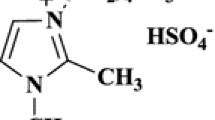
Abstract
An efficient and green strategy for the regioselective synthesis of highly functionalized pyranopyrazole via one-pot condensation of 3-methyl-1-phenyl-5-pyrazolone or EAA and hydrazine hydrate, substituted aromatic aldehydes with NMSM [(E)-N-Methyl-1-(methylthio)-2-nitro-ethenamine] in the existence of IL [(EMIM)Ac] as catalyst with solvent-free condition (SFC) is described. This domino protocol produces biologically substantial heterocycles through Knoevenagel condensation proceeded by Michael addition and O-cyclization with an eradication of methanethiol group, which create the one stereo-center and creation of “C–C, C–N, C–O, C=C, C=N, bonds.” The final product is produced by exceptionally easy filtering after the reaction mass was triturated with ethanol. The strategy's noteworthy features include the use of biodegradable IL catalyst, excellent to exceptional yield with rapid reaction times, applicability to a wide range of substrate, clear reaction profile, and straightforward workup process.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
New routes for synthesizing simple and highly functionalized bioactive compounds by adopting a green strategy are highly sought in the current era of research and technology [1, 2]. MCR is one of the most effective strategies used in green synthesis [3]. In MCR, at least three reactants are brought together in a single reactor to generate a complex molecular cascade. Each reactant's structural features are incorporated into the produced molecules. MCRs have many strengths, including good selectivity, atom economy, and simplicity. MCRs are at the heart of combinatorial chemistry and diversity-oriented synthesis (DOS) also it has played a crucial part in developing today's synthetic approach to drug discovery [4,5,6,7,8].
Solvent-free reactions emerged as a valuable technique in synthetic organic chemistry for producing a large variety of bioactive heterocycles in more environmentally friendly routes [9]. Solvent-free processes also have advantages, including rapidity of response, shorter reaction times, reduction in energy use, easier isolation, and high purity with a good yield of products [10, 11]. Ionic liquids (ILs) as a catalyst is an eco-friendly technique in chemical synthesis [12]. Researchers have focused a lot of emphasis on ILs as a solvent and catalyst that is gentle on the environment [13]. ILs have a wide spectrum of solubilities, low viscosity, good thermal stability, and are non-flammable. Ionic liquids (ILs) have the capability to revolutionize synthetic processes by substituting traditional reagents [14].
Pyranopyrazoles are valuable, naturally occurring, fused heterocyclic scaffolds featuring powerful pharmacological and biological effects as well as industrial significance [15]. Anticancer [16], potential Chk1 inhibitor [17, 18], antifungal, antibacterial [19], anti-inflammatory [20], analgesic [21], antiplatelet [22], herbicidal [23], molluscicidal, vasodilator, prostaglandin inhibitory, and anticonvulsant [24] action are only a few of the biological and pharmacological properties of pyranopyrazole (Fig. 1). Furthermore, it works amazingly well in industrial procedures like inhibitors of steel corrosion and antioxidants for lubricating oil [25].
In recent times, many approaches have been reported to synthesize pyranopyrazole concerning the reaction of 1,3 di-carbonyl molecules, hydrazines, malononitrile, and carbonyl compound utilizing a diverse catalysts [26,27,28,29,30]. However, Dr R. R. Kumar and his co-workers are the first to report the synthesis of highly functionalized pyranopyrazole by using NMSM reagent [31]. NMSM is an impactful and valuable synthetic counterpart for a number of synthons because its ethene molecule possesses four operating sites along with three functional groups (Fig. 2).
The nitro group in the NMSM is a potent electron-withdrawing group. In contrast, it works so well as a Michael donor due to the electron-donating methylamino group. The SNV (Substitution Nucleophilic Vinyl) mechanism makes use of the thiomethyl as an excellent leaving group that many other nucleophiles may replace. The ethylene moiety is a nucleophilic and electrophilic extremely polarized push–pull alkene. These characteristics demonstrate that NMSM has the potential to develop innovative strategies for synthesizing bioactive nucleuses.
There are only four strategies documented for the synthesis of highly functionalized pyranopyrazole, as per a comprehensive review of the literature by cyclo-condensation of various hydrazines, different esters, carbonyl compounds, and NMSM. R. R. Kumar with his co-workers prepared novel pyranopyrazoles using DIPEA catalyst [31], T. P. Paramasivan and K. Jayabal used piperidine catalyst under SFC [32], V. B. Helavi used Lewis’s acid catalyst indium chloride (InCl3) [33], Md. M. Khan and his coworker’s carried out catalyst-free synthesis in high-to-exceptional yield [34]. In many cases, the results of these described approaches have been outstanding. However, some synthetic strategies have drawbacks such as low yield, harsh reaction conditions, high quantity of catalyst, and long reaction times.
IL [(EMIM)Ac] has recently been utilized as a catalyst for a variety of organic conversions [35,36,37,38]. In furtherance of our study on the development of green pathways for the creation of bioactive heterocycles [39,40,41,42] in this instance, we have disclosed a one-pot preparation of extremely functionalized pyranopyrazole by equimolar condensation of hydrazine hydrate, EAA, aromatic aldehydes, and NMSM using 20 mol% of [(EMIM)Ac] at 115–120 °C under solvent-free condition and condensation of 3-methyl-1-phenyl-5-pyrazolone, aromatic aldehydes, and NMSM using 20 mol% of [(EMIM)Ac] at 80–85 °C with SFC in high-to-exceptional yield (Scheme 1).
Result and discussion
A representative reaction utilizing an equimolar quantity of NMSM, 2,4 dichlorobenzaldehyde, and 3-methyl-1-phenyl-5-pyrazolone was carried in lacking catalyst at the room temperature, and at reflux condition in ethanol, no desired product was observed after 12 and 10 h (Table 1, entries 1 & 2). Similarly, a representative reaction was conducted in methanol at ambient and reflux condition for 12 and 11 h. In the absence of a catalyst, it failed to start the reaction and bring about the expected outcome. (Table 1, entries 3, 4). Subsequently, when the reaction was continued in a neat condition at an ambient temperature and 80 °C, the reaction mixture was unchanged (Table 1, entries 5 & 6). These preceding experiment results promoted us to test the use of a catalyst to complete this reaction.
