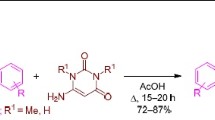
Abstract
5-Arylidene-1-methyl-2-thiohydantoins undergo [3+2]-cycloaddition reaction with nitrile imines, generated in situ from hydrazonyl chlorides, at C=C and C=S dipolarophiles in the thiohydantoin moiety to afford thioxo-tetraazaspiro[4.4]nonenones and thia-tetraazaspiro[4.4]nonenones in moderate to good yields. The stereochemistry of these spiroheterocycles has been confirmed by X-ray diffraction studies.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The creation of molecular complexity and diversity in potential drug candidates and biologically important molecules from common starting materials is a challenge in modern organic chemistry from both academic and industrial viewpoints [1, 2]. A protocol to achieve these goals involves the use of molecular hybridization approach that involves coupling of different pharmacophores with varied bioactivities in one molecular framework. Such hybrid molecules have been used for treatment of metabolic disorders, malaria, inflammation, and ischemia [3, 4].
The construction of spirocycles, besides facilitating the expedient creation of chemical libraries of structurally diverse compounds, plays a key role in combinatorial synthesis. Interest in spirocyclic structures stems not only from their structural properties but also from their biological activities and their occurrence in a wide range of natural products [5,6,7,8,9,10].
Arylidene-thioxoimidazolidinone skeleton, commonly known as “arylidenethiohydantoin,” is an important structural unit, which can be used as building blocks in spiroheterocycles preparation [11,12,13,14]. The cycloaddition reactions of nitrile imines with arylidenethiohydantoins afford nitrogen-containing spiroheterocycles.
Nitrile imines, generally prepared from hydrazonyl halides, are important transient 1,3-dipolar species and have been utilized as useful synthons of spiroheterocycles in organic synthesis [15,16,17]. In arylidenethiohydantoins, both the exocyclic C=C and C=S bonds could be considered as the potential dipolarophilic units. The 1,3-dipolar cycloaddition of nitrile imines to arylidenethiohydantoins is poorly studied in the literature. In 1995, Hassaneen et al. reported three examples of 1,3-dipolar cycloaddition of nitrile imines to arylidenethiohydantoins [18]. However, the reaction in these reported three examples was claimed to be chemoselective to the C=C dipolarophile (see Scheme 1). In 2007, Jakse et al. reported two examples of 1,3-dipolar cycloaddition of nitrile imines to arylidenethiohydantoins which were chemoselective to the C=S dipolarophile [19]. As a part of our interest in the cycloaddition reaction of nitrile imines with various dipolarophiles [20,21,22,23], we reinvestigated the 1,3-dipolar cycloaddition of nitrile imines to arylidenethiohydantoins using 5-arylidene-1-methyl-2-thiohydantoins 2 as potential reaction partners of nitrile imines 1. As shown in Scheme 1, 1,3-dipolar cycloaddition reaction took place at the C=C and C=S dipolarophiles in the imidazole moiety to afford the corresponding spiroproducts.
Results and discussion
The 5-arylidene-1-methyl-2-thiohydantoins 2 were prepared by refluxing a mixture of benzaldehydes, appropriate isothiocyanates, and sarcosine in ethanol containing Et3N, according to a previously reported method [12]. In a test experiment, we conducted the reaction between N-phenylbenzohydrazonyl chloride (1a) and 1-methyl-5-(4-methylbenzylidene)-3-phenyl-2-thioxoimidazolidin-4-one (2a) in the presence of Et3N in MeCN at room temperature. After 3 h, two products, namely 6-methyl-1,3,8-triphenyl-7-thioxo-4-(p-tolyl)-1,2,6,8-tetraazaspiro[4.4]non-2-en-9-one (3a) and 9-methyl-8-(4-methylbenzylidene)-1,3,6-triphenyl-4-thia-1,2,6,9-tetraazaspiro[4.4]non-2-en-7-one (4a), were isolated in 38% and 49% yields, respectively. Then, the reaction conditions, including the base and solvent, were optimized. As shown in Table 1, comparison of results obtained in the presence of different bases and solvents shows that Et3N and MeCN were superior to the others.
With the optimal reaction conditions in hand, the scope of the substrates was investigated (see Table 2). Various hydrazonyl chlorides having electron-donating or withdrawing groups at different positions of the benzene ring performed the reaction well to give products 3 and 4 in moderate to good yields. In general, substrates with electron-withdrawing groups on the benzene ring exhibited higher reactivity than those with electron-donating groups. Next, we carried out an investigation on precursors 2 with different substituents at 4-position of the aryl moiety. The electronic properties of the substituents on the benzene ring seem to have no significant effect on the reaction with the exception of 4-NMe2 substituted precursor (2i), leading to product 4i.
The structures of spiroadducts 3 and 4 resulting from the cycloaddition were deduced from their IR, 1H NMR, and 13C NMR spectra and their mass spectrometric data. The 13C NMR spectra of 3a and 4a each exhibit 23 signals in agreement with the proposed structures. The mass spectra of products 3a and 4a displayed the molecular ion peak at m/z = 502. The regio- and the stereochemical outcome of the cycloaddition was furthermore ascertained by X-ray analysis of the crystal structure of cycloadducts 3a and 4g, whose ORTEP presentations are shown in Fig. 1. The same structures were assumed for the other derivatives on the basis of their NMR spectroscopic similarities. As shown by NMR and X-ray analysis, the regioisomeric configurations of cycloadducts 3 are opposite compared to the products reported by Hasseneen et al. (see Scheme 1).
To explain the formation of products 3 and 4, the following mechanistic pathway is proposed (Scheme 1). The hydrazonyl chlorides 1, under basic conditions, produce the nitrile imine species 5. This 1,3-dipolar system undergoes chemoselective @@@[3+2]-cycloaddition reactions with the exocyclic C=C and C=S dipolarophiles of thiohydantoin 2 to afford thioxo-tetraazaspiro[4.4]nonenones 3 and thia-tetraazaspiro[4.4]nonenones 4, respectively (Scheme 2).
Conclusion
In summary, we have reinvestigated the 1,3-dipolar cycloaddition of 5-arylidene-1-methyl-2-thiohydantoins as potential reaction partners of nitrile imines. The 1,3-dipolar cycloaddition reaction took place at both the C=C and C=S double bonds of thiohydantoin moiety to afford spiroadducts thioxo-tetraazaspiro[4.4]nonenones and thia-tetraazaspiro[4.4]nonenones in moderate to good yields. A range of thiohydantoins and nitrile imines are compatible with the mild reaction conditions. Thus, the 1,3-dipolar cycloaddition reaction of nitrile imines is found to be chemoselective at both C=C and C=S dipolarophile moieties of arylidenethiohydantoins.
