Abstract
The present study was designed to evaluate the effects of matrine (MAT) on scopolamine (SCOP)-induced learning and memory impairment. After successive oral administration of MAT to mice for three days at doses of 0.4, 2, and 10 mg/kg, we assessed improvements in learning and memory and investigated the mechanism of action of SCOP-induced amnesia. Donepezil at a dose of 3 mg/kg was used as a standard memory enhancer. MAT significantly improved SCOP-induced learning and memory impairment in novel object recognition and Y-maze tests at doses of 0.4, 2, and 10 mg/kg. Furthermore, MAT inhibited acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) activities and decreased oxidative stress in the brain, as evidenced by increased total antioxidant capacity, total superoxide dismutase levels, and catalase activities as well as decreased malondialdehyde levels. Additionally, there was a significant negative correlation between the percentage of spontaneous alternation in the Y maze and AChE activity in the cortex and hippocampus. MAT ameliorated SCOP-induced amnesia by the inhibition of both AChE/BuChE activities and oxidative stress. This study provides further evidence to encourage the development of MAT as a drug for the prevention or treatment of Alzheimer’s disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disease and the fifth leading cause of death in Americans aged ≥65 years. The total cost for the care and treatment of these individuals was estimated to be $236 billion in 2016 (Alzheimer's Association 2016). The pathophysiology of AD includes neurofibrillary tangles and amyloid plaque formation, increased oxidative stress and neuroinflammation, and reduced acetylcholine levels (Banks 2012). Choline acetyltransferase levels, cholinergic neuron numbers, and nAChR levels decrease and cholinergic neuron and axon are abnormal in AD brains (Bartus et al. 1982). Changes in acetylcholinesterase (AChE) are strongly correlated with learning and memory impairments (Araujo et al. 2005). In addition, AChE accelerates amyloid β (Aβ) fibril formation, and the neurotoxicity induced by Aβ–AChE complexes is greater than that induced by the Aβ peptide alone (Inestrosa et al. 2008). Until now, the most successful approach for AD treatment has been the inhibition of AChE activity with drugs such as tacrine and donepezil (DON). Such inhibitors can increase the availability of acetylcholine (ACh) and slow the progression of dementia symptoms. The Food and Drug Administration has approved them to treat mild AD (Lleó et al. 2006); however, their clinical value is limited because of disadvantages such as hepatotoxicity and peripheral side effects (Lleó et al. 2006; Russ and Morling 2012). Thus, researchers are actively developing novel compounds with better therapeutic efficacies and fewer side effects from natural products (Haider et al. 2018; Li et al. 2016).
Scopolamine (SCOP), a well-known muscarinic cholinergic receptor antagonist, induces a reversible impairment of learning and memory not only in mice and rats (Haider et al. 2018; Li et al. 2016) but also in humans (Alvarez-Jimenez et al. 2016). Hence, SCOP-induced learning and memory impairment models are widely used to evaluate anti-amnesic effects (Xu et al. 2016; Xiao et al. 2014; Liu et al. 2017; Qu et al. 2017). Studies have shown that SCOP-induced memory impairment is associated with cholinesterase (ChE) activity and oxidative stress increases in the brain (Xu et al. 2016; Xiao et al. 2014; Liu et al. 2017; Qu et al. 2017). Oxidative stress plays an essential role in regulating signaling pathways that lead to cell death (Luque-Contreras et al. 2014), and high-level oxidative stress in the brain is a factor that leads to AD. Malondialdehyde (MDA) and superoxide dismutase (SOD) are positive correlation in the AD and MCI population, so they are valuable biomarkers for the diagnosis of AD (López et al. 2013).
Matrine (MAT) is the major alkaloid extracted from the traditional Chinese medicinal plant Sophora flavescens. Previous studies have shown that MAT exhibits a wide range of pharmacological effects, including antiviral, antitumor, neuroprotective, and procognitive effects (Chen et al. 2016; Li et al. 2015; Meng et al. 2017; Liu et al. 2016; Cui et al. 2017). It has been demonstrated that MAT not only attenuates focal cerebral ischemic injury by decreasing MDA levels and increasing SOD levels, catalase (CAT) activity, and total antioxidant capacity (T-AOC) (Zhao et al. 2015) but also produces an antinociceptive effect by increasing cholinergic activation (Yin and Zhu 2005). However, the question of whether MAT protects against SCOP-induced amnesia has not yet been addressed. To further understand the effect of MAT on learning and memory and also evaluate it as a candidate for drug development, we investigated whether MAT has an impact on cognitive impairment and memory deficits as well as on ChE and oxidative stress levels in the brain.