Footnote 1bReaction failed to produce product
In our subsequent investigation, we decided to conduct a model reaction using an acidic catalyst Amberlite IR— 120 (50 mg). At first, the model reaction was done in ethanol under conditions of reflux, and after 9 h it resulted in 42% of the intended product. (Table 1, entry 7). The obtained product is validated by spectral data. After that, an identical reaction was performed in methanol at reflux conditions which delivers the desired output in 40% of yield in 9 h (Table 1, entry 8). Later, we decided to perform a reaction in SFC as progression of the reaction in the solvent-free condition has fascinated significant attention due to their various green chemistry applications. The solvent-free reaction was performed at 80 °C, and it delivers the desired output in 52% yield in 2 h. (Table 1, entry 9). These results demonstrate that Amberlite IR-120 may initiate the reaction, but it fails to produce the intended product in a reasonable yield.
Afterward, we decided to switch to the glycine catalyst. We performed the model reaction with it, in ethanol and methanol under reflux environment, which affords the desired product in 26% and 30% after 6 and 7 h, respectively (Table 1, entries 10 & 11). With these unsatisfactory results, we moved on to the next acidic catalyst, p-Toluene sulfonic acid (p-TSA). We conducted the model reaction at reflux condition for 10 h with ethanol, producing the required product in a lower yield of 45% (Table 1, entry 12). An identical procedure was carried out in methanol, offering the required product in 46% in 10 h (Table 1, entry 13). To get a good yield, A comparable process was conducted in a solvent-free environment at 80–85 °C for 2 h, offering a noticeable 59% of yield (Table 1, entry 14).
In the quest of improved outcomes, we turned to the Lewis acid catalyst SnCl2 and performed the model reaction using SnCl2 as catalyst. The Standard reaction was done in ethanol and methanol at reflux conditions for 6 h, offering the final products in optimal yield of 40% and 33%, respectively (Table 1, entries 15 & 16). A similar reaction was conducted in neat condition at 80–85 °C for 2 h, offering the necessary product in substantial outcome of 52% (Table 1, entry 17). Referring to the inadequate findings obtained with the acidic catalyst as it initiate the reaction but failed to give cyclized product because acid catalyst is able to do Knoevenagel condensation of 3-methyl-1-phenyl-5-pyrazolone with NMSM and 1,4 Michael addition reaction of another NMSM molecule with formed adduct but acidic catalyst are not able to activate to OH group easily which result in low yield of the desired product or no formation of desired product, we decided to employ a basic catalyst potassium tertiary-butoxide to execute a model reaction in THF as the solvent, which failed to begin the reaction and produce the required product (Table 1, entry 18). Since most basic catalysts have been described in the literature, we chose to execute a model reaction employing a basic ionic liquid catalyst [EMIM(Ac)].
Herein, we investigated the chemistry of ionic liquid [(EMIM)Ac] for the synthesis of pyranopyrazole. A standard reaction was carried out in ethanol with an IL catalyst [EMIM(Ac)] that produced bispyrazolone instead of the expected product in 5 min (Table 1, entry 19). Similar results were obtained when the process was repeated in methanol and acetonitrile (Table 1, entries 20 & 21). The reaction with the catalyst [EMIM(Ac)] does not start at room temperature without solvent. (Table 1, entry 22). Then, we executed the reaction neatly at 75–80 °C and obtained a decent yield of the target product in 45 min. (Table 1, entry 23). We were ecstatic to discover that the reaction went rapidly and delivered excellent results as 93% yield of final molecule in 45 min at 80–85 °C. (Table 1, entry 24). We repeated a similar reaction at a higher temperature. Still, the final product's yield did not rise, and the time required to complete the reaction was unaffected (Table 1, entries 25 to 28).
We evaluated the amount of catalyst necessary to execute a reaction by running multiple experiments by adjusting the proportion of catalyst [(EMIM)Ac] on a model reaction at 80–85 °C, utilizing these pleasant findings produced by employing catalyst [EMIM(Ac)]. Initially, a model reaction was performed at 80–85 °C using 10 mol% of catalyst, producing the desired product in 55% yield within 70 min. Increasing the concentration of the catalyst to 15 mol% desired product is obtained in 73% of yield within 60 min. After that, increasing the catalyst quantity to 20 mol% offers the product in 93% of yield. Furthermore, raising the catalyst concentration to 25 mol% and 30 mol% did not affect the rate or yield of the reaction. It is clear from the preceding observations that using 20 mol% catalysts [(EMIM)Ac] produces excellent results (Fig. 3).
Additionally, to examine the universality and application of the procedure as mentioned earlier, we performed the reaction by employing substituted aromatic aldehydes under identical optimal circumstances. This resulted in excellent yields of the required pyranopyrazole being produced. Aldehydes containing groups that donate and pull electrons produced products without difficulty (Table 2). In the case of substituted hydroxy aldehydes rate of reaction was slowed down, and aldehydes bearing hydroxy groups like 1d, 1h, 1i, & 1k failed to produce the final product even after 75 min. as reaction stuck at intermediate level which is validated by spectral data (See SI).
Similarly, to identify the optimum reaction condition for the preparation of pyranopyrazole using hydrazine hydrate and ethyl acetoacetate instead of 3-methyl-1-phenyl-5-pyrazolone, a model reaction of hydrazine hydrate, ethyl acetoacetate, p-chlorobenzaldehyde, and NMSM is investigated using 20 mol% of IL catalyst [(EMIM)Ac]. Initially, a model reaction is performed in ethanol at reflux, which offers 61% of the desired product in 7 h. Solvents like methanol and acetonitrile give 54% and 51% of yield in 8 and 9 h, respectively (Table 3, Entries 1 to 3).
Later, we attempted the reaction in neat condition at ambient temperature, which failed to commence the reaction after 3 h of stirring (Table 3, Entry 4). Then the reaction was conducted at 80–85 °C, offering a considerable product with 64% yield in 65 min (Table 3, Entry 5). To get a decent result, the influence of temperature on the reaction was assessed. Initially, we raised the temperature of reaction to 85–90 °C, which generated a 69% yield in 50 min. (Table 3, Entry 6). This outcome has given us hope; therefore, we continued the reaction at high temperatures such as 90–95 °C, 95–100 °C, 100–105 °C, and 105–110 °C, offering the desired product in 73% in 45 min., 78% in 45 min., 81% in 40 min., and 86% in 30 min., respectively. (Table 3, Entries 7 to 10). As good results are observed by raising the reaction temperature again, we performed reactions at a higher temperature, which are 110–115 °C, 115–120 °C, 120–125 °C, and 125–130 °C, which resulted in 89% yield in 30 min., 94% in 20 min., 84% in 20 min., and 70% yield in 20 min. (Table 3, Entries 11 to 14). It is clear from the above observations that boosting the temperature of the reaction increased the reaction rate and the extent of the final products and that 115–120 °C is the optimal temperature for executing this reaction. However, raising the temperature further did not change the speed of reaction, and the extent of required product abruptly decreased (Fig. 4).
