Abstract
In this study, we model the long-term effect of climate change on commercially important teak (Tectona grandis) and its productivity in India. This modelling assessment is based on climate projections of the regional climate model of the Hadley Center (HadRM3) and the dynamic vegetation model, IBIS. According to the model projections, 30% of teak grids in India are vulnerable to climate change under both A2 and B2 SRES scenarios because the future climate may not be optimal for teak at these grids. However, the net primary productivity and biomass are expected to increase because of elevated levels of CO2. Given these directions of likely impacts, it is crucial to further investigate the climate change impacts on teak and incorporate such findings into long-term teak plantation programs. This study also demonstrates the feasibility and limitations of assessing the impact of projected climate change at the species level in the tropics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recent studies indicate that the Indian subcontinent could be subjected to an average of over 4°C rise in temperature by 2085 for SRES A2 scenario (Rupakumar et al. 2006). These studies also project an increase in precipitation over India but other studies suggest that monsoons over India might actually weaken significantly in the short term (Zickfeld et al. 2005). Such a decrease in precipitation could have a large impact on the health of forests. For example, it was shown that forest patches subjected to just a 100 mm decrease in rainfall under the effects of the 2005 drought in the Amazon released (on average) 5.3 tonnes of carbon per hectare as trees in the area died (Phillips et al. 2009). We do not know of any similar focussed studies for India and believe that this is a gap in research with respect to regional impacts of climate change.
India has been implementing a large afforestation program at rates of over 1 million hectares annually (Ravindranath et al. 2008) dominated largely by a few species such as teak, eucalyptus and acacia. Such monoculture plantations are likely to dominate plantation forests in India. India has a current forest cover of 23% of total geographic area and would like to increase it to 33% in the coming decades (NAPCC 2008). Thus, there is a need to assess the potential implications of climate change on such forest plantations. In this study, climate change impacts are assessed specifically for teak plantations.
Teak is primarily a native of India and south–east Asia and is known and cherished for its strength, durability and fine grain. Teak is the second-most important plantation tree species in India after sal (Shorea robusta). Teak forests cover an area of 6.3 Mha in the country. Indeed, teak from central India (CP teak) is comparable to the best varieties of teak. It would not be wrong to maintain that teak wood is probably the most thoroughly tested and investigated timber in India. That all of India’s teak production is processed domestically and that India is also a significant net importer of teak is a testimony to the demand for teak-wood in the country (Pandey and Brown 2000). Indeed, India ranks among the top four manufacturers of teak products. There are, thus, compelling reasons for a comprehensive assessment of impacts of potential climate change on the teak species in India. The main goal of this paper is to assess the impacts of climate change at the species level.
The vulnerability of the dominant timber-wood species of Europe and USA to climate change has been explored to a large extent. As far as timber production is concerned, some of the most important species of the US are douglas-fir, ponderosa pine, birch, maple and loblolly pine (Howard 2003). Investigations on the effects of climate change on all of these are being carried out (Littell and Peterson 2005; DeLucia et al. 1994; Riikonen et al. 2004; Norby et al. 2003; McNulty et al. 1996). Investigations of the impacts of climate change on several species of a region collectively have also been performed (Iverson and Prasad 1998). Similar studies have also been done for Europe (Sykes and Prentice 1996; Gessler et al. 2007). Meanwhile, some species-level diebacks are already being observed: for example, quaking aspen in certain parts of USA (Worrall et al. 2008). However, no systematic study has been done on the effect of climate change on the important forest species in India. This study is a first step towards filling this gap in research.
In this study, we modified a global dynamic vegetation model (IBIS, detailed below) so that teak (as a species) is better represented in it. Then, we forced this vegetation model with climate data from a GCM (global circulation model) centred around the year 2085. This allowed us to project the effect of climate change on teak in a spatially explicit manner which helps in making our assessment policy-relevant.
2 Experimental data and methods
2.1 Location of teak in India
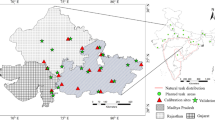
The spatial distribution of teak forests were assessed using a digital forest map prepared by the Forest Survey of India (FSI). The geographic area of India was divided into 165,000 grids (each of 2.5’ by 2.5’). Out of these, 3329 were classified as “teak grids”—grids dominated by teak forests. The location of these grids is given in Fig. 1. Teak is grown in diverse agro-ecological zones in India. It is naturally distributed mainly in the peninsular region. But teak also has been planted in non-traditional areas in northern and north eastern states.