Materials and methods
Drugs and chemicals
MAT, SCOP hydrobromide, and DON HCl were purchased from Aladdin Reagent company (Shanghai, China). All other materials were the highest grade available.
Animals and drug administration
Male ICR mice (8-week-old, 30–35 g) were purchased from Shanxi Medical University (Shanxi, China). Mice were housed 5 per cage, allowed free access to food and water, and maintained at a constant temperature (23 °C ± 1 °C) and humidity (55% ± 5%) under a 12-h light/dark cycle (lights on between 08:00 and 20:00 h). The investigation conformed to the International Guiding Principles for Biomedical Research Involving Animals (1985). The same mice were used in each experiment to minimize both the number of animals used and their suffering. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Shanxi Agricultural University.
The mice were randomly divided into six groups (12 mice per group): control group (2% ethanol in saline + saline), SCOP group (2% ethanol in saline + SCOP 2 mg/kg), DON group (DON 3 mg/kg + SCOP 2 mg/kg), MAT low-dose group (MAT 0.4 mg/kg + SCOP 2 mg/kg), MAT middle-dose group (MAT 2 mg/kg + SCOP 2 mg/kg), and MAT high-dose group (MAT 10 mg/kg + SCOP 2 mg/kg). The mice were orally given MAT or solvent (2% ethanol in saline) daily from three days before the behavior test to the dissection of mice. The control group was treated with saline, and the remaining groups were treated with SCOP hydrochloride (2 mg/kg, i.p.) 30 min before the behavior test.
Novel object recognition (NOR) task
Learning and memory ability was assessed by the NOR task as described previously (Botton et al. 2010; Milić et al. 2013). The dimensions of the apparatus were 40 cm × 25 cm × 20 cm (length × width × height). The mice were individually placed into the apparatus for 5 min in the absence of any objects once per day for two days. The apparatus was cleaned using 70% ethanol to eliminate any residual odor, and the next mouse was placed into the apparatus once it was dry. On the third day, the mice were tested by comparing two sessions: the training and test sessions. In the training session, the mice were placed into the apparatus containing two identical objects and allowed to explore for 5 min. The objects were placed in opposite adjacent corners, 5 cm away from the walls. The test session was performed 60 min after the training session, and one object was replaced by a novel one before the test session start. Exploration was defined as pointing of the head or nose toward the object within 1 cm or actual touching of the object. However, if the mouse sat on the object, this was not considered to be exploration. The discrimination index of each mouse was defined as (TN − TF) / (TN + TF), where TF = time exploring the familiar object and TN = time exploring the novel object (Antunes and Biala 2012).
Spontaneous alternation behavior Y-maze test
The Y-maze test performed after the NOR test as described previously (Sarter et al. 1988). The Y-maze was composed of three dark, polyvinyl plastic arms (30 cm × 5 cm × 12 cm, 120°). Mice were initially placed at the end of one arm and allowed to explore the Y maze freely. The number of arm entries and the sequence of arm visits was recorded manually for each mouse over 8 min. The mouse was then removed from the Y-maze, and the Y-maze was cleaned with 70% ethanol to remove any odor. The next mouse was placed into the Y-maze once it had dried. Consecutive entry into the three different arms was defined as spontaneous alternation, i.e., BCA, ABC, or CAB but not ABB, and the alternation percentage was calculated as the number of spontaneous alterations × 100 / total number of entries.
Biochemical analysis
After the Y-maze test, mice were sacrificed by cervical dislocation 30 min after injection of SCOP at a dose of 2 mg/kg, and the brain was immediately removed. The brain was bisected along the sagittal plane, and the hippocampus and cortex were dissected from one half and stored at −20 °C until needed for biochemical analysis. Tissues were rapidly homogenized in ice-cold saline (9 times tissue weight), and the homogenates were centrifuged at 4500 g at 4 °C for 15 min, and the supernatants were collected for determination of AChE, BuChE, T-AOC, SOD, and CAT activities as well as MDA level. Protein concentrations were determined using a Bradford protein assay kit and BSA was used as the standard (Bio-Rad Laboratories, Hercules, USA). 2 uL of a 10-fold dilution of the homogenate was used for protein concentration determination.