With these pleasing results achieved by catalyst [EMIM(Ac), we figured out how much catalyst was needed for a reaction by doing several tests with different concentrations of catalyst [(EMIM)Ac] on a model reaction at 115–120 °C. Apparently, a model reaction was conducted around 115–120 °C with 10 mol% catalysts, offering the required product in 57% in 80 min. Boosting catalyst proportion to 15 mol% desired products in 78% yield is attained within 50 min. After that, raising the catalyst amount to 20 mol%, 94% yield of the desired product in 20 min is obtained. Furthermore, enhancing catalyst intensity to 25 mol% and 30 mol% did not influence the reaction rate or yield. We may conclude that utilizing 20 mol% [(EMIM)Ac] delivers good results based on the above observation (Fig. 5).
To evaluate the generality and application of the proposed strategy, we repeated the reaction using substituted aromatic aldehydes under identical optimum circumstances. As a result, high yields of the required pyranopyrazole have been obtained. Aldehydes have both electron-withdrawing and electron-donating groups, easily generated compounds (Table 4).
We compared [(EMIM)Ac] ionic liquid's catalytic activity to previously described methods to synthesize highly functionalized pyranopyrazoles. The reactant is easily converted into the desired product by the ionic liquid [(EMIM)Ac], with a yield of up to 96%. (Table 5).
Taking into consideration of entire outcomes, Fig. 6 illustrates the proposed mechanism for synthesizing pyranopyrazole utilizing [(EMIM)Ac] as a catalyst. The likely process includes polarization of the aromatic aldehyde and activation of the 1,3-dinuleophilic molecule by the IL to entail a Knoevenagel condensation reaction in between and provide an adduct. This newly produced adduct functions as a Michael acceptor and conducts a 1,4 Michael addition reaction with NMSM to produce an intermediate, which is then subjected to O-cyclization and methanethiol elimination to produce pyranopyrazole. (Fig. 6).
Conclusion
As a result, we designed a regioselective, green, and efficient strategy for producing extremely functionalized pyranopyrazole from readily accessible starting ingredients. The reaction was carried out in one-pot routes in the presence of a catalytic quantity of IL [EMIM]Ac under proper reaction conditions in all cases. We feel that the strategy outlined here provides an environmentally benign route to these valuable heterocyclic motifs. Furthermore, these processes have a number of benefits, including a rapid reaction time, high atom economy, modestly high yields, operational friendliness, and the lack of column chromatography.
Experimental
TCI was the source of the NMSM. Many aromatic aldehydes were purchased from Sigma-Aldrich, and a few were purchased from sd-fine or Loba chemical firm, respectively; Sigma-Aldrich supplies the ionic liquid [EMIM]Ac that is used as a catalyst in this process. Loba chemicals produced the solvents needed. Using TLC, we were able to monitor the progress of the reaction. Melting points, IR spectra, and HRMS spectra may be recorded using the Digital MP instrument, KBr FT-IR Spectrometer, and Waters Aquity UPLC-SYNAPT G2-HDMS Q-tof mass spectrometer. An NMR spectrum of 1H and 13C was recorded at SAIF P. U. Chandigarh using Bruker's Advanced Neo 500 MHz NMR Spectrometer with TMS as an internal Standard and DMSO-d6 & CDCl3 as the solvent. Values for chemical shifts are expressed in terms of TMS in the unit of δ (ppm).
General procedure for synthesis of highly functionalized pyranopyrazole
In a clean and dry 10 mL, R.B.F. equipped with a magnetic stirrer charged an equimolar mixture of 3-methyl-1-phenyl-5-pyrazolone/ethyl acetoacetate & hydrazine hydrate, aromatic aldehyde, NMSM, and catalyst ionic liquid [EMIM]Ac (20 mol%). The above mixture was heated at 80–85 °C/115–120 °C under solvent-free conditions for an appropriate time. The progress of the reaction was monitored by TLC; after the accomplishment of the reaction, which was confirmed by TLC, the reaction mass was cooled gradually, and the precipitated solid product was triturated with 2–3 mL ethanol and stirred for 20 min. The resultant reaction mass was filtered and washed with 2–3 mL of cold ethanol. The obtained product was pure enough for spectral investigation.
Spectral data of novel and some of the selected compounds
N,3-dimethyl-5-nitro-4-(3-nitrophenyl)-1-phenyl-1,4-dihydropyrano[2,3-c]pyrazol-6-amine (4b)
Yield – 92%, Yellow Solid, M. P.: 226–227 °C, IR (KBr) cm−1:758.02, 1051.20, 1128.36, 1346.31, 1388.75, 1521.84, 1653.00, 1869.02, 2866.22, 2945.30, 3446.79. 1H NMR (500 MHz, CDCl3) δ: 1.97 (s, 3H), 3.28 (d, 3H), 5.36 (s, 1H), 7.34–7.38 (m, 1H), 7.47–7.53 (m, 3H), 7.67–7.69 (td, 2H), 7.74–7.76 (td, 1H), 8.08–8.10 (m, 1H), 10.57 (d, 1H). 13C NMR (DMSO-d6, 125 MHz), δC (ppm): 28.58, 37.32, 106.58, 107.70, 112.52, 116.49, 122.73, 124.91, 126.95, 127.95, 128.35, 133.05, 141.14, 151.88, 156.68, 159.08. HRMS, Calcd. For C20H17N5O5: 407.10; found 408.1307 [M + H]+.
4-(2,4-dichlorophenyl)-N,3-dimethyl-5-nitro-1-phenyl-1,4-dihydropyrano[2,3-c]pyrazol-6-amine (4c)
Yield – 93%, White Solid, M. P.: 228–230 °C, IR (KBr) cm−1: 759.95, 842.89, 1058.92, 1271.09, 1363.67, 1453.18, 1662.64, 2927.94, 3066.82, 3446.79. 1H NMR (500 MHz, CDCl3) δ: 2.01 (s, 3H), 3.25 (d, 3H), 5.62 (s, 1H), 7.17–7.19 (dd, 1H), 7.24 (d, 1H), 7.33–7.36 (m, 2H), 7.48–7.51 (t, 2H), 7.64–7.66 (dd, 2H), 10.63 (d, 1H). 13C NMR (DMSO-d6, 125 MHz), δC (ppm): 12.83, 28.54, 36.34, 120, 0.95, 127.07, 127.28, 129.46, 129.84, 130.21, 133.38, 137.17, 137.35, 142.20, 146.34, 159.52. HRMS, Calcd. For C20H16Cl2N4O3: 430.06; found 431.0680 [M + H]+.
