Abstract
A novel series of 1,2,3-triazolo-benzodiazepine derivatives 6a–o has been synthesized and evaluated in vivo for their anticonvulsant activities using by pentylenetetrazole (PTZ)- and maximal electroshock (MES)-induced seizures in mice. The synthetic approach started with diazotizing 2-aminobenzoic acids 1 to produce 2-azidobenzoic acids 2. Next, reaction of the latter compounds with propargylamine 3, benzaldehyde 4, and isocyanides 5 led to the formation of the title compounds 6a–o, in good yields. All the synthesized compounds exhibited high anticonvulsant activity in the PTZ test, comparable to or better than the standard drug diazepam. Among the tested compounds, N-(tert-butyl)-2-(9-chloro-6-oxo-4H-[1,2,3]triazolo[1,5-a][1,4]benzodiazepin-5(6H)-yl)-2-(3-bromophenyl)acetamide 6h was the most potent compound in this assay. Moreover, compounds 6i and 6k showed excellent activity in MES test. Loss of the anticonvulsant effect of compound 6h in the presence of flumazenil in the PTZ test and appropriate interaction of this compound in the active site of benzodiazepine (BZD)-binding site of GABAA receptor confirm involvement of BZD receptors in the anticonvulsant activity of compound 6h.
Graphical abstract
A novel series of 1,2,3-triazolo-benzodiazepine derivatives 6a–o have been synthesized and evaluated in vivo for their anticonvulsant activities using by pentylenetetrazole (PTZ)- and maximal electroshock (MES)-induced seizures in mice. All the synthesized compounds exhibited high anticonvulsant activity, comparable to or better than the standard drug diazepam in the PTZ test and compounds 6i and 6k showed excellent activity in MES test. Flumazenil test and in silico docking study confirm involvement of benzodiazepine receptors in the anticonvulsant activity of these compounds.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
After cerebrovascular disease and dementia, epilepsy is the third most frequent neurological disorder affecting approximately 50 million people worldwide [1]. This disease is caused by the abnormal discharge of cerebral neurons and is associated with the periodic and unpredictable occurrence of seizures. Several antiepileptic drugs such as phenobarbital, sodium valproate, carbamazepine, phenytoin, and diazepam are available but they fail to control seizures in about 30% of epileptic patients [2]. In addition, some of these drugs display several severe side effects [3]. Thus, there is continuing demand to discover and develop new effective and safe anticonvulsant agents.
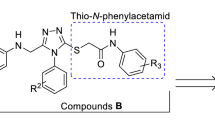
Benzodiazepines (BZDs) such as diazepam (Valium), lorazepam (Ativan), clonazepam (Klonopin) and alprazolam (Xanax) are a popular class of drugs that are used to treat anxiety, insomnia, agitation, and seizures [4]. BZDs enhance the effect of the neurotransmitter GABA through agonist binding to a specific domain of GABAA receptor known as BZD pocket [5]. With minor changes in structures of BZDs, agents with the mentioned therapeutic effects and or different biologically active compounds with novel applications can be achieved. For example, alprazolam and estazolam are two effective derivatives of BZDs with 1,2,4-triazole-benzodiazepine scaffold [6]. Biagia et al. in 1996 reported that derivatives of 1,2,3-triazole fused to benzodiazepine acted as potent agonists for BZD receptor (Fig. 1, A) [7]. Fused 1,2,3-triazolo-benzodiazepine derivatives such as compounds B and C were also introduced that acted as antitumor and serine protease inhibitor agents, respectively (Fig. 1) [8, 9]. Due to the importance of BZD derivatives, diverse routes have been reported for the synthesis of these compounds [10,11,12,13].
In this work, taking into account the anticonvulsant structures D and E, a novel series of fused 1,2,3-triazolo-benzodiazepine derivatives 6a–o were designed using a hybrid approach to achieve new scaffolds as potent anticonvulsant drugs (Fig. 1) [14]. Figure 2 shows the pharmacophore model of designed compounds for anticonvulsant activity [15]. Fused 1,2,3-triazolo-benzodiazepines 6a–o were tested for their in vivo anticonvulsant activity. Furthermore, to evaluate the mechanism action of these compounds as BZD receptor agonists, flumazenil test and in silico molecular docking studies were also performed.
Chemistry
Scheme 1 shows the synthetic route for the synthesis of fused triazolo-benzodiazepines. In the first step, 2-azidobenzoic acids 2 were obtained from azidation of 2-aminobenzoic acids 1 in the presence of NaNO2, HCl, and NaN3. Next, 2-Azidobenzoic acids 2 reacted with propargylamine 3, benzaldehyde 4, and isocyanide 5 in methanol at reflux to give desired triazolo-benzodiazepines 6a–o in good yields (65–79%).
Pharmacology
Anticonvulsant activity
Anticonvulsant activity against PTZ-induced seizures
Percentage of clonic seizure threshold in the PTZ test of target compounds revealed that all of these compounds had excellent anticonvulsant activity, comparable or more than the standard drug diazepam (Table 1). Among 1,2,3-triazolo-benzodiazepines 6a–o, compounds 6g, 6h, 6n, and 6o were the most potent compounds with activity more than diazepam.

Synthesized derivatives 6a–o, structurally, can be divided to two series: tert-butyl derivatives 6a–h and cyclohexyl derivatives 6i–o.
