Abstract
Post-operative cognitive dysfunction (POCD) is associated with elderly patients undergoing surgery. However, pharmacological treatments for POCD are limited. In this study, we found that curcumin, an active compound derived from Curcuma longa, ameliorated the cognitive dysfunction following abdominal surgery in aged mice. Further, curcumin prevented surgery-induced anti-oxidant enzyme activity. Curcumin also increased brain-derived neurotrophic factor (BDNF)-positive area and expression of pAkt in the brain, suggesting that curcumin activated BDNF signaling in aged mice. Furthermore, curcumin neutralized cholinergic dysfunction involving choline acetyltransferase expression induced by surgery. These results strongly suggested that curcumin prevented cognitive impairments via multiple targets, possibly by increasing the activity of anti-oxidant enzymes, activation of BDNF signaling, and neutralization of cholinergic dysfunction, concurrently. Based on these novel findings, curcumin might be a potential agent in POCD prophylaxis and treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Post-operative cognitive dysfunction (POCD) is one of the most common clinical syndromes in elderly patients after surgery, resulting in variant duration of cognitive impairments from few days to even months. Although POCD is common in cardiac surgery, it is still reported that 41.4% of elderly patients (older than 60 years) were diagnosed as POCD after non-cardiac surgery (Monk et al. 2008). In addition to short-term cognitive disruption, POCD also increases long-term disability and mortality in aged patients (Mason et al. 2010). Therefore, POCD dramatically disrupts the quality of daily life in aged patients, and enhances healthcare burden. Pharmacological treatments for POCD are urgently needed (Bilotta et al. 2013). Although the detailed mechanisms underlying the incidence of POCD remain unknown, factors such as increased oxidative stress, reduced neurotrophin expression, and cholinergic dysfunction mediate the pathophysiology of POCD (Fan et al. 2016b; Kalb et al. 2013; Yuan et al. 2014). Surgery triggers free radical synthesis by acting on superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) (Yuan et al. 2014). Further, surgery decreases the level of neuroprotective brain-derived neurotrophic factor (BDNF) in the brain (Fan et al. 2016b). Surgery and anesthesia decrease acetylcholine levels in the brain, resulting in defective cognitive function (Kalb et al. 2013). Therefore, the treatment of POCD entails engagement of multiple targets, concurrently.
Curcumin, an active compound derived from Curcuma longa, exhibits a wide spectrum of biological activities, especially anti-oxidative effects (Fan et al. 2016a; Fu et al. 2016). In animal models, curcumin could prevent cognitive impairments. For example, curcumin prevented the decrease of episodic memory in β amyloid-treated mice (Zhang et al. 2015b). Moreover, curcumin ameliorated heavy ion irradiation-induced deficits in learning and memory in mice (Xie et al. 2014). Recent studies also show that curcumin prevents cholinergic dysfunction in Alzheimer’s disease transgenic mice (Millington et al. 2014; Seo et al. 2010). However, it is still uncertain whether curcumin prevents cognitive dysfunction following surgery.
Previous studies have shown that abdominal surgery lead to cognitive impairments from 3 to 30 days post-surgery in mice (Wang et al. 2016b; Xu et al. 2014). In this study, we tested the effects of curcumin on cognitive impairments induced by abdominal surgery in aged mice. We also aimed to explore how curcumin regulated cognitive functions in this animal model.
Methods
Chemicals and reagents
Fentanyl (Fentanyl citrate injection, 0.05 mg/mL) was procured from Humanwell Pharmaceutical Co. Ltd. (Yichang, Hubei, China). Droperidol (Droperidol injection, 2.5 mg/mL) was obtained from SunRise Pharmaceutical Co. Ltd. (Shanghai, China). Curcumin (pure curcumin ≥80%) was supplied by Sigma-Aldrich (St. Louis, MO, USA). Carboxymethyl cellulose was obtained from Aladdin (Shanghai, China).
Animal experiments
Male ICR mice aged 12 months and weighing 30 to 40 g each were supplied by Zhejiang Academy of Medical Sciences. Mice were exposed to a 12 h light/dark cycle under humidity (50 ± 10%) and controlled temperature (22 ± 2 °C). Four to five animals were kept in one cage. Animals were given free access to normal animal food (Shanghai Slac Laboratory Animal Co. LTD, Shanghai, China) and water. All the procedures were performed according to the guidelines recommended by the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23, revised 1996) and approved by the Animal Care and Use Committee of Ningbo University.