3-(3-methyl-6-(methylamino)-5-nitro-1-phenyl-1,4-dihydropyrano[2,3-c]pyrazol-4-yl)phenol (4e)
Yield – 87%, White Solid, M. P.: 240–241 °C, IR (KBr) cm−1:775.38, 800.46, 1053.13, 1267.23, 1392.61, 1487.12, 1517.98, 1651.07, 1870.95, 2619.33, 2945.30, 3018.60, 3186.40, 3288.63. 1H NMR (500 MHz, CDCl3) δ: 1.96 (s, 3H), 3.17 (d, 3H), 5.14 (s, 1H), 6.52–6.63 (m, 1H), 6.68–6.69 (t, 1H), 6.74–6.75 (dd, 1H), 7.05–7.11 (m, 2H), 7.34–7.37 (t, 1H), 7.52–7.55 (t, 1H),7.73–7.75 (dd, 1H), 9.29 (s, 1H), 10.55–10.58 (q, 1H). 13C NMR (DMSO-d6, 125 MHz), δC (ppm): 12.51, 28.62, 37.76, 100.72, 108.79, 113.52, 114.54, 118.48, 120.31, 126.55, 128.94, 129.43, 137.07, 141.65, 144.48, 145.31, 157.07, 158.60. HRMS, Calcd. For C20H18N4O4: 378.13; found 379.1411 [M + H]+.
4-(4-fluorophenyl)-N,3-dimethyl-5-nitro-1-phenyl-1,4-dihydropyrano[2,3-c]pyrazol-6-amine (4f)
Yield – 92%, White Solid, M. P.: 223–224 °C, IR (KBr) cm−1: 758.02, 835.18, 1049.28, 1217.08, 1392.61, 1508.33, 1654.92, 1907.06, 2945.30, 3066.82, 3446.79. 1H NMR (500 MHz, CDCl3) δ: 1.98 (s, 3H), 3.23 (d, 3H), 5.23 (s, 1H), 6.94–6.98 (tt, 2H), 7.25–7.26 (t, 1H), 7.26–7.27 (t, 1H), 7.32–7.35 (td, 1H), 7.47—7.51 (td, 2H), 7.66–7.68 (dt, 2H), 10.56 (q, 1H). 13C NMR (DMSO-d6, 125 MHz), δC (ppm): 12.74, 28.53, 37.69, 100.86, 110.43, 115.04, 115.21, 120.79, 126.96, 128.73, 129.73, 137.43, 137.63, 141.96, 146.38, 159.69, 160.70, 162.73. HRMS, Calcd. For C20H17FN4O3: 380.13; found 381.1359 [M + H]+.
4-(4-isopropylphenyl)-N,3-dimethyl-5-nitro-1-phenyl-1,4-dihydropyrano[2,3-c]pyrazol-6-amine (4 g)
Yield – 88%, White Solid, M. P.: 214–216 °C, IR (KBr) cm−1:827.46, 1045.42, 1124.50, 1359.82, 1517.98, 1654.92, 2868.15, 2956.87, 3462.22. 1H NMR (500 MHz, CDCl3) δ: 1.19 (d, 6H), 2.01 (s, 3H), 2.82–2.86 (d, 3H), 3.23–3.24 (d, 3H), 5.23 (sept, 1H), 7.11 (d, 2H), 7.18–7.20 (d, 2H), 7.31–7.34 (t, 1H), 7.47–7.50 (t, 2H), 7.66–7.68 (dd, 2H), 10.56 (d, 1H). 13C NMR (DMSO-d6, 125 MHz), δC (ppm):12.62, 23.87, 23.93, 23.95, 28.50, 33.69, 37.90, 101.41, 120.78, 126.33, 126.83, 127.00, 127.92, 129.42, 137.53, 139.15, 141.98, 146.50, 147.49, 159.27. HRMS, Calcd. For C23H24N4O3: 404.18; found 409.1920 [M + H]+.
4-(2-chlorophenyl)-N,3-dimethyl-5-nitro-1-phenyl-1,4-dihydropyrano[2,3-c]pyrazol-6-amine (4j)
Yield – 85%, White Solid, M. P.: 248–249 °C, IR (KBr) cm−1:831.32, 854.47, 908.47, 1056.99, 1138.00, 1361.74, 1394.53, 1519.91, 1662.64, 2949.16, 3068.75, 3178.69, 3446.79. 1H NMR (500 MHz, CDCl3) δ: 2.00 (s, 3H), 3.26 (d, 3H), 5.65 (s, 1H), 7.13–7.16 (m, 1H), 7.17–7.21 (m, 1H), 7.32–7.35 (m, 1H), 7.47–7.51 (t, 2H), 7.65–7.67 (dt, 2H), 10. 64 (d, 1H). 13C NMR (CDCl3, 125 MHz), δC (ppm): 12.41, 28.63, 99.21, 108.08, 120.42, 126.66, 127.22, 127.62, 128.28, 129.25, 129.44, 129.90, 132.33, 132.55, 136.98, 142.02, 145.11, 158.77. HRMS, Calcd. For C20H17ClN4O3: 396.10; found 419.0885 [M + Na]+.
4-(3,5-bis(trifluoromethyl)phenyl)-N,3-dimethyl-5-nitro-1-phenyl-1,4-dihydropyrano[2,3-c]pyrazol-6-amine (4 l)
Yield – 93%, White Solid, M. P.: 243–245 °C, IR (KBr) cm−1: 837.11, 1008.77, 1056.99, 1340.53, 1373.32, 1525.69, 1676.14, 1734.01, 2945.30, 2978.09, 3261.63, 3462.22. 1H NMR (500 MHz, CDCl3) δ: 1.96 (s, 3H), 3.28 (d, 3H), 5.39 (s, 1H), 7.35–7.38 (t, 1H), 7.49–7.53 (t, 2H), 7.68–7.70 (d, 2H), 7.75 (s, 3H), 10.56 (d, 1H). 13C NMR (CDCl3, 125 MHz), δC (ppm): 12.39, 28.71, 98.70, 98.70, 107.72, 120.42, 124.37, 126.65, 128.69, 128.85, 129.37, 129.73, 129.99, 136.98, 141.81, 145.27, 146.71, 158.68. HRMS, Calcd. For C22H16F6N4O3: 498.11; found 499.1211 [M + H]+.
(E)-4-(4-hydroxy-3-methoxy-5-nitrobenzylidene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one (4i-Intermediate)
IR (KBr) cm−1: 883.40, 937.40, 999.13, 1062.78, 1138.00,1280.73, 1541.72, 1653.00, 1683.86, 2841.15, 3099.61, 3446.79 1H NMR (500 MHz, CDCl3) δ: 2.34 (s, 3H), 4.08 (s, 3H), 6.32 (s, 1H), 6.99–7.00 (d, 1H), 7.19 (t, 1H), 7.31 (s, 1H), 7.39–7.43 (t, 2H), 7.50–7.52 (dd, 1H), 7.93–7.95 (dd, 2H), 9.20 (s, 1H).