Experimental section
General remarks
All purchased solvents and chemicals were of analytical grade and used without further purification. Melting points were measured on an Electrothermal 9100 capillary melting point apparatus and are uncorrected. IR spectra were taken on a IR-460 Shimadzu spectrometer in KBr pellets and reported in cm−1. The 1H and 13C spectra were obtained with a BRUKER DRX-500 AVANCE instrument using CDCl3 as applied solvent and TMS as internal standard at 500.1 and 125.7 MHz, respectively. The chemical shift (δ) is given in ppm (s = singlet, d = doublet, t = triplet, brs = broad singlet, m = multiplet), coupling constant in Hz. Mass spectra were recorded on a FINNIGAN-MAT 8430 mass spectrometer operating at an ionization potential of 70 eV. Elemental analyses for C, H and N were performed using a Heraeus CHN-O-Rapid analyzer. X-Ray crystallographic analysis was performed with a STOE IPDS 2T diffractometer.
General procedure for the synthesis of compounds 3 and 4
A mixture of hydrazonyl chloride derivative 1 (1 mmol) and Et3N (0.101 g, 1 mmol) in MeCN (3 mL) was stirred at r.t. for 15 min. Then, 5-arylidene-1-methyl-2-thiohydantoins 2 (1 mmol) was added to the above mixture, and the reaction was stirred at r.t. for 3 h. After completion of the reaction (the progress of the reaction was followed by TLC), the solvent was removed under reduced pressure. The crude residue was purified by column chromatography [silica gel (230–400 mesh; Merck, n-hexane/AcOEt 7:1] to give the products 3 and 4. Retention factor (Rf) values of products 4 (Rf = 0.70–0.75) were found to be higher than those of compounds 3 (Rf = 0.60–0.65).
8-Methyl-1,3,6-triphenyl-7-thioxo-4-(p-tolyl)-1,2,6,8-tetraazaspiro[4.4]non-2-en-9-one (3a)
Colorless powder; yield: 0.19 g (38%); mp: 143–145 °C; IR (KBr) (νmax/cm−1): 1749, 1660, 1595; 1H NMR (500 MHz, CDCl3): δH = 2.33 (3 H, s, Me), 3.43 (3 H, s, Me), 5.20 (1 H, s, CH), 6.87 (2 H, d, 3J = 7.4 Hz, CH), 7.07 (1 H, t, 3J = 7.4 Hz, CH), 7.11–7.15 (4 H, m, CH), 7.19 (2 H, d, 3J = 8.1 Hz, CH), 7.28 (2 H, t, 3J = 7.2 Hz, CH), 7.34–7.37 (6 H, m, CH), 7.67 (2 H, d, 3J = 7.4 Hz, CH); 13C NMR (125.7 MHz, CDCl3): δC = 21.3 (Me), 29.8 (Me), 61.1 (CH), 89.5 (C), 115.5 (2 CH), 122.7 (CH), 126.7 (2 CH), 128.0 (2 CH), 128.4 (CH), 128.5 (CH), 128.6 (2 CH), 129.0 (2 CH), 129.4 (2 CH), 129.6 (2 CH), 129.7 (2 CH), 130.6 (C), 133.7 (C), 133.8 (C), 138.7 (C),142.7 (C), 148.4 (C=N), 166.8 (C=O), 181.0 (C=S); MS (EI, 70 eV): m/z (%) = 502 (M+, 1), 429 (8), 396 (5), 367 (22), 337 (42), 309 (23), 261 (15), 194 (60), 169 (10), 135 (22), 91 (100), 51 (15); Anal. Calcd for C31H26N4OS (502.63): C, 74.08; H, 5.21; N, 11.15%. Found: C, 74.44; H, 5.23; N, 11.47%.
4-(4-Chlorophenyl)-6-methyl-1,3,8-triphenyl-7-thioxo-1,2,6,8-tetraazaspiro[4.4]non-2-en-9-one (3b)
Colorless powder; yield: 0.21 g (40%); mp: 91–93 °C; IR (KBr) (νmax/cm−1): 1756, 1586, 1520; 1H NMR (500 MHz, CDCl3): δH = 3.42 (3 H, s, Me), 5.17 (1 H, s, CH), 6.89 (2 H, d, 3J = 7.4 Hz, CH), 7.08 (1 H, t, 3J = 7.3 Hz, CH), 7.16–7.18 (4 H, m, CH), 7.32 (2 H, d, 3J = 8.3 Hz, CH), 7.33–7.37 (5 H, m, CH),. 7.38–7.41 (3 H, m, CH), 7.63 (2 H, d, 3J = 7.3 Hz, CH); 13C NMR (125.7 MHz, CDCl3): δC = 29.9 (Me), 60.7 (CH), 89.1 (C), 115.7 (2 CH), 123.1 (CH), 126.6 (2 CH), 127.7 (2 CH), 128.7 (2 CH), 129.1 (2 CH), 129.2 (CH), 129.3 (2 CH), 129.6 (2 CH), 129.6 (2 CH), 130.1 (C), 130.7 (C), 130.8 (CH), 132.5 (C), 135.0 (C),142.4 (C), 147.8 (C=N), 166.8 (C=O), 180.9 (C=S); MS (EI, 70 eV): m/z (%) = 522 (M+, 1), 449 (9), 416 (50), 357 (60), 329 (15), 381 (100), 281 (18), 227 (14), 194 (60), 169 (55), 150 (11), 133 (48), 107 (8), 91 (100), 64 (3); Anal. Calcd for C30H23ClN4OS (522.13): C, 68.89; H, 4.43; N, 10.71%. Found: C, 68.98; H, 4.45; N, 10.97%.
4-(4-Chlorophenyl)-3-(4-fluorophenyl)-6-methyl-1,8-diphenyl-7-thioxo-1,2,6,8-tetraazaspiro[4.4]non-2-en-9-one (3c)
Colorless powder; yield: 0.21 g (39%); mp: 149–151 °C; IR (KBr) (νmax/cm−1): 1758, 1597,1500; 1H NMR (500 MHz, CDCl3): δH = 3.43 (3 H, s, Me), 5.14 (1 H, s, CH), 6.85 (2 H, d, 3J = 7.3 Hz, CH), 7.04 (2 H, t, 3J = 8.6 Hz, CH), 7.08 (1 H, t, 3J = 7.4 Hz, CH), 7.15–7.17 (4 H, m, CH), 7.32 (2 H, d, 3J = 8.4 Hz, CH), 7.36 (2 H, d, 3J = 7.4 Hz, CH), 7.38–7.40 (3 H, m, CH), 7.61 (2 H, d, 3J = 8.6 Hz, CH); 13C NMR (125.7 MHz, CDCl3): δC = 29.9 (Me), 60.6 (CH), 89.3 (C), 115.7 (2 CH), 115.9 (d, 2JC-F = 21.4 Hz), 123.2 (CH), 126.8 (d, 4JC-F = 3.8 Hz), 127.8 (2 CH), 128.5 (d, 3JC-F = 8.8 Hz), 129.2 (2 CH), 129.3 (2 CH), 129.6 (4 CH), 130.4 (C), 130.8 (CH), 132.5 (C), 135.2 (C), 142.4 (C), 146.9 (C=N), 163.4 (d, 1JC-F = 250 Hz), 166.6 (C=O), 181.0 (C=S); MS (EI, 70 eV): m/z (%) = 540 (M+, 1), 328 (50), 271 (13), 212 (15), 150 (100), 123 (53), 77 (80), 51 (25); Anal. Calcd for C30H22ClFN4OS (540.12): C, 66.60; H, 4.10; N, 10.36%. Found: C, 66.91; H, 4.12; N, 10.75%.
