Abstract
Emotional interference on behavior is commonly observed when task-irrelevant negative stimuli appear before behavioral targets. One explanation postulates that affect-laden stimuli readily capture attention, interfering with the processing of the upcoming target. Emotional stimuli might also preactivate motor programs incompatible with the demanded response. Using a cued go/no-go procedure we showed that task-irrelevant unpleasant stimuli cause interference or facilitation depending on their onset asynchrony relative to the target. We observed interference with short (200 ms) stimulus-target asynchronies and facilitation for longer ones (600 ms), both for key press (Experiment 1) and key release (Experiment 2) responses. The interference effect is compatible with an attentional explanation, but the behavioral facilitation is hard to accommodate within either attentional or motor accounts. This interference-facilitation pattern can be explained assuming that once the attentional effect subsides, emotional processing may enhance the perceptual processing of the stimuli, or lower the decision threshold, thereby facilitating the response selection process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mutual influences between cognitive and emotional processing have been the subject of a recent surge of scientific interest (Dolan 2002; Zald et al. 2002). Studies on the emotional modulation of cognitive processes frequently adapt classical behavioral tasks by including some sort of emotional manipulation. Usually, the targets in the behavioral task are simply replaced by affect-laden stimuli (words or images of positive or negative valence). Many experimental studies, thus, introduce emotion just as the target of discrimination in a discrimination task: e.g., the participant has to decide whether a word shown on a screen has negative or positive affective value. In those experiments, there is no explicit aim to change the emotional state of the participant, i.e., to make the participant feel anything. In other words, the emotional value is treated rather as an attribute of the discriminandum than as a state of the subject. In such circumstances, it is hard to tell whether the effects observed are attributable to emotional processing or to the fact that the discrimination task is a peculiar one (Goldstein et al. 2007). Only a few studies have addressed the question of how emotional content affects behavior when it is incidental to the task at hand. To make emotional processing incidental to the task, some of them have instructed participants to discriminate low level features (such as typeface) of emotion-laden words (Goldstein et al. 2007). Others have showed the participants task-irrelevant emotional stimuli (Pereira et al. 2004, 2006; Phelps et al. 2006; Bocanegra and Zeelenberg 2009; Becker 2009). The second strategy mimics circumstances encountered in a great number of situations in everyday life (i.e.: driving after having watched a road accident) (Megías et al. 2011a, b).
Using an implicit emotional task, Pereira et al. (2004) found that visual detection times were slower after watching negatively-valenced task-irrelevant images than after positive or neutral ones (the targets themselves being emotionally neutral). They attributed this effect to the interference caused by the motor program triggered by the emotional stimuli (avoidance response for negative pictures) on the response demanded by the task (keystroke, that they considered an approach response). Using a similar procedure, Pereira et al. (2006) have observed both transient and sustained emotional modulation of visual detection of targets after watching task-irrelevant mutilation pictures. The sustained effect lingered several seconds after image offset, and was apparent when the pictures were blocked according to their emotional value. Conversely, when the emotional value of the pictures was randomized in the temporal sequence, only transient effects—affecting just the first trial after image offset—were observed. The authors believed that the former effect depended on the link between the induced emotional state and approach/withdrawal processes, so that watching mutilation images engaged the subjects in avoidance behaviors incompatible with the approach response demanded by the task (keystroke). An alternative explanation for the sustained effect suggested in that study was based on the idea that affect-laden stimuli may produce different degrees of freezing. A freezing response is incompatible with any other response, and, therefore, the same size of effect should be observed either for approach or withdrawal responses. For the transient effect, however, these authors favored an attentional account: unpleasant pictures reduced the available processing resources as they have a greater capability for capturing attention.
Buodo et al. (2002) found transient emotional interference effects in an auditory discrimination task. In their experiment, a task-irrelevant image was shown in each trial for 6 s, with its onset 1 or 4 s before the target sound was played. They observed that erotic and mutilation images caused an increase in discrimination reaction times when the pictures appeared 1 s before the target, but not when they appeared 4 s before. Similarly, using a rapid serial visual presentation procedure, Most et al. (2005) have also observed that target detection accuracy was lower after emotional pictures relative to non-emotional ones when the SOA was 200 ms, but there were no differences when the SOA was 800 ms.
These remarkable results show that the way task-irrelevant emotional stimuli interfere with the response to the target may depend on the picture-target onset asynchrony (SOA). As mentioned, emotional interference has been attributed to the allocation of attentional resources to the emotional stimuli or, alternatively, to the preactivation of motor processes by them. An attentional account predicts that the emotional interference will fade shortly after attention is disengaged from the emotional stimuli. Since the attentional dwell time estimated from visual search experiments is believed to be around 250 ms (Theeuwes et al. 2004), it is reasonable to expect that attention should be away from the emotional stimuli at around 500–800 ms (Pereira et al. 2006), even if it is assumed that disengaging attention from emotional stimuli takes longer than disengaging it from neutral ones. On the other hand, the motor hypothesis predicts that response facilitation or response interference will be observed depending on whether the motor response demanded by the task (e.g.: keystroke) is compatible or incompatible with the motor response presumably preactivated by the emotional stimulus (e.g.: withdrawal). An integration of these two accounts predicts maximal interference when the demanded response is incompatible with the one preactivated by a task-irrelevant emotional stimulus and the emotional stimulus is shown shortly before the target (attention is still engaged in the emotional stimuli and the preactivated motor response must be inhibited). If the response demanded is incompatible with the emotional stimulus valence but the emotional stimulus is shown long before the target, only motor interference should occur, and the observed effect should be weaker.
