Abstract
Obsessive-compulsive disorder (OCD) is a prevalent and debilitating condition, characterized by intrusive thoughts and repetitive behavior. Animal models of OCD arguably have the potential to contribute to our understanding of the condition. Deer mice (Permomyscus maniculatus bairdii) are characterized by stereotypic behavior which is reminiscent of OCD symptomology, and which may serve as a naturalistic animal model of this disorder. Moreover, a range of deer mouse repetitive behaviors may be representative of different compulsive-like phenotypes. This paper will review work on deer mouse behavior, and evaluate the extent to which this serves as a valid and useful model of OCD. We argue that findings over the past decade indicate that the deer mouse model has face, construct and predictive validity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obsessive-compulsive disorder (OCD) is a debilitating psychiatric disorder, with lifetime prevalence estimates of 2.6% (Ruscio et al. 2010). Obsessions are intrusive thoughts or ideas that are often associated with compulsive, or repetitive and rigid, behavior (American Psychiatric Association 2013). On factor analyses of OCD symptoms, several symptom dimensions emerge, with varying content of obsessions and/or compulsions (O/C) (Table 1). The symptoms of OCD are time-consuming, distressing, and result in significant interference in occupational and social function (American Psychiatric Association 2013). Since the publication of the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), OCD is included with body dysmorphic disorder, hoarding disorder, trichotillomania, and excoriation disorder in a single diagnostic chapter on ‘Obsessive-Compulsive and Related Disorders’ (OCRDs; American Psychiatric Association 2013). OCD is not only often co-morbid with conditions classified in the OCRD cluster, but also with mood and anxiety disorders (Torres et al. 2016).
Animal models may be useful for investigating the underlying etiopathology of psychiatric illness. Not only can they contribute to understanding underlying neurobiology, but they may also allow investigation of novel treatments (Fineberg et al. 2011). In the past, a number of animal models of OCD have been proposed. Given that obsessions are difficult to demonstrate in animals, models of OCD have focused on repetitive and rigid behavior that is reminiscent of the symptoms of OCRDs. Such behavior includes excessive lever-pressing (Joel 2006) and nest building behavior (Hoffman and Rueda Morales 2009; Li et al. 2006; Wolmarans et al. 2016a; Greene-Schloesser et al. 2011), rigid locomotive patterns (Szechtman et al. 1998; Wolmarans et al. 2013; Yadin et al. 1991), aberrant grooming and hair pulling behavior (Greer and Capecchi 2002; Kinnear et al. 2000; Welch et al. 2007), compulsive-like chewing (Chou-Green et al. 2003a, b), compulsive-like marble burying (Greene-Schloesser et al. 2011) and hoarding (Andersen et al. 2010). Together, these models conceivably represent different OC-like phenotypes that may contribute to our understanding of the mechanisms underlying different symptoms. Animal models of OCD can be based on naturalistic or conditioned behavior, pharmacological challenges, or genetic manipulation (Alonso et al. 2015). Whereas naturalistic and conditioned models may provide more insight into the behavioral triggers and course of OC-like behavior, pharmacological and genetic models may provide targeted frameworks for studying specific neurobiological and genetic mechanisms underlying O/Cs (Alonso et al. 2015).
The deer mouse (Peromyscus maniculatus bairdii) model of OCD (Korff et al. 2008) can be regarded as a naturalistic model characterized by spontaneous OC-like behavior (Hoffman 2011; Wolmarans et al. 2013). Over the past decade, our group has published a number of investigations of this model. The current paper will review progress to date.
The deer mouse model of OCD
Peromyscus maniculatus (deer mouse) and congeneric species are the most common mammals native to the North-American continent (Shorter et al. 2012). As opposed to rats, dogs, cows, sheep, and laboratory mice (Mus) where selective breeding has led to genomic alterations (Vrana 2007), deer mice represent a true wild-type mammalian model system, although bred in captivity at the Peromyscus genetic stock center of the University of South Carolina (Joyner et al. 1998). Wild-type strains differ from inbred strains in being genetically more diverse (Yang et al. 2011), and deer mice have proven to be useful in the study of genetic variability and epi-genetic influences underlying different behavioral phenotypes. One subspecies, viz. the tall grass prairie P. maniculatus bairdii strain (hereafter only referred to as deer mice), of which stock animals have been derived from 40 wild-type ancestors caught in Washtenaw County, Michigan, has been used in studies of spontaneous repetitive behavior (Hadley et al. 2006; Presti et al. 2004; Shorter et al. 2014).
Notably, the repetitive and stereotypic behavior observed in these animals, i.e. jumping, backward somersaulting and pattern running, is expressed in varying frequencies across the population in laboratory settings, suggesting that these behaviors are influenced by a combination of genetic and environmental factors (Shorter et al. 2014). While repetitive motor patterns are not necessarily indicative of pathology (Eilam et al. 2006; Langen et al. 2011), the seemingly purposeless and time-consuming stereotypies of varying forms and intensity in deer mice, have been proposed to resemble the repetitive and rigid symptomology of OCD (Korff et al. 2008) and autism (Lewis et al. 2007). That said, given that stereotypic behavior is seen in deer mice in naturalistic settings also (Shorter et al. 2012, 2014), it is likely that this may be an adaptive response.
Spontaneous stereotypy in deer mice as a phenotype of persistent, but compulsive behavior
Jumping, backward somersaulting, and pattern running in deer mice were originally studied based on their resemblance to stereotypical movement disorders, in particular the motor manifestations of autism (Powell et al. 1999). Compared to animals housed in standard cages, individuals maintained in enriched cages express lower levels of stereotypy (most notably pattern running), characterized by a delayed onset and a lower incidence (Bechard et al. 2016; Hadley et al. 2006; Powell et al. 1999). However, it has been suggested that up to 62% of deer mice housed in standard laboratory cages developed stereotypy.
In early work, behavioral categorization was performed by means of visual observation (Powell et al. 1999), with each animal observed twice weekly for 5 min. Subsequently, the same group introduced automated screening (Presti et al. 2004), and animals were classified into low- (L) and high- (H) stereotypical groups based on the mean stereotypy score, i.e. the number of distinct stereotypical movements, generated over an eighteen-hour-long session (Presti et al. 2004). Further, to obtain accurate results from neurochemical investigations, only animals expressing the lowest and highest levels of stereotypy were included in follow-up studies, excluding a grey margin of animals and that yielded a more distinct separation of the two behavioural phenotypes. This approach has since been adopted by others (Wolmarans et al. 2013, 2016a, b, 2017a).
Korff et al. (2008) classified deer mice into non- (N), low- (L), and high- (H) stereotypic groups on the basis of a mean stereotypy score obtained during three individual one-hour long behavioral screening sessions, each one week apart. Deer mouse stereotypy was variable within the population with 45% of animals classified as H, 41% as L, and 14% as N, irrespective of sex. As deer mice are nocturnal animals, follow-up investigations measured the time spent executing stereotypy over 12-h during the dark cycle (Wolmarans et al. 2013); this allowed us to demonstrate that the stereotypy frequency and intensity vary across subjects and between different assessments, and that H deer mice express time-consuming stereotypy during specific bouts of the dark cycle only (Fig. 1, two of the normal 5 baseline stereotypy trials shown). This is arguably reminiscent of the waxing and waning nature of OC symptomology (American Psychiatric Association 2013; Wolmarans et al. 2013).
A central question to modeling OCD in animals is whether it is possible to characterize stereotypy not simply as a motoric phenomenon, but rather as representing an underlying cognitive-affective alteration (Tanimura et al. 2009, 2008; Wolmarans et al. 2017a). In this regard, previous findings must be considered. Repeated administration of psychostimulants induces stereotypy and facilitates the transition of goal-directed to habitual responses (Burguière et al. 2015; Graybiel 2008). However, since deer mouse stereotypy is not subject to amphetamine-induced behavioral sensitization (Tanimura et al. 2009), it is likely that the stereotypical phenotype described in deer mice differs from a purely habitual phenomenon.
