Abstract
Extracellular deposition of Beta-amyloid peptide (Aβ) is the main finding in the pathophysiology of Alzheimer’s disease (AD), which damages cholinergic neurons through oxidative stress and reduces the cholinergic neurotransmission. Satureja bachtiarica is a medicinal plant from the Lamiaceae family which was widely used in Iranian traditional medicine. The aim of the present study was to investigate possible protective effects of S. bachtiarica methanolic extract on Aβ induced spatial memory impairment in Morris Water Maze (MWM), oxidative stress and cholinergic neuron degeneration. Pre- aggregated Aβ was injected into the hippocampus of each rat bilaterally (10 μg/rat) and MWM task was performed 14 days later to evaluate learning and memory function. Methanolic extract of S.bachtiarica (10, 50 and 100 mg/Kg) was injected intraperitoneally for 19 consecutive days, after Aβ injection. After the probe test the brain tissue were collected and lipid peroxidation, Acetylcholinesterase (AChE) activity and Cholin Acetyl Transferees (ChAT) immunorectivity were measured in the hippocampus. Intrahipocampal injection of Aβ impaired learning and memory in MWM in training days and probe trail. Methanolic extract of S. bachtiarica (50 and 100 mg/Kg) could attenuate Aβ-induced memory deficit. ChAT immunostaining revealed that cholinergic neurons were loss in Aβ- injected group and S. bachtiarica (100 mg/Kg) could ameliorate Aβ- induced ChAT reduction in the hippocampus. Also S. bachtiarica could ameliorate Aβ-induced lipid peroxidation and AChE activity increase in the hippocampus. In conclusion our study represent that S.bachtiarica methanolic extract can improve Aβ-induced memory impairment and cholinergic loss then we recommended this extract as a candidate for further investigation in treatment of AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease predominantly characterized by memory deficits. The impairment of memory in AD is associated with hypofunction of cholinergic system in forebrain resulted from the loss of basal forebrain cholinergic neurons which play an important role in memory formation and cognition. (Schliebs and Arendt 2006, Kar et al. 2004) Extracellular deposition of beta-amyloid peptid (Aβ) is the main finding in the pathophysiology of AD and is considered a critical event in initiating the synaptic loss and finally neuronal cell death (Carter and Lippa 2001, Hardy and Selkoe 2002).Several mechanisms were proposed for Aβ- induced neurotoxicity (Cavallucci et al. 2012, Luquin et al. 1997). but much evidence suggested that oxidative damage plays a pivotal role in Aβ -induced toxicity (Chen and Zhong 2014). Increased production of reactive oxygen species (ROS) was reported in the brain of animal model of AD. Oxidative damage induced by ROS, includes protein, DNA, RNA oxidation and lipid peroxidation, have been described in the AD brain also treatment with antioxidant could ameliorate Aβ –induced memory deficit and antioxidants such as vitamin C and natural polyphenols were considered in AD therapy (Heo et al. 2013, Butterfield 1997). Current curative for treatment of AD are the acetylcholinesterase inhibitors such as donepezil and N-methyl-D-aspartate (NMDA) receptor antagonist such as memantine. These medicines have mild to moderate effectiveness and cannot prevent of disease progression (Birks 2006).
The therapeutic limitations and pharmacological side effects of these medicines along with this fact that prevalence of AD is steadily increasing, have led to further studies for finding new agents for the management of AD. Because of complex pathology of AD, new research are focused on therapeutic agent that can prevent disease progress through different pathways (Tayeb et al. 2012). Herbal medicines which contain several components with different pharmacological effects are considered for treatment of AD by the several studies and also traditional remedies have been used for treatment of dementia for several years (Perry and Howes 2011, Howes and Houghton 2012).