(E)-4-(4-hydroxy-3,5-dimethoxybenzylidene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one (4d-Intermediate)
1H NMR (500 MHz, CDCl3) δ: 2.34 (s, 3H), 4.02 (s, 6H), 7.17 (q, 1H), 7.28 (s, 1H), 7.39–7.43 (t, 2H), 7.93–7.95 (dd, 1H), 8.01 (s, 2H). 13C NMR (CDCl3, 125 MHz), δC (ppm): 13.39, 56.64, 111.73, 119.52, 124.88, 125.15, 125.23, 128.81, 138.52, 140.30, 146.84, 147.49, 150.85, 162.33.
N,3-dimethyl-5-nitro-4-(2-nitrophenyl)-1,4-dihydropyrano[2,3-c]pyrazol-6-amine (7d)
Yield – 84%, Yellow Solid, M. P.: 248–249 °C, IR (KBr) cm−1: 792.74, 1008.77, 1072.42, 1217.08, 1354.03, 1525.69, 1647.21, 3028.24, 3066.82, 3273.20. 1H NMR (500 MHz, DMSO-d6) δ: 1.97 (s, 3H), 3.15 (d, 3H), 5.74 (s, 1H), 7.24–7.26 (dd, 1H), 7.39–7.42 (dd, 1H), 7.54–7.57 (ddd, 1H), 7.81–7.83 (dd, 1H), 10.63 (d, 1H), 12.47 (s, 1H). 13C NMR (DMSO-d6, 125 MHz), δC (ppm):9.63, 28.18, 97.29, 109.06, 122.93, 127.49, 130.53, 132.96, 136.49, 137.51, 149.68, 153.62, 159.25. HRMS, Calcd. For C14H13N5O5: 331.0917; found 332.0988 [M + H]+.
4-(1,3-dihydroisobenzofuran-5-yl)-N,3-dimethyl-5-nitro-1,4-dihydropyrano[2,3-c]pyrazol-6-amine (7 g)
Yield – 74%, White Solid, M. P.: 264–266 0C, IR (KBr) cm−1: 810.10, 921.97, 1055.06, 1363.67, 1444.68, 1502.55, 1637.56, 2887.44, 2972.31, 3130.47, 3209.55. 1H NMR (500 MHz, DMSO-d6) δ: 1.96 (s, 3H), 3.14 (d, 3H), 5.12 (s, 1H), 5.94 (s, 2H), 6.68–6.70 (dd, 1H), 6.77–6.78 (t, 2H), 10.65 (d, 1H), 12.29 (s, 1H). 13C NMR (DMSO-d6, 125 MHz), δC (ppm):9.63, 28.11, 99.04, 100.65, 101.60, 105.87, 107.63, 107.73, 108.46, 108.87, 120.29, 124.98, 135.97, 137.99, 145.39, 146.87, 153.01, 159.71, 160.56. HRMS, Calcd. For C15H14N4O5: 330.09; found 331.1048 [M + H]+.
4-(3,4-dimethoxyphenyl)-N,3-dimethyl-5-nitro-1,4-dihydropyrano[2,3-c]pyrazol-6-amine (7 h)
Yield – 91%, Pale Solid, M. P.: 260–261 °C, IR (KBr) cm−1: 813.96, 1062.78, 1141.86, 1238.30, 1336.67, 1521.84, 1635.64, 2833.43, 3080.32, 3169.04, 3313.71. 1H NMR (500 MHz, DMSO-d6) δ: 1.98 (s, 3H), 3.14 (d, 3H), 3.68 (s, 3H), 3.70 (s, 3H), 5.14 (s, 1H), 6.63–6.66 (dd, 1H), 6.80–6.81 (d, 1H), 6.85 (d, 1H), 10.63 (d, 1H), 12.26 (s, 1H). 13C NMR (DMSO-d6, 125 MHz), δC (ppm): 9.68, 28.10, 55.36, 55.48, 99.20, 108.81, 111.58, 118.68, 118.77, 135.91, 136.61, 147.13, 148.16, 150.02, 159.75 HRMS, Calcd. For C16H18N4O5: 346.13; found 347.1364 [M + H]+.
4-(2,4-dichlorophenyl)-N,3-dimethyl-5-nitro-1,4-dihydropyrano[2,3-c]pyrazol-6-amine (7i)
Yield –93%, Pale Yellow Solid, M. P.: 250–253 °C, IR (KBr) cm−1: 702.09, 864.11, 1074.35, 1361.74, 1467.83, 1641.42, 2947.23, 3016.67, 3061.03, 3089.96, 3273.20. 1H NMR (500 MHz, DMSO-d6) δ: 1.91 (s, 3H), 3.14 (d, 3H), 5.53 (s, 1H), 7.30–7.34 (m, 2H), 7.50 (d, 1H), 10.69 (q, 1H), 12.37 (s, 1H). 13C NMR (DMSO-d6, 125 MHz), δC (ppm): 9.60, 28.16, 97.13, 107.76, 127.35, 128.54, 131.44, 131.64, 132.82, 136.18, 139.13, 153.21, 153.85. HRMS, Calcd. For C14H12Cl2N4O3: 354.03; found 355.0370 [M + H]+.
2,6-dimethoxy-4-(3-methyl-6-(methylamino)-5-nitro-1,4-dihydropyrano[2,3-c]pyrazol-4-yl)phenol (7j)
Yield – 82%, Yellow Solid, M. P.: 265–266 °C, IR (KBr) cm−1: 810.10, 1060.85, 1215.15, 1361.74, 1463.97, 1639.49, 2756.28, 2833.43, 2972.31, 3170.97. 1H NMR (500 MHz, DMSO-d6) δ: 2.01 (s, 3H), 3.14 (d, 3H), 3.68 (s, 6H), 5.53 (s, 1H), 6.4 (s, 2H), 8.13 (s, 1H), 10.65 (d, 1H), 12.25 (s, 1H). 13C NMR (DMSO-d6, 125 MHz), δC (ppm): 9.80, 28.13, 55.91, 56.01, 99.21, 104.80, 108.66, 134.12, 134.25, 135.98, 147.62, 153.02, 159.02. HRMS, Calcd. For C16H18N4O6: 362.12; found 363.1309 [M + H]+.