In addition, we have noted differences in sociability between H and N deer mice, both within- and between cohorts (Wolmarans et al. 2017a), with changes in sociability after administration of escitalopram. This again suggests that stereotypic behavior in deer mice is not merely a motoric phenomenon, but reflects more broad-spread mechanisms. By investigating group interactions between three animals in different social paradigms, viz. HHH, NNN, HHN and NNH, we demonstrated that H animals group together in the presence of an N conspecific (HHN paradigm), while being marginalized by animals of the N cohort (NNH paradigm). Our data relating to the sociability of deer mice has key implications. Notably, P. maniculatus bairdii engage in fewer social interactions compared to P. polionotus subgriseus, another species within the genus (Shorter et al. 2014). However, stereotypical behavior in P. polionotus subgriseus is negligible. Taken together, results from the Shorter et al. (2014) and Wolmarans et al. (2017a) investigations may indicate that the sociability of P. maniculatus bairdii is modified by the level of stereotypy displayed by conspecifics, although it is premature to draw strong conclusions about a causal relationship between stereotypy and altered social competence. This is consistent with clinical evidence regarding the social behavior of OCD patients and their social experiences in the presence of healthy peers (Berrocal et al. 2006; Kim et al. 2012; Rosa et al. 2012; Storch et al. 2006).
Taken together, deer mouse stereotypy resembles OC behavior in that 1) it is repetitive, persistent and time consuming, 2) it manifests as a narrow range of phenotypes, i.e. jumping, pattern running and backward somersaulting that can possibly be differentiated at a neurobiological level, 3) it demonstrates within- and between-subject variance in frequency and intensity, 4) it is expressed in a waxing and waning pattern, 5) it is resistant to behavioral sensitization and so may represent a form of abnormally regulated goal-directed behavior, rather than a habit, 6) it is characterized by social deficits, and 7) it is influenced by both environmental and genetic factors.
Parallels between the treatment response of deer mouse stereotypy and OCD
Response to chronic, but not sub-chronic selective serotonin reuptake inhibitors (SSRIs)
During the initial stages of investigating deer mouse stereotypy as a putative animal model of OCD, Korff et al. (2008) demonstrated that chronic (21-day) intraperitoneal treatment with a high dose SSRI, i.e. fluoxetine 20 mg/kg/day, but not the noradrenaline reuptake inhibitor (NRI), desipramine, attenuated stereotypy intensity in H and L animals without affecting normal locomotion. As chronic and high dose SSRI treatment is the first-line pharmacological treatment for OCD, while noradrenergic compounds are ineffective (Fineberg and Craig 2007), these findings accredit deer mouse stereotypy with valuable predictive validity as an animal model of OCD. Subsequent work extended these initial findings. To account for individual fluctuations in stereotypy over the course of a single dark cycle and considering that the distinct forms of stereotypy do not demonstrate any significant association with one another, a focus was established on time and stereotypy intensity (Fig. 2) (Wolmarans et al. 2013). This classification system also allows for genetic predisposition and epigenetic influence in the stock colony as it appraises deer mouse behavior on a continuum from normal to severe manifestations of stereotypy. As opposed to the use of cut-off criteria, the balance may shift in any direction without changing the fundamental goal of the investigation, viz. comparing normal and stereotypic animals.
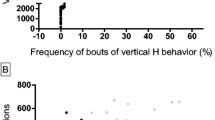
(a) Mean of highest individual daily vertical activity scores across the first five baseline behavioral trials versus time spent engaging in H activity. (b) Mean of highest individual daily cage revolution scores over the first five baseline behavioral trials versus time spent engaging in H activity. Sex symbols indicate selected male and female H animals
To exclude the effect of injection stress on the manifestation of stereotypy, oral dosing with high dose escitalopram (50 mg/kg/day), a highly potent selective serotonin reuptake inhibitor (Owens et al. 2001), was introduced, together with a comparison between chronic (4 weeks) and sub-chronic (1 week) treatment. Subsequently, while adjusting H bouts to periods of normal rodent activity, we demonstrated that chronic but not sub-chronic escitalopram treatment reduced the time spent executing stereotypy (Wolmarans et al. 2013). Notably however, such animals still engaged in bouts of spontaneous H behavior (Wolmarans et al. 2013), arguably not unlike that observed in OCD (Overduin and Furnham 2012). These observations are important as we have previously suggested that by increasing the number of bouts of normal rodent activity, escitalopram engenders control over the urge to engage in H behavior (Wolmarans et al. 2013). Instead of persistently expressing less severe compulsive-like behavior, H animals can engage in normal rodent activities for a greater part of their wake cycle, although not being able to abstain entirely from OC bouts.
Overlaps between deer mouse behavior and treatment resistance in OCD
Treatment resistance remains a clinical obstacle in approximately 30% - 50% of OCD patients who remain unresponsive to SSRI monotherapy (Fineberg et al. 2006). The treatment of refractory OCD may include an increase in the dose of the SSRI and a longer duration of treatment (Bejerot and Bodlund 1998), or switching treatment to another SSRI (Fineberg et al. 2006). A third strategy is to augment SSRI therapy with a low dose D2 blocker (Erzegovesi et al. 2005; Hollander et al. 2003; Ipser et al. 2006; McDougle et al. 2000). Recently, we investigated marble burying (MB) behavior in deer mice as a measure of anxiety- and/or compulsive-like behavior (Dixit et al. 2014; Kedia and Chattarji 2014). We identified persistent burying behavior in 11% of deer mice; such behavior was found independent of stereotypy levels or sex (Wolmarans et al. 2016b). Moreover, in contrast with previous work in different species (Ichimaru et al. 1995; Li et al. 2006), chronic high dose oral escitalopram (50 mg/kg/day) failed to attenuate this behavior. We therefore speculate that MB in deer mice may be useful in modeling treatment resistant OCD; however, this remains to be proven.
Together with findings that backward somersaulting, but not jumping and pattern running, may reflect a behavior that is resistant to change (Tanimura et al. 2008), it is possible that some phenotypes of deer mouse behavior may be representative of different underlying psychobiological processes that may respond differentially to OCD treatment strategies. Although backward somersaulting does respond to escitalopram treatment, it may be useful to establish whether it demonstrates differential response to augmentation treatment strategies, compared to vertical jumping and pattern running. There is clinical evidence to suggest that different psychobiological mechanisms are variably affected in different patients with OCD, perhaps influencing treatment outcomes (Mataix-Cols et al. 1999; Rufer et al. 2006). To date, little work on animal models of OCD has focused on the issue of treatment-resistance, and this may be a useful focus for future investigation.
Biobehavioral overlaps between deer mouse stereotypy and OCD
Cortico-striatal-thalamic-cortical (CSTC) circuitry in OCD
OCD may reflect underlying disruption in cortico-striatal-thalamic-cortical (CSTC) signaling (Ahmari 2016; Tanimura et al. 2008; Wolmarans et al. 2013). Abnormal regulation of goal-directed behavior may be central to OCD symptomology (Gillan et al. 2011). Brain areas implicated in OCD mediate goal-directed behavior. These include the prefrontal cortex, striatum and thalamic nuclei which communicate with each other via different pathways (Evans et al. 2004; Nambu 2008). The CSTC circuit (Fig. 3) is organized in such a manner that the anterior cingulate cortex (ACC), via innervation of the ventral striatum, exerts feedback through the thalamus to the orbitofrontal cortex (OFC) (Haber and Knutson 2010). Consisting of a direct (behaviorally activating) and an indirect (behaviorally inactivating) pathway, the CSTC circuit is important for planning, executing and terminating complex motor responses and to facilitate reward learning (Morein-Zamir et al. 2014; Stocco et al. 2010). A relative bias in favor of the direct over the indirect pathway may underlie OC symptomology (Saxena and Rauch 2000; van den Heuvel et al. 2005). This not only results in an overactive OFC, resulting in dysfunctional reward processing (Haber and Knutson 2010), but also increases the activity in the CSTC circuit as a whole (Bartz and Hollander 2006; Saxena and Rauch 2000).