3-(4-Fluorophenyl)-6-methyl-1,8-diphenyl-7-thioxo-4-(p-tolyl)-1,2,6,8 tetraazaspiro[4.4]non-2-en-9-one (3d)
Colorless powder; yield: 0.18 g (35%); mp: 162–164 °C; IR (KBr) (νmax/cm−1): 1756, 1598, 1494; 1H NMR (500 MHz, CDCl3): δH = 2.33 (3 H, s, Me), 3.45 (3 H, s, Me), 5.17 (1 H, s, CH), 6.83 (2 H, d, 3J = 6.5 Hz, CH), 7.02 (2 H, t, 3J = 8.6 Hz, CH), 7.06 (1 H, t, 3J = 7.6 Hz, CH), 7.10 (2 H, d, 3J = 7.9 Hz, CH), 7.14–7.17 (4 H, m, CH), 7.34–7.38 (5 H, m, CH), 7.64 (2 H, d, 3J = 8.6 Hz, CH); 13C NMR (125.7 MHz, CDCl3): δH = 2.87 (3 H, s, Me), 5.78 (1 H, s, CH), 7.07 (2 H, t, 3J = 8.6 Hz, Ar), 7.12 (1 H, m, Ar), 7.23 (2 H, d, 3J = 8.2 Hz, Ar), 7.30 (5 H, m, Ar), 7.36 (4 H, m, Ar), 7.56 (2 H, d, 3J = 8.7 Hz, Ar), 7.78 (2 H, d, 3J = 8.6 Hz, Ar); 13C NMR (125.7 MHz, CDCl3): δC = 21.2 (Me), 29.8 (Me), 60.9 (CH), 89.7 (C), 115.5 (2 CH), 115.8 (d, 2JC-F = 22.7 Hz), 122.8 (CH), 126.8 (d, 4JC-F = 2.8 Hz), 127.9 (2 CH), 128.6 (d, 3JC-F = 8.8 Hz), 128.7 (C), 129.0 (2 CH), 129.2 (2 CH), 129.4 (CH), 129.6 (2 CH), 129.8 (2 CH), 132.7 (C), 138.9 (C), 142.7 (C), 147.4 (C=N), 163.3 (d, 1JC-F = 250 Hz), 166.7 (C=O), 181.1 (C=S); MS (EI, 70 eV): m/z (%) = 520 (M+, 1), 428 (20), 368(9), 353 (50), 325 (8), 148 (70), 120 (100), 105 (25); Anal. Calcd for C31H25FN4OS (520.17): C, 71.52; H, 4.84; N, 10.76%. Found: C, 71.85; H, 4.86; N, 10.95%.
3-(4-Chlorophenyl)-6-methyl-1,8-diphenyl-7-thioxo-4-(p-tolyl)-1,2,6,8-tetraazaspiro[4.4]non-2-en-9-one (3e)
Colorless powder; yield: 0.23 g (42%); mp: 149–151 °C; IR (KBr) (νmax/cm−1): 1760, 1596, 1500; 1H NMR (500 MHz, CDCl3): δH = 2.33 (3 H, s, Me), 3.44 (3 H, s, Me), 5.16 (1 H, s, CH), 6.82 (2 H, d, 3J = 6.5 Hz, CH), 7.05–7.09 (3 H, m, CH), 7.13–7.17 (4 H, m, CH), 7.30 (2 H, d, 3J = 8.6 Hz, CH), 7.34–7.37 (5 H, m, CH), 7.58 (2 H, d, 3J = 8.6 Hz, CH); 13C NMR (125.7 MHz, CDCl3): δC = 21.2 (Me), 29.8 (Me), 60.7 (CH), 89.7 (C), 115.5 (2 CH), 122.9 (CH), 127.8 (2 CH), 127.9 (2 CH), 128.6 (C), 128.9 (2 CH), 129.0 (2 CH), 129.1 (C), 129.2 (2 CH), 129.3 (CH), 129.6 (2 CH), 129.8 (2 CH), 132.6 (C), 135.3 (C), 138.9 (C),142.6 (C), 147.3 (C=N), 166.7 (C=O), 181.1 (C=S); MS (EI, 70 eV): m/z (%) = 536 (M+, 1), 370(9), 281 (50), 182 (8), 143 (70), 106 (100), 91 (25), 77 (10), 43 (4); Anal. Calcd for C31H25ClN4OS (536.14): C, 69.33; H, 4.69; N, 10.43%. Found: C, 69.76; H, 4.71; N, 10.78%.
3-(3-Chlorophenyl)-6-methyl-1,8-diphenyl-7-thioxo-4-(p-tolyl)-1,2,6,8-tetraazaspiro[4.4]non-2-en-9-one (3g)
Colorless powder; yield: 0.23 g (43%); mp: 131–132 °C; IR (KBr) (νmax/cm−1): 1749, 1594, 1492; 1H NMR (500 MHz, CDCl3): δH = 2.33 (3 H, s, Me), 3.43 (3 H, s, Me), 5.15 (1 H, s, CH), 6.86 (2 H, d, 3J = 6.5 Hz, CH), 7.06–7.10 (3 H, m, CH), 7.15 (2 H, d, 3J = 8.3 Hz, CH), 7.17 (2 H, d, 3J = 8.6 Hz, CH), 7.23 (1 H, t, 3J = 8.6 Hz, CH),. 7.31 (1 H, d, 3J = 8.0 Hz, CH), 7.35–7.38 (6 H, m, CH), 7.79 (1 H, s, CH); 13C NMR (125.7 MHz, CDCl3): δC = 21.3 (Me), 29.8 (Me), 60.7 (CH), 89.6 (C), 115.6 (2 CH), 123.1 (CH), 124.8 (CH), 126.4 (CH), 127.9 (2 CH), 128.3 (C), 128.5 (CH), 129.0 (2 CH), 129.1 (CH), 129.2 (CH), 129.3 (2 CH), 129.6 (2 CH), 129.8 (2 CH), 132.4 (C), 132.6 (C), 134.7 (C), 138.9 (C), 142.4 (C), 147.0 (C=N), 166.6 (C=O), 181.1 (C=S); MS (EI, 70 eV): m/z (%) = 536 (M+, 1), 427 (10), 308 (40), 228 (80), 130 (50), 91 (100), 64 (10); Anal. Calcd for C31H25ClN4OS (536.14): C, 69.33; H, 4.69; N, 10.43%. Found: C, 69.74; H, 4.72; N, 10.80%.