In our study, we used a cued emotional go/no-go task to specifically investigate the emotional modulation of behavioral responses by the visualization of task-irrelevant emotional stimuli shown at different SOAs before the target. Originally introduced by Donders (1868/1969), the go/no-go task requires the participant to discriminate between two types of stimuli presented in random sequence—go and no-go targets—and respond only to the former type. In a typical experiment, go targets are more frequent than no-go ones, and this is assumed to create a predisposition to respond in every trial, which has to be overcome in the rare cases when a no-go target appears. When go and no-go emotionally charged stimuli are used, it is possible to measure the modulation of performance by emotional processing. Differences between the reaction times for neutral and emotional go targets have been interpreted as reflecting an emotional bias on the tendency to approach or avoid the target (Hare et al. 2005). Differences in error rates for emotional and neutral no-go targets are commonly interpreted as the result of the emotional modulation of the ability to inhibit motor responses (Schultz et al. 2007). In our experiments, targets were, however, emotionally neutral. The emotionally-charged stimuli were instead task-irrelevant pictures shown before target onset.
In a cued go/no-go procedure, a warning signal indicates the probable identity of the upcoming target. Anticipatory activity throughout the foreperiod—the interval between the warning cue and the target—leads to faster responses to the target (Niemi and Näätänen 1981). It has been shown that warning signals trigger motor activation (as measured by electromyography) in an automatic way and that the probability of commission errors depends on the strength of this activation (Boulinguez et al. 2008, see also Nobre et al. 2007). In our experiments, a predictive cue was shown before the emotional picture in each trial. Thus, two forces could contribute independently to create a proneness to respond in our experiments: the predictive cue, and—according to the motor preactivation hypothesis—the emotional picture, depending on its valence. We also manipulated the onset asynchrony between the emotional image and the target that followed it, providing enough time for the disengagement of attention from the emotional stimuli in some experimental conditions but not in others. Moreover, we manipulated the cue-target onset asynchrony, to explore the effect on the response of the interval between the cue and the task-irrelevant emotional stimuli. Lastly, the response demands were manipulated between experiments, requiring from the participant two types of responses: key strokes (Experiment 1) and key releases (Experiment 2). If visualizing emotional pictures causes the activation of specific motor programs, a change in the demanded response might alter or even reverse the effect of the emotional pictures on reaction time. We also expected a tendency towards interference when the emotional picture was shown shortly before the target (for delays within the attentional dwell time), but not when this interval was long enough to allow attention to disengage from the picture.
Experiment 1
In this experiment we investigated the effect of the incidental visualization of emotional pictures on the speed and accuracy of keystroke responses in a cued go/no-go task. A bias to respond or withhold motor responses was elicited by a highly predictive cue, which was displayed 800 or 400 ms before the target. Task-irrelevant emotional distractors (pictures) were presented at two different time lags (200 or 600 ms before the target) within the cue-target interval in order to affect the ongoing mental activity (attention engagement and motor pre-activation) elicited by the cue. The shorter picture-target interval (200 ms) occurred for trials with cue-target asynchrony of 400 or 800 ms whereas the longer picture-target interval (600 ms) only happened in trials with 800 ms cue-target asynchrony. A no-picture condition served as the baseline for measuring the interference produced by the distractor pictures.
Method
Participants
Twenty (2 male) undergraduate students at the Faculty of Psychology of the University of Granada (Spain) ranging in age from 18 to 25 (mean 20.8) took part in this study, in exchange for course credit.
Apparatus and stimuli
The stimuli were displayed on a 15-inch LCD monitor, at 1,024 × 768 pixels resolution, 32-bit color depth. Refresh rate was 60 Hz (6 frames ≈ 100 ms). The task was coded in Java using PXLab library for psychophysical experiments (Irtel 2006). The predictive cue was a white bar, either vertical (100 × 300 pixels, which was predictive of a go target) or horizontal (300 × 100 pixels, predicting a no-go target), shown at the screen center. The target was a bar whose size, location, and orientation were identical to those of the cue presented before, but colored in blue (go target) or green (no-go target). Affective pictures belonging to four categories were selected from the International Affective Picture System (IAPS) (Lang et al. 2005): mutilation (average valence: 1.32, max = 1.42, min = 1.18, average arousal: 7.55), babies and children (average valence: 8.34, max = 8.43, min = 8.18, average arousal: 3.76), neutral common objects (a towel, a stool, a cabinet, a plate, average valence = 5.25, max = 5.69, min = 4.7, average arousal = 2.43), and erotic (couples, average valence = 7.21, max = 7.42, min = 7.06, average arousal = 6.67). Four images were selected for each of the four categories. Valence and arousal values are taken from the Spanish female population tables (Moltó et al. 1999). The original IAPS pictures were scaled down so that their height was 384 pixels or their width was 512 pixels, whichever was more restrictive, but maintaining their original aspect ratio. All visual stimuli were shown over a black background. Participant responses were recorded using a standard PC keyboard.
Design and procedure
All participants gave written consent to their participation before the beginning of the experiment, after being warned that the task involved briefly watching some images that could be deemed offensive, and being also reminded of their right to withdraw their participation without giving up any of the benefits derived from it. Then the instructions asked them to press the space bar as fast as they could whenever they saw a blue bar and to refrain to respond when they saw a green bar.
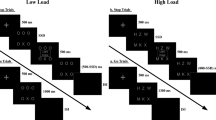
Each trial started with a fixation point (a 50 × 50 pixel cross on the screen center) that lasted for 800 ms, immediately followed by a blank screen for 500 ms (see Fig. 1). The predictive cue (either a vertical or a horizontal white bar) was then shown for 100 ms. Half of the trials had a go (vertical) cue, and the other half a no-go (horizontal) cue. Go cues were followed by a go target (blue) in most cases (80%), and by no-go targets in the rest of the trials (20%). Likewise, a no-go cue was followed by a no-go target in 80% of the occasions, and a go target in the remaining 20%. The stimulus onset asynchrony (SOA) between cue and target was 400 ms in one third of the trials and 800 ms in the rest. The target remained on-screen for a maximum of 1,000 ms or until the participant responded. After the offset of the target a message was shown on the center of the screen for 700 ms with the word “Correcto” (the Spanish word for “correct”) and, for go trials, the reaction time in ms below it, or the word “Fallaste!” (“You failed!”).