Solid lines , no cortical activation of pathways; dotted lines , cortically activated pathways; crosses , no considerable neurotransmitter release; minus signs , GABAergic inhibition; plus signs , disinhibition of target / glutamatergic activation; GPi / SNr , globus pallidus interna/substantia nigra pars reticulata; GPe , globus pallidus externa; STN , subthalamic nucleus; GABA , gamma-amino butyric acid
The striatum mainly consists of GABAergic projections that divide into two subgroups, i.e. striato-nigral (SN) neurons of the direct pathway (projecting to and inhibiting the GPi/SNr), and striato-pallidal (SP) neurons constituting the indirect pathway (projecting to and inhibiting the GPe) (Rymar et al. 2004; Yelnik et al. 1991). Further, both pathways are tonically inhibited under resting conditions (Wilson and Groves 1981). However, upon initiating a behavioral action, signaling in both the activating (direct) and inactivating (indirect) pathways are triggered. This functional antagonism is resolved by the substantia nigra pars compacta (SNc) that modulates both pathways via dopaminergic signaling. Whereas the SN neurons of the direct pathway express Gs associated dopamine-1 (D1) receptors, the SP neurons of the indirect pathway express Gi associated D2 receptors (Tanimura et al. 2010). As such, stimulation of D1 elevates cAMP and increases GABA release, resulting in the activation of the direct pathway (Fig. 3). Conversely, activation of the SP D2 receptors decreases cAMP concentrations and inhibits the release of GABA, thereby inhibiting the indirect pathway (Gerfen et al. 1990; Tepper and Bolam 2004). Striatal dopamine release will therefore initiate motor behavior by shifting the executive balance to the direct pathway (Beiser et al. 1997; Chesselet and Delfs 1996).
Hyperactivity of the CSTC circuitry in OCD is hypothesized to be related to deficits in reward processing (Ferreira et al. 2017; Figee et al. 2011; Palminteri et al. 2012; Pinto et al. 2014), a process that is closely correlated with cortico-striatal dopaminergic signaling (Ljungberg et al. 1991; Mirenowicz and Schultz 1994; Schultz et al. 1993). Briefly, initial anticipation of a possible reward activates nearly 75% of the dopaminergic neurons in the basal ganglia (Ljungberg et al. 1991). However, repetitive exposure to the same stimulus facilitates a process of reward conditioning that enables the brain to evaluate future confrontations with the same set of factors it was conditioned to (Romo and Schultz 1990). It has been shown that the basal ganglia code differences between predicted and actual rewards with ‘reward prediction errors’ (Schultz et al. 1997; Schultz 2002). These are important for reward- and punishment based learning, as it is applied to implement sensorimotor changes to either keep experiencing the same reward in the case of a positive error, or achieving a better outcome in the case of a negative error. Therefore, the relative lack of a significant dopaminergic response after the manifestation of a fully predicted reward may account in part for inadequate closure after task completion in patients with OCD (Figee et al. 2011). It can thus be hypothesized that a dysfunctional reward system and altered dopaminergic signaling plays a role in OCD (Denys et al. 2004; Gillan et al. 2011; Husted et al. 2006).
Although dopamine plays a prominent role to facilitate and maintain motor behavior, it is drugs that target serotonergic and not dopaminergic signaling that have proved most useful in the first-line treatment of OCD (Fineberg and Craig 2007). The fact that behavioral inhibition has been associated with serotonergic neurotransmission (Cools et al. 2008; Daw et al. 2002) may be relevant. In a review of the opponent interactions between serotonin and dopamine (Daw et al. 2002), the term ‘opponency’ describes a paradigm in which more than one system codes for different affective events. While it is known that the dopaminergic system codes rewarding stimuli, serotonin is activated during the experience of aversive stimuli (Fletcher 1995; Fletcher and Korth 1999; Fletcher et al. 1999; Kapur and Remington 1996). Indeed, by enhancing serotonergic signaling, both conditioned behaviors (such as lever pressing for food) and unconditioned behaviors (such as feeding) normally associated with dopaminergic signaling, are inhibited. Consequently, the opposite effect is achieved when antagonizing serotonin or stimulating dopamine. This corresponds with data demonstrating that serotonin antagonizes the effects of dopamine in the SN neurons of the direct pathway (Daw et al. 2002; Kapur and Remington 1996). Therefore, with respect to OCD, it is possible that the balance between reward seeking behavior and aversive reactions is related to the balance between dopaminergic and serotonergic signaling (Ferreira et al. 2017).
Deer mouse stereotypy and aberrant CSTC signaling
A strong body of evidence confirms cortico-striatal involvement in deer mouse stereotypy. First, a bias in favor of the direct SN pathway has been demonstrated by findings that the phenotypic expression of deer mouse stereotypy, but not normal patterns of motor behavior, can be inhibited via selective blockade of striatal D1 and N-methyl-D aspartate (NMDA) receptors (Presti et al. 2003). Moreover, this is supported by a significantly higher SN-dynorphin / SP-enkephalin ratio (Presti and Lewis 2005) as well as reduced activity in the subthalamic nuclei (STN) of H, compared to N animals (Tanimura et al. 2010). There is also evidence that such striatal dysfunction underlies an association between deer mouse stereotypy and deficits in cognitive ability (Bechard et al. 2016; Tanimura et al. 2008). As alluded to earlier, rearing deer mice in EE cages improves procedural learning ability in jumpers and pattern runners, but not in backward somersaulters, implicating a possible role for different psychobiological mechanisms underlying unique forms of stereotypy. Importantly, these cognitive changes were linked to striatal rather than hippocampal mechanisms, the former normally associated with age-related deficits in learning ability (Frick and Fernandez 2003; Frick et al. 2003). In addition, in line with findings demonstrating increased EE-induced neuronal firing in the indirect SP pathway (Bechard et al. 2016), a positive correlation was found between the rate of stereotypy and cognitive rigidity in animals that benefited from EE. Thus, improvements in procedural learning ability occurred in parallel with striatally-mediated adaptations in expression of stereotypy. Although CSTC-associated deficits in learning ability have been demonstrated in OCD patients (Eng et al. 2015; Olley et al. 2007), these are not specific to OCD (Colomer et al. 2017; Lewis et al. 2007; Palminteri et al. 2009). While behavioral rigidity in jumpers and pattern runners responds to intervention, backward somersaulting may represent a behavior that is resistant to change (Tanimura et al. 2008).
Dopamine in deer mouse stereotypy
Although dopamine is known to be a pivotal role player in the CSTC circuitry, its role in the pathogenesis of deer mouse stereotypy remains to be clarified. Most parameters of dopaminergic signaling remain unaltered when comparing H vs. N mice, including striatal D1 and D2 receptor density (Powell et al. 1999) and regional brain levels of dopamine (Güldenpfennig et al. 2011; Powell et al. 1999; Presti et al. 2004), 3,4-dihydroxyphenylacetic acid (DOPAC), and homovanillic acid (HVA) (Güldenpfennig et al. 2011; Powell et al. 1999). Also, while systemic and intrastriatal administration of the D1/D2 receptor agonist, apomorphine, elicits typical rodent stereotypies, e.g. hyperlocomotion, gnawing and excessive grooming, it fails to exacerbate the characteristic deer mouse stereotypies, i.e. jumping, pattern running and backward somersaulting (Presti et al. 2002).