Compound 6a with 4-fluoro substituent on pendant phenyl group is the weakest derivative among synthesized compounds. The presence of a chlorine atom on pendant phenyl ring, especially in 2-position, led to a significant increase in activity as observed in compounds 6b and 6c. On the other hand, adding a second chlorine atom to 3-position of pendant phenyl group in 2-chloro derivative 6b, as in compound 6d, decreased anticonvulsant activity. Moreover, 3 or 4-bromo derivatives 6e and 6f showed activity similar to 4-chloro derivative 6c. Introduction of 9-chloro substituent on benzodiazepine moiety improve anticonvulsant potency in tert-butyl derivatives. In this regard, the comparison of percentage of clonic seizure threshold of 9-chloro derivatives 6g and 6h with their analogs 6a and 6e revealed that the chlorine atom had an important role in the anticonvulsant activities obtained.
In the cyclohexyl series, the 9-unsubstituented derivatives 6i–m showed approximately the same anticonvulsant activity (Table 1). In this series, similar to tert-butyl series, the most potent compounds were derivatives with 9-chloro substituent on benzodiazepine moiety (6n and 6o). A comparison of anticonvulsant activity of tert-butyl derivatives with their corresponding cyclohexyl analogs revealed that cyclohexyl analogs except 6i and 6l were as active as their tert-butyl analogs. In the case of compounds 6i and 6l, the anticonvulsant activity of these cyclohexyl derivatives was more than their tert-butyl analogs 6a and 6d, respectively.
Anticonvulsant activity against MES-induced seizures
The target compounds 6a–o were also screened for their anticonvulsant activities against maximal electroshock (MES)-induced seizure in mice [17]. Among the synthesized compounds, cyclohexyl derivative 6k showed 100% protection against MES-induced seizure while diazepam (standard drug) showed 87.5% protection in this assay (Table 2). Moreover, compound 6i showed protection equal with diazepam. Other synthesized compounds exhibited less protective percentage of diazepam in the MES-induced seizure assay.
In vivo neurotoxicity
Compounds 6h and 6k as most potent compounds, respectively, in PTZ and maximal electroshock (MES) tests were screened for their neurotoxicity in mice by using rotarod test [18]. Rotarod test is used widely to evaluate neurotoxicity of new anticonvulsant agents in mice. As shown in Table 3, these compounds showed neurological deficits less than diazepam.
Study on mechanism of action
To study the mechanism of action, effect of flumazenil as an antagonist of BZD receptor on anticonvulsant activity of compound 6h was evaluated. In this assay, flumazenil antagonized anticonvulsant activity of the most potent compound 6h in the PTZ test. This finding confirms that title compounds can act as agonists for BZD receptors.
Docking study
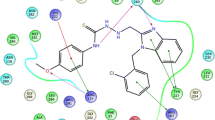
To study the interaction mode of the title compounds in the BZD-binding pocket of GABAA receptor (α1β2 γ2), a docking study was performed using Auto Dock Tools (version 1.5.6) [19]. The superposed structure of diazepam and the most potent compound 6h in the binding pocket is shown in Fig. 3. The detailed binding mode of diazepam showed that benzodiazepine moiety interacted with α1 Tyr159 (π–π) and α1 Tyr 209 (π–π), and α1 Thr206 (hydrogen bond) (Fig. 4a). Pendant phenyl group of this compound formed a π–π and a π–anion interactions with γ2 Phe77 and α1 His101. Furthermore, a weak hydrophobic interaction between chlorine atom and α1 Val211 was also observed.
The most active compound 6h established π interactions with γ2Glu189 and α1 His101 through benzodiazepine and 1,2,3-triazole moieties (Fig. 4b). A hydrogen bond between carbonyl unit attached to N-tert-butyl moiety and α1 Tyr159 was formed. Several π interactions were also observed between pendant phenyl ring of compound 6h and BZD-binding pocket residues α1 His101 and α1 Tyr 209. 3-Bromo of pendant phenyl group and 9-chloro of benzodiazepine moiety of this compound, interacted with α1 Phe99 and γ2 Val190, respectively.
The comparison of interaction modes of the most potent compound 6h (Fig. 4b) with it 9-unsubstituented analog 6e (Fig. 5) showed that compound 6h interacted with six residues—γ2Glu189, α1 His101, α1 Tyr159, α1 Tyr 209, α1 Phe99, and γ2 Val190—while compound 6e displayed interactions with four residues—γ2Glu189, α1 His101, α1 Tyr159, and α1 Phe99—in the BZD-binding pocket. It appears that the difference in the anticonvulsant activity of these two compounds can be reasonably explained by two additional interactions of compound 6h with BZD-binding pocket (α1 Tyr 209 and γ2 Val190) in comparison with compound 6e.
Conclusion
In conclusion, we presented design, synthesis, in vivo anticonvulsant activity of a novel series of 1,2,3-triazolo-benzodiazepine derivatives 6a–n. All synthesized compounds 6a–o showed high anticonvulsant activity in the PTZ test, comparable to or more than diazepam as the standard drug. Among synthesized compounds, the 9-chloro-benzodiazepin derivatives 6g, 6h, 6n, and 6o displayed more potent activity than diazepam in the PTZ test. Among the synthesized compounds, compounds 6k and 6i were most potent compounds in MES test. The promising compounds 6h and 6k showed neurological deficits less than diazepam in the rotarod test. The experimental investigation of action of mechanism compound 6h by flumazenil and docking study of this compound showed this compound can act as BZD receptor agonist.
Experimental
Chemistry
Melting points of target compounds 6a–o were measured by a Kofler hot stage apparatus and are uncorrected. A Bruker FT-500 spectrometer was used to record 1H and 13C NMR spectra, using CDCl3 as a solvent and TMS as an internal standard. The IR spectra were obtained by a Nicolet Magna FTIR 550 spectrometer (in KBr disks). High-resolution mass spectra were determined with an Agilent Technology (HP) mass spectrometer operating at an ionization potential of 70 eV. All reagents and solvents used in this study were purchased from Aldrich or Merck Company without the requirement of any purification.