Fifty mice were randomly allocated to five groups, each containing 10 mice, as follows: control (Con), surgery, and surgery plus low (50 mg/kg in 0.4% carboxymethyl cellulose), medium (100 mg/kg in 0.4% carboxymethyl cellulose), and high (200 mg/kg in 0.4% carboxymethyl cellulose) dose of curcumin. Mice in the surgery group were treated with 0.4% carboxymethyl cellulose as the vehicle. Drugs or vehicle were administered by oral gavage once daily for 32 consecutive days.
Anesthesia and surgical procedure
Mice were injected intraperitoneally (i.p.) with a mixture of fentanyl (0.02 mg/kg) and droperidol (5.0 mg/kg). The blink reflexes were used to determine the anesthetic effect. All surgeries were performed using a standardized procedure. Briefly, the fur of the mice in the surgical site was shaved. A laparotomy was conducted using a 1.5-cm midline incision. Approximately 5 cm of the small intestine was removed from the peritoneal cavity, covered with clean and moist gauze. After 3 min, the small intestine was replaced in the peritoneal cavity, and two layers of the abdominal wall were closed by sutures. All the procedures lasted approximately 10 min, each.
Novel object recognition (NOR) test
The NOR test was conducted in an open-field arena (30 × 30 × 30 cm) constructed with polyvinyl chloride, plywood, and acrylic, as described previously (Bevins and Besheer 2006). The task included acclimation, training, and retention over three consecutive days. On day 1, the animals were acclimated to the experimental area for 5 min without exposure to any behavioral stimulus. On day 2, the animals explored two identical objects (black plastic cubes, 5 × 5 × 5 cm) for 5 min. On day 3, one of the objects was replaced with a new shape and color (a gray plastic square pyramid, 5 × 5 × 7 cm), and the animals were again acclimated to the area for 5 min. The field was decontaminated with 70% ethanol solution and dry cloth between the tests. The animals explored the test area by sniffing or touching the objects with their nose and/or forepaws at a distance of less than 2 cm. Sitting or turning around the objects was not considered exploratory behavior. The exploratory behavior was evaluated manually using a video camera by an observer blinded to the test conditions. Total exploration time refers to the amount of time devoted to location of the two objects. The cognitive function was measured using a recognition index, which is the exploration time involving either of the two objects (training session) or the novel object (retention session) compared with the total exploration time.
Morris water maze (MWM)
Spatial memory was tested using the MWM as described previously (Morris 1981). The water maze comprises a circular pool measuring 110 cm in diameter, and filled with water at 23 ± 2 °C to immerse a platform. The platform was always positioned in the middle of the northwest quadrant except on the last day. Swimming was recorded by a video camera linked to a computer-based image system. Learning was evaluated for four consecutive days. Each mouse was trained to locate the platform during four trials daily. The time required to enter the hidden platform was measured. On day 5, a probe trial was conducted by removing the platform and training the mice to swim for 90 s to locate it. Swimming time in the four quadrants of the pool was calculated. Preference for a previous quadrant occupied by the platform indicated spatial memory.
Brain tissue harvest
One day after MWM test, animals were deeply anesthetized and perfused transcardially with ice-cold saline. Brains were removed quickly. Proteins were exacted for Western blot analysis (3 mice per group) and measurements of anti-oxidative enzyme activity (4 mice per group), and stored at −80 °C before use. Other brain tissues (3 mice per group) were used for IHC staining.
Western blot
Western blot was conducted as described previously (Cui et al. 2014). Briefly, brain tissue was extracted at 4 °C for 1 min using a lysis buffer, and centrifuged at 16000 g for 10 min. The protein levels in the supernatant were estimated by Bradford assay, followed by SDS-PAGE of tissue samples (40 μg), and transfer to polyvinylidene fluoride membrane. The membranes were blocked with 5% non-fat milk in TBST for 2 h, and incubated overnight at 4 °C with primary antibodies against pAkt, Akt and β-actin (Cell Signaling Technology, Beverly, MA, USA). After washing the samples three times with TBST, the membranes were incubated with a secondary antibody. Blots were developed using enhanced chemiluminescence as instructed by the manufacturer (Amersham Bioscience, Aylesbury, UK). All data were representative of three independent experiments. Data were expressed as ratios of optical density (OD) compared with controls for statistical analyses.
Measurement of anti-oxidant enzyme activity
The specific markers of oxidative stress, including SOD, GPx and CAT were measured using specific reagents and kits according to the manufacturers’ guidelines (Nanjing Jiancheng Biotechnology Institute, Nanjing, Jiangsu, China). The absorbance of the anti-oxidant enzymes inhibiting the hydroxy radicals was measured at 550 nm, 240 nm, and 412 nm, for SOD, GPx and CAT, respectively. For the kit of SOD measurements, the concentration of superoxide anion radicals degraded by SOD in the tissue was directly detected in the solution (pH = 7.8). For the kit of GPx measurements, the concentration of glutathione degraded by GPx in the tissue was directly detected in the solution (pH = 7.0). For the kit of CAT measurements, the concentration of H2O2 degraded by CAT in the tissue was directly detected in the solution (pH = 7.0). The coefficient of variation of standards is smaller than 2% by using these kits.