AChE and BuChE assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) were initially used to measure the AChE or BuChE activities in the hippocampus and cortex according to the manufacturer’s instructions. Up to 6 μL of the homogenate was optimal for activity detection. AChE and BuChE activities were expressed as U/mg protein.
Additionally, SOD and CAT activities, T-AOC, and MDA levels were measured with Total Superoxide Dismutase, CAT, Total Antioxidant Capacity, and MDA Detection Kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), respectively. Sixty, 10, or 40 μL of the 10% tissue homogenate was used for T-AOC, CAT, and MDA levels, respectively. 12 μL of a 20-fold dilution of the homogenate was used to detect the SOD activity. The activities of T-AOC, SOD, and CAT were expressed in U/mg protein, and MDA level was expressed in nmol/mg protein.
Statistical analyses
The results of the NOR task, the Y-maze, enzyme activity, and MDA level were expressed as mean ± SEM. Statistical analysis was performed using the SPSS 16 software. Data were analyzed by one-way ANOVA followed by Tukey statistical post hoc analysis. Statistical significance was set at p < 0.05. Pearson’s correlation coefficient and regression analysis were used to evaluate the correlation between biochemical parameters and behavioral responses.
Results
Effect of MAT on the SCOP-induced cognitive impairment in the object recognition task
The chemical structure of MAT and experimental protocol in this study are shown in the Fig. 1. SCOP caused a decline in the object recognition index (p < 0.01), whereas pre-administration of DON (3 mg/kg) or MAT (0.4, 2, and 10 mg/kg, oral) abolished the partial amnesic effect of SCOP (Fig. 2b; p < 0.05 for all). SCOP caused memory deficit in the NOR task, which was prevented by pretreatment with DON or MAT (0.4, 2, and 10 mg/kg). No significant differences were seen in total time spent on the object among all groups (Fig. 2a). These results suggest that MAT can improve the reference memory impairment caused by SCOP.
MAT effects on SCOP-induced memory impairment in the novel object recognition test. MAT (0.4, 2 10 mg/kg) was administrated orally to SCOP-treated mice. Thirty min before the test, the mice were treated with SCOP (2 mg/kg, i.p.). The time spent on the object in seconds (a) and discrimination index (b) were measured. Results are presented as mean ± SEM, (n = 12). Data were analyzed by one-way ANOVA followed by Tukey statistical post hoc analysis: ##p < 0.01 vs. the vehicle control group and *p < 0.05 vs. the SCOP-treated group. DON 3 received 3 mg/kg donepezil, MAT 0.4 received 0.4 mg/kg matrine, MAT 2 received 2 mg/kg matrine, MAT 10 received 10 mg/kg matrine
Effect of MAT on the SCOP-induced learning and memory decline in the Y-maze
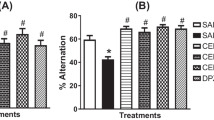
The impact of MAT on working memory was investigated with the Y-maze test. The SCOP-treated group exhibited a dramatic decrease in spontaneous alternation and an increased number of arm entries compared to the control group (Fig. 3; p < 0.001, p < 0.001). DON and MAT (0.4, 2 and 10 mg/kg) both significantly reversed the spontaneous alternation decline caused by the SCOP (Fig. 3b; p < 0.01 for all). However, DON at 3 mg/kg or MAT at all three doses did not change the number of arm entries exhibited by the SCOP-treated group (Fig. 3a). The data suggest that MAT can improve the working and short-term memory of SCOP-treated mice.
MAT effects on the Y-maze test. The effect of MAT on (a) the total number of entries and (b) alternation behavior in SCOP-induced working memory deficit in a Y-maze. Values are expressed as mean ± SEM (n = 12). Data were analyzed by one-way ANOVA followed by Tukey statistical post hoc analysis: ###p < 0.001 vs. the vehicle control group and **p < 0.01 vs. the SCOP-treated group
Effect of MAT on AChE and BuChE activities in the hippocampus and cortex
We examined the influence of MAT on AChE and BuChE activities in the hippocampus and cortex. SCOP significantly increased AChE activity in both tissues relative to the control group (Fig. 4a, b; p < 0.05 for both tissues). Pre-administration of DON (3 mg/kg) and MAT (0.4, 2 and 10 mg/kg) significantly inhibited AChE activity in the hippocampus (Fig. 4a; p < 0.05, p < 0.05, p < 0.05, and p < 0.01, respectively), as well as DON (3 mg/kg) and MAT (2 and 10 mg/kg) decreased AChE activity in the cortex (Fig. 4b p < 0.05, p < 0.05 and p < 0.01, respectively), compared with the SCOP-treated group. However, SCOP did not affect BuChE activity in the hippocampus and cortex, and treatment with MAT decreased BuChE activity only at a dose of 0.4 mg/kg in the hippocampus (Fig. 4c; p < 0.001). DON at 3 mg/kg did not affect the BuChE activity increased elicited by SCOP treatment (Fig. 4c, d).