General procedure for the synthesis of 2-azidobenzoic acids 2
A suspension of 2-aminobenzoic acids 1 (21.78 mmol) in water (15 mL) and concentrated hydrochloric acid (5.55 mL) was cooled to − 5 °C. Then, a solution of sodium nitrite (22.8 mmol) dissolved in water (4.5 mL) was prepared and added dropwise to the suspension and the resulting mixture was stirred for 30 min at − 5 °C. The reaction mixture (without diazonium salt isolation) was poured into a solution of sodium azide (24.5 mmol) in water (4.5 mL) and ice (20 g), and a pale yellow precipitate formed immediately. The reaction mixture was set aside overnight. Next, the precipitate was isolated by filtration, washed with water and dried under reduced pressure to provide compounds 2 (yield 93%).
General procedure for the synthesis of N-(alkyl)-2-(9-chloro-6-oxo-4H-[1,2,3]triazolo[1,5-a][1,4]benzodiazepin-5(6H)-yl)-2-(substituted phenyl)acetamide ( 6a – o )
2-azidobenzoic acids 2 (1 mmol), propargylamine 3 (1 mmol), substituted benzaldehydes 4 (1 mmol), and isocyanides 5 (1 mmol) were refluxed in MeOH (10 mL) for 24 h. Then, the reaction mixture was poured into crushed ice, and the precipitated products were filtered and dried at 60 °C to obtain pure compounds 6a–o (64–79%).
N-(tert-butyl)-2-(6-oxo-4H-[1,2,3]triazolo[1,5-a][1,4]benzodiazepin-5(6H)-yl)-2-(4-fluorophenyl)acetamide(6a)
White solid; yield: 79%, mp 232–235 °C. IR (KBr) (νmax/cm−1): 3302, 3061, 2965, 1673, 1611, 1546.1H NMR (CDCl3, 500 MHz): δ = 1.33 (9H, s), 4.43 (1H, d, J = 15 Hz, CHdiastrotopic), 4.67 (1H, d, J = 15 Hz, CHdiastrotopic), 6.27 (1H, s, CHChiral), 6.80 (1H, s, CHtriazole), 7.04 (2H, t, J = 8.5 Hz), 7.19–7.32 (2H, m), 7.54 (1H, td, J = 7.7, 1.2 Hz), 7.67 (1H, td, J = 7.8, 1.6 Hz), 7.94 (1H, d, J = 8.1 Hz), 8.08 (1H, dd, J = 7.9, 1.5 Hz), 8.13 (1H, s) ppm; 13C NMR (CDCl3, 125 MHz): δ = 28.58, 36.53, 28.58, 36.53, 52.11, 59.98, 116.00, 116.17, 122.57, 126.51, 128.84, 130.59, 130.86, 132.74, 133.15, 135.28, 138.05, 161.86, 163.84, 166.87, 167.22 ppm. Anal. Calcd for C22H22FN5O2 (407.44): C, 64.85; H, 5.44; N, 17.19. Found: C, 64.71; H, 5.63; N, 17.25.
N-(tert-butyl)-2-(6-oxo-4H-[1,2,3]triazolo[1,5-a][1,4]benzodiazepin-5(6H)-yl)-2-(2-chlorophenyl)acetamide (6b)
White solid; yield: 78%, mp 273–275 °C. IR (KBr) (νmax/cm−1): 3305, 3066, 2961, 1675, 1615, 1549. 1H NMR (CDCl3, 500 MHz): δ = 1.36 (9H, s), 4.42 (1H, d, J = 15 Hz, CHdiastrotopic), 4.64 (1H, d, J = 15 Hz, CHdiastrotopic), 6.40 (1H, s, CHChiral), 6.91 (1H, s, CHtriazole), 7.30–7.34 (1H, m), 7.38–7.44 (2H, m), 7.55 (1H, t, J = 7 Hz), 7.63–7.71 (2H, m), 7.95(1H, d, J = 8 Hz), 8.13 (1H, d, J = 8 Hz), 8.24 (1H, s) ppm; 13C NMR (CDCl3, 125 MHz): δ = 28.58, 36.89, 52.10, 59.15, 122.59, 126.41, 127.13, 128.73, 130.32, 130.43, 130.56, 132.27, 132.91, 132.97, 133.04, 134.83, 166.09, 166.34 ppm. Anal. Calcd for C22H22ClN5O2 (423.9): C, 62.34; H, 5.23; N, 16.52. Found: C, 62.51; H, 5.48; N, 16.39.
N-(tert-butyl)-2-(6-oxo-4H-[1,2,3]triazolo[1,5-a][1,4]benzodiazepin-5(6H)-yl)-2-(4-chlorophenyl)acetamide(6c)
White solid; yield: 76%, mp 242–243 °C. IR (KBr) (νmax/cm−1): 3301, 3065, 2959, 1678, 1613, 1544. 1H NMR (CDCl3, 500 MHz): δ = 1.35 (9H, s), 4.46 (1H, d, J = 15 Hz, CHdiastrotopic), 4.82 (1H, d, J = 15 Hz, CHdiastrotopic), 6.30 (1H, s, CHChiral), 6.77 (1H, s, CHtriazole), 7.17–7.30 (2H, m), 7.35 (2H, d, J = 8.0 Hz), 7.56 (1H, t, J = 7.7 Hz), 7.69 (1H, t, J = 7.5, Hz), 7.96(1H, d, J = 8.1 Hz), 8.09 (1H, d, J = 7.9 Hz), 8.24 (1H, s) ppm; 13C NMR (CDCl3, 125 MHz): δ = 28.57, 29.65, 36.59, 47.32, 62.12, 122.59, 126.44, 128.86, 129.24, 130.30, 132.72, 133.20, 135.01, 139.49, 165.78, 166.92 ppm. Anal. Calcd for C22H22ClN5O2 (423.9): C, 62.34; H, 5.23; N, 16.52. Found: C, 62.47; H, 5.11; N, 16.65.