Immunohistochemical (IHC) staining
Briefly, after the behavioral tests, brains were dissected and incubated with 4% paraformaldehyde for 1 day. The brain specimens were dehydrated, embedded in paraffin, and cut into 4-μM-thick sections. The sections were dewaxed and rehydrated. Treatment with 3% H2O2 for 10 min, inhibits the cellular peroxidase activity. The sections were left overnight with primary antibodies against choline acetyltransferase (ChAT) and BDNF (Santa Cruz Biotechnology, Santa Cruz, CA, USA), at 4 °C. The specimens were rinsed, and treated with secondary antibodies at 37 °C for 30 min. The sections were labeled with DAB and analyzed colorimetrically. Image Pro 6.0 (Media Cybernetics Inc., MD, USA) was used to analyze the IHC index, defined as the average of integral optical density. The protocol was modified according to a previous publication (Koga et al. 2013).
Data analysis
The data represent means ± standard deviation (SD). One-way ANOVA was used to determine the statistical significance and Tukey’s or Dunnett’s test was used for post hoc multiple comparison. Significant differences were accepted at p < 0.05.
Results
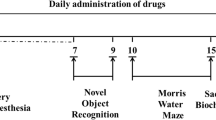
The timeline of experimental design is displayed in Fig. 1. Curcumin or vehicle was administered daily to aged animals for 32 consecutive days. The bodyweight of mice were not significantly changed among different groups during the experiments and at sacrifice. The effect of curcumin on cognition was tested. We evaluated the learning and memory of aged mice at the early (days 5–15 post-surgery) and the late (day 16–32 post-surgery) stages, respectively.
Chronological sequence of experimental design. At day 1, aged mice were i.p. injected with a mixture of 0.02 mg/kg fentanyl and 5.0 mg/kg droperidol to induce anesthesia. A laparotomy was then conducted. Curcumin or vehicle was further administered daily by oral gavage for 32 consecutive days. Mice were allowed to recover from surgery for 4 days. The effect of curcumin on cognition was tested by NOR or MWM at the early (days 5–15 post-surgery) and the late (day 16–32 post-surgery) stages, respectively. At day 32, animals were sacrificed for biochemical study
Curcumin significantly attenuates surgical impairment of cognition in aged mice
NOR tests were used to examine the role of curcumin in improving spatial recognition in animals. In early stages, the recognition index in different groups was similar to that of groups under training (one-way ANOVA, F (4, 45) = 1.048, p > 0.05, Fig. 2a). However, in the retention session, the recognition index varied in different groups (one-way ANOVA, F (4, 45) = 5.285, p < 0.01, Fig. 2b). Further, the surgery group exhibited a significantly higher recognition index than the control group (one-way ANOVA, Tukey’s test, p < 0.05). Curcumin (100 and 200 mg/kg) significantly abrogated surgery-induced decrease of the recognition index when compared to the surgery group (one-way ANOVA, Tukey’s test, p < 0.05). However, the recognition index in 50 mg/kg curcumin group and the surgery group was similar during the retention session in the late stage.
Curcumin attenuates surgery-induced recognition impairment. The NOR tests were performed on day 5 (the early stage) and day 24 (the late stage) after surgery, respectively. a In the early stage, all the groups exhibited similar recognition index during the training session of NOR. b In the early stage, curcumin significantly attenuated the decrease in recognition index during the retention session of NOR. c In the late stage, all the groups exhibited similar recognition index during the training session of NOR. d In the late stage, curcumin significantly attenuated the decrease in recognition index during the retention session of NOR. Data represent the mean ± SD (n = 10). # p < 0.05 and ## p < 0.01 vs. control group, * p < 0.05 and ** p < 0.01 vs. surgery group (one way ANOVA and Tukey’s test)
The results were similar in the late stage. The recognition index in different groups was similar during the training session (one-way ANOVA, F (4, 45) = 1.471, p > 0.05, Fig. 2c). In the retention session, the recognition index varied across different groups (one-way ANOVA, F (4, 45) = 6.821, p < 0.01, Fig. 2d).The recognition index in the surgery group was significantly higher than in the control group (one-way ANOVA, Tukey’s test, p < 0.01). Curcumin (100 and 200 mg/kg) significantly attenuated the decreased recognition index when compared to the surgery group (one way ANOVA, Tukey’s test, p < 0.05). However, the recognition index in 50 mg/kg curcumin group and the surgery group was similar during the retention session in the late stage.