On 4 out of every 5 trials an affective image from IAPS (Lang et al. 2005) was shown between cue and target for 100 ms. The image belonged to one of the four aforementioned categories (mutilation, babies, categories, neutral). On the remaining trials, no image was shown. We manipulated the time onset of the image in order to explore the temporal course of the affective interference. When the cue-target SOA was short (SOA400 condition), the target onset was 200 ms after the image onset. However, when the cue-target SOA was long, the image onset could be delayed either 200 ms (SOA800-early) or 600 ms (SOA800-late) from cue onset.
There were 5 affective (mutilation, babies, erotic, neutral and no-picture) × 3 timing (SOA400, SOA800-early, SOA800-late) conditions. Each affective × timing condition comprised 40 trials, 20 with go target and 20 with no-go target. On 80% of the trials, the cue was congruent with the target. Therefore, of the 40 trials in each condition, 16 were congruent go trials, 16 were congruent no-go trials, 4 were incongruent go trials, and the remaining 4 were incongruent no-go trials. As there were only 4 incongruent go and 4 incongruent no-go trials in each affective × timing condition, each affective picture category (babies, mutilations, erotic, neutral) contained just four pictures, so that every picture was shown at least once in each kind of trial. This meant that each image was shown 10 times in each affective × timing condition, 8 of them in congruent trials (four go, four no-go), and 2 in incongruent trials (one go, one no-go), totalling 30 repetitions in the whole task (ten in each of the three timing conditions with pictures, namely SOA400, SOA800-early, SOA800-late). Trials were blocked according to timing condition, giving rise to three blocks for conditions with task-irrelevant image (SOA800-early, SOA800-late and SOA400), and two blocks for conditions without picture, one with long (800 ms) and another with short (400 ms) SOA. Within each block, the order of the trials and images was randomized for each participant. The blocks without pictures had a total of 40 trials. The blocks with pictures contained 40 trials of each affective condition, totalling 160 trials per block. The total number of trials was 560.
The experiment was carried out according to one of the following sequences of blocks: 1) SOA400 without pictures, SOA400 with pictures, SOA800 without pictures, SOA800-late (or SOA800-early), SOA800-early (or SOA800-late); 2) SOA800 without pictures, SOA800-late (or SOA800-early), SOA800-early (or SOA800-late), SOA400 without pictures, SOA400 with pictures. Blocks of trials with the same SOA were always placed together, and blocks without pictures came always before blocks with pictures. These sequences were chosen so that the sequential effects caused by changes of SOA were minimal in the blocks with images, since they always came after one block with identical SOA. The sequence for each participant was randomly selected.
The interval between the participant response and the beginning of the following trial was adjusted to compensate for the variations in RT, so that a constant interval of 2,400 ms elapsed between the onset of the target on one trial and the appearance of the fixation cross on the following trial. In this way the whole trial lasted always either 4.1 or 4.5 s (depending on whether the cue-target SOA was 400 or 800 ms). There was a 1-min break after each two consecutive blocks, making the duration of the whole experiment about 50 min.
Data analysis
Median RTs for correct go trials were computed for each participant and condition. Commission error rates were computed for no-go trials in each condition. Averages across subjects of the medians of reactions times and error percentages are displayed in Table 1. Data analysis was organized in two sets of repeated-measures ANOVAs. In the first set we explored differences between the conditions without emotional pictures (Baseline analysis), while in the second one we examined the differences between the conditions with pictures (Emotion analysis). In the baseline analysis, for the no-picture conditions, we performed a 2 (SOA: 400 and 800 ms) × 2 (Cue-target congruence: congruent and incongruent) repeated-measures ANOVA to explore the differences in reaction time and commission errors. For the Emotion analysis, median reaction times for the conditions with pictures were submitted to a repeated-measures ANOVA with timing condition (SOA400, SOA800-early and SOA800-late), affective content of the images (babies, erotic, mutilations, and neutral) and cue-target congruence (congruent and incongruent) as within-subject factors. The percentages of commission errors for no-go trials were submitted to a repeated-measures ANOVA with timing condition (SOA800-late, SOA800-early and SOA400), affective content (babies, erotic, neutral, and mutilation), and cue-target congruence (congruent, incongruent) as within-subject factors. To further explore significant effects we first checked the differences between SOA400 and SOA800-late, as they shared the same short picture-target onset asynchrony (200 ms). These two conditions were then pooled together if no differences were observed. Accordingly, we will use the term “short asynchrony” for the pool SOA400-SOA800-late and “long asynchrony” for SOA800-early. Finally, we performed post-hoc LSD comparisons. A significance level of 0.05 was set up for all statistical decisions.
Results and discussion
Go trials
Omission errors occurred in only 0.1% of the go trials, so no attempt to analyze omission errors was made. The baseline analysis for reaction times revealed significant main effects of cue-target SOA, F (1, 19) = 6.184, p = .022, and cue-target congruence, F (1, 19) = 10.68, p = .004, but no interaction. Responses were slower for the long (800 ms) than for the short (400 ms) SOA (319 and 301 ms, respectively). Also, responses were faster for the cue-target-congruent condition than for the incongruent condition (301 and 318 ms, respectively), indicating that the participants used the cue for preparing their response to the target.
Emotion analysis (see Fig. 2). The ANOVA showed significant main effects of affective content, F (3, 57) = 5.04, p = .003, R 2 = .21, and timing condition, F (2, 38) = 15.91, p < .001, R 2 = .46, and a significant interaction between affective content and timing, F (6, 114) = 3.09, p = .007, R 2 = .14. We also observed a significant effect of congruence, F (1, 19) = 8.07, p = .01, R 2 = .29. No other effects were significant.