N-(tert-butyl)-2-(6-oxo-4H-[1,2,3]triazolo[1,5-a][1,4]benzodiazepin-5(6H)-yl)-2-(2,3-dichlorophenyl)acetamide(6d)
White solid; yield: 69%, mp 270–272 °C. IR (KBr) (νmax/cm−1): 3302, 3064, 2959, 1676, 1614, 1547. 1H NMR (CDCl3, 500 MHz): δ = 1.34 (9H, s), 4.42 (1H, d, J = 15 Hz, CHdiastrotopic), 4.78 (1H, d, J = 15 Hz, CHdiastrotopic), 6.45 (1H, s, CHChiral), 6.97 (1H, s, CHtriazole), 7.37 (1H, t, J = 7.9 Hz), 7.49–7.63 (3H, m), 7.69 (1H, td, J = 7.8, 1.6 Hz), 7.96 (1H, d, J = 7.5 Hz), 8.11 (1H, d, J = 7.5 Hz), 8.20 (1H, s) ppm; 13C NMR (CDCl3, 125 MHz): δ = 28.54, 36.87, 49.20, 59.26, 118.56, 119.07, 121.74, 123.13, 126.40, 128.41, 128.79, 131.28, 132.74, 132.80, 133.18, 136.99, 166.84, 167.59 ppm. Anal. Calcd for C22H21Cl2N5O2 (458.34): C, 57.65; H, 4.62; N, 15.28. Found: C, 57.46; H, 4.39; N, 15.09.
N-(tert-butyl)-2-(6-oxo-4H-[1,2,3]triazolo[1,5-a][1,4]benzodiazepin-5(6H)-yl)-2-(3-bromophenyl)acetamide(6e)
White solid; yield: 68%, mp 207–209 °C. IR (KBr) (νmax/cm−1): 3300, 3064, 2967, 1674, 1612, 1544 cm−1. 1H NMR (CDCl3, 500 MHz): δ = 1.37 (9H, s), 4.27 (1H, d, J = 15 Hz, CHdiastrotopic), 4.78 (1H, d, J = 15 Hz, CHdiastrotopic), 6.30 (1H, s), 6.89 (1H, s), 7.15–7.27 (2H, m), 7.48 (1H, s), 7.54 (1H, d, J = 8 Hz), 7.58 (1H, t, J = 7.7 Hz), 7.71(1H, t, J = 7.5 Hz), 7.99 (1H, d, J = 8.0 Hz), 8.05 (1H, s), 8.12 (1H, d, J = 8.0 Hz). 13C NMR (CDCl3, 125 MHz): δ = 28.59, 36.68, 48.22, 60.06, 118.78, 120.13, 122.61, 123.07, 126.40, 128.86, 130.56, 132.11, 132.74, 132.80, 133.21, 137.03, 166.97, 167.90. EIMS, m/z (%): 470 (M+81Br, 9), 468 (M+79Br, 9), 368 (100), 339 (54), 261 (20), 185 (28), 171 (62), 155 (67), 130 (72), 115 (39), 57 (82), 41 (25). Anal. Calcd for C22H22BrN5O2 (468.35): C, 56.42; H, 4.73; N, 14.95. Found: C, 56.27; H, 4.91; N, 15.12.
N-(tert-butyl)-2-(6-oxo-4H-[1,2,3]triazolo[1,5-a][1,4]benzodiazepin-5(6H)-yl)-2-(4-bromophenyl)acetamide(6f)
White solid; yield: 77%, mp 208–210 °C. IR (KBr) (νmax/cm−1): 3275, 3089, 1683, 2966, 1644, 1564. 1H NMR (CDCl3, 500 MHz): δ = 1.37 (9H, s), 4.14 (1H, d, J = 15 Hz, CHdiastrotopic), 4.73 (1H, d, J = 15 Hz, CHdiastrotopic), 6.26 (1H, s, CHChiral), 6.75 (1H, s, CHtriazole), 7.09–7.25 (2H, m), 7.49–7.55 (2H, m), 7.58 (1H, t, J = 7.7 Hz), 7.72(1H, t, J = 7.7 Hz), 7.98 (1H, d, J = 8.1 Hz), 8.12 (1H, d, J = 7.9 Hz), 8.18 (1H, s) ppm; 13C NMR (CDCl3, 125 MHz): δ = 28.59, 36.61, 52.18, 60.16, 116.91, 118.02, 122.61, 126.41, 128.90, 130.62, 132.25, 132.75, 133.24, 139.39, 161.32, 166.93 ppm. EIMS, m/z (%): 470 (M+81Br, 7), 468 (M+79Br, 7), 368 (100), 339 (51), 260 (11), 186 (22), 171 (69), 155 (41), 130 (53), 115 (27), 89 (18), 57 (53), 41 (19). Anal. Calcd for C22H22BrN5O2 (468.35): C, 56.42; H, 4.73; N, 14.95. Found: C, 56.22; H, 4.87; N, 15.06.