Curcumin significantly attenuates surgery-induced impairment in spatial learning and memory in aged mice
MWM tests were used to determine the role of curcumin in improving spatial learning and memory. In the early stage, the surgery group took longer to locate the platform than the control group (one-way ANOVA, Tukey’s test, p < 0.05). Curcumin at 200 mg/kg significantly reduced the escape latency, suggesting that curcumin attenuated surgery-induced defects in spatial learning (one-way ANOVA, Tukey’s test, p < 0.05). The spatial memory was tested during the probe trial. The swimming duration in the specific quadrant varied across the groups (one-way ANOVA, F (4, 45) = 7.111, p < 0.01, Fig. 3b). Curcumin at 200 mg/kg significantly lowered the time spent in the target quadrant (one-way ANOVA, Tukey’s test, p < 0.01), suggesting that curcumin prevented impairment in spatial memory.
Curcumin attenuates impaired spatial learning and memory. The MWM tests were performed on days 8 to 12 post-surgery (the early stage), or day 27 to 31 (the late stage), respectively. a Curcumin decreased the escape latency during the training trials in the early stage. b Curcumin increased the duration in the target quadrant during the probe trial in the early stage. c Curcumin decreased escape latency during the training trials in the late stage. d Curcumin increased the duration in the target quadrant during the probe trial in the late stage. Data represent the mean ± SD (n = 10). # p < 0.05 and ## p < 0.01 vs. control group, * p < 0.05 and ** p < 0.01 vs. surgery group (one-way ANOVA and Tukey’s test)
Similar results were observed during the late stage: the surgery group took longer to reach the platform than the control group (one-way ANOVA, Tukey’s test, p < 0.05). Curcumin at 100 and 200 mg/kg also reduced the increase in escape latency during the late stage (one-way ANOVA, Tukey’s test, p < 0.05). The spatial memory was tested in the probe trial of the MWM test. The time spent by the mice in the target quadrant varied across different groups in the late stage (one way ANOVA, F (4, 45) = 4.241, p < 0.01, Fig. 3d). Curcumin at 200 mg/kg significantly prevented the decrease of the time in the target quadrant (one-way ANOVA, Tukey’s test, p < 0.05).
Curcumin significantly increases the activity of GPx, SOD and CAT
We investigated the effects of curcumin on the anti-oxidant enzyme activity. We found that surgery significantly increased the activities of GPx, SOD and CAT when compared to the control group (Table 1, one-way ANOVA, Tukey’s test, p < 0.05). Curcumin at 100 and 200 mg/kg inhibited the altered activities of enzymes when compared to the surgery group (one-way ANOVA, Tukey’s test, p < 0.05).
Curcumin significantly attenuates surgery-induced decrease of BDNF
The BDNF-positive area in the brain of the surgery group is significantly lower than that of the control group. Curcumin at 50–200 mg/kg significantly increased BDNF-positive area when compared to the surgery group, indicating that curcumin attenuated cognitive dysfunction by increasing BDNF expression (Fig. 4).
Curcumin increases BDNF-positive area in aged mice. a Representative images of IHC staining of BDNF in various groups as indicated. Arrows indicate a cell with positive expression of BDNF. b Quantitative results showed that curcumin increases BDNF-positive area. Data represent mean ± SD (n = 3). ## p < 0.01 vs. control group, ** p < 0.01 vs. surgery group (one way ANOVA and Tukey’s test)
Curcumin activates Akt
BDNF binds to TrkB and activates the Akt pathway. In our study, we used Western blot assay to analyze the expression of pAkt. It was demonstrated that surgery downregulated the expression of pAkt in the brain when compared to the control group (Fig. 5). Curcumin at 50–200 mg/kg significantly reversed the decrease in pAkt expression when compared to the surgery group, suggesting that curcumin activated BDNF-related Akt signaling in the brain of aged mice.
Curcumin significantly attenuates surgery-induced decrease of ChAT-positive area
ChAT is a marker of cholinergic neurons. To investigate whether curcumin affected cholinergic neurons, IHC staining of ChAT was performed in the brain sections. Surgery significantly reduced ChAT-positive area, leading to cholinergic dysfunction. Curcumin increased the ChAT-positive area, and prevented surgical dysfunction of cholinergic system (Fig. 6).
Curcumin increases ChAT-positive area in aged mice. a Representative images of IHC staining of ChAT in various groups are indicated. Arrows indicate ChAT-positive expression. b Quantitative results showed that curcumin increases surgery-induced decrease of ChAT-positive area. Data represent the mean ± SD (n = 3). ## p < 0.01 vs. control group, ** p < 0.01 vs. surgery group (one-way ANOVA and Tukey’s test)
Discussion
Curcumin effectively prevented surgical impairment of cognitive function in mice. Our results suggest that curcumin increased the activity of anti-oxidant enzymes, activated BDNF signaling, and neutralized cholinergic dysfunction.