2.2 The dynamic vegetation model
The impact of projected climate change on teak grids was assessed using the global dynamic vegetation model IBIS (Kucharik et al. 2000). The model was configured to study the changes in survival and productivity of teak in India. The model runs were conducted with climatological data centered around year 2085 for A2 and B2 SRES scenarios derived from the HadRM3 model (Rupakumar et al. 2006). These were compared with model projections for the current climate (termed “baseline” or “control” scenario) obtained by forcing the IBIS model with observed climatological data centered around 1975 obtained from CRU (climate research unit), University of East Anglia. The HadRM3 climate model output data was only available for the time periods of 1960–1990 and 2070–2100. Therefore, we restricted our analysis to climate data centered around 1975 and 2085.
2.3 Parameterization of IBIS for teak species
The IBIS model groups all species of vegetation into twelve distinct “plant functional types” (PFTs). Teak falls into PFT type 2, the tropical broadleaf drought-deciduous trees. The main physiological parameters that characterize a particular PFT in IBIS are: 1) Maximum rubisco activity at 15°C at top of canopy, 2) The specific leaf area (SLA), 3) The residence time of carbon in the root, leaf and stem (Rroot, Rleaf and Rwood), and 4) The fractional allocation of carbon in root, leaf and the stem. We assumed that the only species of PFT 2 that would be present in a grid is teak. Hence, the values of these parameters for PFT 2 were changed from the default model values to values corresponding to the teak species. Values for other PFTs were unchanged. In summary, we modified the generic “tropical broadleaf drought-deciduous” PFT in IBIS (represented by PFT 2) to represent teak species.
As we could not get any single source for physiological parameters for teak, we obtained the values for the above parameters from several sources. Since we did not have laboratory access to measure the Rubisco activity of teak, we assumed that it is same as the default value in the model (65.0 * 10−06 mol[CO2] /m2/s).
From our field studies in the Western Ghats regions, we obtained a value of 113 tC/ha for Biomasswood and 5.2 tC/ha/yr for mean annual increment (MAI). This gives a value of 21.73 years for Rwood. Rroot was calculated from values taken from Jha (2003). The value for below ground biomass (BGB) is 11.89 tC/ha and the value of MAI is 10.35 tC/ha/yr, yielding avalue of 1.14 years for Rroot. The value of Rleaf for teak is given as 1 year in Jha (2003) and we use this values in our study
Further, the fractional carbon allocation to leaf, root and stem for teakwere obtained from field measurements. First, the diameter and heights of teak stands were measured. Then, both above ground and below-ground biomass values were estimated using standard volume equations, and subsequently, the allocation coefficients were also derived. The values thus derived for Teak are detailed in Table 1.
3 Model validation
Out of the 3,329 grid points over India, IBIS classified 1,193 as forests and the rest as grasslands in the baseline simulation. This is an under-estimation for forest cover over India when compared to observations. This under-representation of forests in the tropics by IBIS is documented in the literature (Bonan et al. 2003) where it is stated that IBIS simulates higher (than observed) grass coverage in the great plains, southern South America, Africa, and India. In this study, we restrict our analysis to the grids that IBIS simulates as forests.
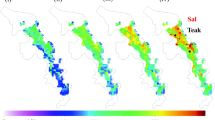
Biomass distribution, as simulated by the IBIS model (parameterized for teak) is shown in Fig. 2. It averages around 2–6 kg/m2 which translates to a biomass of 20–60 tons/ha. The teak-stand biomass reported in literature varies from 50–350 tons/ha (Tewari 1992). Our belief is that IBIS simulates less biomass due to two reasons: 1) The biomass reported in the literature is for plantations raised in optimal and near-optimal conditions. Selection of suitable sites for raising plantations, good water availability and the practise of “thinning” to eliminate substandard tree-individuals are reasons for higher biomass production at plantations. Indeed, the MAI (proportional to biomass) reported for best sites is five times that for poor sites (Ball et al. 1999); 2) IBIS predicts less productivity in the tropics which might be another reason for its under prediction of forest cover in the tropics (Bonan et al. 2003).
The distribution of IBIS-simulated Net Primary Productivity (NPP) is shown in Fig. 3. NPP averages around 0.3–0.5 kg/m2/year or 3–5 tons/ha/year. Typical values reported in the literature are 0.47–0.64 kg/m2/year (Pande 2005). As before, we believe that actual observed values are higher due to near-optimal conditions in teak plantations.