Interference caused by pictures of different affective value for each picture-target asynchrony (short: 200 ms, long: 600 ms). Vertical dimension represents the difference between the RT in the neutral condition and the RT in each affective condition. Positive values indicate interference and negative ones facilitation. Vertical bars represent the standard error of the difference. The left panel represents the results of Experiment 1 (demanded response: key press), and the right panel, Experiment 2 (demanded response: key release). The pattern of interference and facilitation is similar in both experiments. Significant differences are indicated using * for p < .05 and ** for p < .01. An asterisk above or below of a bar indicates a significant difference between the RTs to that type of pictures and to neutral ones, while over a bracket, it indicates a significant difference between the two conditions connected by the bracket
SOA800-late and SOA400 conditions showed no interaction with affective content either in median RTs or in errors, both F (3, 57) < 1, so they were pooled together to obtain the short asynchrony reaction times. Post-hoc LSD analyses of the affective content by timing interaction revealed than in the short asynchrony conditions, reaction times for mutilation pictures were slower than those in the remaining conditions, all p’s < .035, t(19) > 2.28, and reactions after babies pictures were faster than for all other pictures, all p’s < .012, t(19) > 2.80, but erotic pictures did not differ from neutral ones, p > .30. In stark contrast, in the long asynchrony condition reaction times for mutilation pictures were faster than for neutral ones, p = .044, t(19) = 2.16, but not different from those for babies or erotic pictures, both p’s > .20. Moreover, reaction times for positive pictures (either erotic or babies) did not differ from those for neutral ones (p’s > .28) or between them (p = .81). On the other hand, reaction times in the long asynchrony conditions were faster than those in the short asynchrony ones for all emotional contents, all p’s < .01, t(19) > 2.89. Interestingly, the difference between the short and the long asynchronies was larger for mutilation than for all other pictures, all p’s < 0.02, t(19) > 2.59.
In summary, relative to the neutral condition mutilation pictures interfered with the response if the picture preceded the target by 200 ms (short asynchrony), but facilitated the same response when the picture came 600 ms before the onset of the target (long asynchrony). Babies pictures facilitated the response for the short picture-target asynchrony, but caused no effect for the long asynchrony. Erotic pictures did not differ from the neutral ones. These results support the idea that mutilations have qualitatively different effects in these two time lags rather than one transient effect that fades in less than 600 ms. To further characterize those two separate effects, it is important to complement the measures of reaction speed with those of decision errors. Decision errors on go trials cannot be observed directly, and their best available estimate are response error rates on no-go trials. The estimate is reliable if we assume the decision process is symmetric for both types of target (go and no-go), despite the fact that the motor responses are not (Gómez et al. 2007).
No-go trials
Commission errors occurred on 9.2% of no-go trials. No differences were observed in the baseline analysis. Emotion analysis showed only main effects of timing, F (2, 38) = 5.89, p = .006, R 2 = .19. Error rates were higher in the short asynchrony conditions (SOA800-late, 10.50, and SOA400, 12.94) than in the long asynchrony one (SOA800-early, 4.87), which indicates that errors depended on the picture-target interval, but not on the affective content.
These results indicate that mutilation pictures shown near the target onset may interfere with motor responses. Crucially, response facilitation, rather than interference, was observed when mutilation pictures appeared 600 ms before the target. This last result is in apparent contrast with Pereira et al. (2006) results showing that interference effects lasted for 500–700 ms. However given that their pictures were displayed for 500 and 2,000 ms, it is difficult to compare both results. At any rate, Pereira et al. (2006) results agree with ours in that, even with longer viewing times, the effect of mutilation pictures fades very fast. Thus, briefly watching mutilation pictures had two opposite effects on the speed of response, depending on the picture-target asynchrony. The result observed in the short asynchrony conditions is consistent both with the attentional and the motor preactivation hypothesis. However, the facilitation effect for the longer asynchrony is problematic both to the attentional and the motor pre-activation hypotheses since they predict interference or no effect when mutilation pictures are displayed some time (600 ms) before the target. Neither the reduction in attentional resources nor the preactivation of withdrawal response tendencies (as expected for mutilation pictures) explains the speed-up of responses observed in that condition, unless we assume that watching a mutilation picture preactivates the response of pressing a key. Testing this last possibility was the purpose of Experiment 2, in which we asked the participants to release the key instead of pressing it.
In the general discussion we put forward two possible explanations for this late facilitation of responses by mutilation pictures, in terms of perceptual enhancement (Pessoa 2008; Phelps et al. 2006; Bocanegra and Zeelenberg 2009; Becker 2009) or a relaxation of the decision criterion (Simen et al. 2006).
Experiment 2
In this experiment we changed the response demands to further discriminate the motor preactivation and the attentional accounts. Several studies have reported modulations of reaction time attributable to the emotional content of the target stimuli in speeded reaction tasks, that could be reversed by changing the motor response demanded from the participant. For instance, Solarz (1960) asked students to evaluate affect-laden words as positive or negative by pulling or pushing a lever, and he found they were faster to do the evaluation if the response mapping was pulling-positive and pushing-negative than when the reverse mapping was used. Chen and Bargh (1999) replicated Solarz (1960) results, and extended them to a simple detection task, where participants had to always pull (or always push) a lever as fast as possible whenever a stimulus (word) appeared. They found the participants to be faster to react to positive stimuli if the response demanded was pulling but faster to react to negative ones if the response demanded was pushing, which suggests that the crucial aspect is the congruence between the stimulus valence and the motor action, rather than the correspondence between each action and its meaning for the task (the response mapping).
Duckworth et al. (2002) obtained the same results using completely novel visual stimuli—created specifically for the experiment—showing that the motor effect of pleasant or unpleasant stimulus does not require a previous learning of the association between the stimulus and a motor response. The actual motor response, however, may be selected by the brain by virtue of a previously learned arbitrary association between valence and response, and this has been supported by the literature (e.g., McCall et al. 2011).