Effect of MAT on the cholinergic nerve system of SCOP-induced mice. Effect of MAT on AChE in the hippocampus and cortex (a and b) and BuChE activities in the hippocampus and cortex (c and d) of SCOP-treated mice. The animals were dislocated after the Y-maze test, and the hippocampus and cortex dissected from the brains for determination of AChE and BuChE activities. The values represent mean ± SEM, (n = 6). Data were analyzed by one-way ANOVA followed by Tukey statistical post hoc analysis: #p < 0.05 vs. the vehicle control group and *p < 0.05, ***p < 0.001 vs. the SCOP-treated group
More importantly, linear regression was determined showed that there was a significant negative correlation between the percentage of the spontaneous alternation and AChE activity in the hippocampus (n = 36, r = −0.399, p < 0.05) and cortex (n = 36, r = −0.629, p < 0.05; Fig. 5).
Pearson’s correlation between the percentage of the spontaneous alternation versus AChE levels. There was a significant negative correlation between the percentage of spontaneous alternation and AChE levels in the hippocampus (a) and cortex (b). Control (hollow circle), SCOP (2 mg/kg scopolamine, inverted hollow triangle), DON-3 (3 mg/kg donepezil +2 mg/kg scopolamine, filled square), MAT-0.4 (0.4 mg/kg matrine +2 mg/kg scopolamine MAT, filled triangle), MAT-2 (2 mg/kg matrine +2 mg/kg scopolamine MAT, hollow triangle), and MAT-10 (10 mg/kg matrine +2 mg/kg scopolamine, inverted filled triangle); n = 36
The anti-oxidative effect of MAT in the hippocampus and cortex of SCOP-induced amnesiac mice
We also examined the anti-oxidative effect of MAT on SCOP-treated mice. T-AOC was first analyzed in the brain. The SCOP-treated group exhibited a significant decrease in brain T-AOC compared with the control group (Fig. 6; p < 0.05), while DON and MAT at 2 and 10 mg/kg treatment increased T-AOC compared with the SCOP-treated group (Fig. 6; p < 0.05, p < 0.05 and p < 0.001). T-SOD decreased in the hippocampus (Fig. 7a; p < 0.05) and cortex (Fig. 7b; p < 0.05) in the SCOP-treated group, and increased upon pretreatment with DON and all concentrations of MAT in both the hippocampus (Fig. 7a; p < 0.001, p < 0.001, p < 0.001 and p < 0.001, respectively) and cortex (Fig. 7b; p < 0.05, p < 0.05, p < 0.05 and p < 0.001, respectively); meanwhile, MDA level increased in the SCOP-treated group in both the hippocampus (Fig. 7c; p < 0.05) and cortex (Fig. 7d; p < 0.05), whereas it decreased in the hippocampus upon treatment with DON at 3 mg/kg and MAT at 10 mg/kg (Fig. 7c; p < 0.05) and in the cortex upon treatment with DON at 3 mg/kg and MAT at 2 and 10 mg/kg (Fig. 7d; p < 0.05). CAT activity reduced in the cortex in SCOP group (Fig. 7f; p < 0.001) but was significantly higher upon treatment with DON and MAT at all concentrations compared with the SCOP group (Fig. 7f; p < 0.05, p < 0.05, p < 0.05, and p < 0.001, respectively).
MAT reduces oxidative stress in the hippocampus and cortex of SCOP-treated mice. Changes in T-SOD activity (a and b), MDA levels (c and d) and CAT activity (e and f) in the hippocampus and cortex after MAT treatment. The values represent mean ± SEM, (n = 6). Data were analyzed by one-way ANOVA followed by Tukey statistical post hoc analysis: #p < 0.05 and ###p < 0.001 vs. the control group; *p < 0.05 and ***p < 0.001 vs. the SCOP-treated control group
Discussion
The present study was designed to assess the neuroprotective activity of MAT. Treatment with MAT significantly improved SCOP-induced learning and memory deficits via inhibition of AChE/BuChE and oxidative stress mechanisms.