3-(3-Chlorophenyl)-4-(4-chlorophenyl)-6-methyl-1,8-diphenyl-7-thioxo-1,2,6,8-tetraazaspiro[4.4]non-2-en-9-one (3h)
Colorless powder; yield: 0.23 g (41%); mp: 153–155 °C; IR (KBr) (νmax/cm−1): 1752, 1593, 1493; 1H NMR (500 MHz, CDCl3): δH = 3.42 (3 H, s, Me), 5.14 (1 H, s, CH), 6.87 (2 H, d, 3J = 7.4 Hz, Ar), 7.10 (1 H, t, 3J = 7.4 Hz, Ar), 7.16–7.18 (4 H, m, Ar), 7.25 (1 H, d, 3J = 7.9 Hz, Ar), 7.32–7.36 (5 H, m, Ar), 7.37–7.40 (4 H, m, Ar), 7.76 (1 H, s, Ar); 13C NMR (125.7 MHz, CDCl3): δC = 29.7 (Me), 60.3 (CH), 89.3 (C), 115.8 (2 CH), 123.4 (CH), 124.7 (CH), 126.4 (CH), 127.9 (2 CH), 128.5 (CH), 128.6 (C), 129.2 (2 CH), 129.4 (2 CH), 129.5 (2 CH), 129.7 (2 CH), 129.9 (CH), 130.2 (CH), 132.1 (C), 132.5 (C), 134.9 (C), 135.2 (C), 142.2 (C), 147.5 (C=N), 166.5 (C=O), 180.9 (C=S); MS (EI, 70 eV): m/z (%) = 556 (M+, 1), 483 (11), 450 (13), 391 (50), 363 (10), 315 (12), 295 (100), 228 (30), 169 (46), 150 (21), 133 (63), 91 (100), 64 (8); Anal. Calcd for C30H22Cl2N4OS (556.09): C, 64.63; H, 3.98; N, 10.05%. Found: C, 64.88; H, 4.01; N, 10.46%.
4-(4-Chlorophenyl)-6-methyl-1,8-diphenyl-7-thioxo-3-(p-tolyl)-1,2,6,8-tetraazaspiro[4.4]non-2-en-9-one (3j)
Colorless powder; yield: 0.23 g (43%); mp: 163–165 °C; IR (KBr) (νmax/cm−1): 1757, 1596, 1498; 1H NMR (500 MHz, CDCl3): δH = 2.36 (3 H, s, Me), 3.40 (3 H, s, Me), 5.14 (1 H, s, CH), 6.89 (2 H, d, 3J = 7.6 Hz, CH), 7.06 (1 H, t, 3J = 7.4 Hz, CH), 7.14–7.18 (5 H, m, CH), 7.31 (2 H, d, 3J = 8.4 Hz, CH), 7.34–7.37 (3 H, m, CH), 7.38–7.40 (3 H, m, CH), 7.51 (2 H, d, 3J = 8.2 Hz, CH); 13C NMR (125.7 MHz, CDCl3): δC = 21.4 (Me), 29.9 (Me), 60.8 (CH), 89.1 (C), 115.6 (2 CH), 122.9 (CH), 126.6 (2 CH), 127.9 (2 CH), 128.4 (CH) 128.5 (C), 129.1 (2 CH), 129.2 (2 CH), 129.5 (2 CH), 129.6 (2 CH), 130.8 (2 CH), 132.0 (C), 132.1 (C), 134.9 (C), 139.9 (C), 142.5 (C), 148.0 (C=N), 166.1 (C=O), 180.9 (C=S); MS (EI, 70 eV): m/z (%) = 536 (M+, 1), 363 (8), 350 (10), 322 (22), 134 (25), 106 (100), 91 (10), 59 (23); Anal. Calcd for C31H25ClN4OS (536.14): C, 69.33; H, 4.69; N, 10.43%. Found: C, 69.77; H, 4.73; N, 10.69%.
(E)-9-Methyl-8-(4-methylbenzylidene)-1,3,6-triphenyl-4-thia-1,2,6,9-tetraazaspiro[4.4]non-2-en-7-one (4a)
Colorless powder; yield: 0.25 g (49%); mp: 145–147 °C; IR (KBr) (νmax/cm−1): 1723, 1633, 1595; 1H NMR (500 MHz, CDCl3): δH = 2.36 (3 H, s, Me), 2.86 (3 H, s, Me), 5.85 (1 H, s, CH), 7.11 (1 H, t, 3J = 7.2 Hz, CH), 7.15 (2 H, d, 3J = 7.4 Hz, CH), 7.26–7.30 (3 H, m, CH), 7.32–7.40 (4 H, m, CH), 7.49 (2 H, d, 3J = 7.2 Hz, CH), 7.56–7.59 (3 H, m, CH), 7.70 (2 H, t, 3J = 8.2 Hz, CH), (2 H, d, 3J = 7.2 Hz, CH); 13C NMR (125.7 MHz, CDCl3): δC = 21.3 (Me), 28.6 (Me), 108.6 (CH), 116.4 (C) 117.3 (2 CH), 123.1 (CH), 126.0 (2 CH), 128.1 (2 CH), 128.4 (CH), 128.5 (CH), 128.6 (2 CH), 128.7 (2 CH), 129.0 (2 CH), 129.3 (2 CH), 129.6 (C), 129.8 (2 CH), 131.1 (C), 132.0 (C), 132.1 (C), 133.7 (C),137.0 (C), 141.5 (C=N), 160.7 (C=O); MS (EI, 70 eV): m/z (%) = 502 (M+, 1), 318 (8), 305 (10), 274 (22), 218 (25), 100 (23), 72 (100), 59 (23); Anal. Calcd for C31H26N4OS (502.63): C, 74.08; H, 5.21; N, 11.15%. Found: C, 74.35; H, 5.25; N, 11.36%.