Wentura et al. (2000) found effects analogous to those just described in a task in which the participants were demanded a much simpler response (either pressing or releasing a key): the reaction was faster when subjects had to press a key in response to a positive word, and also when they had to release the key in response to a negative one. In all the studies mentioned so far, the affect-laden stimulus was the target, but, as mentioned in the introduction, other studies have recorded effects on reaction times of valenced stimuli that were incidental to the experimental task, and at least some of them attributed these effects to the activation of motor programs by those stimuli (Pereira et al. 2004, 2006). On the other hand, general links between affective states (fear, anger) and action tendencies (freezing, flight, attack) have been widely documented across species in the ethological and neurophysiological literature (Blanchard and Blanchard 1988; Lang et al. 1998; LeDoux and Phelps 2000).
Therefore, at least in principle, even the slowdown of responses observed for the short picture-target asynchrony might be caused by some form of motor interference due to emotional processing. Changing the response demanded in the task may help rule out that possibility: if the effect of mutilation images on reaction times arose just because they primed a particular motor response, we would expect a change in the pattern of results if the response demanded now is antagonistic to the one demanded before. For example, it might happen that reaction times were longer when a mutilation picture appeared 200 ms before the target simply because processing the picture preactivates certain motor programs, which are incompatible with pressing a key. In this case, the effect of image processing on reaction times should be quite the opposite if the participant was required to release the key for go targets, instead of pressing it. The same can be said about the effect of mutilation pictures when they are shown 600 ms before the target: if the decrease in reaction times occurs because, for some reason, mutilation pictures preactivate keystroke responses in those circumstances, replacing the demanded response by its antagonist should cause a reversal in the effect. The attentional account, on the other hand, predicts no change in the pattern of results for a change in the demanded response.
Method
Participants
Twenty undergraduate students (4 male) at the Faculty of Psychology of the University of Granada, ranging in age from 18 to 26 (mean 20.5) took part in this study in exchange for course credit.
Stimuli and apparatus
The stimuli and the apparatus used in this experiment were identical to those in Experiment 1.
Design, procedure and data analyses
After giving written informed consent, the participants read the instructions of the task. The design and the data analyses of this experiment were the same as those of Experiment 1. The only difference was the response demanded for go trials. As in Experiment 1, only those trials with a correct response were included in RT analyses. Participants had to keep the space bar pressed during the whole session and release it only when a go target was displayed. After releasing the bar, they should press it again. If a participant forgot to press the space bar after having released it, the fixation point of the following trial stayed on screen, and the trial did not start until the participant pressed the space bar again. Averaged median RTs and error percentages are displayed in Table 1.
In order to test for differences in the response patterns between Experiments 1 and 2, the RTs of both experiments were submitted to an ANOVA with response demanded (key press vs key release) as between-groups factor and timing, affective content, and cue-target congruence as within-subject factors.
Results and discussion
Go trials
Omission errors occurred in 0.5% of the go trials. Baseline RT analysis showed only a main effect of congruence, F (1, 19) = 9.78, p = .006, R 2 = 0.34, with faster reaction times for congruent (322 ms) than for incongruent trials (342 ms). Emotion analysis showed significant main effects of congruence, F (1, 19) = 19.58, p < .001, R 2 = 0.51, and timing condition, F (2, 38) = 6.29, p < .004, R 2 = .25, and a significant interaction between affective contents and timing, F (6, 114) = 4.22, p < .001, R 2 = 0.18. No other effects were significant.
As in Experiment 1, SOA800-late and SOA400 conditions showed no interaction with affective content, so we pooled them together in a single Short picture-target asynchrony condition. The a posteriori LSD analysis of the affective content by timing interaction showed that reaction times were slower for mutilation images than for neutral ones in the short asynchrony condition (p = .044, t(19) = 2.16). Again, responses were faster for mutilation than for neutral pictures in the long asynchrony condition (p = .018, t(19) = 2.596) (see Fig. 2). On the other hand, differences between short and long asynchronies were significant in mutilation, babies and erotic pictures (all p’s < .03, t(19) > 2.41), but not in neutral ones (p = 0.259, t(19) = 1.16).
The comparison between the two experiments yielded a marginally significant main effect of response demanded, F (1, 38) = 3.42, p = .072, which can be attributed to a difference in how hard is to perform each of the responses that were demanded. Also significant was the interaction between response demanded and congruence, F (1, 38) = .046. The Picture x SOA interaction was significant, F (6, 228) = 6.11, p < 0.01, as expected. Crucially, however, the Picture x SOA x response effect was not significant (p = .21), which means that the combined effect on RTs of timing and picture content was similar in both experiments. There were no other significant effects.
In summary, relative to mutilation pictures, and despite the change in the required response, the results of Experiment 2 are similar to those of Experiment 1, as responses were slowed down at the short picture-target asynchrony, but they were speeded-up at the long asynchrony. Leaving aside the question of whether pressing/releasing a keyboard key can be considered as real approach/withdrawal responses, these are motor responses with very different motor programs, and therefore, it seems unlikely that the emotional effects on reaction times can be attributed to the preactivation of specific motor responses.
No-go trials
Commission errors occurred on 11.93% of no-go trials. Higher error rates were observed for the short than for the long cue-target interval in the no-pictures conditions. Emotion analysis showed a significant main effect of affective content, F (3, 57) = 3.90, p = .013, R 2 = 0.18, and significant main effect of timing, F (2, 38) = 4.12, p = .024, R 2 = 0.17. Error percentages were higher for mutilation (15.62%) than for the remaining pictures types (neutral: 11.56%, erotic: 10%, babies: 9.84%), as revealed by post-hoc LSD comparisons. Error rates were higher for the short asynchrony conditions (SOA800-late, 12.66, and SOA400, 14.96) than for the long asynchrony one (SOA800-early, 7.66), which indicates again that response errors depended on the picture-target interval.
The results obtained in this experiment replicate the pattern of facilitation and interference observed in Experiment 1 for mutilation images relative to neutral ones. Commission errors were higher for mutilation images than for the rest of the images.