Cholinergic dysfunction is one of leading causes of deterioration of learning and memory processing in AD (Fodale et al. 2006; Yue et al. 2015), and blocking cholinergic function in young subjects can artificially induce learning and memory deficits (Bartus et al. 1982). SCOP can block ACh muscarinic receptors and leads to a substantial increase in AChE activity in the hippocampus and cortex (Ben-Barak and Dudai 1980). In this study, we employed a SCOP-treated mouse model to evaluate the neuroprotective activity of MAT. Behavioral tests showed that MAT could protect against SCOP-induced learning and memory impairment.
Reduced levels of acetylcholine is an important pathophysiology of AD (Banks 2012). AChE is a hydrolytic enzyme that rapidly hydrolyzes ACh (Ladner and Lee 1998). AChE accelerates the accumulation of fibrils by binding to Aβ-associated proteins, and the neurotoxicity induced by Aβ-AChE complexes is higher than that of Aβ peptide alone. (Inestrosa et al. 2008). In this study. Pre-administration of DON and MAT significantly inhibited AChE activity in the hippocampus and cortex relative to the SCOP group, (Fig. 4). These findings are consistent with the behavioral data. Changes in AChE activity are strongly correlated with learning and memory impairments (Araujo et al. 2005), and our results showed that there was a significant negative correlation between the percentage of spontaneous alternation in a Y maze with AChE activity in the cortex and hippocampus (Fig. 5).
BuChE serves as a co-regulator of cholinergic neurotransmission by hydrolyzing ACh (Mesulam et al. 2002a, b). AChE levels decrease gradually in the brains of patients with AD; however, BuChE activity is either unchanged or increases to 165% of normal values (Dighe et al. 2016; Perry et al. 1978). Additionally, extracellular ACh levels in the hippocampus are elevated 60-fold in AChE−/− mice compared with wild-type (AChE+/+) animals, while BuChE controls ACh levels in the AChE deficient brain (Hartmann et al. 2007). This means that BuChE is a viable target for AD treatment. BuChE levels in the hippocampus decreased after treatment with MAT at 0.4 mg/kg compared with SCOP group (Fig. 4c). In a previous study, MAT exhibited an antinociceptive effect through increased cholinergic activation (Yin and Zhu 2005). Thus, MAT is a dual AChE/BuChE inhibitor and so a potential neuroprotective agent, since these results suggest that MAT may improve reference and spatial memory by modulating the choline system.
In a previous study, pretreatment with SCOP at 1 mg/kg increased BuChE levels in the frontal cortex but did not alter its activity in the hippocampus (Liu et al. 2017). In this study, BuChE levels increased in the hippocampus with SCOP treatment at 2 mg/kg, albeit not significantly, but did not change in the cortex. This may be due to differences in both the locations and treatments between the studies, and SCOP did not take effect on the whole cortex under our treatment conditions because the distribution pattern of BuChE in the brain is distinct from that of AChE (Darvesh 2013). Additionally, MAT inhibited the BuChE only at doses of 0.4 mg/kg in the hippocampus, which may be due to a feedback regulation effect, as reported previously when BuChE levels in the cerebrospinal fluid of AD patients treated with rivastigmine was not dose-dependent (Giacobini et al. 2002). MAT only inhibited SCOP-induced BuChE in the hippocampus, however, it did not change the BuChE in the cortex, this means that MAT only affect the SCOP-induced the BuChE increase. DON treatment did not alter brain BuChE levels compared with the SCOP group (Fig. 4b) since DON is a selective AChE inhibitor and does not alter BuChE activity in AChE−/− mice (Naik et al. 2009).