The severity of POCD is modulated by factors such as the type of surgery, the anesthetics used, and the patients’ age (Deiner and Silverstein 2009). Although cardiac surgery is common to induce POCD, non-cardiac surgery could also lead to cognitive impairments in elderly patients (Monk et al. 2008). Exploratory laparotomy in mice simulated abdominal surgery in humans, and is widely used in animal models of POCD (Zhang et al. 2016). Fentanyl-droperidol combination is traditionally used for surgical anesthesia (Zanette et al. 2010). Exploratory laparotomy under fentanyl-droperidol anesthesia triggered cognitive impairment in aged but not young mice (unpublished data), suggesting that the rodent model was an appropriate tool to investigate pharmacological treatment of POCD in aged patients.
The NOR and MWM test results are reliable for the evaluation of cognitive performance. In our study, the cognitive performance of mice in the surgery group was worse than in the control group during the early (days 1–15 post-surgery) and the late (days 16–31 post-surgery) stages, suggesting that the cognitive dysfunction persisted at least one month post-surgery. The results are consistent with a previous study showing that surgery induced long-term dysfunction of hippocampus in aged mice (Wang et al. 2016a).
Chronic administration of curcumin prevented cognitive derangement induced by neurotoxins, such as β amyloid, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and lipopolysaccharide (He et al. 2015; Jangra et al. 2016; Zhang et al. 2015a). Curcumin exhibits excellent safety profile, and therefore, is considered therapeutic against several neurodegenerative disorders (Ringman et al. 2005). Most importantly, curcumin decreased the serum levels of TNF-α after surgery, and improved aging-related cerebrovascular dysfunction in mice (Jomezadeh et al. 2012; Pu et al. 2013). These results suggested that curcumin might be effective in aging-related post-operative management. In our study, daily administration of curcumin (50–200 mg/kg) significantly prevented surgery-induced impairments in cognitive function, suggesting that curcumin might play a role in the treatment of POCD. Interestingly, curcumin improved cognitive performance not only in the early stage, but also in the late stage post-surgery, suggesting that curcumin might reverse long-term alteration in the brain.
Could curcumin exert its effects via directly acting on the targets in the brain? A previous study showed that 50 mg/kg curcumin could not result in measurable curcumin in the brain after oral gavages (Schiborr et al. 2010). We found that curcumin could not be detected in the brain at 15 min to 2 h after oral gavage of curcumin at 100 or 200 mg/kg (unpublished data). These results suggested that the anti-POCD effects of curcumin might not via the interaction between curcumin and its targets in the brain. Curcumin could form many metabolites, including dihydro-curcumin, tetrahydro-curcumin and bicyclopentadione after reductive or conjugative metabolisms in the body (Metzler et al. 2013). Some of these metabolites might cross the brain blood-barrier. We speculated that brain-permeable metabolites of curcumin might act on molecules in the brain, and improve cognitive performance in mice.
What are the potential pathways involved in curcumin-mediated cognitive improvement after surgery? Surgery triggers the release of oxidative free radicals and inhibits anti-oxidant mechanisms. In our study, we showed that curcumin significantly prevented the decrease of anti-oxidant enzyme activity in aged mice, which is consistent with previous studies of curcumin demonstrating an increase in the transcription of anti-oxidant enzymes (Huang et al. 2016; Tvrda et al. 2016). How could curcumin increase the activity of anti-oxidant enzymes? Most anti-oxidant hydroxyl groups of circulating curcumin were conjugated with glucuronic acid or sulphate. Therefore, the anti-oxidant activity of curcumin may not be due to its anti-oxidant hydroxyl groups. Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) is a transcription factor that increases the expression of anti-oxidant proteins. We further found that curcumin could reverse surgery-induced decrease of Nrf2 expression (unpublished data), suggesting that curcumin might act on anti-oxidant signaling pathways to increase the expression of anti-oxidant enzymes, and eventually lead to its anti-oxidant activity.