N-(tert-butyl)-2-(9-chloro-6-oxo-4H-[1,2,3]triazolo[1,5-a][1,4]benzodiazepin-5(6H)-yl)-2-(4-fluorophenyl)acetamide (6g)
White solid; yield: 67%, mp 298–300 °C. IR (KBr) (νmax/cm−1): 3279, 3085, 1681, 2968, 1647, 1562. 1H NMR (CDCl3, 500 MHz): δ = 1.35 (9H, s), 4.46 (1H, d, J = 15 Hz, CHdiastrotopic), 4.68 (1H, d, J = 15 Hz, CHdiastrotopic), 6.25 (1H, s, CHChiral), 6.83 (1H, s, CHtriazole), 7.06 (2H, t, J = 8.5 Hz), 7.22–7.31 (2H, m), 7.51 (1H, dd, J = 8.5, 2.1 Hz), 7.99 (1H, d, J = 2.1 Hz), 8.04 (1H, d, J = 8.5 Hz), 8.20 (1H, s) ppm. 13C NMR (CDCl3, 125 MHz): δ = 28.60, 36.44, 52.18, 60.43, 116.07, 116.24, 119.12 122.57, 124.71, 129.08, 133.49, 134.20, 135.08, 139.36, 161.19, 163.16, 166.07, 166.57 ppm. Anal. Calcd for C22H21ClFN5O2 (441.89): C, 59.80; H, 4.79; N, 15.85. Found: C, 59.69; H, 4.57; N, 16.01.
N-(tert-butyl)-2-(9-chloro-6-oxo-4H-[1,2,3]triazolo[1,5-a][1,4]benzodiazepin-5(6H)-yl)-2-(3-bromophenyl)acetamide (6h)
White solid; yield: 66%, mp 209–211 °C. IR (KBr) (νmax/cm−1): 3278, 3083, 1682, 2969, 1645, 1564. 1H NMR (CDCl3, 500 MHz): δ = 1.37 (9H, s), 4.45 (1H, d, J = 15 Hz, CHdiastrotopic), 4.73 (1H, d, J = 15 Hz, CHdiastrotopic), 6.25 (1H, s, CHChiral), 6.90 (1H, s, CHtriazole), 7.16–7.27 (2H, m), 7.46 (1H, s), 7.51–7.56 (2H, m), 8.01 (1H, d, J = 2.1 Hz), 8.07 (1H, d, J = 8.5 Hz), 8.20 (1H, s)ppm; 13C NMR (CDCl3, 125 MHz): δ = 28.59, 36.60, 52.26, 60.30, 118.96, 122.60, 123.13, 124.61, 127.50, 129.09, 130.60, 131.82, 132.22, 133.52, 134.20, 136.84, 139.43, 166.16, 166.84 ppm. Anal. Calcd for C22H21BrClN5O2 (502.79): C, 52.55; H, 4.21; N, 13.93. Found: C, 52.61; H, 4.35; N, 14.07.
N-(cyclohexyl)-2-(6-oxo-4H-[1,2,3]triazolo[1,5-a][1,4]benzodiazepin-5(6H)-yl)-2-(4-fluorophenyl)acetamide(6i)
White solid; yield: 76%, mp 249–251 °C. IR (KBr) (νmax/cm−1): 3235, 3073, 2934, 2858, 1637, 1569. 1H NMR (CDCl3, 500 MHz): δ = 1.00–1.99 (10H, m), 3.80 (1H, m), 4.36 (1H, d, J = 15 Hz, CHdiastrotopic), 4.82 (1H, d, J = 15 Hz, CHdiastrotopic), 6.41 (1H, s, CHChiral), 6.76 (1H, s, CHChiral), 6.87 (1H, d, J = 8.0 Hz), 7.05 (2H, t, J = 8.3 Hz), 7.16–7.34 (2H, m), 7.54 (1H, t, J = 7.7 Hz), 7.69(1H, t, J = 7.7 Hz), 7.97(1H, d, J = 8.1 Hz), 8.05(1H, d, J = 7.9 Hz) ppm; 13C NMR (CDCl3, 125 MHz): δ = 24.64, 24.66, 25.33, 32.61, 32.77, 36.57, 48.71, 59.65, 115.91, 116.08, 122.57, 126.44, 128.77, 130.56, 132.61, 132.76, 133.14, 135.06, 161.83, 163.77, 166.92, 167.27 ppm. EIMS, m/z (%):434 (M+, 11), 334 (5), 308 (100), 279 (71), 235 (17), 206 (21), 155 (32), 130 (41), 109 (50), 83 (19), 55 (24), 41 (10). Anal. Calcd for C24H24FN5O2 (433.48): C, 66.50; H, 5.58; N, 16.16. Found: C, 66.31; H, 5.62; N, 16.26.
N-(cyclohexyl)-2-(6-oxo-4H-[1,2,3]triazolo[1,5-a][1,4]benzodiazepin-5(6H)-yl)-2-(2-chlorophenyl)acetamide(6j)
White solid; yield: 75%, mp 240–242 °C. IR (KBr) (νmax/cm−1): 3233, 3075, 2937, 2861, 1636, 1565. 1H NMR (CDCl3, 500 MHz): δ = 1.00–2.02 (10H, m), 3.83 (1H, m), 4.32–4.75 (2H, m, CH2diastrotopic), 6.45 (1H, s, CHChiral), 6.83 (1H, s, CHtriazole), 7.03 (1H, d, J = 8 Hz), 7.29–7.36 (1H, m), 7.37–7.44 (2H, m), 7.56 (1H, td, J = 7.7, 1.2 Hz), 7.64 (1H, dd, J = 7.5, 2.6 Hz), 7.70(1H, td, J = 7.8, 1.6 Hz), 7.96(1H, d, J = 8 Hz), 8.12(1H, d, J = 7.5 Hz) ppm; 13C NMR (CDCl3, 125 MHz): δ = 24.62, 24.67, 25.36, 32.63, 32.70, 48.05, 59.79, 119.55, 121.06, 122.61, 126.43, 127.13, 128.77, 130.41, 130.56, 132.08, 132.91, 133.07, 165.81, 166.33 ppm. Anal. Calcd for C24H24ClN5O2 (449.93): C, 64.07; H, 5.38; N, 15.57. Found: C, 63.91; H, 5.22; N, 15.73.