Oxidative stress significantly contributes to cell apoptosis and death, which is commonly observed in AD patients (Luque-Contreras et al. 2014); oxidative stress is also considered to be a decisive factor in AD (López et al. 2013). Supplementation with antioxidants attenuates the cell apoptosis and death caused by oxidative stress (Schroeter et al. 2000). In previous studies, SCOP increased oxidative stress by a number of mechanisms, including decreasing T-AOC, SOD and CAT and increasing MDA levels (Xiao et al. 2014; Haider et al. 2018). It has been demonstrated that blockage of muscarinic receptors inhibits the expression of nuclear factor-E2 related factor-2 (Nrf2), a transcription factor that regulates the expression of proteins that collectively constitute antioxidant cellular responses (Espada et al. 2009). Hence, it has been proposed that scopolamine-induced cholinergic dysfunction leads to downregulation of expression of antioxidant enzymes via decreased Nrf2 expression (Venkatesan et al. 2016). In our studies, MAT and DON could significantly increase T-AOC in the brain, T-SOD in the hippocampus and cortex, and CAT in the cortex. MDA is a hallmark of lipid peroxidation in the cortex and hippocampus, and it is an important biomarker for the diagnosis of AD (López et al. 2013). MAT significantly inhibited MDA levels in the cortex and hippocampus. Our results suggest that the neuroprotective effects of MAT may be due to an anti-oxidative stress effect. This is consistent with previous reports that MAT has neuroprotective effects in focal cerebral ischemic injury (Cui et al. 2017) and MPTP-induced Parkinson’s disease via anti-oxidative mechanisms (Meng et al. 2017), and that DON can inhibit oxidative stress in SCOP-induced amnesiac mice (Li et al. 2016; Xu et al. 2016).
In conclusion, the present study provides evidence that MAT ameliorates the SCOP-induced cognitive impairment and memory deficits highlighted by NOR and Y-maze tasks in mice. In addition, the potent neuroprotective effects of MAT may be partly linked to anti-ChE and antioxidant activities in the brain. These results suggest that MAT may be a candidate for a pharmaceutical agent for the prevention or treatment of the cognitive dysfunction observed in many disorders, including AD.
Abbreviations
- Aβ:
-
amyloid-β
- AChE:
-
acetylcholinesterase
- Ach:
-
acetylcholine
- AD:
-
Alzheimer’s disease
- BuChE:
-
butyrylcholinesterase
- CAT:
-
catalase
- ChE:
-
cholinesterase
- DON:
-
donepezil
- MAT:
-
matrine
- MDA:
-
malondialdehyde
- NOR:
-
novel object recognition
- Nrf2:
-
nuclear factor-E2 related factor-2
- SCOP:
-
scopolamine
- T-AOC:
-
total anti-oxidantant capacity
- T-SOD:
-
total superoxide dismutase
References
Alvarez-Jimenez R, Groeneveld GJ, van Gerven JM, Goulooze SC, Baakman AC, Hay JL, Stevens J (2016) Model-based exposure-response analysis to quantify age related differences in the response to scopolamine in healthy subjects. Br J Clin Pharmacol 82:1011–1021
Alzheimer's Association (2016) 2016 Alzheimer's disease facts and figures. Alzheimers Dement 12:459–509
Antunes M, Biala G (2012) The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13:93–110
Araujo JA, Studzinski CM, Milgram NW (2005) Further evidence for the cholinergic hypothesis of aging and dementia from the canine model of aging. Prog Neuro-Psychopharmacol Biol Psychiatry 29:411–422
Banks WA (2012) Drug delivery to the brain in Alzheimer's disease: consideration of the blood-brain barrier. Adv Drug Deliv Rev 64:629–639
Bartus RT, Dean RL, Ljubic B, Lippa A (1982) The cholinergic hypothesis of geriatric memory dysfunction. Science 217:408–414
Ben-Barak J, Dudai Y (1980) Scopolamine induces an increase in muscarinic receptor level in rat hippocampus. Brain Res 193:309–313
Botton PH, Costa MS, Ardais AP, Mioranzza S, Souza DO, da Rocha JB, Porciúncula LO (2010) Caffeine prevents disruption of memory consolidation in the inhibitory avoidance and novel object recognition tasks by scopolamine in adult mice. Behav Brain Res 214:254–259