Furthermore, we demonstrated that curcumin could increase BDNF expression and activate Akt pathway, a downstream signaling pathway of BDNF. These results are in consistence with previous studies that curcumin could activate BDNF and Akt signaling (Nam et al. 2014; Wu et al. 2013; Zhu et al. 2014). BDNF contributes not only to neuronal survival and neurogenesis, but also to cognition and stress. Therefore, the effects of curcumin in improving cognitive performance might be partially mediated via activation of BDNF signaling. Moreover, Akt phosphorylation could facilitate the translocation of Nrf2. Curcumin is reported to produce anti-oxidative stress effects via Nrf2 in an Akt-dependent manner (Kang et al. 2007). Therefore, curcumin might improve cognitive performance from increasing Nrf2-mediated anti-oxidant pathway, possible via acting on BDNF and its downstream molecule Akt in our study. How could curcumin act on BDNF? A recent study suggested that surgical stress might reduce the expression of BDNF via phosphorylation of glucocorticoid receptor in aged mice (Tian et al. 2015). Chronic administration of curcumin decreased the levels of cortisol, the primary ligand of glucocorticoid receptor (Yu et al. 2015). Therefore, we inferred that curcumin inhibits the decrease in BDNF expression following surgery, by reducing the cortisol/glucocorticoid receptor system.
Dysfunction of cholinergic system leads to cognitive dysfunction in mice (Shin and Dixon 2015). In our study, the decreased ChAT-positive area in the brains of mice in the surgery group suggested altered cholinergic system by inducing the loss of cholinergic neurons. Further, curcumin significantly reversed the decrease in ChAT-positive area, suggesting that curcumin potentially restores the cholinergic function and enhances cognitive performance. Oxidative stress triggers the loss of neurons; and BDNF signaling is neuroprotective (Fan et al. 2016b). Therefore, we speculated that curcumin prevented the decrease of ChAT-positive area by reducing oxidative stress and enhancing BDNF expression, concurrently. Additional investigations are ongoing into the mechanisms of curcumin against ChAT.
Besides anti-oxidant enzymes, BDNF signaling and cholinergic system, curcumin might act on other targets to improve cognitive performance. For example, dietary curcumin could enhance energy availability and mitochondrial function, leading to the improved learning and memory (Eckert et al. 2013; Hagl et al. 2015). Moreover, curcumin might act on cannabinoid receptor and dopaminergic receptor to increase cognitions in animals (Hassanzadeh and Hassanzadeh 2012; Kumar et al. 2010). However, the involvement of these potential targets in curcumin-mediated cognitive improvements in our study remains to be exclusive.
Finally, it was recently reported that certain nanoparticle formulations could increase the distribution of curcumin in the brain (Dutzmann et al. 2016; Mulik et al. 2012; Tsai et al. 2011). For example, the half-life of curcumin-loaded poly(lactic-co-glycolic acid) in brain tissues is largely increased when compared to curcumin alone (Tsai et al. 2011). Moreover, curcumin-loaded poly(butyl)cyanoacrylate nanoparticles could use apolipoprotein E3 to cross the brain blood-barrier (Mulik et al. 2012). Furthermore, oral administration of micellar curcumin led to quantifiable level of curcumin in the brain (Dutzmann et al. 2016). These studies suggested that curcumin could be an interesting compound in the prevention of POCD, if administered in a bioavailable form.
In conclusion, we have demonstrated that curcumin significantly prevented cognitive dysfunction in aged mice undergoing surgery. The possible mechanisms include enhancement of anti-oxidant enzymes, activation of BDNF signaling, and neutralization of cholinergic defects. These results suggest that curcumin therapy might be developed as a potential intervention for prevention and treatment of POCD.
Abbreviations
- BDNF:
-
brain-derived neurotrophic factor
- CAT:
-
catalase
- ChAT:
-
choline acetyltransferase
- GPx:
-
glutathione peroxidase
- IHC:
-
Immunohistochemical
- MWM:
-
Morris water maze
- NIH:
-
National Institutes of Health
- NOR:
-
Novel object recognition
- Nrf2:
-
Nuclear factor (erythroid-derived 2)-like 2
- OD:
-
optical density
- POCD:
-
post-operative cognitive
- SOD:
-
superoxide dismutase.