N-(cyclohexyl)-2-(6-oxo-4H-[1,2,3]triazolo[1,5-a][1,4]benzodiazepin-5(6H)-yl)-2-(4-chlorophenyl)acetamide(6k)
White solid; yield: 79%, mp 248–250 °C. IR (KBr) (νmax/cm−1): 3234, 3086, 2930, 2857, 1638, 1569. 1H NMR (CDCl3, 500 MHz): δ = 1.01–2.02 (10H, m), 3.82 (1H, m), 4.42 (1H, d, J = 15 Hz, CHdiastrotopic), 4.88 (1H, d, J = 15 Hz, CHdiastrotopic), 6.36 (1H, s, CHChiral), 6.83 (1H, s, CHtriazole), 7.11 (1H, d, J = 7.9 Hz), 7.19–7.30 (2H, m), 7.32–7.43 (2H, m), 7.57 (1H, t, J = 7.7 Hz), 7.71(1H, t, J = 7.7 Hz), 7.99(1H, d, J = 8 Hz), 8.10(1H, d, J = 8 Hz) ppm; 13C NMR (CDCl3, 125 MHz): δ = 24.66, 25.33, 32.63, 32.81, 36.64, 48.76, 59.73, 119.06, 122.62, 126.37, 128.84, 129.23, 130.27, 132.66, 132.78, 133.23, 134.98, 164.64, 166.98 ppm. EIMS, m/z (%): 452 (M+37Cl, 7), 450 (M+35Cl, 25), 350 (9), 324 (100), 295 (67), 155 (43), 125 (59), 98 (12), 83 (18), 55 (21), 41 (16). Anal. Calcd for C24H24ClN5O2 (449.93): C, 64.07; H, 5.38; N, 15.57. Found: C, 64.26; H, 5.45; N, 15.73.
N-(cyclohexyl)-2-(6-oxo-4H-[1,2,3]triazolo[1,5-a][1,4]benzodiazepin-5(6H)-yl)-2-(2,3-dichlorophenyl)acetamide(6l)
White solid; yield: 70%, mp 241–243 °C. IR (KBr) (νmax/cm−1): 3233, 3084, 2931, 2859, 1635, 1566. 1H NMR (CDCl3, 500 MHz): δ = 1.01–1.94 (10H, m), 3.80 (1H, m), 4.46 (1H, d, J = 15 Hz, CHdiastrotopic), 4.62 (1H, d, J = 15 Hz, CHdiastrotopic), 6.29 (1H, d, J = 3 Hz, CHChiral), 6.81(1H, s, CHtriazole), 7.15 (1H, d, J = 7.9 Hz) 7.35(1H, t, J = 7.9 Hz), 7.50–7.61 (3H, m), 7.70 (1H, t, J = 7.5 Hz), 7.97 (1H, d, J = 8.1 Hz), 8.08(1H, d, J = 7.9 Hz) ppm; 13C NMR (CDCl3, 125 MHz): δ = 24.60, 24.65, 25.33, 32.53, 32.64, 36.87, 48.89, 59.46, 118.34, 122.68, 126.27, 127.50, 128.50, 128.79, 131.18, 132.77, 132.88, 133.18, 134.45, 134.69, 137.28, 166.47, 167.39 ppm. Anal. Calcd for C24H23Cl2N5O2 (484.38): C, 59.51; H, 4.79; N, 14.46. Found: C, 59.33; H, 4.48; N, 14.57.
N-(cyclohexyl)-2-(6-oxo-4H-[1,2,3]triazolo[1,5-a][1,4]benzodiazepin-5(6H)-yl)-2-(3-bromophenyl)acetamide(6m)
White solid; yield: 67%, mp 211–213 °C. IR (KBr) (νmax/cm−1): 3231, 3084, 2933, 2862, 1632, 1563.1H NMR (CDCl3, 500 MHz): δ = 0.92–2.03 (10H, m), 3.79 (1H, m), 4.47 (1H, d, J = 15 Hz, CHdiastrotopic), 4.78 (1H, d, J = 15 Hz, CHdiastrotopic), 6.43 (1H, s, CHChiral), 6.78(1H, s, CHtriazole), 7.10–7.24 (3H, m),7.45 (1H, s), 7.50 (1H, d, J = 7.7 Hz), 7.53(1H, t, J = 7.7 Hz), 7.69(1H, t, J = 7.7 Hz), 7.96 (1H, d, J = 8.1 Hz), 8.02 (1H, d, J = 7.9 Hz) ppm; 13C NMR (CDCl3, 125 MHz): δ = 24.65, 25.34, 32.60, 32.75, 36.74, 48.75, 59.76, 119.95, 122.61, 123.03, 126.34, 127.47, 128.80, 130.52, 131.67, 132.06, 132.65, 132.80, 133.22, 136.99, 162.29, 167.04 ppm. Anal. Calcd for C24H24BrN5O2 (494.38): C, 58.31; H, 4.89; N, 14.17. Found: C, 58.52; H, 4.78; N, 14.05.
N-(cyclohexyl)-2-(9-chloro-6-oxo-4H-[1,2,3]triazolo[1,5-a][1,4]benzodiazepin-5(6H)-yl)-2-(4-fluorophenyl)acetamide (6n)
White solid; yield: 69%, mp 239–241 °C. IR (KBr) (νmax/cm−1): 3234, 3085, 2931, 2865, 1633, 1567. 1H NMR (CDCl3, 500 MHz): δ = 1.02–1.99 (10H, m), 3.68–3.92 (1H, m), 4.32–4.70 (2H, m, CH2diastrotopic), 6.27 (1H, s, CHChiral), 6.86 (1H, s, CHtriazole), 7.06 (2H, t, J = 8.4 Hz), 7.20–7.25 (2H, m), 7.52 (1H, dd, J = 8.8, 2.0 Hz), 7.97–8.02 (1H, m), 8.06 (1H, d, J = 8.5 Hz), 8.23 (1H, d, J = 2.5 Hz) ppm; 13C NMR (CDCl3, 125 MHz): δ = 24.67, 25.34, 32.69, 32.84, 36.56, 48.83, 62.20, 116.05, 116.22, 122.59, 124.66, 129.08, 133.51, 134.08, 134.19, 135.05, 139.40, 160.33, 162.34, 166.10, 167.14 ppm. Anal. Calcd for C24H23ClFN5O2 (467.92): C, 61.60; H, 4.95; N, 14.97. Found: C, 61.56; H, 4.81; N, 15.06.