3-(3-methyl-6-(methylamino)-5-nitro-1,4-dihydropyrano[2,3-c]pyrazol-4-yl)phenol (7 k)
Yield – 89%, White Solid, M. P.: 269–270 °C, IR (KBr) cm−1: 781.17, 1008.77, 1066.64, 1213.23, 1249.87, 1363.67, 1458.18, 1608.63, 1641.42, 2885.51, 2947.23, 2916.76, 3253.91. 1H NMR (500 MHz, DMSO-d6) δ: 1.97 (s, 3H), 3.14 (d, 3H), 5.09 (s, 1H), 6.52–6.53 (m, 1H), 6.54–6.56 (m, 1H), 6.63–6.65 (d, 1H), 7.01–7.04 (t, 1H), 9.22 (s, 1H), 10.63 (d, 1H), 12.28 (s, 1H). 13C NMR (DMSO-d6, 125 MHz), δC (ppm):9.68, 28.11, 99.07, 108.07, 113.20, 113.78, 117.91, 128.89, 135.92, 145.34, 153.06, 157.07, 159.78. HRMS, Calcd. For C14H14N4O4: 302.10; found 303.1100 [M + H]+.
4-(4-isopropylphenyl)-N,3-dimethyl-5-nitro-1,4-dihydropyrano[2,3-c]pyrazol-6-amine (7 l)
Yield – 85%, White Solid, M. P.: 260–62 °C, IR (KBr) cm−1: 702.09, 823.60, 1060.85, 1074.35, 1354.03, 1467.83, 1647.21, 2870.08, 2889.37, 2958.80, 3182.55, 3298.28. 1H NMR (500 MHz, DMSO-d6) δ: 1.14 (d, 6H), 1.94 (s, 3H), 2.76–2.85 (sept, 1H), 3.02 (d, 3H), 5.15 (s, 1H), 7.10 (s, 4H), 10.63 (d, 1H), 12.28 (s, 1H). 13C NMR (DMSO-d6, 125 MHz), δC (ppm):9.68, 23.77, 23.80, 28.16, 32.89, 99.27, 108.99, 125.94, 127.03, 135.90, 141.37, 146.11, 153.14, 159.86. HRMS, Calcd. For C17H20N4O3: 328.15; found 329.1664 [M + H]+.
2-methoxy-4-(3-methyl-6-(methylamino)-5-nitro-1,4-dihydropyrano[2,3-c]pyrazol-4-yl)phenol (7 m)
Yield – 88%, Pale Yellow Solid, M. P.: 274–276 °C, IR (KBr) cm−1: 748.38, 817.82, 1064.71, 1269.16, 1338.60, 1392.61, 1514.12, 1635.64, 2937.59, 3026.31, 3250.05, 3419.79. 1H NMR (500 MHz, DMSO-d6) δ: 1.99 (s, 3H), 3.14 (d, 3H), 3.72 (s, 3H), 5.10 (s, 1H), 6.51–6.53 (dd, 1H), 6.63–6.65 (d, 1H), 6.82 (d, 1H), 8.74 (s, 1H), 10.65 (d, 1H), 12.26 (s, 1H). 13C NMR (DMSO-d6, 125 MHz), δC (ppm): 9.70, 28.11, 55.65, 99.46, 108.99, 111.95, 115.17, 119.09, 135.06, 135.90, 144.85, 146.96, 153.07, 159.80. HRMS, Calcd. For C15H16N4O5: 332.1121; found 333.1216 [M + H]+.
2-methoxy-4-(3-methyl-6-(methylamino)-5-nitro-1,4-dihydropyrano[2,3-c]pyrazol-4-yl)-6-nitrophenol (7n)
Yield – 90%, Brown Red Solid, M. P.: 280–81 °C, IR (KBr) cm−1: 779.24, 1068.56, 1257.59, 1338.60, 1359.82, 1541.12, 1639.49, 2953.02, 2987.74, 3032.10, 3136.25, 3184.48, 3566.38, 3618.46. 1H NMR (500 MHz, DMSO-d6) δ: 1.99 (s, 3H), 3.15 (d, 3H), 3.82 (s, 3H), 5.22 (s, 1H), 7.19 (d, 1H), 7.22 (d, 1H), 9.74 (s, 1H), 10.68 (d, 1H), 12.34 (s, 1H). 13C NMR (DMSO-d6, 125 MHz), δC (ppm):9.76, 28.18, 56.61, 98.17, 108.08, 113.74, 115.77, 134.34, 136.33, 136.62, 141.57, 149.18, 152.97, 159.76. HRMS, Calcd. For C15H15N5O7: 377.10; found 378.1057 [M + H]+.
4-(2-chlorophenyl)-N,3-dimethyl-5-nitro-1,4-dihydropyrano[2,3-c]pyrazol-6-amine (7o)
Yield – 74%, White Solid, M. P.: 255–256 °C, IR (KBr) cm−1: 758.02, 1033.85, 1078.21, 1213.23, 1373.32, 1396.46, 1460.11, 1608.63, 1637.56, 2949.16, 3142.04, 3261.63. 1H NMR (500 MHz, DMSO-d6) δ: 1.90 (s, 3H), 3.15 (d, 3H), 5.34 (s, 1H), 7.17–7.20 (td, 1H), 7.21–7.24 (td, 1H), 7.27–7.28 (d, 1H), 7.33–7.35 (dd, 1H),10.70 (d, 1H), 12.33 (s, 1H). 13C NMR (DMSO-d6, 125 MHz), δC (ppm):9.60, 28.14, 97.63, 108.07, 127.14, 127.95, 129.25, 130.23, 131.88, 136.05, 140.40, 153.25, 159.52. HRMS For C14H12ClN4O3: 320.05; found 321.0755 [M + H]+.
4-(4-chlorophenyl)-N,3-dimethyl-5-nitro-1,4-dihydropyrano[2,3-c]pyrazol-6-amine (7p)
Yield – 93%, White Solid, M. P.: 244–246 °C, IR (KBr) cm−1: 817.82, 1006.84, 1209.37, 1363.67, 1604.77, 1643.35, 2819.93, 2981.95, 3022.45, 3238.48. 1H NMR (500 MHz, DMSO-d6) δ: 1.93 (s, 3H), 3.14 (d, 3H), 5.20 (s, 1H), 7.24–7.27 (dt, 2H), 7.29–7.31 (dt, 2H), 10.66 (d, 1H), 12.33 (s, 1H). 13C NMR (DMSO-d6, 125 MHz), δC (ppm): 9.58, 28.14, 98.49, 108.51, 137.93, 128.97, 129.12, 129.90, 130.61, 136.15, 142.96, 152.97, 159.69, 160.47. HRMS, For C14H12ClN4O3: 320.07; found 321.0752 [M + H]+.