Lamiaceae family is the one of the largest family between plants family. There is 81 genus in this family used as herbal medicine. These plants were widely used in Iranian traditional medicine for neurological, cardiovascular and gastrointestinal disorders. Several plants from this family have been studied for their effectiveness in AD. Neuroprotection and memory-inhancing activity have been reported for some plants in this family (Orhan et al. 2012, Singhal et al. 2012, Topcu and Kusman 2014, Soodi et al. 2014, Sepand et al. 2013). Satureja species are medicinal plants from the Lamiaceae family which were widely used in Iranian traditional medicine as a tonic, carminative and digestive, also in several studies anti-inflammatory, anti-oxidant, anti-diabetic, anti-bacterial, anti-viral, anti-leishmanial and anti-hyperlipedimic activity have been reported for plants of Satureja species (Naghibi et al. 2005; Tepe 2015). S. bachtiarica is one of the endemic species from Iran. There are few studies about the pharmacological effect of this plant. Anti-bacterial and anti-leschmania activity was reported for essential oil of S.bachtiarica (Mohammadpour et al. 2012, Ahanjan et al. 2011). In our previous study we screened the methanolic extract of some plants from Lamiaceae family against Aβ –induced toxicity in cell culture. Our results indicated that S. bachtiarica methanolic extract significantly ameliorated Aβ –induced toxicity in PC12 cells (Balali et al. 2012). This finding implies to neroprotective activity of this extract and possible effectiveness in AD. In the present study, the protective effect of S. bachtiarica methanolic extract on Aβ-induced spatial memory impairment in Morris water maze(MWM) and against Aβ –induced toxicity in primary neuron culture were investigated,in addition the effect of this extract on oxidative stress and cholinergic system were studied.
Materials and methods
Materials
Dulbecco’s modified Eagle’s Medium (DMEM), fetal bovine serum (FBS), penicillin–streptomycin (10,000 U/ml) and trypsin (%0.25) were taken from GIBCO, VectaStain Elite ABC Kit obtained from Vector Laboratories, Rabbit anti-Goat IgG biotin conjugate and Goat Anti-Choline Acetyltransferase (ChAT) antibody were purchased from Millipore. Aβ (25–35) was obtained from Alexis Biochemicals. All other chemicals were obtained from Sigma-Aldrich.
The plant materials (flowered aerial parts) were collected from wild growing areas of Iran (Chaharmahal-va-Bakhtiari Province) in June (2008) during full flowering stage. The plants were identified by Dr. Yousef Ajani. The voucher herbarium specimens were deposited at the Herbarium of Institute of Medicinal Plants, ACECR.
Preparation of plant extract
Extraction was performed by percolation method. Dried and milled aerial part of plant was extract with ethyl acetate and methanol respectively. At each step, after 24 h, the mixture was filtered and new solvent was added to the plant powder. The combined methanolic extracts were concentrated in rotary evaporator and dried in freeze drier. Methanolic extract was used in this study.
Preparation of stock solution for extract and Aβ
The Aβ (25–35) was dissolve in sterilized and deionized distilled water, and was stored in sterile microtubes at −20° C until use. Before injection aliquots were incubated for 4 days at 37° C for producing aggregated form of peptide.
Stock solution of methanolic extract was prepared by dissolving of 1 g in DMSO and further diluted by PBS before each experiment. The final concentrations of DMSO in behavioral test and cell culture were 1 % and 0.1 % respectively.
Antioxidant activity evaluation (FRAP assay)
The key solutions for performing FRAP assay were prepared as followed: a) Acetate buffer 300 mM pH 3.6; b) TPTZ (2, 4, 6-tripyridyl-striazine):10 mM in 40 mM HCl; c) FeCl3. 6H2O:20 mM. The FRAP solution were prepared by mixing a, b and c in the ratio of 10:1:1 just before testing. Standard was FeSO4.7H2O: 0.1–1.5 mM in methanol. FRAP solution (3.6 mL) was incubated at 37 °C for 5 min. Then the solution was mixed with 0.4 mL distilled water and certain concentration of the plant extract (80 μL) and incubated at 37 °C for 10 min. The absorbance of the reaction mixture was measured at 593 nm. For plotting of the calibration curve, five concentrations of FeSO4.7H2O (0.1, 0.4, 0.8, 1, 1.5 mM) were used and the absorbance values were measured as for sample solutions. Vitamin C was used as positive control (Hajimehdipoor et al. 2014a).
Determination of total phenolics content
The total phenolics contents of the extracts were determined spectrophotometrically according to the Folin-Ciocalteu method using chlorogenic acid (concentrations, 0.05, 0.1, 0.2, 0.3, 0.4 and 0.5 mg/mL) as the standard. The reaction mixture was prepared by mixing the methanolic solution (1 mL) of the extract, distilled water (9 mL), Folin-Ciocalteu’s reagent (1 mL) and sodium carbonate (10 mL, 7 %). Incubation at room temperature was down for 90 min and the absorbance was determined at 765 nm. The total phenolics content was expressed as chlorogenic acid equivalent in milligram per 100 g dried extract (Manayi et al. 2012).