4 Results
4.1 Vulnerability of teak
The vegetation of a particular location is strongly dependent on the local climate (Kirschbaum et al. 1996). It follows that if the climate of a particular location changes significantly, the regional vegetation may be significantly changed (Solomon 1986). However, a “significant” change in climate is hard to define and may be a function vegetation type. Hence, to quantify this effect, we considered the vegetation types predicted by IBIS for all grid points. If, for any grid point, there was a change in the vegetation type predicted by IBIS between baseline and future years, that grid point was marked as “vulnerable”. This implies that the future climate is not conducive to the present vegetation at that grid, and this unsuitability of future climate may cause a shift in vegetation type. This is similar to the approach adopted in Ravindranath et al. (2006). For the sake of simplicity, we assumed that there would not be significant migration (range shift) of teak. We also believe that such migrations would be limited by competing land-use requirements and fragmentation of land use patterns.
Figure 4 shows the distribution of vulnerable grids for both A2 and B2 climate scenario. The distribution is almost same for both scenarios, with 33.3% grids being vulnerable in the A2 scenario and 30.3% grids being vulnerable in the B2 scenario. Around 25.5% of the grids are vulnerable in both the scenarios, suggesting large overlap between the two scenarios.. Hence, the impacts from scenario B2 are not a small subset of A2 but there is a high degree of overlap.
4.2 Impact of climate change on teak plantations
The model simulations project an increase in biomass, NPP and soil organic carbon (SOC) in all the teak grids. Figures 5, 6 and 7 show the percentage increase in biomass, NPP and SOC, respectively, in comparison to the baseline simulation. In the A2 scenario, the percentage increase in biomass averages around 130–150%, while it is around 90–110% in the B2 scenario. These large increases are primarily due to the CO2 fertilization effect: previous studies have shown that IBIS simulates a higher fertilization effect compared to other models (McGuire et al. 2001). The higher sensitivity of IBIS to CO2-fertilization is due to the following reason: Currently, IBIS simulates the effects of changes in the supply of sunlight, water and CO2 to vegetation—limitations of important nutrients like nitrogen and phosphorous in the tropical soils are not considered. The addition of nitrogen and phosphorous cycles might diminish the magnitude of response to elevated CO2 (Hungate et al. 2003). For teak, presence of calcium in the soil is important, followed by nitrogen, potassium, magnesium and phosphorous (Tewari 1992).
Under the National Action Plan on Climate Change, India has announced the Greening India mission which endeavours to bring an additional 6 Mha under forest cover by the year 2020 (NAPCC 2008). This is over and above the current afforestation rate of about 1.1 Mha per annum. One of the positive benefits of such a large scale afforestation programme is its possible contribution to climate change mitigation through carbon sequestration. Therefore, it is essential that long-term climatic and species-related factors that may affect the efficacy of such carbon sinks be studied.
Also, as per the latest document on green-india mission (MoEF 2010), the government of India has detailed its plan to bring 1.50 mha of degraded agricultural lands and fallows under agro forestry. Protection and enhancement of forests with relatively dense forest cover is also envisaged in the document. This work, by identifying critical areas, would help in prioritizing regions for such agro-forestry and forest-enhancement measures.
5 Limitations in modelling and future research needs
In this section, we briefly discuss some shortcomings of the present study. It is known that GCMs are quite robust in their projections for mean temperature change at global levels. However, downscaling of GCM projections to the regional level (as done by HadRM3) has uncertainties associated with it. There is also a lack of consensus among models even as to the sign of future regional precipitation changes predicted (whether it will increase or decrease) for parts of the tropics (IPCC 2007); and annual rainfall and relative humidity are the most important climatic factors influencing the growth of teak (Ball et al. 1999).
Fire is another process that is not accounted for in IBIS, the terrestrial model used in this study. The Intergovernmental Panel on Climate Change (IPCC) report concluded that global climate change will increase the risk of extreme fire events (IPCC 2007). Teak plantations are particularly susceptible to annual fires, as leaf-fall during the early dry season results in the accumulation of fuel on the ground. While teak is often described as fire resistant because trees are seldom killed by fires, annual burning reduces timber quality and growth rate. About 5% of dry deciduous forests in India (e.g. teak) are susceptible to frequent fires and 35% of them are susceptible to occasional fires (FAO 2001).