Chen JX, Shen HH, Niu M, Guo YM, Liu XQ, Han YZ, Zhang YM, Zhao YL, Bai BK, Zhou WJ, Xiao XH (2016) Anti-hepatitis B virus effect of matrine-type alkaloid and involvement of p38 mitogen-activated protein kinase and tumor necrosis factor receptor-associated factor 6. Virus Res 215:104–113
Cui LL, Cai YJ, Cheng WW, Liu G, Zhao JG, Cao H, Tao H, Wang Y, Yin MK, Liu TT, Liu Y, Huang PR, Liu Z, Li KS, Zhao B (2017) A novel, multi-target natural drug candidate, Matrine, Improves Cognitive Deficits in Alzheimer's Disease Transgenic Mice by Inhibiting Aβ Aggregation and Blocking the RAGE/Aβ Axis. Mol Neurobiol 54:1939–1952
Darvesh S (2013) Butyrylcholinesterase radioligands to image Alzheimer's disease brain. Chem Biol Interact 203:354–357
Dighe SN, Deora GS, DelaMora E, Nachon F, Chan S, Parat MO, Brazzolotto X, Ross BP (2016) Discovery and structure-activity relationships of a highly selective butyrylcholinesterase inhibitor by structure-based virtual screening. J Med Chem 59:7683–7689
Espada S, Rojo AI, Salinas M, Cuadrado A (2009) The muscarinic M1 receptor activates Nrf2 through a signaling cascade that involves protein kinase C and inhibition of GSK-3beta: connecting neuro transmission with neuroprotection. J Neurochem 110:1107–1119
Fodale V, Quattrone D, Trecroci C, Caminiti V, Santamaria LB (2006) Alzheimer's disease and anaesthesia: implications for the central cholinergic system. Br J Anaesth 97:445–452
Giacobini E, Spiegel R, Enz A, Veroff AE, Cutler NR (2002) Inhibition of acetyl- and butyryl-cholinesterase in the cerebrospinal fluid of patients with Alzheimer's disease by rivastigmine: correlation with cognitive benefit. J Neural Transm (Vienna) 109:1053–1065
Haider S, Batool Z, Ahmad S, Siddiqui RA, Haleem DJ (2018) Walnut supplementation reverses the scopolamine-induced memory impairment by restoration of cholinergic function via mitigating oxidative stress in rats: a potential therapeutic intervention for age related neurodegenerative disorders. Metab Brain Dis 33:39–51
Hartmann J, Kiewert C, Duysen EG, Lockridge O, Greig NH, Klein J (2007) Excessive hippocampal acetylcholine levels in acetylcholinesterase-deficient mice are moderated by butyrylcholinesterase activity. J Neurochem 100:1421–1429
Inestrosa NC, Dinamarca MC, Alvarez A (2008) Amyloid-cholinesterase interactions. Implications for Alzheimer's disease. FEBS J 275:625–632
Ladner CJ, Lee JM (1998) Pharmacological drug treatment of Alzheimer disease: the cholinergic hypothesis revisited. J Neuropathol Exp Neurol 57:719–731
Li HJ, Li XJ, Bai ML, Suo YE, Zhang GH, Cao XY (2015) Matrine inhibited proliferation and increased apoptosis in human breast cancer MCF-7 cells via upregulation of Bax and downregulation of Bcl-2. Int J Clin Exp Pathol 8:14793–14799
Li J, Gao L, Sun K, Xiao D, Li W, Xiang L, Qi J (2016) Benzoate fraction from Gentiana rigescens Franch alleviates scopolamine-induced impaired memory in mice model in vivo. J Ethnopharmacol 193:107–116
Liu X, Zhang X, Ma K, Zhang R, Hou P, Sun B, Yuan S, Wang Z, Liu Z (2016) Matrine alleviates early brain injury after experimental subarachnoid hemorrhage in rats: possible involvement of PI3K/Akt-mediated NF-κB inhibition and Keap1/Nrf2-dependent HO-1 induction. Cell Mol Biol (Noisy-le-grand) 62:38–44
Liu W, Rabinovich A, Nash Y, Frenke D, WangY YMBH, Weinreb O (2017) Anti-inflammatory and protective effects of MT-031, a novel multitarget MAO-A and AChE/BuChE inhibitor in scopolamine mouse model and inflammatory cells. Neuropharmacology 113:445–456
Lleó A, Greenberg SM, Growdon JH (2006) Current pharmacotherapy for Alzheimer's disease. Annu Rev Med 57:513–533
López N, Tormo C, Blas ID, Llinares I, Alom J (2013) Oxidative stress in Alzheimer’s disease and mild cognitive impairment with high sensitivity and specificity. J Alzheimers Dis 33:823–829
Luque-Contreras D, Carvaja K, Toral-Rios D, Franco-Bocanegra D, Campos-Peña V (2014) Oxidative stress and metabolic syndrome: cause or consequence of Alzheimer's disease? Oxidative Med Cell Longev 2014:497802