References
Bevins RA, Besheer J (2006) Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study 'recognition memory'. Nat Protoc 1:1306–1311
Bilotta F et al (2013) Pharmacological perioperative brain neuroprotection: a qualitative review of randomized clinical trials. Br J Anaesth 110(Suppl 1):i113–i120
Cui W et al (2014) Sunitinib produces neuroprotective effect via inhibiting nitric oxide overproduction. CNS Neurosci Ther 20:244–252
Deiner S, Silverstein JH (2009) Postoperative delirium and cognitive dysfunction. Br J Anaesth 103(Suppl 1):i41–i46
Dutzmann S et al (2016) Intratumoral concentrations and effects of orally administered micellar Curcuminoids in Glioblastoma patients. Nutr Cancer 68:943–948
Eckert GP et al (2013) Curcumin prevents mitochondrial dysfunction in the brain of the senescence-accelerated mouse-prone 8. Neurochem Int 62:595–602
Fan CD, et al. (2016a) Reversal of Beta-amyloid-induced neurotoxicity in PC12 cells by curcumin, the important role of ROS-mediated signaling and ERK pathway. Cell Mol Neurobiol
Fan D, Li J, Zheng B, Hua L, Zuo Z (2016b) Enriched environment attenuates surgery-induced impairment of learning, memory, and neurogenesis possibly by preserving BDNF expression. Mol Neurobiol 53:344–354
Fu XY et al (2016) Strategy to suppress oxidative damage-induced neurotoxicity in PC12 cells by curcumin: the role of ROS-mediated DNA damage and the MAPK and AKT pathways. Mol Neurobiol 53:369–378
Hagl S et al (2015) Curcumin micelles improve mitochondrial function in neuronal PC12 cells and brains of NMRI mice - impact on bioavailability. Neurochem Int 89:234–242
Hassanzadeh P, Hassanzadeh A (2012) The CB1 receptor-mediated Endocannabinoid signaling and NGF: the novel targets of curcumin. Neurochem Res 37:1112–1120
He XJ, Uchida K, Megumi C, Tsuge N, Nakayama H (2015) Dietary curcumin supplementation attenuates 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) neurotoxicity in C57BL mice. J Toxicol Pathol 28:197–206
Huang HC et al (2016) Antioxidative and neuroprotective effects of curcumin in an Alzheimer's disease rat model Co-treated with Intracerebroventricular streptozotocin and subcutaneous D-Galactose. J Alzheimers Dis 52:899–911
Jangra A et al (2016) Piperine augments the protective effect of curcumin against lipopolysaccharide-induced neurobehavioral and neurochemical deficits in mice. Inflammation 39:1025–1038
Jomezadeh V, Mohammadpour AH, Rajabi O, Tavassoli A, Maddah G (2012) Evaluation of curcumin effects on post-operative peritoneal adhesion in rats. Iran J Basic Med Sci 15:1162–1167
Kalb A et al (2013) Acetylcholinesterase inhibitors reduce neuroinflammation and -degeneration in the cortex and hippocampus of a surgery stress rat model. PLoS One 8:e62679
Kang ES et al (2007) Up-regulation of aldose reductase expression mediated by phosphatidylinositol 3-kinase/Akt and Nrf2 is involved in the protective effect of curcumin against oxidative damage. Free Radical Bio Med 43:535–545
Koga T, Bellier JP, Kimura H, Tooyama I (2013) Immunoreactivity for choline acetyltransferase of peripheral-type (pChAT) in the trigeminal ganglion neurons of the non-human primate Macaca fascicularis. Acta Histochem Cytoc 46:59–64
Kumar TP, Antony S, Gireesh G, George N, Paulose CS (2010) Curcumin modulates dopaminergic receptor, CREB and phospholipase c gene expression in the cerebral cortex and cerebellum of streptozotocin induced diabetic rats. J Biomed Sci 17
Mason SE, Noel-Storr A, Ritchie CW (2010) The impact of general and regional anesthesia on the incidence of post-operative cognitive dysfunction and post-operative delirium: a systematic review with meta-analysis. J Alzheimers Dis 22(Suppl 3):67–79
Metzler M, Pfeiffer E, Schulz SI, Dempe JS (2013) Curcumin uptake and metabolism. Biofactors 39:14–20
Millington C et al (2014) Chronic neuroinflammation in Alzheimer's disease: new perspectives on animal models and promising candidate drugs. Biomed Res Int 2014:309129
Monk TG et al (2008) Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology 108:18–30
Morris R (1981) Spatial localization does not require the presence of local cues. Learn Motiv 2:239–260
Mulik RS, Monkkonen J, Juvonen RO, Mahadik KR, Paradkar AR (2012) ApoE3 mediated polymeric nanoparticles containing curcumin: apoptosis induced in vitro anticancer activity against neuroblastoma cells. Int J Pharmaceut 437:29–41
Nam SM et al (2014) Effects of curcumin (Curcuma longa) on learning and spatial memory as well as cell proliferation and neuroblast differentiation in adult and aged mice by Upregulating brain-derived neurotrophic factor and CREB signaling. J Med Food 17:641–649