As previously noted, several elemental and nutrient cycles such as nitrogen, phosphorous, calcium, potassium and magnesium are not represented in IBIS. Their inclusion would reduce the large increases seen in biomass, NPP and SOC. Indeed, other studies have predicted a loss in productivity of teak—for example, from 5.4 m3/ha to 5.07 m3/ha (Achanta and Kanetkar 1996).
India has high genetic variability of teak (Tewari 1992) and significant climatic and soil variations within the country. For example, the allocation fractions of biomass between various compartments vary significantly during the age of the tree. According to Tewari (1992), the fraction of the stem biomass varies from a low of 23.0% to a high of 54.2%. This highlights the limitations of using physiological parameters obtained from only a few site studies to characterize teak stands (of varying ages) over different agro-ecological zones.
Another factor not taken into consideration is the variability in properties of soil. The physical and chemical properties of soil like texture, depth, porosity and drainage are important factors in determining how well teak can grow in a region. Besides being sensitive to the amount of calcium, phosphorous, potassium and nitrogen (C/N ratio), Teak is also sensitive to the pH of the soil (Tewari 1992). The soil data that we used was too coarsely gridded to account all these factors.
6 Concluding remarks
In assessing the vulnerability of the forest sector, there is a strong need for species level assessment. However, only a few species level assessments have been made in the past. In this paper, the effects of climate change on teak in India have been investigated. . Using climate projections from the HadRM3 and IBIS models, we show that the future climate may not be optimal to the present vegetation in about 30% of teak grids. Biomass and NPP is projected to rise but there is a high uncertainty in the magnitude of these increments. Various gaps in the modelling methodology have been identified, indicating the need for more sustained research effort in this direction. Further studies should take into account the current planting practices: for example, the present trend of using a species mixture (such as teak with bamboo, acacia or sissoo) and its future impacts. Dissemination of findings from investigations is also important, especially in the light of growing investment in teak plantations from the private sector and farming community.
References
Achanta AN, Kanetkar R (1996) Impact of climate change on forest productivity: a case study of Kerala, India. Paper presented at the Asian and Pacific Workshop on Climate Change Vulnerability and Adaptation Assessment, Manila, 15–19 January 1996
Ball JB, Pandey D, Hirai S (1999) Global overview of teak plantations. Paper presented at seminar on technology and productivity of teak plantations, Chiang Mai, Thailand, 26–29 January 1999
Bonan GB, Levis S, Sitch S, Vertenstein M, Oleson KW (2003) A dynamic global vegetation model for use with climate models: concepts and description of simulated vegetation dynamics. Glob Chang Biol 9:1543–1566
DeLucia EH, Callaway RM, Schlesinger WH (1994) Offsetting changes in biomass allocation and photosynthesis in ponderosa pine (Pinus ponderosa) in response to climate change. Tree Physiol 14:669–677
FAO (2001) Global forest fire assessment 1990–2000, FAO (Forest Resources Assessment)
Gessler A, Keitel C, Kreuzwieser J, Matyssek R, Seiler W, Rennenberg H (2007) Potential risks for European beech (Fagus sylvatica L.) in a changing climate. Trees 21:1–11
Howard JL (2003) U.S. timber production, trade, consumption, and price statistics, 1965–2002, US Department of Agriculture. Available at: http://permanent.access.gpo.gov/lps5730/1965-2002/fplrp615.pdf . Cited 26th Jan 2010
Hungate BA, Dukes JS, Shaw R, Luo Y, Field CB (2003) Nitrogen and climate change. Science 302:1512–1513
IPCC (2007) Climate change 2007: the physical science basis, contribution of working group I to the fourth assessment report of the IPCC. Cambridge Univ. Press, Cambridge, 2007
Iverson LR, Prasad AM (1998) Predicting abundance of 80 tree species following climate change in the Eastern United States. Ecol Monogr 68:465–485
Jha KK (2003) Temporal pattern of dry matter and nutrient dynamics in young teak plantations, XII world forestry congress, 2003. Available via: http://www.fao.org/DOCREP/ARTICLE/WFC/XII/0029-B1.HTM. Cited on 26th Feb. 2010