(E)-8-(4-Chlorobenzylidene)-9-methyl-1,3,6-triphenyl-4-thia-1,2,6,9-tetraazaspiro[4.4]non-2-en-7-one (4b)
Colorless powder; yield: 0.22 g (43%); mp: 91–93 °C; IR (KBr) (νmax/cm−1): 1726, 1593, 1491; 1H NMR (500 MHz, CDCl3): δH = 5.77 (1 H, s, CH), 7.11 (1 H, t, 3J = 7.1 Hz, CH), 7.16 (2 H, d, 3J = 8.0 Hz, CH), 7.24 (2 H, d, 3J = 8.2 Hz, CH), 7.28–7.31 (3 H, m, CH), 7.33–7.36 (4 H, m, CH), 7.39 (2 H, d, 3J = 7.7 Hz, CH), 7.51 (2 H, d, 3J = 8.5 Hz, CH), 7.75 (2 H, d, 3J = 8.1 Hz, CH); 13C NMR (125.7 MHz, CDCl3): δC = 28.6 (Me), 106.7 (CH), 116.1 (C) 117.4 (2 CH), 123.3 (CH), 126.1 (2 CH), 126.2 (CH), 126.9 (CH), 127.2 (C), 128.0 (2 CH), 128.1 (2 CH), 128.6 (2 CH), 128.8 (C), 129.1 (2 CH), 129.4 (2 CH), 129.5 (C), 129.8 (C), 131.2 (2 CH), 132.6 (C), 141.0 (C),141.4 (C=N), 160.6 (C=O); MS (EI, 70 eV): m/z (%) = 522 (M+, 1), 413(9), 327 (15), 194 (100), 165 (35), 139 (40), 111 (20), 91 (90), 64 (4); Anal. Calcd for C30H23ClN4OS (522.13): C, 68.89; H, 4.43; N, 10.71%. Found: C, 69.21; H, 4.46; N, 10.96%.
(E)-8-(4-Chlorobenzylidene)-3-(4-fluorophenyl)-9-methyl-1,6-diphenyl-4-thia-1,2,6,9-tetraazaspiro[4.4]non-2-en-7-one (4c)
Colorless powder; yield: 0.24 g (45%); mp: 149–151 °C; IR (KBr) (νmax/cm−1): 1733, 1596, 1493; 1H NMR (500 MHz, CDCl3): δH = 2.87 (3 H, s, Me), 5.78 (1 H, s, CH), 7.07 (2 H, t, 3J = 8.6 Hz, CH), 7.12 (1 H, m, CH), 7.24 (2 H, d, 3J = 8.2 Hz, CH), 7.28–7.33 (5 H, m, CH), 7.34–7.37 (4 H, m, CH), 7.56 (2 H, d, 3J = 8.7 Hz, CH), 7.78 (2 H, d, 3J = 8.6 Hz, CH); 13C NMR (125.7 MHz, CDCl3): δC = 28.6 (Me), 106.9 (CH), 115.7 (d, 2JC-F = 22.2 Hz), 116.5 (C), 117.3 (2 CH), 123.3 (CH), 127.7 (d, 4JC-F = 3.2 Hz), 127.8 (d, 3JC-F = 8.6 Hz), 128.0 (2 CH), 128.1 (2 CH), 128.7 (CH), 129.1 (2 CH), 129.4 (2 CH), 131.2 (2 CH), 131.9 (C), 132.5 (C), 132.6 (C), 133.4 (C), 139.9 (C), 141.3 (C=N), 160.5 (C=O),163.4 (d, 1JC-F = 250 Hz); MS (EI, 70 eV): m/z (%) = 540 (M+, 1), 431(8), 327 (15), 296 (10), 212 (60), 162 (40), 135 (35), 119 (10), 91 (100), 51 (20); Anal. Calcd for C30H22ClFN4OS (540.12): C, 66.60; H, 4.10; N, 10.36%. Found: C, 66.93; H, 4.13; N, 10.76%.
(E)-3-(4-Fluorophenyl)-9-methyl-8-(4-methylbenzylidene)-1,6-diphenyl-4-thia-1,2,6,9-tetraazaspiro[4.4]non-2-en-7-one (4d)
Colorless powder; yield: 0.23 g (44%); mp: 161–164 °C; IR (KBr) (νmax/cm−1): 1725, 1600, 1493; 1H NMR (500 MHz, CDCl3): δH = 2.37 (3 H, s, Me), 2.87 (3 H, s, Me), 5.87 (1 H, s, CH), 7.07 (2 H, t, 3J = 8.6 Hz, CH), 7.11 (1 H, t, 3J = 7.1 Hz, CH), 7.16 (2 H, d, 3J = 8.1 Hz, CH), 7.25 (2 H, d, 3J = 8.3 Hz, CH), 7.29–7.33 (3 H, m, CH), 7.35 (2 H, t, 3J = 7.7 Hz, CH), 7.39 (2 H, d, 3J = 8.1 Hz, CH), 7.57 (2 H, d, 3J = 8.5 Hz, CH), 7.76 (2 H, d, 3J = 8.1 Hz, CH); 13C NMR (125.7 MHz, CDCl3): δC = 21.3 (Me), 28.6 (Me), 108.8 (CH), 115.7 (d, 2JC-F = 22.1 Hz), 116.7 (C), 117.3 (2 CH), 123.1 (CH), 127.7 (d, 4JC-F = 3.2 Hz), 127.8 (d, 3JC-F = 8.4 Hz), 128.2 (2 CH), 128.5 (CH), 128.7 (2 CH), 129.1 (2 CH), 129.4 (2 CH), 129.9 (2 CH), 131.0 (2 C), 133.7 (C), 137.1 (C), 139.9 (C), 141.4 (C=N), 160.7 (C=O),163.4 (d, 1JC-F = 250.7 Hz); MS (EI, 70 eV): m/z (%) = 520 (M+, 1), 411 (13), 307(8), 212 (80), 183 (8), 130 (30), 91 (100), 64 (15); Anal. Calcd for C31H25FN4OS (520.17): C, 71.52; H, 4.84; N, 10.76%. Found: C, 71.87; H, 4.86; N, 10.93%.
(E)-3-(4-Chlorophenyl)-9-methyl-8-(4-methylbenzylidene)-1,6-diphenyl-4-thia-1,2,6,9-tetraazaspiro[4.4]non-2-en-7-one (4e)
Colorless powder; yield: 0.25 g (46%); mp: 149–151 °C; IR (KBr) (νmax/cm−1): 1721, 1635, 1591; 1H NMR (500 MHz, CDCl3): δH = 2.36 (3 H, s, Me), 2.86 (3 H, s, Me), 5.87 (1 H, s, CH), 7.11 (1 H, t, 3J = 7.1 Hz, CH), 7.16 (2 H, d, 3J = 8.0 Hz, CH), 7.24 (2 H, d, 3J = 8.2 Hz, CH), 7.28–7.31 (3 H, m, CH), 7.33–7.36 (4 H, m, CH), 7.39 (2 H, d, 3J = 7.7 Hz, CH), 7.51 (2 H, d, 3J = 8.5 Hz, CH), 7.75 (2 H, d, 3J = 8.1 Hz, CH); 13C NMR (125.7 MHz, CDCl3): δC = 21.3 (Me), 28.7 (Me), 108.9 (CH), 116.8 (C), 117.3 (2 CH), 123.3 (CH), 127.2 (2 CH), 128.2 (2 CH), 128.5 (CH), 128.7 (2 CH), 128.8 (2 CH), 129.1 (2 CH), 129.4 (2 CH), 129.9 (2 CH), 130.0 (C), 130.9 (C), 131.0 (C), 133.6 (C), 135.4 (C), 137.1 (C), 139.9 (C), 141.4 (C=N), 160.7 (C=O); MS (EI, 70 eV): m/z (%) = 536 (M+, 1), 370(9), 281 (50), 182 (8), 143 (70), 106 (100), 91 (25), 77 (10), 43 (4); Anal. Calcd for C31H25ClN4OS (536.14): C, 69.33; H, 4.69; N, 10.43%. Found: C, 69.76; H, 4.73; N, 10.72%.