In vitro AChE activity assay
The experiment was conducted according to modified microplate method. The sample was dissolved in methanol in concentration of 3 mg/mL. In the 96-well plates, 125 μL of 3 mM DTNB, 25 μL of 15 mM ATCH and 50 μL of phosphate buffer (pH 8), 25 μL of sample dissolved in methanol were added to the wells. The absorbance was read at 405 nm every 13 s for 65 s. 25 μL of 0.22 U/mL of AChE enzyme was then added and the absorbance was again measured every 13 s for 104 s using a microplate reader (BIOTEK) at 405 nm. Absorbance was plotted against time and enzyme activity was calculated from the slope of the line and expressed as a percentage compared to an assay using a methanol without any inhibitor. Any increase in absorbance due to the spontaneous hydrolysis of the substrate was corrected by subtracting the rate of the reaction before adding the enzyme, from the rate after adding the enzyme. Inhibition percentage was obtained by comparing the rates for the sample to the blank (methanol). Donepezil was used as the positive control (Hajimehdipoor et al. 2014b).
Assessment of protective effect of extract in cell culture
Primary cultures of cerebellar granule neurons (CGNs) were prepared from 7-day-old mouse pups, as previously described (Kramer and Minichiello 2010). Briefly, cerebella freshly dissected and briefly minced then were incubated with 0.025 % trypsin solution for 15 min at 37 °C. After inhibition of trypsin by serum, single cell suspension prepared by mechanically disruption of digested tissue. Then, they were cultured on PDL coated cell culture plates in DMEM containing 10 % FBS,4.5 g/l glucose, 25 mM KCl, insulin (100 mU/L), penicillin and streptomycine 1 % (v/v). Cells were maintained at 37 °C in a humidified atmosphere with 5%CO2. The growth of non-neuronal cells was inhibited by addition of 20 μm cytosine arabinofuranoside 48 h after seeding and the medium was not changed during the culture period. After 7 days of growth in vitro (DIV7), more than 95 % of cells in culture were neurons which characterized by MAP2 protein immunostaining. Then experiments done on DIV 7.The CGNs plated onto PDL coated 96 well plate (1 × 105cell/well) were pre-incubated with different concentration of extract (0.1-100 μg/ml for 24 h, then Aβ peptide was added to the medium at final concentration of 20 μM.cell viability was assessed after 48 h by MTT assay. Briefly, after incubation the medium was replaced with fresh medium containing MTT solution (final concentration 0.5 mg/ml) and incubated for 4 h in 37 °C. Then the medium was removed and 100 μl DMSO was added to each well and mixed properly until blue formazan product completely dissolved. Absorbance was measured at 570 nm in an automated plate reader (BIOTEK) against 630 nm as reference wavelength. The results were reported as percentage of control.
Behavioral tests
Animals
Male Albino Wistar rats (200–220 g) were obtained from faculty of pharmacy, Tehran University of Medical Sciences. Animals were housed in cages (five/cage), having access to food and water ad libitum. They were maintained on a 12 h-light-dark cycle at 20–22 °C temperature. All efforts were made to avoid animal suffering and reduce the number of animal used and all animal experiments were approved by ethical committee of the Tarbiat Modares University.
Treatments
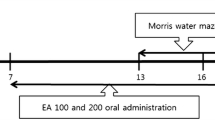
The animals were randomly divided into the following groups, with 8 rats in each group: the control group, the Aβ injected group, Aβ + extract groups and extract group. The Aβ was bilaterally injected into hippocampus through Stereotaxic surgery and the animals were trained in Morris Water Maze 14 days after injection. Three doses of extract (10, 50 and 100 mg/Kg) were daily injected intraperitoneally. Extract injection were began on the day of surgery and continued until the end of training (19 days) The control group received DMSO (1 %) similarly. In order to investigate just extract effects, highest dose of extract (100 mg/Kg) were administrated to non-surgical animals in a similar manner.
Stereotaxic surgery
Before surgery rats were anesthetized by interaperitoneal injection of Ketamin (100 mg/Kg) and Xylazine (10 mg/Kg). They were placed into stereotaxic device (Steolting, USA). Lidocaine and epinephrine solution (0.2 ml) was injected around the midline and a section was made along the midline. Bilateral injection was made by the 10-μl Hamilton syringe at the CA1 area of hippocampus,3.8 mm posterior, 2.2 mm lateral to bregma and 2.7 mm ventral to the surface of the skull, consistent with the atlas of Paxinos and Watson (1997). During 10 min, rats received volume of 2 μl/side of Aβ (10 μg/rat). After injection, the incisions were sutured.