Kirschbaum MUF, Cannell MGR, Cruz RVO, Galinski W, Cramer WP (1996) Climate change impacts on forests. In: Climate Change 1995, Impacts, Adaptation and Mitigation of Climate Change: Scientific–Technical Analyses, Cambridge University Press, Cambridge, 1996
Kucharik CJ, Foley JA, Delire C, Fisher VA, Coe MT, Lenters J, Young-Molling C, Ramankutty N, Norman JM, Gower ST (2000) Testing the performance of a dynamic global ecosystem model: Water balance, carbon balance and vegetation structure. Glob Biogeochem Cycles 14(3):795–825
Littell JS, Peterson DL (2005) A method for estimating vulnerability of Douglas-fir growth to climate change in the Northwestern U.S. For Chron 81(3):369–374
McGuire AD, Sitch S, Clein JS, Dargaville R, Esser G et al (2001) Carbon balance of the terrestrial biosphere in the twentieth century: analyses of CO2, climate and land-use effects with four process-based ecosystem models. Glob Biogeochem Cycles 15:183–206
McNulty SG, James MV, Wayne TS (1996) Potential climate change effects on loblolly pine forest productivity and drainage across the Southern United States. Ambio 25(7):449–453
Ministry of environment and forests (MoEF) (2010) Govt. of India, “National mission for a green India (version 1.0)”, Available via: http://moef.nic.in/downloads/public-information/green-india-mission.pdf. Cited on 25th may 2010
National Action Plan on Climate Change (NAPCC) (2008) Govt. of India. Available via http://pmindia.nic.in/Pg01-52.pdf. Cited 25th may 2010
Norby RJ, Hartz-Rubin JS, Verbrugge MJ (2003) Phenological responses in maple to experimental atmospheric warming and CO2 enrichment. Glob Chang Biol 9(12):1792–1801
Pande PK (2005) Biomass and productivity in some disturbed tropical dry deciduous teak forests of Satpura plateau, Madhya Pradesh. Trop Ecol 46:229–39
Pandey D, Brown C (2000) Teak: a global overview, Unasylva, No. 201. Available via: http://www.fao.org/docrep/x4565e/x4565e03.htm#P0_0 . Cited on 26th May 2010
Phillips OL, Aragao LEOC, Lewis SL, Fisher JB, Lloyd J, Lopez-Gonzalez G, Malhi Y, Monteagudo A, Peacock J, Quesada CA et al (2009) Drought sensitivity of the Amazon rainforest. Science 323:1344–1347
Ravindranath NH, Joshi NV, Sukumar R, Saxena A (2006) Impact of climate change on forests in India. Curr Sci 90(3):354–361
Ravindranath NH, Chaturvedi RK, Murthy IK (2008) Forest conservation, afforestation and reforestation in India: implications for forest carbon stocks. Curr Sci 95(2):216–222
Riikonen J, Lindsberg MM, Holopainen T, Oksanen E, Lappi J, Peltonen P, Vapaavuori E (2004) Silver birch and climate change: variable growth and carbon allocation responses to elevated concentrations of carbon dioxide and ozone. Tree Physiol 24(11):1227–1237. doi:10.1093/treephys/24.11.1227
Rupakumar K, Sahai AK, Krishnakumar K, Patwardhan SK, Mishra PK, Revadekar JV, Kamala K, Pant GB (2006) High-resolution climate change scenarios for India for the 21st century. Curr Sci 90(3):334–344
Solomon AM (1986) Transient responses of forests to CO2-induced climate change: simulating modelling experiments in eastern North America. Oecologia 68:567–579
Sykes MT, Prentice IC (1996) Climate change, tree species distributions and forest dynamics: a case study in the mixed conifer/northern hardwoods zone of northern Europe. Clim Change 34(2):161–77
Tewari DN (1992) Monograph on teak, International Book Distributors
Worrall JJ, Egeland L, Eager T, Mask RA, Johnson EW, Kemp PA, Shepperd WD (2008) Rapid mortality of Populus tremuloides in southwestern Colorado, USA. For Ecol Manage 255:686–696
Zickfeld K, Knopf B, Petoukhov V, Schellnhuber HJ (2005) Is the Indian summer monsoon stable against global change? Geophys Res Lett 32:L15707. doi:10.1029/2005GL022771
Acknowledgements
Research work for this publication was conducted under the project “Impact of climate change on tropical forest ecosystems and biodiversity in India”, funded by the Royal Norwegian Embassy in collaboration with CICERO, Oslo. We thank the Royal Norwegian Embassy and CICERO for this support. We also thank IITM, Pune, and in particular K Krishna Kumar and Savita Patwardhan for providing HadRM3 climate projections under the NATCOM project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gopalakrishnan, R., Jayaraman, M., Swarnim, S. et al. Impact of climate change at species level: a case study of teak in India. Mitig Adapt Strateg Glob Change 16, 199–209 (2011). https://doi.org/10.1007/s11027-010-9258-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11027-010-9258-6