(E)-3-(4-Chlorophenyl)-8-(4-(dimethylamino)benzylidene)-9-methyl-1,6-diphenyl-4-thia-1,2,6,9-tetraazaspiro[4.4]non-2-en-7-one (4f)
Colorless powder; yield: 0.50 g (89%); mp: 181–183 °C; IR (KBr) (νmax/cm−1): 1720, 1651, 1601, 1489; 1H NMR (500 MHz, CDCl3): δH = 2.79 (3 H, s, Me), 2.98 (6 H, s, 2 Me), 6.67 (2 H, d, 3J = 8.7 Hz, CH), 6.71 (1 H, s, CH), 7.13 (2 H, d, 3J = 8.5 Hz, CH), 7.28–7.30 (3 H, m, CH), 7.32–7.35 (5 H, m, CH), 7.38–7.40 (4 H, m, CH), 7.50 (2 H, d, 3J = 8.5 Hz, CH); 13C NMR (125.7 MHz, CDCl3): δC = 31.5 (Me), 40.4 (2 Me), 104.9 (CH), 111.6 (2 CH), 117.2 (2 CH), 117.9 (C), 123.2 (CH), 127.2 (2 CH), 127.9 (2 CH), 128.4 (CH), 128.7 (2 CH), 129.1(2 CH), 129.4 (2 CH), 129.5 (C), 129.9 (C), 130.5 (2 CH), 131.3 (C), 134.1 (C), 135.3 (C), 139.8 (C), 141.4 (C=N), 149.5 (C), 162.6 (C=O); MS (EI, 70 eV): m/z (%) = 565 (M+, 1), 394(9), 334 (50), 319 (8), 232 (70), 106 (100), 91 (25), 77 (10), 59 (4); Anal. Calcd for C32H28ClN5OS (565.17): C, 67.89; H, 4.99; N, 12.37%. Found: C, 68.19; H, 5.02; N, 12.67%.
(E)-3-(3-Chlorophenyl)-9-methyl-8-(4-methylbenzylidene)-1,6-diphenyl-4-thia-1,2,6,9-tetraazaspiro[4.4]non-2-en-7-one (4g)
Colorless powder; yield: 0.24 g (44%); mp: 131–133 °C; IR (KBr) (νmax/cm−1): 1718, 1639, 1591, 1552; 1H NMR (500 MHz, CDCl3): δH = 2.38 (3 H, s, Me), 2.87 (3 H, s, Me), 5.89 (1 H, s, CH), 7.13 (1 H, t, 3J = 7.2 Hz, Ar), 7.17 (2 H, d, 3J = 8.0 Hz, Ar), 7.26 (2 H, d, 3J = 6.8 Hz, Ar), 7.29 (1 H, t, 3J = 7.1 Hz, Ar), 7.30–7.34 (4 H, m, Ar),. 7.37 (2 H, t, 3J = 7.8 Hz, Ar), 7.41–7.44 (3 H, m, Ar), 7.63 (1 H, s, Ar), 7.77 (2 H, d, 3J = 8.1 Hz, Ar); 13C NMR (125.7 MHz, CDCl3): δC = 21.3 (Me), 28.7 (Me), 109.0 (CH), 116.8 (C), 117.4 (2 CH), 123.4 (CH), 124.2 (CH), 125.8 (CH), 128.2 (2 CH), 128.6 (C), 128.7 (2 CH), 129.1 (2 CH), 129.4 (2 CH), 129.5 (CH), 129.8 (CH), 129.9 (2 CH), 130.9 (C), 131.0 (C), 133.2 (C),133.6 (C), 134.7 (C), 137.1 (C), 139.5 (C), 141.3 (C=N), 160.7 (C=O); MS (EI, 70 eV): m/z (%) = 536 (M+, 1), 456(5), 427 (7), 401 (15), 371 (8), 308 (90), 279 (10), 228 (60), 130 (80), 91 (100), 51 (3); Anal. Calcd for C31H25ClN4OS (536.14): C, 69.33; H, 4.69; N, 10.43%. Found: C, 69.69; H, 4.72; N, 10.90%.
(E)-8-(4-Chlorobenzylidene)-3-(3-chlorophenyl)-9-methyl-1,6-diphenyl-4-thia-1,2,6,9-tetraazaspiro[4.4]non-2-en-7-one (4h)
Colorless powder; yield: 0.25 g (45%); mp: 153–155 °C; IR (KBr) (νmax/cm−1): 1725, 1634, 1593; 1H NMR (500 MHz, CDCl3): δH = 2.87 (3 H, s, Me), 5.79 (1 H, s, CH), 7.13 (1 H, m, CH), 7.23 (2 H, d, 3J = 7.9 Hz, CH), 7.28 (2 H, d, 3J = 8.5 Hz, CH), 7.30–7.32 (4 H, m, CH), 7.36–7.38 (5 H, m, CH), 7.41 (1 H, d, 3J = 8.5 Hz, CH), 7.60 (1 H, s, CH), 7.78 (2 H, d, 3J = 8.5 Hz, CH); 13C NMR (125.7 MHz, CDCl3): δC = 28.7 (Me), 107.1 (CH), 116.5 (C), 117.5 (2 CH), 123.6 (CH), 124.2 (CH), 125.8 (CH), 128.0 (2 CH) 128.1 (2 CH), 128.7 (CH), 129.2 (2 CH), 129.4 (2 CH), 129.5 (CH), 129.8 (CH), 131.2 (2 CH), 131.9 (C), 132.4 (C), 132.7 (C), 133.0 (C), 133.4 (C), 134.7 (C), 139.5 (C), 141.2 (C=N), 160.5 (C=O); MS (EI, 70 eV): m/z (%) = 556 (M+, 1), 339 (11), 311 (13), 300 (21), 252 (10), 100 (50), 72 (100), 59 (44); Anal. Calcd for C30H22Cl2N4OS (556.09): C, 64.63; H, 3.98; N, 10.05%. Found: C, 64.97; H, 4.01; N, 10.45%.