Behavioral training and testing
Fourteen day after surgery all experimental groups were trained in Morris Water Maze, for four consecutive days, as described in our previous studies (Soodi et al. 2007). Black-painted water maze was a circular pool by 136 cm diameter and 60 cm height, filled by 22 ± 2 °C water to a height of 40 cm. It divided to four equal quadrants, hypothetically. A hidden platform by 10 cm diameter was located in center of North-west quadrant (target quadrant) in order to be 1 cm under the water surface. Four trials in one block were done in each training day, at about the same time of morning. In each trial the rat was randomly placed in one of quadrants facing the nearest wall. The animal was allowed to swim in maze during a period of 90 s to find the platform, using three visual ques. mounted on the walls of the room. If an animal could not find the platform within this period, it was manually guided to platform by researcher and was allowed to rest 30 s between two consecutive trials. Directions of the animals were recorded by a video camera located just above the center of maze. The camera was linked to a computer. Spatial acquisition was evaluated by Ethovision video tracking system (Noldus Information Technology, Wageningen, Natherlands) through measuring escape latency (time to find the platform) and swimming speed (Azami et al. 2010). The probe test was performed on the fifth day. In the probe test, the hidden platform was removed, and the animal was released from the opposite of target quadrant and allowed to swim freely for 90 s. Time spent in target quadrant was measured. The visible test was performed in order to assess motivation and vasomotor coordination. For visible test evaluation, the platform was elevated above water in the center of opposite quadrant and the same measures for acquisition evaluation were assessed in this test.
Immunohistochemical staining procedure
Brain tissue was obtained and processed according to standard protocols (Hosseini-Sharifabad et al. 2011). Briefly, animals were deeply anesthetized and then transcardially perfused with 100 mL of phosphate-buffered saline (PBS), followed by 300 mL of 4 % paraformaldehyde in 0.1 M phosphate buffer. The brains were then postfixed in the same fixative overnight followed by incubation in a PBS solution containing 30 % sucrose. After being embedded in optimal cutting temperature (OCT), the brains were sectioned at 40 μm intervals. These tissue sections were immunostained to determine the expression of ChAT protein. Free-floating tissue sections were made permeable in PBS containing 0.4 % TX-100 and 1 % normal rabbit serum for 45–60 min followed by incubation for 30 min in PBS containing 0.3 % hydrogen peroxide solution. After washing the tissue in PBS the sections were blocked for 60 min in PBS containing 0.3 % TX-100 and 3 % normal rabbit serum. The sections were then incubated for 48 h on a shaking platform at 4 °C with Anti-ChAT polyclonal antibody (diluted 1:100 in PBS containing 1 % normal rabbit serum and 0.3 % TX-100). The sections were then washed extensively 8 times for 5 min each with 1 % normal rabbit serum in PBS, followed by incubation with biotinylated anti-goat IgG secondary antibody diluted 1:200 in PBS and 1 % normal rabbit serum for 60 min at room temperature. The sections were incubated for 45 min at room temperature in avidin-biotin complex detection solution (ABC Elite Kit, Vector Labs) diluted 1:150 in PBS then the sections were incubated for 5–15 min with a PBS solution containing 0.02 % diaminobenzidine–4HCl (Sigma), 0.3 % nickel sulfate, and 0.03 % hydrogen peroxide until the desired staining intensity was achieved. Staining was stopped by washing the sections 3 times with PBS. The sections were then mounted on gelatin-coated glass slides and coverslipped. These tissue sections were analyzed with a BX51 Olympus microscope.
Preparation of brain tissue samples for biochemical analysis
After training, the animals were sacrificed by decapitation under ether anesthesia, and the hippocampuses were dissected out and homogenized in ice cold 0.1 M phosphate buffer saline (pH 7.4) with 1 % TritonX. The homogenates were then centrifuged at 3000 × g for 10 min at 4 °C, and the supernatant was used.
Measurement of brain AChE activity
The AChE activity was measured as described earlier by Ellman (Ellman et al. 1961). Briefly, 0.1 M phosphate buffer saline (pH 8.0), acetyl thiocholine iodide (ATCh, 75 mM) as a substrate, and 5, 5-dithiobis (2-nitrobenzioc) acid (DTNB,10 mM) in a ratio of 150:2:5 were mixed. Absorbance was then measured immediately after the enzyme source (10 μl) was added to the reaction mixture during 6 min at 412 nm using a microplate reader. The supernatant of brain homogenate was used as the enzyme source. Protein concentration in the supernatant was measured using the Bradford method (Bradford 1976).