Further, deer mouse stereotypy seems to be unrelated to selective interference by D1 or D2 receptor modulators, which neither trigger nor exacerbate its expression (Korff et al. 2008; Presti et al. 2004). Korff et al. (2008) demonstrated attenuation of stereotypical behavior following administration of the selective D2 agonist, quinpirole (5 mg/kg/day × 4 days), while Presti et al. (2004) demonstrated no significant behavioral alteration. While Korff et al. (2008) used a 4-day intraperitoneal dosing and administration schedule, Presti et al. (2004) administered quinpirole intra-striatally at a dose of 5 μg/side over 60 s, which may account for the disparate results. However, findings from both these investigations seem inconsistent with the quinpirole compulsive-like checking model of OCD (Szechtman et al. 2001, 1998). Still, given important phenotypical differences between pharmacologically induced and spontaneous stereotypy in deer mice (Presti et al. 2002) and considering that neither the Korff et al. (2008), nor the Presti et al. (2004) investigations administered quinpirole for a duration comparable to that of the Szcechtman group (1998, 2001), both these models may be useful in understand the range of mechanisms that may underlie OC-like behavior (Szechtman et al. 2017). The dopaminergic system may play a role in processing context, salience, or reward which differ across these models. Indeed, recent work related to different OC phenotypes and its association with context related deficits in reward and punishment processing (Ferreira et al. 2017; Figee et al. 2011; Palminteri et al. 2012; Pinto et al. 2014), have to be considered.
Altered serotonergic signaling and deer mouse behavior
Deer mouse stereotypy is associated with significant evidence for serotonergic involvement, namely selective response to an SSRI but not an NRI, and significantly reduced striatal SERT density in H-animals (Korff et al. 2008; Wolmarans et al. 2013). Regional brain analysis of the cyclic adenosine monophosphate (cAMP) - phosphodiesterase type 4 (PDE4) cascade in deer mice may assist in learning more on receptor signaling in the CSTC in these animals, and possibly in OCD. The intensity of stereotypy expressed in deer mice is positively correlated to frontal-cortical cAMP concentrations, while being inversely related to PDE4 activity (Korff et al. 2009). Furthermore, chronic SSRI treatment attenuated this response (Korff et al. 2009). This not only supports frontal-cortical dysfunction in deer mouse stereotypy at a neurobiological level (Evans et al. 2004), but suggests involvement of the adenylate cyclase-cAMP-PDE4 cascade. The inverse correlation between PDE4 activity and the intensity of stereotypy may indicate that H-associated increased cAMP is related to increased post-synaptic 5HT1A adenylate cyclase (AS)-cAMP activity (Korff et al. 2009). This hypothesis is strengthened by the demonstration that stimulation of pre-synaptic 5HT1A/1B/1D auto-receptors induce perseverative locomotor paths (Shanahan et al. 2011; Yadin et al. 1991), while their desensitization is thought to mediate some of the ameliorative effects of the SSRIs (Blier et al. 1996). Desensitization of frontal-cortical 5HT1A/1B/D auto-receptors results in increased release of serotonin which in turn is associated with anti-compulsive effects (El Mansari and Blier 2006; Goddard et al. 2008). This may have relevance to earlier studies describing the attenuation of deer mouse stereotypy to meta-chlorophenylpiperazine (mCPP), a non-selective serotonergic agonist (Korff et al. 2008). Although some of the human literature on mCPP indicates an association with exacerbation of OC-symptoms (Aouizerate et al. 2005), mCPP attenuates quinpirole-induced compulsive checking (Tucci et al. 2013; Tucci et al. 2015) providing congruence across at least two animal models of OCD. However, that selective PDE4 inhibition with rolipram decreases methamphetamine-induced stereotypy (Iyo et al. 1995) hints at a possible causal role for disordered PDE4 activity in deer mouse stereotypy.
Disturbances in SERT are well-described in OCD (Hesse et al. 2005; Reimold et al. 2007; Zitterl et al. 2008), while this protein represents an important biological target for the SSRI group of drugs (El Mansari and Blier 2006; Fineberg and Craig 2007). To test the hypothesis that hyposerotonergic signaling underlies OC behavior, we determined frontal-cortical and striatal serotonin transporter (SERT) densities in H and N deer mice (Wolmarans et al. 2013). In line with the theory of behavioral opponency between dopamine and serotonin (Daw et al. 2002) and consistent with findings that deer mouse stereotypy involves a relative bias in favor of the direct SN pathway, we found a significant reduction in striatal but not frontal-cortical SERT density in H, compared to N animals (Wolmarans et al. 2013). This is consistent with clinical (Hesse et al. 2005) and pre-clinical (Vermeire et al. 2012) literature and supports the hypothesis that the biobehavioral effects of a relative increase in SN dopaminergic signaling in deer mice (Presti and Lewis 2005; Presti et al. 2003) are not sufficiently countered by serotonin (Daw et al. 2002).
Deer mouse stereotypy and oxidative stress
Recent clinical studies have indicated oxidative stress in OCD (Behl et al. 2010; Chakraborty et al. 2009a, b; Selek et al. 2008), as well as effective augmentation of standard SSRI treatment with the glutathione precursor, N-acetyl cysteine (NAC) (Camfield et al. 2011; Lafleur et al. 2006; Sayyah et al. 2010). Consistent with these findings, we have demonstrated that H deer mice present with a disturbed frontal-cortical redox balance, i.e. reduced activity of the glutathione system as evinced by diminished concentrations of reduced (GSH) and oxidized glutathione (GSSG) in frontal cortical circuits with these deficits correlated with stereotypy severity (Güldenpfennig et al. 2011). A positive correlation was found between the intensity of stereotypy and the glutathione redox balance (Güldenpfennig et al. 2011), possibly suggesting a relative protective upregulation of glutathione synthesis as a function of stereotypy. Similar findings were demonstrated in deer mice exposed to low levels of environmental toxins (Wu et al. 2009) perhaps suggesting that P. maniculatus bairdii is able to counter the effects of low to moderate degrees of oxidative stress.
Taken together, these findings possibly indicate that H behavior is associated with levels of oxidative stress akin to that of mild pathology. However, caution must be applied when drawing causal relationships between deer mouse stereotypy and OCD, and it is notable that oxidative stress has been found in a number of psychiatric disorders (Berk et al. 2011; Chauhan and Chauhan 2006; Sarandol et al. 2007; Wang et al. 2009). In addition, the response of OC symptoms to NAC augmentation may be related to its modulation of NMDA receptor signaling, rather than specific effects on oxidative stress (Lafleur et al. 2006). Further study of the association between deer mouse stereotypy and oxidative stress, and of the response of such stereotypy to anti-oxidants is needed.
Peromyscus maniculatus bairdii as a model of heterogeneous OC behavior
OCD is characterized by a narrow range of different symptoms. The most prevalent obsessions are concerns about contamination (55%), inappropriate aggressive and sexual thoughts (50% and 32% respectively), and concerns about symmetry and order (36%). The most common compulsions are ritualistic checking (80%), cleaning and decontamination rituals (46%) and counting (21%) (Abramowitz et al. 2010). Recently, we began to investigate whether P. maniculatus bairdii may express different OC-related phenotypes in addition to spontaneous stereotypy. We studied MB, previously proposed as a measure of compulsive activity (Albelda and Joel 2012) and nest building (NB) (Greene-Schloesser et al. 2011), which represents normal rodent activity but with between- and within-species variance (Jirkof 2014; Smithers 1983). As referred to earlier, high MB behavior was observed in 11% of our deer mouse cohort, independent of stereotypy or sex (Wolmarans et al. 2016a), while 30% of animals, again independent of stereotypy or sex, displayed persistent large NB behavior. Escitalopram had no effect on high MB, but reduced high NB (Wolmarans et al. 2016a, b). Further work is needed to determine whether such observations are analogous to clinical findings (including differential response of different symptom dimensions to stressors and to SSRIs). However, that large NB but not high MB responded to escitalopram, and that neither behavior was associated with a specific stereotypical cohort, suggests that NB and MB in the deer mouse reflect different underlying neurobiological mechanisms. Further, it is possible that such neurobiological differences may be species specific (Greene-Schloesser et al. 2011). While large NB also occurs naturally, mainly in response to environmental change (Jirkof 2014), the persistent and severe nature of large NB in some laboratory houseddeer mice only, suggests that in this sub-group, large NB may not be goal-directed or adaptive. This tentatively establishes a degree of face and predictive validity for large NB as reflecting a different, but also naturalistic OC-like phenotype in deer mice.