General discussion
In two experiments we investigated the effects of viewing task-irrelevant emotional pictures on the speed and accuracy of a subsequent non-emotional go/no-go task. With a short (200 ms) picture-target asynchrony, mutilation pictures induced a slowdown of reaction times both for key press (Experiment 1) and key release (Experiment 2) responses. Critically, with a longer (600 ms) picture-target asynchrony, facilitation rather than interference was observed for mutilation pictures (Experiments 1 and 2). Moreover, there was a trend for error rates to be higher for mutilation pictures than for neutral ones. Taken together, these results suggest the existence of an immediate (200 ms) emotional effect, interference, as observed in the SOA800-late and SOA400 conditions, and a delayed (600 ms) emotional effect, facilitation, as revealed by the SOA800-early condition.
Mechanisms for the immediate emotional effect
Interference effects have been accounted for by two different mechanisms. First, emotional stimuli, especially those of negative valence, appear to capture attention (Bradley et al. 1997; Eastwood et al. 2001; White 1996) thereby reducing the amount of attentional resources allocated to the processing of the target. The slowdown of responses and the error rates observed in the immediate emotional effect (SOA800-late and SOA400, the short asynchrony conditions) are compatible with this hypothesis. It is suggestive that the time interval (200 ms) at which the effect arises matches closely that of attentional blink (Nieuwenhuis et al. 2005), that has been shown to occur also when unconditionally aversive stimuli (as were the mutilation pictures in our case) appear briefly before the target, hindering its detection (Most et al. 2005). Interestingly, this attentional blink effect fades completely for distractor–target SOAs of around 800 ms and longer.
Second, it has also been suggested that interference effects can be the result of the preactivation of motor programs incompatible with the response demanded by the task (Pereira et al. 2004, 2006). According to this idea, positive and negative stimuli should activate approach and withdrawal responses, respectively. There is ample experimental support for this proposal. For example, Duckworth et al. (2002) demonstrated that pulling a lever is faster than pushing it when responding to positive stimuli, but the converse is true when responding to negative ones. In a similar study, Wentura et al. (2000) showed that go/no-go lexical decisions tended to be faster when subjects had to press a key in response to a positive word, but also when they had to release the key in response to a negative one. Hare et al. (2005) observed a slowdown of an approach response (keystroke) when subjects responded to fearful faces, and that the activity in the amygdala correlated well with reaction time. In our procedure the preactivation elicited by the emotional pictures adds to that triggered by the warning cue. However, the ‘Emotion analysis’ we performed should be free of the effect of the triggering cue—at least if we assume that the effects of the cue and the emotional content on response facilitation or interference are additive—since we are comparing among emotional conditions that share the same trial structure. Therefore, in Experiment 1, a purely motor account would predict response facilitation for positive images relative to neutral ones, and interference for negative pictures, as the response demanded was, if anything, an approach one (keystroke). Conversely, for Experiment 2 such explanation would predict interference for positive images, and facilitation for negative images, as the response demanded there have been considered by some (Wentura et al. 2000) a withdrawal one (key release). Since the effects observed were approximately the same in both experiments, our results favor the attentional account over the motor pre-activation account as an explanation of the immediate emotional effect.
Mechanisms for the delayed emotional effect
The emotional modulation of attention accounts for the response slowdown in the immediate emotional effect (SOA800-late and SOA400 conditions), but it seems difficult to accommodate to explain the facilitation of the response in the delayed effect (SOA800-early condition). Apparently, once the effect on attention has gone by, emotion acts by speeding up the behavioral response, irrespective of its direction (key press or key release). This facilitation may be explained by two mechanisms. First, it is well known that emotional stimuli modulate the activity of visual areas in the cortex (see Pessoa 2008, for a recent review) possibly by means of direct or indirect projections from the amygdala to the visual cortex (Freese and Amaral 2005; Phelps and LeDoux 2005). This effect of emotion on perception has been shown to occur even when the emotional stimulus is task-irrelevant (Phelps et al. 2006). Bocanegra and Zeelenberg (2009) showed that task-irrelevant fearful faces improve the visual sensitivity to some stimuli (low spatial frequency Gabor gratings) but impair the sensitivity to others (high spatial frequency Gabor gratings). Padmala and Pessoa (2008) observed increased sensitivity in a simple visual detection task to targets that had been paired to mild electrical shocks compared to the same physical targets but devoid of affective value (see Lim and Pessoa 2008, for a similar result using more complex emotional stimuli). Moreover, enhanced detection of affectively charged targets was associated to increased BOLD activity in areas of primary visual cortex, whereas detection of affectively neutral targets had no relation to BOLD activity in the corresponding early visual areas. The areas of increased BOLD activity for emotional targets matched retinotopically their spatial location. Interestingly, Lim et al. (2008) have shown that this enhanced processing for emotional stimuli depends on the attentional resources available. Under low-load attentional conditions, task-irrelevant fearful faces paired to aversive electrical stimulation elicited stronger activations in the amygdala and the fusiform gyrus than unpaired fearful faces. However, neither activation in these brain areas nor behavioral performance were different for both types of stimuli under high-load attentional conditions, indicating that facilitation depends on the availability of attentional resources. Thus, if we assume that the attentional load of our go/no-go task is low, and that attention can be disengaged from the emotional stimuli in a few hundred milliseconds (Koster et al. 2006), our facilitation effect may well be explained by this perceptual enhancement mechanism, given the evidence that affect-laden stimuli, specially mutilation ones, increase visual brain areas’ sensitivity to the target.
Emotional processing could also affect later stages of the response selection process, such as decision-making. The speed-up of responses observed in the delayed emotional effect is reminiscent of a recent theoretical proposal (Simen et al. 2006) that posits that average reward rate provides a global signal that controls the decision threshold during the task: the larger the reward rate, the lower the selected decision threshold. This idea has been supported by experimental results indicating that participants adjust their decision threshold in order to maximize reward rate in decision-making tasks (Simen et al. 2009). It has been suggested that not only the average rate of recently received rewards but also the opportunity to escape from an aversive situation may lower the decision threshold (Niv et al. 2007). Thus, we can speculate that an unpleasant stimulus might cause the lowering of the threshold for a decision made around that time, consequently speeding up responses regardless of their direction. This explanation, moreover, is consistent with the observed increase of error rates in Experiment 2.