Pu YF et al (2013) Dietary curcumin ameliorates aging-related cerebrovascular dysfunction through the AMPK/uncoupling protein 2 pathway. Cell Physiol Biochem 32:1167–1177
Ringman JM, Frautschy SA, Cole GM, Masterman DL, Cummings JL (2005) A potential role of the curry spice curcumin in Alzheimer's disease. Curr Alzheimer Res 2:131–136
Schiborr C, Eckert GP, Rimbach G, Frank J (2010) A validated method for the quantification of curcumin in plasma and brain tissue by fast narrow-bore high-performance liquid chromatography with fluorescence detection. Anal Bioanal Chem 397:1917–1925
Seo JS et al (2010) Severe motor neuron degeneration in the spinal cord of the Tg2576 mouse model of Alzheimer disease. J Alzheimers Dis 21:263–276
Shin SS, Dixon CE (2015) Alterations in cholinergic pathways and therapeutic strategies targeting cholinergic system after traumatic brain injury. J Neurotrauma 32:1429–1440
Tian XS et al (2015) Surgical stress induces brain-derived neurotrophic factor reduction and postoperative cognitive dysfunction via glucocorticoid receptor phosphorylation in aged mice. CNS Neurosci Ther 21:398–409
Tsai YM, Chien CF, Lin LC, Tsai TH (2011) Curcumin and its nano-formulation: the kinetics of tissue distribution and blood-brain barrier penetration. Int J Pharmaceut 416:331–338
Tvrda E, et al. (2016) Curcumin has protective and antioxidant properties on bull spermatozoa subjected to induced oxidative stress. Anim Reprod Sci
Wang HL, Liu H, Xue ZG, Liao QW, Fang H (2016a) Minocycline attenuates post-operative cognitive impairment in aged mice by inhibiting microglia activation. J Cell Mol Med
Wang HL, Liu H, Xue ZG, Liao QW, Fang H (2016b) Minocycline attenuates post-operative cognitive impairment in aged mice by inhibiting microglia activation. J Cell Mol Med 20:1632–1639
Wu JX et al (2013) Neuroprotection by curcumin in ischemic brain injury involves the Akt/Nrf2 pathway. PLoS One 8
Xie Y et al (2014) Curcumin ameliorates cognitive deficits heavy ion irradiation-induced learning and memory deficits through enhancing of Nrf2 antioxidant signaling pathways. Pharmacol Biochem Be 126:181–186
Xu ZP, et al. (2014) Age-dependent postoperative cognitive impairment and Alzheimer-related neuropathology in mice Sci Rep-Uk 4
Yu JJ, Pei LB, Zhang Y, Wen ZY, Yang JL (2015) Chronic supplementation of curcumin enhances the efficacy of antidepressants in major depressive disorder: a randomized, double-blind, placebo-controlled pilot study. J Clin Psychopharmacol 35:406–410
Yuan S et al (2014) The effects of electroacupuncture treatment on the postoperative cognitive function in aged rats with acute myocardial ischemia-reperfusion. Brain Res 1593:19–29
Zanette G, Micaglio M, Zanette L, Manani G, Facco E (2010) Comparison between ketamine and fentanyl-droperidol for rectal premedication in children: a randomized placebo controlled trial. J Anesth 24:197–203
Zhang L et al (2015a) Curcumin improves amyloid beta-peptide (1-42) induced spatial memory deficits through BDNF-ERK signaling pathway. PLoS One 10:e0131525
Zhang L, et al. (2015b) Curcumin Improves Amyloid beta-Peptide (1–42) Induced Spatial Memory Deficits through BDNF-ERK Signaling Pathway. Plos One 10
Zhang Z, Li X, Li F, An L (2016) Berberine alleviates postoperative cognitive dysfunction by suppressing neuroinflammation in aged mice. Int Immunopharmacol 38:426–433
Zhu XY, et al. (2014) Curcumin Alleviates Neuropathic Pain by Inhibiting p300/CBP Histone Acetyltransferase Activity-Regulated Expression of BDNF and Cox-2 in a Rat Model. Plos One 9
Acknowledgements
This work was supported by Ningbo Natural Science Foundation (2015A610219, 2013A610221), the National Natural Science Foundation of China (U1503223, 81673407), the Natural Science Foundation of Zhejiang Province (LY15H310007), the Applied Research Project on Nonprofit Technology of Zhejiang Province (2016C37110), Medicine and Drugs Research of Zhejiang Province (2014KYB232), the Ningbo International Science and Technology Cooperation Project (2014D10019), Ningbo municipal innovation team of life science and health (2015C110026), the Scientific Research Foundation for the Returned Overseas Chinese Scholars, the State Education Ministry, and the K. C. Wong Magna Fund in Ningbo University.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xiang Wu and Huixin Chen contribute equally as co-first authors.
Rights and permissions
About this article
Cite this article
Wu, X., Chen, H., Huang, C. et al. Curcumin attenuates surgery-induced cognitive dysfunction in aged mice. Metab Brain Dis 32, 789–798 (2017). https://doi.org/10.1007/s11011-017-9970-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-017-9970-y