Conclusion
The deer mouse (Peromyscus maniculatus bairdii) offers an opportunity to study the neurobiology of OC-like behavior within a naturalistic framework. The species presents with stereotypies that are reminiscent of OCRD symptoms, while such stereotypies appear to share, at least in part, underlying psychobiological mechanisms and treatment response typical of OCD (Table 2). Given that deer mice display a narrow range of stereotypical behaviors, it is possible that some of these may be useful for studying specific symptom dimensions found in OCRDs. Further research on the genetic and epigenetic associations of stereotypies in the deer mouse model may be useful.
References
Abramowitz JS et al (2010) Assessment of Obsessive-Compulsive Symptom Dimensions: Development and Evaluation of the Dimensional Obsessive-Compulsive Scale. Psychol Assess 22:180–198
Abramovitch A, Cooperman A (2015) The cognitive neuropsychology of obsessive-compulsivedisorder: A critical review Journal of Obsessive-Compulsive and Related Disorders 5:24-36http://dx.doi.org.nwulib.nwu.ac.za/10.1016/j.jocrd.2015.01.002
Ahmari SE (2016) Using mice to model Obsessive Compulsive Disorder: From genes to circuits. Neuroscience 321:121–137. https://doi.org/10.1016/j.neuroscience.2015.11.009
Albelda N, Joel D (2012) Animal models of obsessive-compulsive disorder: Exploring pharmacology and neural substrates. Neurosci Biobehav Rev 36:47–63. https://doi.org/10.1016/j.neubiorev.2011.04.006
Alonso P, López-Solà C, Real E, Segalàs C, Menchón JM (2015) Animal models of obsessive–compulsive disorder: Utility and limitations. Neuropsychiatr Dis Treat 11:1939–1955. https://doi.org/10.2147/NDT.S62785
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders vol 5. vol Book, Whole. American Psychiatric Association, Washington
Andersen SL, Greene-Colozzi EA, Sonntag KC (2010) A novel, multiple symptom model of obsessive-compulsive-like behaviors in animals. Biol Psychiatry 68:741–747
Aouizerate B, Guehl D, Cuny E, Rougier A, Burbaud P, Tignol J, Bioulac B (2005) Updated overview of the putative role of the serotoninergic system in obsessive-compulsive disorder. Neuropsychiatr Dis Treat 1:231
Bartz JA, Hollander E (2006) Is obsessive–compulsive disorder an anxiety disorder? Prog Neuro-Psychopharmacol Biol Psychiatry 30:338–352. https://doi.org/10.1016/j.pnpbp.2005.11.003
Bechard AR, Cacodcar N, King MA, Lewis MH (2016) How does environmental enrichment reduce repetitive motor behaviors? Neuronal activation and dendritic morphology in the indirect basal ganglia pathway of a mouse model. Behav Brain Res 299:122–131
Behl A, Swami G, Sircar SS, Bhatia MS, Banerjee BD (2010) Relationship of possible stress-related biochemical markers to oxidative/antioxidative status in obsessive-compulsive disorder. Neuropsychobiology 61:210–214
Beiser DG, Hua SE, Houk JC (1997) Network models of the basal ganglia. Curr Opin Neurobiol 7:185–190
Bejerot S, Bodlund O (1998) Response to high doses of citalopram in treatment-resistant obsessive- compulsive disorder. Acta Psychiatr Scand 98:423–424
Berk M et al (2011) Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev 35:804–817
Berrocal C, Ruiz Moreno MA, Montero M, Rando MA, Rucci P, Cassano GB (2006) Social anxiety and obsessive-compulsive spectra: Validation of the SHY-SR and the OBS-SR among the Spanish population. Psychiatry Res 142:241–251. https://doi.org/10.1016/j.psychres. 2005.07.025
Blier P, Ei Mansari M, Ducharme V, Bouchard C (1996) Role of the terminal 5-HT autoreceptor in the orbitofrontal cortex in the mechanism of action of 5-HT reuptake inhibitors in obsessive compulsive disorder. Eur Neuropsychopharmacol 6:91–91. https://doi.org/10.1016/0924-977X(96)87719-4
Burguière E, Monteiro P, Mallet L, Feng G, Graybiel AM (2015) Striatal circuits, habits, and implications for obsessive-compulsive disorder. Curr Opin Neurobiol 30:59–65. https://doi.org/10.1016/j.conb.2014.08.008
Camfield DA, Sarris J, Berk M (2011) Nutraceuticals in the treatment of Obsessive Compulsive Disorder (OCD): A review of mechanistic and clinical evidence. Prog Neuro-Psychopharmacol Biol Psychiatry 35:887–895
Chakraborty S, Dasgupta A, Das HN, Singh OP, Mandal AK, Mandal N (2009a) Study of oxidative stress in obsessive compulsive disorder in response to treatment with Fluoxetine. Indian J Clin Biochem 24:194–197
Chakraborty S, Singh OP, Dasgupta A, Mandal N, Das HN (2009b) Correlation between lipid peroxidation-induced TBARS level and disease severity in obsessive-compulsive disorder. Prog Neuro-Psychopharmacol Biol Psychiatry 33:363–366
Chauhan A, Chauhan V (2006) Oxidative stress in autism. Pathophysiology 13:171–181
Chesselet MF, Delfs JM (1996) Basal ganglia and movement disorders: An update. Trends Neurosci 19:417–422
Chou-Green JM, Holscher TD, Dallman MF, Akana SF (2003a) Compulsive behavior in the 5-HT2C receptor knockout mouse. Physiol Behav 78:641–649
Chou-Green JM, Holscher TD, Dallman MF, Akana SF (2003b) Repeated stress in young and old 5-HT2C receptor knockout mice. Physiol Behav 79:217–226. https://doi.org/10.1016/S0031-9384(03)00096-9
Colomer C, Berenguer C, Roselló B, Baixauli I, Miranda A (2017) The impact of inattention, hyperactivity/impulsivity symptoms, and executive functions on learning behaviors of children with ADHD. Front Psychol 8:540. https://doi.org/10.3389/fpsyg.2017.00540
Cools R, Roberts AC, Robbins TW (2008) Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn Sci 12:31–40
Daw ND, Kakade S, Dayan P (2002) Opponent interactions between serotonin and dopamine. Neural Netw 15:603–616. https://doi.org/10.1016/S0893-6080(02)00052-7
Denys D, Zohar J, Westenberg HGM (2004) The role of dopamine in obsessive-compulsive disorder: Preclinical and clinical evidence. J Clin Psychiatry 65:11–17
Dixit MP, Thakre PP, Pannase AS, Aglawe MM, Taksande BG, Kotagale NR (2014) Imidazoline binding sites mediates anticompulsive-like effect of agmatine in marble-burying behavior in mice. Eur J Pharmacol 732:26–31. https://doi.org/10.1016/j.ejphar.2014.02.045
Eilam D, Zor R, Szechtman H, Hermesh H (2006) Rituals, stereotypy and compulsive behavior in animals and humans. Neurosci Biobehav Rev 30:456–471. https://doi.org/10.1016/j.neubiorev.2005.08.003
El Mansari M, Blier P (2006) Mechanisms of action of current and potential pharmacotherapies of obsessive-compulsive disorder. Prog Neuro-Psychopharmacol Biol Psychiatry 30:362–373. https://doi.org/10.1016/j.pnpbp.2005.11.005
Eng GK, Sim K, Chen SHA (2015) Meta-analytic investigations of structural grey matter, executive domain-related functional activations, and white matter diffusivity in obsessive compulsive disorder: An integrative review. Neurosci Biobehav Rev 52:233–257. https://doi.org/10.1016/j.neubiorev.2015.03.002