A direct way to contrast these two hypotheses (perceptual enhancement vs decision threshold lowering) could be to present the emotionally charged stimuli in a sensory modality different from that of the targets (e.g. emotional auditory stimuli in a visual discrimination task or affective visual stimuli in an auditory discrimination task). If the effects are reproduced, that would argue against an explanation in terms of perceptual facilitation.
Conclusions
In summary, the emotional go/no-go procedure we have developed here enabled us to show the emotional modulation of behavior when a very short-lived emotional image preceded the target by several hundred milliseconds. We have also shown how this effect depended on the contents of the emotional image and of its onset asynchrony relative to the target. Our first experiment revealed that, compared to neutral images, mutilation images tended to speed up responses when they were displayed 600 ms before the target onset, but they slowed down the responses when presented 200 ms before the target, while babies facilitated the response for the 200 ms delay, but caused no effect for the longer (600 ms) one, and erotic images did not differ from neutral ones. The immediate (200 ms) emotional effect can be attributed to a deficit in the processing of the target as attention is still allocated to the emotional image, while the delayed (600 ms) effect may be caused by either an emotion induced perceptual enhancement or a reduction in the response threshold. An alternative possible explanation of the delayed effect, namely, that the facilitation observed at the longer picture-target asynchrony was caused by the preactivation of a particular motor program by the mutilation pictures has been ruled out by the results of our second experiment, in which we replaced the demanded response by its antagonist (releasing a key instead of pressing it), but reproduced the results of Experiment 1. The overall results presented here agree with previous findings (Coombes et al. 2007; Most et al. 2005) and further suggest that emotion can modulate typically “cognitive” processes, such as attention and decision-making (McClure et al. 2007; Pessoa 2008).
References
Becker, M. W. (2009). Panic search: fear produces efficient visual search for nonthreatening objects. Psychological Science, 20(4), 435–437.
Blanchard, D. C., & Blanchard, R. J. (1988). Ethoexperimental approaches to the study of emotions. Annual Review of Psychology, 39, 43–68.
Bocanegra, B. R., & Zeelenberg, R. (2009). Emotion improves and impairs early vision. Psychological Science, 20(6), 707–713.
Boulinguez, P., Jaffard, M., Granjon, L., & Benraiss, A. (2008). Warning signals induce automatic EMG activations and proactive volitional inhibition: Evidence from analysis of error distribution in simple RT. Journal of Neurophysiology, 99, 1572–1578.
Bradley, B. P., Mogg, K., & Lee, S. C. (1997). Attentional biases for negative information in induced and naturally occurring dysphoria. Behavior Research & Therapy, 35, 911–927.
Buodo, G., Sarlo, M., & Palomba, D. (2002). Attentional resources measured by reaction times highlight differences within pleasant and unpleasant, high arousing stimuli. Motivation and Emotion, 26(2), 123–138.
Chen, M., & Bargh, J. A. (1999). Consequences of automatic evaluation: immediate behavioral predispositions to approach or avoid the stimulus. Personality and Social Psychology Bulletin, 25, 215–224.
Coombes, S. A., Cauraugh, J. H., & Janelle, Ch M. (2007). Dissociating motivational direction and affective valence: Specific emotions alter central motor processes. Psychological Science, 18(11), 938–942.
Dolan, R. J. (2002). Emotion, cognition, and behavior. Science, 298, 1191–1194.
Donders, F. C. (1969). On the speed of mental processes. Acta Psychologica, 30, 412–431.
Duckworth, K. L., Bargh, J. A., Garcia, M., & Chaiken, S. (2002). The automatic evaluation of novel stimuli. Psychological Science, 13(6), 513–519.
Eastwood, J. D., Smilek, D., & Merikle, P. M. (2001). Differential attentional guidance by unattended faces expressing positive and negative emotion. Perception & Psychophysics, 63, 1004–1013.
Freese, J. L., & Amaral, D. G. (2005). The organization of projections from the amygdala to visual cortical areas TE and V1 in the macaque monkey. Journal of Comparative Neurology, 486, 295–317.
Goldstein, M., Brendel, G., Tuescher, O., Pan, H., Epstein, J., Beutel, M., et al. (2007). Neural substrates of the interaction of emotional stimulus processing and motor inhibitory control: An emotional linguistic go/no-go fMRI study. Neuroimage, 36, 1026–1040.
Gómez, P., Ratcliff, R., & Perea, M. (2007). A model of the go/no-go task. Journal of Experimental Psychology: General, 136, 389–413.
Hare, T. A., Totteham, N., Davidson, M. C., Glover, G. H., & Casey, B. J. (2005). Contributions of amydala and striatal activity in emotion regulation. Biological Psychiatry, 57, 624–632.
Irtel, H. (2006). PXLab: The psychological experiments laboratory [online]. Version 2.1.9. Mannheim (Germany): University of Mannheim. Available from http://www.pxlab.de.
Koster, E. H. W., Crombrez, G., Verschuere, B., Van Damme, S., & Wiersema, J. R. (2006). Components of attentional bias to threat in high trait anxiety: Facilitated engagement, impaired disengagement, and attentional avoidance. Behaviour Research and Therapy, 44, 1757–1771.
Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (1998). Emotion, motivation, and anxiety: Brain mechanisms and psychophysiology. Biological Psychiatry, 44, 1248–1263.
Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (2005). International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical report A-6. Gainesville, FL: University of Florida.
LeDoux, J. E., & Phelps, E. (2000). Emotional networks in the brain. In M. Lewis & J. M. Haviland-Jones (Eds.), Handbook of emotions (2nd ed.). New York: The Guilford Press.