4-(3,5-bis(trifluoromethyl)phenyl)-N,3-dimethyl-5-nitro-1,4-dihydropyrano[2,3-c]pyrazol-6-amine (7q)
Yield – 96%, White Solid, M. P.: 267–269 °C, IR (KBr) cm−1: 902.69, 1074.35, 1130.29, 1166.93, 1278.81, 1352.10, 1465.90, 1641.42, 2974.23, 3024.38, 3066.82, 3246.20.1H NMR (500 MHz, DMSO-d6) δ: 1.88 (s, 3H), 3.16 (d, 3H), 5.51 (s, 1H), 7.91 (s, 1H), 8.02 (s, 2H), 10.75 (d, 1H), 12.41 (s, 1H). 13C NMR (DMSO-d6, 125 MHz), δC (ppm): 9.52, 28.23, 97.28, 107.65, 120.09, 122.17, 124.33, 126.51, 128.19, 129.52, 129.78, 130.03, 136.59, 147.52, 152.86, 159.81. HRMS, Calcd. For C16H12F6N4O3: 422.0814; found 423.0901 [M + H]+.
Notes
aIsolated yield
References
Zuin VG, Eilks I, Elschami M, Kümmerer K (2021) Education in green chemistry and in sustainable chemistry: perspectives towards sustainability. Green Chem 23(4):1594–1608. https://doi.org/10.1039/D0GC03313H
Katariya AP, Bhagat DS, Katariya MV, Pawar RP (2017) Green techniques in organic synthesis and its advantages. J Med Chem Drug Discov 3:62–66
Jiang B, Rajale T, Wever W, Tu SJ, Li G (2010) Multicomponent reactions for the synthesis of heterocycles. Chem Asian J 5(11):2318–2335. https://doi.org/10.1002/asia.201000310
Naresh G, Lakkaniga NR, Kharbanda A, Yan W, Frett B (2019) Li HY (2019) Use of Imidazo [1, 2-a] pyridine as a carbonyl surrogate in a Mannich-Like, catalyst free, one-pot reaction. Eur J Org Chem 4:770–777. https://doi.org/10.1002/ejoc.201801430
Naresh G, Kharbanda A, Lakkaniga NR, Zhang L, Cooper R, Li HY, Frett B (2018) Catalyst free, C-3 functionalization of imidazo [1, 2-a] pyridines to rapidly access new chemical space for drug discovery efforts. Chem Commun 54(92):12954–12957. https://doi.org/10.1021/ol502072k
Naresh G, Kant R, Narender T (2014) Copper (II) catalyzed expeditious synthesis of furoquinoxalines through a one-pot three-component coupling strategy. Org Lett 16(17):4528–4531. https://doi.org/10.1021/ol502072k
Rotstein BH, Zaretsky S, Rai V, Yudin AK (2014) Small heterocycles in multicomponent reactions. Chem Rev 114(16):8323–8359. https://doi.org/10.1021/cr400615v
Boukis AC, Reiter K, Frölich M, Hofheinz D, Meier MAR (2018) Multicomponent reactions provide key molecules for secret communication. Nat Commun 9(1):1439. https://doi.org/10.1038/s41467-018-03784-x
Martins MAP, Frizzo CP, Moreira DN, Buriol L, Machado P (2009) Solvent-free heterocyclic synthesis. Chem Rev 109(9):4140–4182. https://doi.org/10.1021/cr9001098
Zangade S, Patil P (2019) A review on solvent-free methods in organic synthesis. Curr Org Chem 23(21):2295–2318. https://doi.org/10.2174/1385272823666191016165532
Metzger JO (1998) Solvent-free organic syntheses. Angew Chem Int Ed 37(21):2975–2978. https://doi.org/10.1002/(SICI)1521-3773(19981116)37:21%3c2975::AID-ANIE2975%3e3.0.CO;2-A
Olivier-Bourbigou H, Magna L, Morvan D (2010) Ionic liquids and catalysis: recent progress from knowledge to applications. Appl Catal Gen 373(1–2):1–56. https://doi.org/10.1016/j.apcata.2009.10.008
Shi F, Gu Y, Zhang Q, Deng Y (2004) Development of ionic liquids as green reaction media and catalysts. Catal Surv from Asia 8(3):179–186
Zhang Q, Zhang S, Deng Y (2011) Recent advances in ionic liquid catalysis. Green Chem 13(10):2619–2637. https://doi.org/10.1039/c1gc15334j
Das D, Banerjee R, Mitra A (2014) Bioactive and pharmacologically important pyrano[2,3-c]pyrazoles. J Chem Pharm Res 6(11):108–116
Mohamed NR, Khaireldin NY, Fahmy AF, El-Sayed AA (2010) Facile synthesis of fused nitrogen containing heterocycles as anticancer agents. Pharma Chem 2(1):400–417
Foloppe N, Fisher LM, Howes R, Potter A, Robertson AGS, Surgenor AE (2006) Identification of chemically diverse Chk1 inhibitors by receptor-based virtual screening. Bioorg Med Chem 14:4792–4802
Ramtekkar R, Kumarvel K, Vasuki G, Sekar K, Krishna R (2009) Computer-aided drug design of pyranopyrazoles and related compounds for checkpoint kinase-. Lett Drug Des Discovery 6:579–584
Saundane AR, Walmik P, Yarlakatti M, Katkar V, Verma VA (2014) Synthesis and biological activities of some new annulated pyrazolopyranopyrimidines and their derivatives containing indole nucleus: synthesis and biological activities of some new annulated pyrazolopyranopyrimidines and their derivatives containing indole nucleus. J Heterocycl Chem 51(2):303–314. https://doi.org/10.1002/jhet.1582
Ismail MMF, Khalifa NM, Fahmy HH, Nossier ES, Abdulla MM (2014) Design, docking, and synthesis of some new pyrazoline and pyranopyrazole derivatives as anti-inflammatory agents. J Heterocycl Chem 51(2):450–458. https://doi.org/10.1002/jhet.1757
Ueda T, Mase H, Oda N, Ito I (1891) Synthesis of pyrazolone derivatives XXXIX. Synthesis and analgesic activity of pyrano [2, 3-c] pyrazoles. Chem Pharm Bull (Tokyo) 29(12):3522–3528. https://doi.org/10.1248/cpb.29.3522
Capodanno D, Ferreiro JL, Angiolillo DJ (2013) Antiplatelet therapy: new pharmacological agents and changing paradigms. J Thromb Haemost 11:316–329. https://doi.org/10.1111/jth.12219
Tacconi G, Gatti G, Desimoni G, Messori VA (1980) New route to 4H-pyrano[2,3-c]pyrazoles. J Für Prakt Chem 322(5):831–834. https://doi.org/10.1002/prac.19803220519
Abdelrazek FM, Metz P, Metwally NH, El-Mahrouky SF (2006) Synthesis and molluscicidal activity of new cinnoline and pyrano [2,3-c]pyrazole derivatives. Arch Pharm (Weinheim) 339(8):456–460. https://doi.org/10.1002/ardp.200600057