Erzegovesi S, Guglielmo E, Siliprandi F, Bellodi L (2005) Low-dose risperidone augmentation of fluvoxamine treatment in obsessive-compulsive disorder: A double-blind, placebo-controlled study. Eur Neuropsychopharmacol 15:69–74
Evans DW, Lewis MD, Iobst E (2004) The role of the orbitofrontal cortex in normally developing compulsive-like behaviors and obsessive–compulsive disorder. Brain Cogn 55:220–234. https://doi.org/10.1016/S0278-2626(03)00274-4
Ferreira GM, Yücel M, Dawson A, Lorenzetti V, Fontenelle LF (2017) Investigating the role of anticipatory reward and habit strength in obsessive-compulsive disorder. CNS Spectr 22:295–304. https://doi.org/10.1017/S1092852916000535
Figee M, Vink M, De Geus F, Vulink N, Veltman DJ, Westenberg H, Denys D (2011) Dysfunctional reward circuitry in obsessive-compulsive disorder. Biol Psychiatry 69:867–874. https://doi.org/10.1016/j.biopsych.2010.12.003
Fineberg N, Chamberlain S, Hollander E, Boulougouris V, Robbins T (2011) Translational approaches to obsessive-compulsive disorder: from animal models to clinical treatment. Br J Pharmacol 164:1044–1061
Fineberg NA, Craig KJ (2007) Pharmacological treatment for obsessive–compulsive disorder. Anxiety Disord Part 3 of 3 6:234–239. https://doi.org/10.1016/j.mppsy.2007.04.001
Fineberg NA, Nigam A, Sivakumaran T (2006) Pharmacologic strategies for treatment-resistant OCD: A review of the evidence. Psychiatr Ann 36:464–473
Fletcher PJ (1995) Effects of combined or separate 5,7-dihydroxytryptamine lesions of the dorsal and median raphe nuclei on responding maintained by a DRL 20s schedule of food reinforcement. Brain Res 675:45–54
Fletcher PJ, Korth KM (1999) Activation of 5-HT1B receptors in the nucleus accumbens reduces amphetamine-induced enhancement of responding for conditioned reward. Psychopharmacology 142:165–174
Fletcher PJ, Korth KM, Chambers JW (1999) Selective destruction of brain serotonin neurons by 5,7- dihydroxytryptamine increases responding for a conditioned reward. Psychopharmacology 147:291–299
Frick KM, Fernandez SM (2003) Enrichment enhances spatial memory and increases synaptophysin levels in aged female mice. Neurobiol Aging 24:615–626
Frick KM, Stearns NA, Pan J-Y, Berger-Sweeney J (2003) Effects of environmental enrichment on spatial memory and neurochemistry in middle-aged mice. Learn Mem 10:187–198
Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ Jr, Sibley DR (1990) D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250:1429–1432
Gillan CM, Papmeyer M, Morein-Zamir S, Sahakian BJ, Fineberg NA, Robbins TW, De Wit S (2011) Disruption in the balance between goal-directed behavior and habit learning in obsessive-compulsive disorder. Am J Psychiatr 168:718–726. https://doi.org/10.1176/appi.ajp.2011.10071062
Goddard AW, Shekhar A, Whiteman AF, McDougle CJ (2008) Serotoninergic mechanisms in the treatment of obsessive–compulsive disorder. Drug Discov Today 13:325–332. https://doi.org/10.1016/j.drudis.2007.12.009
Graybiel AM (2008) Habits, rituals, and the evaluative brain. Annual review of neuroscience 31:359-387. https://doi.org/10.1146/annurev.neuro.29.051605.112851
Greer JM, Capecchi MR (2002) Hoxb8 Is Required for Normal Grooming Behavior in Mice. Neuron 33:23–34. https://doi.org/10.1016/S0896-6273(01)00564-5
Greene-Schloesser DM et al (2011) Predictive validity of a non-induced mouse model of compulsive-like behavior. Behav Brain Res 221:55–62
Güldenpfennig M, Wolmarans DW, du Preez JL, Stein DJ, Harvey BH (2011) Cortico-striatal oxidative status, dopamine turnover and relation with stereotypy in the deer mouse. Physiol Behav 103:404–411. https://doi.org/10.1016/j.physbeh.2011.03.008
Haber SN, Knutson B (2010) The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35:4
Hadley C, Hadley B, Ephraim S, Yang M, Lewis MH (2006) Spontaneous stereotypy and environmental enrichment in deer mice (Peromyscus maniculatus): Reversibility of experience. Appl Anim Behav Sci 97:312–322. https://doi.org/10.1016/j.applanim.2005.08.006
Hesse S et al (2005) Serotonin and dopamine transporter imaging in patients with obsessive-compulsive disorder. Psychiatry Res Neuroimaging 140:63–72
Hoffman KL (2011) Animal models of obsessive compulsive disorder: Recent findings and future directions. Expert Opin Drug Discovery 6:725–737. https://doi.org/10.1517/17460441.2011.577772
Hoffman KL, Rueda Morales RI (2009) Toward an understanding of the neurobiology of "just right" perceptions: Nest building in the female rabbit as a possible model for compulsive behavior and the perception of task completion. Behav Brain Res 204:182–191
Hollander E, Rossi NB, Sood E, Pallanti S (2003) Risperidone augmentation in treatment-resistant obsessive-compulsive disorder: A double-blind, placebo-controlled study. Int J Neuropsychopharmacol 6:397–401
Husted DS, Shapira NA, Goodman WK (2006) The neurocircuitry of obsessive–compulsive disorder and disgust. Prog Neuro-Psychopharmacol Biol Psychiatry 30:389–399. https://doi.org/10.1016/j.pnpbp.2005.11.024
Ichimaru Y, Egawa T, Sawa A (1995) 5-HT(1A)-receptor subtype mediates the effect of fluvoxamine, a selective serotonin reuptake inhibitor, on marble-burying behavior in mice. Jpn J Pharmacol 68:65–70
Ipser JC, Carey P, Dhansay Y, Fakier N, Seedat S, Stein DJ (2006) Pharmacotherapy augmentation strategies in treatment-resistant anxiety disorders. The Cochrane database of systematic reviews:Cd005473https://doi.org/10.1002/14651858.CD005473.pub2
Iyo M, Maeda Y, Inada T, Kitao Y, Sasaki H, Fukui S (1995) The effects of a selective cAMP phosphodiesterase inhibitor, rolipram, on methamphetamine-induced behavior. Neuropsychopharmacology 13:33–39
Jirkof P (2014) Burrowing and nest building behavior as indicators of well-being in mice. Measuring Behav 234:139–146. https://doi.org/10.1016/j.jneumeth.2014.02.001
Joel D (2006) Current animal models of obsessive compulsive disorder: A critical review. Prog Neuro-Psychopharmacol Biol Psychiatry 30:374–388. https://doi.org/10.1016/j.pnpbp.2005.11.006
Joyner CP, Myrick LC, Crossland JP, Dawson WD (1998) Deer mice as laboratory animals. ILAR J 39:322–330
Kapur S, Remington G (1996) Serotonin-dopamine interaction and its relevance to schizophrenia. Am J Psychiatr 153:466–476
Kedia S, Chattarji S (2014) Marble burying as a test of the delayed anxiogenic effects of acute immobilisation stress in mice. J Neurosci Methods 233:150–154. https://doi.org/10.1016/j.jneumeth.2014.06.012
Kim KL, Reynolds KC, Alfano CA (2012) Social impairment in children with obsessive compulsive disorder: Do comorbid problems of inattention and hyperactivity matter? J Obsessive Compuls Relat Disord 1:228–233. https://doi.org/10.1016/j.jocrd.2012.06.005
Kinnear CJ et al (2000) Obsessive-compulsive disorder and the promoter region polymorphism (5-HTTLPR) in the serotonin transporter gene (SLC6A4): A negative association study in the Afrikaner population. Int J Neuropsychopharmacol, 331 3:327
