–171. doi:10.1093/dnares/9.5.163
Nishio T, Sakamoto K, Yamaguchi J (1994) PCR-RFLP of S locus for identification of breeding lines in cruciferous vegetables. Plant Cell Rep 13:546–550. doi:10.1007/BF00234508
Olmos A, Dasí MA, Candresse T, Cambra M (1996) Print-capture PCR: a simple and highly sensitive method for the detection of Plum pox virus (PPV) in plant tissues. Nucleic Acids Res 24:2192–2193. doi:10.1093/nar/24.11.2192
Romero-Durbán J, Cambra M, Duran-Vila N (1995) A simple imprint-hybridization method for detection of viroids. J Virol Methods 55:37–47. doi:10.1016/0166-0934(95)00043-T
Roy Y, Nassuth A (2005) Detection of plant genes, gene expression and viral RNA from tissue prints on FTA® cards. Plant Mol Biol Rep 23:383–395. doi:10.1007/BF02788886
Sandhu GS, Precup JW, Kline BC (1989) Rapid one-step characterization of recombinant vectors by direct analysis of transformed Escherichia coli colonies. Biotechniques 7:689–690
Shirasawa K, Monna L, Kishitani S, Nishio T (2004) Single nucleotide polymorphisms in randomly selected genes among japonica rice (Oryza sativa L.) varieties identified by PCR-RF-SSCP. DNA Res 11:275–283. doi:10.1093/dnares/11.4.275
Shirasawa K, Shiokai S, Yamaguchi M, Kishitani S, Nishio T (2006) Dot-blot-SNP analysis for practical plant breeding and cultivar identification in rice. Theor Appl Genet 113:147–155. doi:10.1007/s00122-006-0281-7
Steele KA, Price AH, Shashidhar HE, Witcombe JR (2006) Marker-assisted selection to introgress rice QTLs controlling root traits into an Indian upland rice variety. Theor Appl Genet 112:208–221. doi:10.1007/s00122-005-0110-4
Sweeney MT, Thomson MJ, Cho YG, Park JP, Williamson SH, Bustamante CD, McCouch SR (2007) Global dissemination of a single mutation conferring white pericarp in rice. PLoS Genet 3:e133. doi:10.1371/journal.pgen.0030133
Tuberosa R, Salvi S, Sanguineti MC, Landi P, MacCaferri M, Conti S (2002) Mapping QTLs regulating morpho-physiological traits and yield: case studies, shortcomings and perspectives in drought-stressed maize. Ann Bot (Lond) 89:941–963. doi:10.1093/aob/mcf134
Wang H, Qi M, Culter AJ (1993) A simple method of preparing plant samples for PCR. Nucleic Acids Res 21:4153–4154. doi:10.1093/nar/21.17.4153
Xu X, Kawasaki S, Fujimura T, Wang C (2005) A protocol for high-throughput extraction of DNA from rice leaves. Plant Mol Biol Rep 23:291–295. doi:10.1007/BF02772759
Zhang Z, Ober JA, Kliebenstein DJ (2006) The gene controlling the quantitative trait locus EPITHIOSPECIFIER MODIFIER1 alters glucosinolate hydrolysis and insect resistance in Arabidopsis. Plant Cell 8:1524–1536. doi:10.1105/tpc.105.039602
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research (A) (no. 19208001) from the Japan Society for the promotion of science (JSPS). CSSL228 was provided by Rice Genome Resource Center, Japan. S. Shiokai is a recipient of a research fellowship from the Japan Society for the promotion of science for young Scientists.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shiokai, S., Kitashiba, H., Shirasawa, K. et al. Leaf-punch method to prepare a large number of PCR templates from plants for SNP analysis. Mol Breeding 23, 329–336 (2009). https://doi.org/10.1007/s11032-008-9244-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11032-008-9244-9