N-(cyclohexyl)-2-(9-chloro-6-oxo-4H-[1,2,3]triazolo[1,5-a][1,4]benzodiazepin-5(6H)-yl)-2-(4-bromophenyl)acetamide (6o)
White solid; yield: 65%, mp 277–279 °C. IR (KBr) (νmax/cm−1): 3233, 3081, 2932, 2866, 1637, 1564. 1H NMR (CDCl3, 500 MHz): δ = 1.00–2.03 (10H, m), 3.81 (1H, m), 4.25 (1H, d, J = 15 Hz, CHdiastrotopic), 4.90 (1H, d, J = 15 Hz, CHdiastrotopic), 6.29 (1H, s, CHChiral), 6.76 (1H, d, J = 3 Hz), 6.94 (1H, s, CHtriazole), 7.17 (2H, d, J = 8.1 Hz), 7.51–7.54 (3H, m), 8.03–8.06 (2H, m) ppm; 13C NMR (CDCl3, 125 MHz): δ = 24.98, 25.33, 32.68, 32.86, 50.29, 61.08, 122.84, 126.37, 128.62, 128.96, 129.99, 132.32, 132.66, 133.23, 135.08, 139.49, 162.12, 166.69 ppm. Anal. Calcd for C24H23BrClN5O2 (528.83): C, 54.51; H, 4.38; N, 13.24. Found: C, 54.69; H, 4.55; N, 13.12.
Anticonvulsant activity
Animals and drugs
Male mice (Pasteur Institute of Iran) weighing 24–30 g were used as experimental animals. The animals were housed in a temperature-controlled room (22 ± 1 °C) on a 12-h light/dark cycle with free access to water and food for a 24-h period before testing, except during the experiment. Mice were assigned to experimental groups randomly, and each animal was used only once for the experiments. Diazepam (Sigma) was used as a reference drug, and pentylenetetrazole (PTZ, Sigma) was applied to induce convulsions in mice. PTZ was dissolved in physiological saline solution, and diazepam and synthesized compounds 6a–o were dispersed in carboxymethyl cellulose (CMC, 0.5%) [16].
All procedures were carried out in accordance with the institutional guidelines for animal care and use that are in compliance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). Ethical approval ID from ethics committee for involving animals in this work is IR.NIMAD.REC.1397.111.
Determination of seizure threshold
The threshold of PTZ-induced seizure was evaluated by infusing PTZ into the tail vein of the animal using 30-gauge butterfly needle, at a constant rate of 1 mL/min. Infusion was stopped when forelimb clonus followed by full clonus was observed in the body. The minimal dose of PTZ (80 mg/kg of animal weight) needed to induce clonic seizure was determined as an index of seizure threshold.
In addition, flumazenil (0.5 mg/kg) was administered 15 min prior to injection of the vehicle, diazepam (0.1 mg/kg) or most potent compound 6h (0.175 mg/kg). After induction of seizure by PTZ, clonic seizure threshold in mice was evaluated.
Maximal electroshock (MES) induced seizures test
The MES-induced seizure is an electrical test for evaluating anticonvulsant activity [15]. In this assay, MES that induced 100% maximal seizures was found to be 50 mA alternating current of 50 Hz frequency for 1 s, using ECT UNIT (model number 7801, UGO Basile, Varese, Italy) [20]. Equimolar dose of synthesized compounds and standard drug diazepam were injected i.p. 5 min later, mice were restrained by hand and subjected to electric shock (through their ears), and released immediately following electrical stimulation, to permit observation of the maximal seizure [20]. The results were recorded as number of animals protected/number of animals tested.
Rotarod (acute neurotoxicity) test
Male NMRI mice with a weight 20–30 g were used for rotarod test. The selected compounds, diazepam and vehicle were administered i.p (n = 4); and 30 min after administration, the animals were placed for 30 s on the rotating rod (5 rpm) and the numbers of mice falling during this time were recorded [18].
Docking study
Docking study of the compounds 6h and 6e in the BZD-binding pocket of GABAA receptor (α1β2 γ2) was performed by Auto dock Tools (version 1.5.6), using previously described method [19].