Yadav DK, Quraishi MA (2012) Electrochemical investigation of substituted pyranopyrazoles adsorption on mild steel in acid solution. Ind Eng Chem Res 51(24):8194–8210. https://doi.org/10.1021/ie3002155
Vasuki G, Kumaravel K (2008) Rapid four-component reactions in water: synthesis of pyranopyrazoles. Tetrahedron Lett 49(39):5636–5638. https://doi.org/10.1016/j.tetlet.2008.07.055
Kanagaraj K, Pitchumani K (2010) Solvent-free multicomponent synthesis of pyranopyrazoles: Per-6-Amino-β-Cyclodextrin as a remarkable catalyst and host. Tetrahedron Lett 51(25):3312–3316. https://doi.org/10.1016/j.tetlet.2010.04.087
Zolfigol MA, Tavasoli M, Moosavi-Zare AR, Moosavi P, Kruger HG, Shiri M, Khakyzadeh V (2013) Synthesis of pyranopyrazoles using isonicotinic acid as a dual and biological organocatalyst. RSC Adv 3(48):25681. https://doi.org/10.1039/c3ra45289a
Reddy MBM, Jayashankara VP, Pasha MA (2010) Glycine-catalyzed efficient synthesis of pyranopyrazoles via one-pot multicomponent reaction. Synth Commun 40(19):2930–2934. https://doi.org/10.1080/00397910903340686
Katariya AP, Deshmukh SU, Munde SB, Katariya MV, Pawar RP (2019) Green and expeditious one pot synthesis of pyrano[2,3-c]pyrazole using potassium ter-butoxide catalyst in aqueous medium. Int J Green Herb Chem. https://doi.org/10.24214/IJGHC/GC/8/3/79097
Kanchithalaivan S, Sivakumar S, Ranjith Kumar R, Elumalai P, Ahmed QN, Padala AK (2013) Four-component domino strategy for the combinatorial synthesis of novel 1,4-Dihydropyrano[2,3- c ]Pyrazol-6-Amines. ACS Comb Sci 15(12):631–638. https://doi.org/10.1021/co4000997
Jayabal K, Paramasivan TP (2014) An expedient four-component domino protocol for the regioselective synthesis of highly functionalized pyranopyrazoles and chromenopyrazoles via nitroketene-N, S-acetal chemistry under solvent-free condition. Tetrahedron Lett 55(12):2010–2014. https://doi.org/10.1016/j.tetlet.2014.02.019
Survase DN, Chavan HV, Dongare SB, Ganapure SD, Helavi VB (2017) Indium chloride (InCl3) catalysed domino protocol for the regioselective synthesis of highly functionalized pyranopyrazoles under mild conditions. Iran Chem Commun 5:105–114
Khan MdM, Shareef S, Saigal S, Sahoo SCA (2019) Catalyst and solvent-free protocol for the sustainable synthesis of fused 4H-pyran derivatives. RSC Adv 9(45):26393–26401. https://doi.org/10.1039/C9RA04370E
Katariya AP, Yadav AR, Pawar OB, Pisal PM, Sangshetti JN, Katariya MV, Deshmukh SU (2022) An Efficient and green synthesis of tetrahydrobenzo[b]pyan derivatives using [(EMIM)Ac] at room temperature. ChemistrySelect. https://doi.org/10.1002/slct.202104184
Katariya AP, Katariya MV, Sangshetti J, Deshmukh SU (2022) Ionic liquid [(EMIM)Ac] catalyzed green and efficient synthesis of Pyrano[2,3- c ]Pyrazole derivatives. Polycycl Aromat Compd. https://doi.org/10.1080/10406638.2022.2077775
Katariya AP, Gaikwad PB, Kadam GG, Katariya MV, Deshmukh SU (2022) Ionic liquid promoted regio-selective synthesis of 2-Methyl Amino-3-Nitro-pyrano[3,2- c ]chromen-5-ones. ChemistrySelect. https://doi.org/10.1002/slct.202201295
Katariya AP, Deshmukh SU, Tekale SU, Katariya MV, Pawar RP (2021) Amberlite IR-120 catalyzed green and efficient one-pot synthesis of benzylpyrazolyl coumarin in aqueous medium. Lett Appl NanoBioSci 10(3):2525–2534. https://doi.org/10.33263/LIANBS103.25252534
Deshmukh SU, Sangshetti JN, Bhosale SV, Pawar RP (2021) Benzopyranyl phosphonate and β-phosphono malonates derivatives: an exciting breakthrough in chemistry. ChemistrySelect 6(4):617–629. https://doi.org/10.1002/slct.202004159
Deshmukh SU, Kharat KR, Kadam GG, Pawar RP (2018) Synthesis of novel α-aminophosphonate derivatives, biological evaluation as potent antiproliferative agents and molecular docking. ChemistrySelect 3(20):5552–5558. https://doi.org/10.1002/slct.201800798
Thorat VV, Dake SA, Deshmukh SU, RasokkiyamUddin EMF, Pawar RP (2013) Ionic liquid mediated synthesis of novel tetrahydroimidazo [1,2- a]pyrimidine-6-carboxylate derivatives. Lett Org Chem 10(3):178–184
Ansari SA, Deshmukh SU, Patil RB, Damale MG, Patil RH, Alkahtani HM, Almehizia AA, Al-Tuwajiri HM, Aleanizy FS, Alqahtani FY, Pathan SK, Sangshetti JN (2019) Identification of promising biofilm inhibitory and cytotoxic quinazolin-4-one derivatives: synthesis, evaluation molecular docking and ADMET studies. ChemistrySelect 4(12):3559–3566. https://doi.org/10.1002/slct.201803795
Acknowledgements
SAIF-PU, Panjab University, Chandigarh, provided the spectrum data, such as 1H & 13C NMR, to the authors, and they would like to express their gratitude for their assistance in this study. This research work would not have been possible without the general facilities provided by Deogiri College, Aurangabad.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11030_2022_10572_MOESM1_ESM.docx
Supplementary file1 (DOCX 3321 KB) The IR, 1H, 13C NMR, and HRMS spectra of new and a few previously known pyranopyrazole compounds are included in a supporting information also consist of a table shows synthesized compound’s yield and melting point
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Katariya, A.P., Shirsath, P.D., Narode, H. et al. Unraveling the access to the regioselective synthesis of highly functionalized pyranopyrazoles using an ionic liquid catalyst. Mol Divers 27, 2633–2649 (2023). https://doi.org/10.1007/s11030-022-10572-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10572-9