(E)-9-Methyl-8-(4-methylbenzylidene)-3-(4-nitrophenyl)-1,6-diphenyl-4-thia-1,2,6,9-tetraazaspiro[4.4]non-2-en-7-one (4i)
Yellow powder; yield: 0.43 g (79%); mp: 155–157 °C; IR (KBr) (νmax/cm−1): 1722, 1639, 1594, 1547; 1H NMR (500 MHz, CDCl3): δH = 2.37 (3 H, s, Me), 2.89 (3 H, s, Me), 5.92 (1 H, s, CH), 7.17 (2 H, d, 3J = 8.0 Hz, CH), 7.23 (2 H, d, 3J = 6.5 Hz, Ar CH), 7.31 (2 H, d, 3J = 7.6 Hz, CH), 7.34–7.37 (3 H, m, CH),. 7.40–7.43 (3 H, m, CH), 7.72 (2 H, d, 3J = 8.6 Hz, CH), 7.77 (2 H, d, 3J = 8.1 Hz, CH), 8.22 (2 H, d, 3J = 8.7 Hz, CH);13C NMR (125.7 MHz, CDCl3): δC = 21.3 (Me), 28.8 (Me), 109.6 (CH), 117.4 (C), 117.7 (2 CH), 123.9 (2 CH), 124.0 (CH), 126.5 (2 CH), 128.2 (2 CH) 128.5 (CH), 128.7 (2 CH), 129.2 (2 CH), 129.5 (2 CH), 129.9 (2 CH), 130.7 (C), 130.8 (C), 133.7 (C), 133.8 (C), 137.4 (C), 138.6 (C), 140.9 (C=N), 147.8 (C), 160.6 (C=O); MS (EI, 70 eV): m/z (%) = 547 (M+, 1), 448 (6), 420 (10), 353 (22), 309 (25), 252 (10), 140 (100), 125 (50), 73 (41), 45 (22); Anal. Calcd for C31H25N5O3S (547.17): C, 67.99; H, 4.60; N, 12.79%. Found: C, 68.36; H, 4.63; N, 12.95%.
(E)-8-(4-Chlorobenzylidene)-9-methyl-1,6-diphenyl-3-(p-tolyl)-4-thia-1,2,6,9-tetraazaspiro[4.4]non-2-en-7-one (4j)
Colorless powder, yield: 0.24 g (45%); mp: 163–165 °C; IR (KBr) (νmax/cm−1): 1723, 1633, 1595, 1491; 1H NMR (500 MHz, CDCl3): δH = 2.38 (3 H, s, Me), 2.86 (3 H, s, Me), 5.77 (1 H, s, CH), 7.11 (1 H, t, 3J = 7.0 Hz, CH), 7.19 (2 H, d, 3J = 8.0 Hz, CH), 7.25 (2 H, d, 3J = 8.3 Hz, CH), 7.28–7.31 (4 H, m, CH), 7.33–7.38 (5 H, m, CH), 7.48 (2 H, d, 3J = 8.1 Hz, NH), 7.79 (2 H, d, 3J = 8.5 Hz, CH); 13C NMR (125.7 MHz, CDCl3): δC = 21.4 (Me), 28.6 (Me), 106.6 (CH), 116.0 (C), 117.3 (2 CH), 123.1 (CH), 125.9 (2 CH), 128.0 (2 CH), 128.1 (2 CH) 128.6 (CH), 129.1 (2 CH), 129.3 (2 CH), 129.4 (2 CH), 131.2 (2 CH), 132.1 (C), 132.5 (C), 132.6 (C), 133.5 (C), 133.7 (C), 140.0 (C), 141.2 (C), 141.5 (C=N), 160.6 (C=O); MS (EI, 70 eV): m/z (%) = 536 (M+, 1), 463 (8), 401 (22), 371 (22), 328 (28), 208 (100), 165 (25), 150 (30), 123 (15), 91 (90), 64 (10); Anal. Calcd for C31H25ClN4OS (536.14): C, 69.33; H, 4.69; N, 10.43%. Found: C, 69.64; H, 4.72; N, 10.72%.
(E)-8-Benzylidene-6-(4-methoxyphenyl)-9-methyl-1-(4-nitrophenyl)-3-phenyl-4-thia-1,2,6,9-tetraazaspiro[4.4]non-2-en-7-one (4k)
Colorless powder, yield: 0.41 g (73%); mp: 179–181 °C; IR (KBr) (νmax/cm−1): 1724, 1649, 1598, 1544; 1H NMR (500 MHz, CDCl3): δH = 3.12 (3 H, s, Me), 3.72 (3 H, s, Me), 5.87 (1 H, s, CH), 6.72 (2 H, d, 3J = 7.5 Hz, CH), 7.26 (2 H, d, 3J = 7.6 Hz, CH), 7.30–7.36 (3 H, m, CH), 7.46–7.51 (5 H, m, CH), 7.58 (2 H, d, 3J = 7.5 Hz, CH), 7.87 (2 H, d, 3J = 8.9 Hz,CH), 8.29 (2 H, d, 3J = 8.9 Hz, CH); 13C NMR (125.7 MHz, CDCl3): δC = 28.7 (Me), 55.7 (Me), 109.6 (CH), 114.9 (2 CH), 117.4 (C), 117.7 (2 CH), 123.9 (2 CH), 124.0 (CH), 126.5 (2 CH), 128.2 (2 CH) 128.5 (CH), 128.7 (2 CH), 129.2 (2 CH), 129.5 (2 CH), 130.7 (C), 130.8 (C), 133.8 (C), 137.4 (C), 138.6 (C), 140.9 (C=N), 147.8 (C), 157.7 (C), 160.6 (C=O); MS (EI, 70 eV): m/z (%) = 563 (M+, 1), 363 (8), 350 (10), 322 (22), 263 (25), 100 (100), 91 (10), 72 (27); Anal. Calcd for C31H25N5O4S (563.63): C, 66.06; H, 4.47; N, 12.43%. Found: C, 66.41; H, 4.49; N, 12.83%.
X-ray crystal structure determination of 3a and 4g
Crystallographic data for the structure 3a and 4g have been deposited with the Cambridge Crystallographic Data Centre with CCDC–1874156 and CCDC-1881806 which contain the supplementary crystallographic data for compounds 3a and 4g, respectively. Copies of the data can be obtained, free of charge, on application to the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge, CB2 1EZ, UK (fax: +44 (0)1223 336033 or e-mail:deposit@ccdc.cam.ac.uk).