References
Löscher W (1998) New visions in the pharmacology of anticonvulsion. Eur J Pharmacol 342:1–13. https://doi.org/10.1016/S0014-2999(97)01514-8
Meador KJ (2003) Newer anticonvulsants: dosing strategies and cognition in treating patients with mood disorders and epilepsy. J Clin Psychiatry 64:30–34
Lin Z, Kadaba PK (1997) Molecular targets for the rational design of antiepileptic drugs and related neuroprotective agents. Med Res Rev 17:537–572. https://doi.org/10.1002/(SICI)1098-1128(199711)17
Riss J, Cloyd J, Gates J, Collins S (2008) Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand 118:69–86. https://doi.org/10.1111/j.1600-0404.2008.01004.x
Costa E, Guidotti A, Mao CC, Suria A (1975) New concepts on the mechanism of action of benzodiazepines. Life Sci 17:167–185. https://doi.org/10.1016/0024-3205(75)90501-9
Narayana B, Raj KV, Ashalatha BV, Kumari NS (2006) Synthesis of some new substituted triazolo[4,3-a][1,4]benzodiazepine derivatives as potent anticonvulsants. Eur J Med Chem 41:417–422. https://doi.org/10.1016/j.ejmech.2005.12.003
Biagi G, Giorgi I, Livi O, Scartoni V, Velo S, DeSantis B, Martinelli A, Martini C, Senatore G (1996) 1,2,3-Triazolo[1,5-a][1,3] benzodiazepine a new heterocyclic system: synthesis, benzodiazepine receptor binding and theoretical calculations. Il Farmaco 51:13–18
Hemming K, Chambers CS, Hamasharif MS, Joao H, Khan MN, Patel N, Airley R, Day S (2014) Azide based routes to tetrazolo and oxadiazolo derivatives of pyrrolobenzodiazepines and pyrrolobenzothiadiazepines. Tetrahedron 70:7306–7317. https://doi.org/10.1016/j.tet.2014.07.050
Mohapatra DK, Maity PK, Shabab M, Khan MI (2009) Click chemistry based rapid one-pot synthesis and evaluation for protease inhibition of new tetracyclic triazole fused benzodiazepine derivatives. Bioorg Med Chem Lett 19:5241–5245. https://doi.org/10.1016/j.bmcl.2009.06.107
Saeedi M, Mahdavi M, Foroumadi A, Shafiee A (2013) Synthesis of novel fused 4,5-dihydro-1,2,3-triazolo[1,5-a][1,4]benzodiazepine derivatives via four-component Ugi–Smiles-type reaction. Tetrahedron 69:3506–3510. https://doi.org/10.1016/j.tet.2013.02.023
Mahdavi M, Asadi M, Saeedi M, Rezaei Z, Moghbel H, Foroumadi A, Shafiee A (2012) Synthesis of novel 1,4-benzodiazepine-3,5-dione derivatives: reaction of 2-aminobenzamides under Bargellini reaction conditions. Synlett 23:2521–2525. https://doi.org/10.1055/s-0032-1317297
Noushini S, Mahdavi M, Firoozpour L, Moghimi S, Shafiee A, Foroumadi A (2015) Efficient multi-component synthesis of 1,4-benzodiazepine-3,5-diones: a Petasis-based approach. Tetrahedron 71:6272–6275. https://doi.org/10.1016/j.tet.2015.06.060
Donald JR, Martin SF (2011) Synthesis and diversification of 1,2,3-triazole-fused 1,4-benzodiazepine scaffolds. Org Lett 13:852–855. https://doi.org/10.1021/ol1028404
Oluwaseye A, Uzairu A, Shallangwa GA, Abechi SE (2018) Quantum chemical descriptors in the QSAR studies of compounds active in maxima electroshock seizure test. J King Saud Univ Sci. https://doi.org/10.1016/j.jksus.2018.02.009
Azam F, El-gnidi BA, Alkskas IA (2010) Combating oxidative stress in epilepsy: design, synthesis, quantum chemical studies and anticonvulsant evaluation of 1-(substituted benzylidene/ethylidene)-4-(naphthalen-1-yl) semicarbazides. Eur J Med Chem 45:2817–2826. https://doi.org/10.1016/j.ejmech.2010.02.063
Navidpour L, Shafaroodi H, Miri R, Dehpour AR, Shafiee A (2004) Lipophilic 4-imidazoly-1,4-dihydropyridines: synthesis, calcium channel antagonist activity and protection against pentylenetetrazole-induced seizure. Il Farmaco 59:261–269. https://doi.org/10.1016/j.farmac.2003.11.013
Shafaroodi H, Moezi L, Fakhrzad A, Hassanipour M, Rezayat M, Dehpour AR (2012) The involvement of nitric oxide in the anti-seizure effect of acute atorvastatin treatment in mice. Neurol Res 34:847–853. https://doi.org/10.1179/1743132812Y.0000000080
Dehestani L, Ahangar N, Hashemi SM, Irannejad H, Masihi PH, Shakiba A, Emami S (2018) Design, synthesis, in vivo and in silico evaluation of phenacyl triazole hydrazones as new anticonvulsant agents. Bioorg Chem 78:119–129. https://doi.org/10.1016/j.bioorg.2018.03.001
Mohammadi-Khanaposhtani M, Shabani M, Faizi M, Aghaei I, Jahani R, Sharafi Z, Zafarghandi NS, Mahdavi M, Akbarzadeh T, Emami S, Shafiee A (2016) Design, synthesis, pharmacological evaluation, and docking study of new acridone-based 1,2,4-oxadiazoles as potential anticonvulsant agents. Eur J Med Chem 112:91–98. https://doi.org/10.1016/j.ejmech.2016.01.054
El-Subbagh HI, Hassan GS, El-Azab AS, Alaa AM, Kadi AA, Al-Obaid AM, Al-Shabanah OA, Sayed-Ahmed MM (2011) Synthesis and anticonvulsant activity of some new thiazolo[3,2-a][1,3]diazepine, benzo[d]thiazolo[5,2-a][12,6]diazepine and benzo[d]oxazolo[5,2-a][12,6]diazepine analogues. Eur J Med Chem 46:5567–5572. https://doi.org/10.1016/j.ejmech.2011.09.021
Acknowledgements
This work was supported by Grants from the National Institute for Medical Research Development (NIMAD) (Grant Number: 971149).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shafie, A., Mohammadi-Khanaposhtani, M., Asadi, M. et al. Novel fused 1,2,3-triazolo-benzodiazepine derivatives as potent anticonvulsant agents: design, synthesis, in vivo, and in silico evaluations. Mol Divers 24, 179–189 (2020). https://doi.org/10.1007/s11030-019-09940-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-019-09940-9