Korff S, Stein DJ, Harvey BH (2008) Stereotypic behaviour in the deer mouse: Pharmacological validation and relevance for obsessive compulsive disorder. Prog Neuro-Psychopharmacol Biol Psychiatry 32:348–355
Korff S, Stein DJ, Harvey BH (2009) Cortico-striatal cyclic AMP-phosphodiesterase-4 signalling and stereotypy in the deer mouse: Attenuation after chronic fluoxetine treatment. Pharmacol Biochem Behav 92:514–520. https://doi.org/10.1016/j.pbb.2009.01.025
Lafleur DL et al (2006) N-acetylcysteine augmentation in serotonin reuptake inhibitor refractory obsessive-compulsive disorder. Psychopharmacology 184:254–256
Langen M, Kas MJH, Staal WG, van Engeland H, Durston S (2011) The neurobiology of repetitive behavior: Of mice. Neurosci Biobehav Rev 35:345–355. https://doi.org/10.1016/j.neubiorev.2010.02.004
Lewis MH, Tanimura Y, Lee LW, Bodfish JW (2007) Animal models of restricted repetitive behavior in autism. Behav Brain Res 176:66–74. https://doi.org/10.1016/j.bbr.2006.08.023
Li X, Morrow D, Witkin JM (2006) Decreases in nestlet shredding of mice by serotonin uptake inhibitors: Comparison with marble burying. Life Sci 78:1933–1939
Ljungberg T, Apicella P, Schultz W (1991) Responses of monkey midbrain dopamine neurons during delayed alternation performance. Brain Res 567:337–341
Markarian Y et al (2010) Multiple pathways to functional impairment in obsessive–compulsive disorder. Clin Psychol Rev 30:78–88. https://doi.org/10.1016/j.cpr.2009.09.005
Mataix-Cols D, Rauch SL, Manzo PA, Jenike MA, Baer L (1999) Use of factor-analyzed symptom dimensions to predict outcome with serotonin reuptake inhibitors and placebo in the treatment of obsessive-compulsive disorder. Am J Psychiatr 156:1409–1416
McDougle CJ, Epperson CN, Pelton GH, Wasylink S, Price LH (2000) A double-blind, placebo-controlled study of risperidone addition in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder. Arch Gen Psychiatry 57:794–801
Mirenowicz J, Schultz W (1994) Importance of unpredictability for reward responses in primate dopamine neurons. J Neurophysiol 72:1024–1027
Morein-Zamir S et al (2014) The profile of executive function in OCD hoarders and hoarding disorder. Psychiatry Res 215:659–667. https://doi.org/10.1016/j.psychres.2013.12.026
Nambu A (2008) Seven problems on the basal ganglia. Curr Opin Neurobiol 18:595–604. https://doi.org/10.1016/j.conb.2008.11.001
Olley A, Malhi G, Sachdev P (2007) Memory and executive functioning in obsessive-compulsive disorder: A selective review. J Affect Disord 104:15–23. https://doi.org/10.1016/j.jad.2007.02.023
Overduin MK, Furnham A (2012) Assessing obsessive-compulsive disorder (OCD): A review of self-report measures. J Obsessive Compuls Relat Disord 1:312–324
Owens MJ, Knight DL, Nemeroff CB (2001) Second-generation SSRIs: Human monoamine transporter binding profile of escitalopram and R-fluoxetine. Biol Psychiatry 50:345–350
Palminteri S, Clair AH, Mallet L, Pessiglione M (2012) Similar improvement of reward and punishment learning by serotonin reuptake inhibitors in obsessive-compulsive disorder. Biol Psychiatry 72:244–250. https://doi.org/10.1016/j.biopsych.2011.12.028
Palminteri S, Lebreton M, Worbe Y, Grabli D, Hartmann A, Pessiglione M (2009) Pharmacological modulation of subliminal learning in Parkinson's and Tourette's syndromes. Proc Natl Acad Sci U S A 106:19179–19184. https://doi.org/10.1073/pnas.0904035106
Pinto A, Steinglass JE, Greene AL, Weber EU, Simpson HB (2014) Capacity to delay reward differentiates obsessive-compulsive disorder and obsessive-compulsive personality disorder. Biol Psychiatry 75:653–659. https://doi.org/10.1016/j.biopsych.2013.09.007
Powell SB, Newman HA, Pendergast JF, Lewis MH (1999) A Rodent Model of Spontaneous Stereotypy: Initial Characterization of Developmental, Environmental, and Neurobiological Factors. Physiol Behav 66:355–363. https://doi.org/10.1016/S0031-9384(98)00303-5
Presti MF, Gibney BC, Lewis MH (2004) Effects of intrastriatal administration of selective dopaminergic ligands on spontaneous stereotypy in mice. Physiol Behav 80:433–439
Presti MF, Lewis MH (2005) Striatal opioid peptide content in an animal model of spontaneous stereotypic behavior. Behav Brain Res 157:363–368
Presti MF, Mikes HM, Lewis MH (2003) Selective blockade of spontaneous motor stereotypy via intrastriatal pharmacological manipulation. Pharmacol Biochem Behav 74:833–839
Presti MF, Powell SB, Lewis MH (2002) Dissociation between spontaneously emitted and apomorphine-induced stereotypy in Peromyscus maniculatus bairdii. Physiol Behav 75:347–353
Presti MF, Watson CJ, Kennedy RT, Yang M, Lewis MH (2004b) Behavior-related alterations of striatal neurochemistry in a mouse model of stereotyped movement disorder. Pharmacol Biochem Behav 77:501–507
Reimold M et al (2007) Reduced availability of serotonin transporters in obsessive-compulsive disorder correlates with symptom severity - A [11C]DASB PET study. J Neural Transm 114:1603–1609
Romo R, Schultz W (1990) Dopamine neurons of the monkey midbrain: Contingencies of responses to active touch during self-initiated arm movements. J Neurophysiol 63:592–606
Rosa AC et al (2012) Clinical correlates of social adjustment in patients with obsessive-compulsive disorder. J Psychiatr Res 46:1286–1292. https://doi.org/10.1016/ j.jpsychires.2012.05.019
Rufer M, Fricke S, Moritz S, Kloss M, Hand I (2006) Symptom dimensions in obsessive–compulsive disorder: prediction of cognitive-behavior therapy outcome. Acta Psychiatr Scand 113:440–446
Ruscio A, Stein D, Chiu W, Kessler R (2010) The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry 15:53–63
Rymar VV, Sasseville R, Luk KC, Sadikot AF (2004) Neurogenesis and Stereological Morphometry of Calretinin-Immunoreactive GABAergic Interneurons of the Neostriatum. J Comp Neurol 469:325–339
Sarandol A, Sarandol E, Eker SS, Erdinc S, Vatansever E, Kirli S (2007) Major depressive disorder is accompanied with oxidative stress: short-term antidepressant treatment does not alter oxidative–antioxidative systems. Hum Psychopharmacol Clin Exp 22:67–73
Saxena S, Rauch SL (2000) Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatr Clin N Am 23:563–586
Sayyah M, Boostani H, Pakseresht S, Malayeri A (2010) Comparison of Silybum marianum (L.) Gaertn. with fluoxetine in the treatment of Obsessive− Compulsive Disorder. Prog Neuro-Psychopharmacol Biol Psychiatry 34:362–365
Schultz W (2002) Getting Formal with Dopamine and Reward. Neuron 36:241–263. https://doi.org/10.1016/S0896-6273(02)00967-4
Schultz W, Apicella P, Ljungberg T (1993) Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J Neurosci 13:900–913
Schultz W, Dayan P, Montague PR (1997) A neural substrate of prediction and reward. Science 275:1593–1599
Selek S, Herken H, Bulut M, Ceylan MF, Celik H, Savas HA, Erel O (2008) Oxidative imbalance in obsessive compulsive disorder patients: A total evaluation of oxidant-antioxidant status. Prog Neuro-Psychopharmacol Biol Psychiatry 32:487–491