Lim, S. L., Padmala, S., & Pessoa, L. (2008). Affective learning modulates spatial competition during low-load attentional conditions. Neuropsychologia, 46, 1267–1278.
Lim, S. L., & Pessoa, L. (2008). Affective learning increases sensitivity to graded emotional faces. Emotion, 8, 96–103.
McCall, C., Tipper, C. M., Blascovich, J., & Grafton, S. T. (2011). Attitudes trigger motor behavior through conditioned associations: Neural and behavioral evidence. Social Cognitive and Affective Neuroscience. doi:10.1093/scan/nsr057 (advance online publication).
McClure, S. M., Botvinick, M. M., Yeung, N., Greene, J. D., & Cohen, J. D. (2007). Conflict monitoring in cognition-emotion competition. In J. J. Gross (Ed.), Handbook of emotion regulation (pp. 204–228). New York: Guilford Press.
Megías, A., Maldonado, A., Cándido, A., & Catena, A. (2011a). Emotional modulation of urgent and evaluative behaviors in risky driving scenarios. Accident Analysis and Prevention, 43(3), 813–817.
Megías, A., Maldonado, A., Catena, A., Di Stasi, L. L., Serrano, J., & Cándido, A. (2011b). Modulation of attention and urgent decisions by affect-laden roadside advertisement in risky driving scenarios. Safety Science, 49, 1388–1393.
Moltó, J., Montañés, S., Poy, R., Segarra, P., Pastor, M. C., Tormo, M. P., et al. (1999). Un nuevo método para el estudio experimental de las emociones: el International Affective Picture System (IAPS). Adaptación española. Revista de Psicología General y Aplicada, 52, 55–87.
Most, S. B., Chun, M. M., Widders, D. M., & Zald, D. H. (2005). Attentional rubbernecking: Attentional capture by threatening distractors induces blindness for targets. Psychonomic Bulletin & Review, 12, 654–661.
Niemi, P., & Näätänen, R. (1981). Foreperiod and simple reaction time. Psychological Bulletin, 89(1), 133–162.
Nieuwenhuis, S., Gilzenrat, M. S., Holmes, B. D., & Cohen, J. D. (2005). The role of the locus coeruleus in mediating the attentional blink: A neurocomputational theory. Journal of Experimental Psychology: General, 134, 291–307.
Niv, Y., Daw, N. D., Joel, D., & Dayan, P. (2007). Tonic dopamine: Opportunity costs and the control of response vigor. Psychopharmacology (Berl), 191(3), 507–520.
Nobre, A. C., Correa, A., & Coull, J. T. (2007). The hazards of time. Current Opinion in Neurobiology, 17, 465–470.
Padmala, S., & Pessoa, L. (2008). Affective learning enhances visual detection and responses in primary visual cortex. The Journal of Neuroscience, 28(24), 6202–6210.
Pereira, M. G., Volchan, E., Guerra, G., Oliveira, L., Ramos, R., Machado-Pinheiro, W., et al. (2006). Sustained and transient modulation of performance induced by emotional picture viewing. Emotion, 6, 624–634.
Pereira, M. G., Volchan, E., Oliveira, L., Machado-Pinheiro, W., Rodrigues, J. A., Nepomuceno, F. V. P., et al. (2004). Behavioral modulation by mutilation pictures in women. Brazilian Journal of Medical and Biological Research, 37, 353–362.
Pessoa, L. (2008). On the relationship between emotion and cognition. Nature Reviews Neuroscience, 9, 148–158.
Phelps, E. A., & LeDoux, J. E. (2005). Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron, 48, 175–187.
Phelps, E. A., Ling, S., & Carrasco, M. (2006). Emotion facilitates perception and potentiates the perceptual benefits of attention (research support, N.I.H., extramural).
Schultz, K. P., Fan, J., Magidina, O., Marks, D. J., Hahn, B., & Halperin, J. M. (2007). Does the emotional go/no-go task really measure behavioral inhibition? Convergence with measures on a non-emotional analogue. Archives of Clinical Neuropsychology, 22, 151–160.
Simen, P., Cohen, J. D., & Holmes, P. (2006). Rapid decision threshold modulation by reward rate in a neural network. Neural Networks, 19, 1013–1026.
Simen, P., Contreras, D., Buck, C., Hu, P., Holmes, P., & Cohen, J. D. (2009). Reward rate optimization in two-alternative decision making: Empirical tests of theoretical predictions. Journal of Experimental Psychology: Human Perception and Performance, 35, 1865–1897.
Solarz, A. (1960). Latency of instrumental responses as a function of compatibility with the meaning of eliciting verbal signs. Journal of Experimental Psychology, 59, 239–245.
Theeuwes, J., Godijn, R., & Pratt, J. (2004). A new estimation of the duration of attentional dwell time. Psychonomic Bulletin & Review, 11, 60–64.
Wentura, D., Rothermund, K., & Bak, P. (2000). Automatic vigilance: The attention-grabbing power of approach and avoidance-related social information. Journal of Personality and Social Psychology, 78(6), 1024–1037.
White, M. (1996). Anger recognition is independent of spatial attention. New Zealand Journal of Psychology, 25, 30–35.
Zald, D. H., Mattson, D. L., & Pardo, J. V. (2002). Brain activity in ventromedial prefrontal cortex correlates with individual differences in negative affect. PNAS, 99, 2450–2454.
Acknowledgments
This work was supported by the Junta de Andalucía (P06-HUM02375 and P09-SEJ-4752) and the Spanish Ministry of Science and Innovation (SEJ2006-11906/PSIC and PSI2009-12217) grants to Andrés Catena and Antonio Maldonado, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Contreras, D., Megías, A., Maldonado, A. et al. Facilitation and interference of behavioral responses by task-irrelevant affect-laden stimuli. Motiv Emot 37, 496–507 (2013). https://doi.org/10.1007/s11031-012-9327-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11031-012-9327-0