References
Ivasiv V, Albertini C, Gonçalves AE, Rossi M, Bolognesi ML (2019) Molecular hybridization as a tool for designing multitarget drug candidates for complex diseases. Curr Top Med Chem 19:1694–1711. https://doi.org/10.2174/1568026619666190619115735
Bérubé G (2016) An overview of molecular hybrids in drug discovery. Expert Opin Drug Discov 11:281–305. https://doi.org/10.1517/17460441.2016.1135125
Viegas-Junior C, Danuello A, da Silva BV, Barreiro EJ, Fraga CAM (2007) Molecular hybridization: a useful tool in the design of new drug prototypes. Curr Med Chem 14:1829–1852. https://doi.org/10.2174/092986707781058805
Sandhu S, Bansal Y, Silakari O, Bansal G (2014) Coumarin hybrids as novel therapeutic agents. Bioorg Med Chem 22:3806–3814. https://doi.org/10.1016/j.bmc.2014.05.032
Benabdallah M, Talhi O, Nouali F, Choukchou-Braham N, Bachari K, Silva A (2018) Advances in spirocyclic hybrids: chemistry and medicinal actions. Curr Med Chem 25:3748–3767. https://doi.org/10.2174/0929867325666180309124821
Hong X, Küçük HB, Maji MS, Yang YF, Rueping M, Houk KN (2014) Mechanism and selectivity of N-triflylphosphoramide catalyzed [3+2] cycloaddition between hydrazones and alkenes. J Am Chem Soc 136:13769–13780. https://doi.org/10.1021/ja506660c
Xu X, Zavalij PY, Doyle MP (2012) Synthesis of tetrahydropyridazines by a metal–carbene-directed enantioselective vinylogous N–H insertion/lewis acid-catalyzed diastereoselective mannich addition. Angew Chem Int Ed 51:9829–9833. https://doi.org/10.1002/anie.201203962
Kobayashi S, Shimizu H, Yamashita Y, Ishitani H, Kobayashi J (2002) Asymmetric intramolecular [3+2] cycloaddition reactions of acylhydrazones/olefins using a chiral zirconium catalyst. J Am Chem Soc 124:13678–13679. https://doi.org/10.1021/ja027681d
Saraswat P, Jeyabalan G, Hassan MZ, Rahman MU, Nyola NK (2016) Review of synthesis and various biological activities of spiro heterocyclic compounds comprising oxindole and pyrrolidine moities. Synth Commun 46:1643–1664. https://doi.org/10.1080/00397911.2016.1211704
Zheng YJ, Tice CM (2016) The utilization of spirocyclic scaffolds in novel drug discovery. Expert Opin Drug Discov 11:831–834. https://doi.org/10.1080/17460441.2016.1195367
Wang Y, Deng Y, Pang X, Yu J, Fan L, Chen Y, Zhao L (2017) Novel thiohydantoin analogues bearing the 1-hydroxyl-2,2,2-trifluoro-1-ethyl moiety as androgen receptor inhibitors for the potential treatment of castration resistant prostate cancer. RSC Adv 7:31866–31874. https://doi.org/10.1039/c7ra02142a
Bae YS, Choi S, Park JJ, Joo JH, Cui M, Cho H, Lee WJ, Lee SH (2016) Synthesis and biological evaluation of 3-substituted 5-benzylidene-1-methyl-2-thiohydantoins as potent NADPH oxidase (NOX) inhibitors. Bioorg Med Chem 24:4144–4151. https://doi.org/10.1016/j.bmc.2016.06.056
Majumdar P, Bathula C, Basu SM, Das SK, Agarwal R, Hati S, Singh A, Sen S, Das BB (2015) Design, synthesis and evaluation of thiohydantoin derivatives as potent topoisomerase I (Top1) inhibitors with anticancer activity. Eur J Med Chem 102:540–551. https://doi.org/10.1016/j.ejmech.2015.08.032
Chen Y, Su L, Yang X, Pan W, Fang H (2015) Enantioselective synthesis of 3,5-disubstituted thiohydantoins and hydantoins. Tetrahedron 71:9234–9239. https://doi.org/10.1016/j.tet.2015.10.041
Gazzeh H, Boudriga S, Askri M, Khatyr A, Knorr M, Strohmann C, Golz C, Rousselin Y, Kubicki MM (2016) Stoichiometry-controlled cycloaddition of nitrilimines with unsymmetrical exocyclic dienones: microwave-assisted synthesis of novel mono-and dispiropyrazoline derivatives. RSC Adv 6:49868–49875. https://doi.org/10.1039/c6ra09703k
Liu H, Jia H, Wang B, Xiao Y, Guo H (2017) Synthesis of spirobidihydropyrazole through double 1, 3-dipolar cycloaddition of nitrilimines with allenoates. Org Lett 19:4714–4717. https://doi.org/10.1021/acs.orglett.7b01961
Alizadeh A, Moafi L (2016) Convenient one-pot synthesis of spirooxindole derivatives containing a 1,3,4-thiadiazine scaffold. Synlett 27:1828–1831. https://doi.org/10.1055/s-0035-1561618
Hassaneen HM, Daboun HA, Abdelhadi HA, Abdel-Reheim NA (1995) Site selectivity and regiochemistry of nitrilimines. Cycloadditions to 1, 3-diphenyl-2-thiono-4-imidazolidinone and its 5-phenylmethylene derivatives. Phosphorus Sulfur Silicon Relat Elem 107:269–273. https://doi.org/10.1080/10426509508027942
Jakse R, Groselj U, Sorsak G (2007) Synthesis of thioaplysinopsin analogs derived from 5-dimethylaminomethylidene-2-thioxo-1,3-thiazol-4-ones. Heterocycles 73:743–750. https://doi.org/10.3987/COM-07-S(U)55
Yavari I, Khalili G (2010) A diastereoselective synthesis of phosphorylated dihydro-1H-pyrazoles from dialkyl phosphites, acetylenic esters, and hydrazonoyl chlorides. Synlett 12:1862–1864. https://doi.org/10.1055/s-0030-1258118
Yavari I, Nematpour M, Sodagar E (2015) Formation of spiro [indene-2,3′-pyrazole] derivatives from hydrazonyl chlorides and ninhydrin-malononitrile adduct. Monatsh Chem 146:2135–2138. https://doi.org/10.1007/s00706-015-1495-7
Yavari I, Nematpour M, Yavari S, Sadeghizadeh F (2012) Copper-catalyzed one-pot synthesis of tetrasubstituted pyrazoles from sulfonyl azides, terminal alkynes, and hydrazonoyl chlorides. Tetrahedron Lett 53:1889–1890. https://doi.org/10.1016/j.tetlet.2012.01.083
Yavari I, Taheri Z, Naeimabadi M, Bahemmat S, Halvagar MR (2018) A convenient synthesis of tetrasubstituted pyrazoles from nitrile imines and 2-(thioxothiazolidin-5-ylidene) acetates. Synlett 29:918–921. https://doi.org/10.1055/s-0036-1591921
Acknowledgements
We thank the Research Council of Tarbiat Modares University for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yavari, I., Taheri, Z., Sheikhi, S. et al. Synthesis of thia- and thioxo-tetraazaspiro[4.4]nonenones from nitrile imines and arylidenethiohydantoins. Mol Divers 25, 777–785 (2021). https://doi.org/10.1007/s11030-020-10056-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-020-10056-8