Shanahan NA, Velez LP, Masten VL, Dulawa SC (2011) Essential Role for Orbitofrontal Serotonin 1B Receptors in Obsessive-Compulsive Disorder-like Behavior and Serotonin Reuptake Inhibitor Response in Mice Biol Psychiatry. 70:1039-1048. https://doi.org/10.1016/j.biopsych.2011.07.032
Shorter KR, Crossland JP, Webb D, Szalai G, Felder MR, Vrana PB (2012) Peromyscus as a mammalian epigenetic model Genetics research international 2012
Shorter KR et al (2014) Natural genetic variation underlying differences in peromyscus repetitive and social/aggressive behaviors. Behav Genet 44:126–135. https://doi.org/10.1007/s10519-013-9640-8
Smithers RHN (1983) XXIII. Families CRICETIDAE and MURIDAE, Rats and mice. In: The Mammals of the Southern-African Subregion, vol 1. University of Pretoria, Pretoria, South Africa, pp 220–220 - 296
Stocco A, Lebiere C, Anderson JR (2010) Conditional Routing of Information to the Cortex: A Model of the Basal Ganglia's Role in Cognitive Coordination. Psychol Rev 117:541–574
Storch EA, Ledley DR, Lewin AB, Murphy TK, Johns NB, Goodman WK, Geffken GR (2006) Peer victimization in children with obsessive-compulsive disorder: Relations with symptoms of psychopathology. J Clin Child Adolesc Psychol 35:446–455
Szechtman H et al (2001) Compulsive checking behavior of quinpirole-sensitized rats as an animal model Obsessive-Compulsive Disorder(OCD): Form and control. BMC Neurosci 2:4
Szechtman H, Sulis W, Eilam D (1998) Quinpirole induces compulsive checking behavior in rats: A potential animal model of obsessive-compulsive disorder (OCD). Behav Neurosci 112:1475–1485
Szechtman H, Ahmari SE, Beninger RJ, Eilam D, Harvey BH, Edemann-Callesen H, Winter C (2017) Obsessive-compulsive disorder: insights from animal models. Neurosci Biobehav Rev 76:254–279
Tanimura Y, Ogoegbunam FC, Lewis MH (2009) Amphetamine-induced sensitization and spontaneous stereotypy in deer mice. Pharmacol Biochem Behav 92:670–675
Tanimura Y, Vaziri S, Lewis MH (2010) Indirect basal ganglia pathway mediation of repetitive behavior: attenuation by adenosine receptor agonists. Behav Brain Res 210:116–122
Tanimura Y, Yang MC, Lewis MH (2008) Procedural learning and cognitive flexibility in a mouse model of restricted, repetitive behaviour. Behav Brain Res 189:250–256
Tepper JM, Bolam JP (2004) Functional diversity and specificity of neostriatal interneurons. Curr Opin Neurobiol 14:685–692
Torres AR, Fontenelle LF, Shavitt RG, Ferrão YA, do Rosário MC, Storch EA, Miguel EC (2016) Comorbidity variation in patients with obsessive–compulsive disorder according to symptom dimensions: Results from a large multicentre clinical sample. J Affect Disord 190:508–516. https://doi.org/10.1016/j.jad.2015.10.051
Tucci MC et al (2013) Effects of the serotonergic agonist mCPP on male rats in the quinpirole sensitization model of obsessive-compulsive disorder (OCD). Psychopharmacology 227:277–285. https://doi.org/10.1007/s00213-013-2976-1
Tucci MC, Dvorkin-Gheva A, Johnson E, Wong M, Szechtman H (2015) 5-HT2A/C receptors do not mediate the attenuation of compulsive checking by mCPP in the quinpirole sensitization rat model of obsessive-compulsive disorder (OCD). Behav Brain Res 279:211–217. https://doi.org/10.1016/j.bbr.2014.11.017
van den Heuvel OA et al (2005) Frontal-striatal dysfunction during planning in obsessive-compulsive disorder. Arch Gen Psychiatry 62:301–309
Vermeire S et al (2012) Serotonin 2A receptor, serotonin transporter and dopamine transporter alterations in dogs with compulsive behaviour as a promising model for human obsessive-compulsive disorder. Psychiatry Res Neuroimaging 201:78–87. https://doi.org/10.1016/j.pscychresns.2011.06.006
Vrana PB (2007) Genomic imprinting as a mechanism of reproductive isolation in mammals. J Mammal 88:5–23. https://doi.org/10.1644/06-MAMM-S-013R1.1
Wang JF, Shao L, Sun X, Young LT (2009) Increased oxidative stress in the anterior cingulate cortex of subjects with bipolar disorder and schizophrenia. Bipolar Disord 11:523–529
Welch JM et al (2007) Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature 448:894–900. https://doi.org/10.1038/nature06104
Wilson CJ, Groves PM (1981) Spontaneous firing patterns of identified spiny neurons in the rat neostriatum. Brain Res 220:67–80
Wolmarans DW, Brand L, Stein DJ, Harvey BH (2013) Reappraisal of spontaneous stereotypy in the deer mouse as an animal model of obsessive-compulsive disorder (OCD): Response to escitalopram treatment and basal serotonin transporter (SERT) density. Behav Brain Res 256:545–553. https://doi.org/10.1016/j.bbr.2013.08.049
Wolmarans DW, Stein DJ, Harvey BH (2016a) Excessive nest building is a unique behavioural phenotype in the deer mouse model of obsessive-compulsive disorder. J Psychopharmacol 30:867–874. https://doi.org/10.1177/0269881116645554
Wolmarans DW, Stein DJ, Harvey BH (2016b) Of mice and marbles: Novel perspectives on burying behavior as a screening test for psychiatric illness. Cogn Affect Behav Neurosci 16:551–560https://doi.org/10.3758/s13415-016-0413-8
Wolmarans DW, Stein DJ, Harvey BH (2017a) Social behavior in deer mice as a novel interactive paradigm of relevance for obsessive-compulsive disorder (OCD). Soc Neurosci 12:135–149. https://doi.org/10.1080/17470919.2016.1145594
Wolmarans W, Stein DJ, Harvey BH (2017b) If Rodents Could Speak: A Psycho-Behavioral Perspective on modelling Obsessive-Compulsive Disorder (OCD) in Animals: The role of Context. Curr Med Chem. https://doi.org/10.2174/0929867324666170523125256
Wu H, McBride TJ, Isanhart JP, Cox SB, Hooper MJ (2009) Responses of glutamate cysteine ligase and glutathione to oxidants in deer mice (Peromyscus maniculatus). Ecotoxicol Environ Saf 72:1572–1578. https://doi.org/10.1016/j.ecoenv.2009.02.008
Yadin E, Friedman E, Bridger WH (1991) Spontaneous alternation behavior: An animal model for obsessive-compulsive disorder? Pharmacol Biochem Behav 40:311–315
Yang H et al (2011) Subspecific origin and haplotype diversity in the laboratory mouse. Nat Genet 43:648–655. https://doi.org/10.1038/ng.847
Yelnik J, Francois C, Percheron G, Tande D (1991) Morphological taxonomy of the neurons of the primate striatum. J Comp Neurol 313:273–294
Zitterl W et al (2008) Changes in thalamus-hypothalamus serotonin transporter availability during clomipramine administration in patients with obsessive-compulsive disorder. Neuropsychopharmacology 33:3126–3134
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wolmarans, D.W., Scheepers, I.M., Stein, D.J. et al. Peromyscus maniculatus bairdii as a naturalistic mammalian model of obsessive-compulsive disorder: current status and future challenges. Metab Brain Dis 33, 443–455 (2018). https://doi.org/10.1007/s11011-017-0161-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-017-0161-7