Meng F, Wang J, Ding F, Xie Y, Zhang Y, Zhu J (2017) Neuroprotective effect of matrine on MPTP-induced Parkinson's disease and on Nrf2 expression. Oncol Lett 13:296–300
Mesulam MM, Guillozet A, Shaw P, Levey A, Duysen EG, Lockridge O (2002a) Acetylcholinesterase knockouts establish central cholinergic pathways and can use butyrylcholinesterase to hydrolyze acetylcholine. Neuroscience 110:627–639
Mesulam M, Guillozet A, Shaw P, Quinn B (2002b) Widely spread butyrylcholinesterase can hydrolyze acetylcholine in the normal and Alzheimer brain. Neurobiol Dis 9:88–93
Milić M, Timić T, Joksimović S, Biawat P, Rallapalli S, Divljaković J, Radulović T, Cook JM, Savić MM (2013) PWZ-029, an inverse agonist selective for α5 GABAA receptors, improves object recognition, but not water-maze memory in normal and scopolamine-treated rats. Behav Brain Res 241:206–213
Naik RS, Hartmann J, Kiewert C, Duysen EG, Lockridge O, Klein J (2009) Effects of rivastigmine and donepezil on brain acetylcholine levels in acetylcholinesterase deficient mice. J Pharm Pharm Sci 12:79–85
Perry EK, Perry RH, Blessed G, Tomlinson BE (1978) Changes in brain cholinesterases in senile dementia of Alzheimer type. Neuropathol Appl Neurobiol 4:273–277
Qu Z, Zhang JZ, Yang HG, Gao J, Chen H, Liu CX, Gao WY (2017) Prunella vulgaris L, an edible and medicinal plant, attenuates scopolamine-induced memory impairment in rats. J Agric Food Chem 65:291–300
Russ TC, Morling JR (2012) Cholinesterase inhibitors for mild cognitive impairment. Cochrane Database Syst Rev 9:CD009132
Sarter M, Bodewitz G, Stephens DN (1988) Attenuation of scopolamine-induced impairment of spontaneous alteration behaviour by antagonist but not inverse agonist and agonist beta-carbolines. Psychopharmacology 94:491–495
Schroeter H, Williams RJ, Matin R, Iversen L, Rice-Evans CA (2000) Phenolic antioxidants attenuate neuronal cell death following uptake of oxidized low-density lipoprotein. Free Radic Biol Med 29:1222–1233
Venkatesan R, Subedi L, Yeo EJ, Kim SY (2016) Lactucopicrin ameliorates oxidative stress mediated by scopolamine-induced neurotoxicitythroughactivationof the NRF2 pathway. Neurochem Int 99:133–146
Xiao J, Li SY, Sui Y, Wu Q, Li XP, Xie BJ, Zhang MW, Sun ZD, Cui RJ (2014) Lactobacillus casei-01 facilitates the ameliorative effects of Proanthocyanidins extracted from Lotus seedpod on learning and memory impairment in scopolamine-induced amnesia mice. PLoS One 9:e112773
Xu QQ, Xu YJ, Yang C, Tang Y, Li L, Cai HB, Hou BN, Chen HF, Wang Q, Shi XG, Zhang SJ (2016) Sodium Tanshinone IIA sulfonate attenuates scopolamine-induced cognitive dysfunctions via improving cholinergic system. Biomed Res Int 2016:9852536
Yin LL, Zhu XZ (2005) The involvement of central cholinergic system in (+)-matrine-induced antinociception in mice. Pharmacol Biochem Behav 80:419–425
Yue W, Li Y, Zhang T, Jiang M, Qian Y, Zhang M, Sheng N, Feng S, Tang K, Yu X, Shu Y, Yue C, Jing N (2015) ESC-derived basal forebrain cholinergic neurons ameliorate the cognitive symptoms associated with Alzheimer's disease in mouse models. Stem Cell Reports 5:776–790
Zhao P, Zhou R, Zhu XY, Hao YJ, Li N, Wang J, Niu Y, Sun T, Li YX, Yu JQ (2015) Matrine attenuates focal cerebral ischemic injury by improving antioxidant activity and inhibiting apoptosis in mice. Int J Mol Med 36:633–644
Acknowledgements
This work was supported by the grant from Shanxi Agricultural University Science and Technology Innovation Fund (2014YJ04), China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Animals’ use and disposal had been approved by the animal ethics committee of Shanxi Agricultural University.
Conflict of interest
The authors declare no competing financial interest.
Rights and permissions
About this article
Cite this article
Sun, K., Bai, Y., Zhao, R. et al. Neuroprotective effects of matrine on scopolamine-induced amnesia via inhibition of AChE/BuChE and oxidative stress. Metab Brain Dis 34, 173–181 (2019). https://doi.org/10.1007/s11011-018-0335-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-018-0335-y