Measurement of lipid peroxidation
The levels of malonyldialdehyde (MDA), the most abundant lipid peroxidation product, were determined by thiobarbituric acid reaction (TBAR) colorimetric assay. One volume of brain hemogenate was mixed with two volume of TBA reagent (containing 3.75 % TCA and 0.0925 % TBA) and the mixture incubate at 90 °C for 60 min. After cooling, centrifuged at 1000 g for 10 min and optical density of supernatant was measured in 540 nm in plate reader. MDA standard curve was established with using the stable MDA precursor, Malondialdehyde bis (dimethyl acetal).
Statistical analysis
Differences in water maze performance (escape latency and speed) were analyzed by Two-way repeated measures analysis of variance (ANOVA) followed by Benferroni post test. Other data were compared by one-way ANOVA, followed by Newman-Keulz multiple comparison post hoc test.The p values of 0.05 and less were considered as statistically significant.
Results
Antioxidant, total phenol and in vitro AChE activity
The results of FRAP assay, total phenol content and AChE activity of the methanolic extract of S. bachtiarica have been summarized in Table 1. The data were obtained according to calibration curves of FeSO4.7H2O (y = 1.0436×−0.0183, r2 = 0.9972) and chlorogenic acid (y = 2.0912×−0.0227, r2 = 0.9989). The results of in vitro AChE inhibitory assay showed that the methanol extract of this plant displayed no inhibitory activity at 300 μg/ml concentration. It is revealed that polarity and chemical structure of the constituents of the plant extract could make a lot of difference in binding to AChE.
Protective effect of S. bachtiarica extract on Aβ-induced cell toxicity
The effect of S. bachtiarica extract against Aβ-induced toxicity in cultured CGNs was evaluated by MTT assay. Treatment of cultured CGNs in DIV 8 with Aβ for 48 h significantly decreases cell viability as compared with control group. Pretreatment of cultured CGNs with different concentration of S. bachtiarica extract before Aβ incubation protect them dose dependently from Aβ induced toxicity (Fig. 1). The 0.1 μg/ml of extract could slightly increase cell viability however 100 μg/ml of extract completely reverse the Aβ induced toxicity and increase cell viability up to control level. Treatment with extract (0.1-100 μg/ml) alone did not affect the cell viability as compared to control (data not shown).
Effects of S. bachtiarica extract on learning and memory deficits induced by Aβ25–35
According to the results, intra hippocampal injection of 10 μg of aggregated Aβ (25–35) after 14 days could impair learning ability of animals that was shown by increased escape latency during training days. (Fig. 2a) also Aβ-injected group spent less time in target quadrant in the probe test which demonstrate memory impairment in this group in compared to control group (Fig. 2b). Intraperitoneally injection of S. bachtiarica extract after Aβ injection for 19 days, at the dosages of 50 mg/kg and 100 mg/kg significantly protected against Aβ25–35-induced learning and memory deficits, as indicated by the decreased escape latency during training days. Also, these groups spent more time in target quadrant in the probe test, compared with that in the Aβ-injected group (Fig. 2a, b). The learning measures during training day and probe test were not affected by administration of extract (100 mg/kg) in naïve rats alone (data not shown). The swimming speed was not affected in all treated groups of animals (Fig. 2c), as well as, Assessment of visio-motor coordination toward the visible platform showed no significant difference in escape latency between treated groups and the control group (Fig. 3).
The effect of S. bachtiarica methanolic extract on Aβ - induced memory impairment in Morris water maze task: Escape latency during training days (a), Time spent in target quadrant in probe test (b), speed during training days (c). * Represent significant differences versus Aβ-treated group (**p < 0.01, ***p < 0.001). + Represent significant differences versus control group (+++ p < 0.001)
Immunohistochemical evaluation of ChAT expression in hippocampus
The rat brains were collected immediately after behavioral experiments and immunostained with anti-ChAT antibody to evaluate and compare expression of ChAT protein between control, Aβ- injected and Aβ + extract (100 mg/kg)-injected groups. As observed in Fig. 4 expression of ChAT protein has been reduced in Aβ-injected group in compared with the control group, while treatment of animals with extract (100 mg/Kg) after Aβ injection could ameliorate Aβ- induced reduction in ChAT expression.
Effecst of S. bachtiarica extract on brain AChE activity and lipid peroxidation
The AChE activity and lipid peroxidation were measured in the hippocampus of animals in control, Aβ-injected, Aβ + extract (100 mg/kg)-injected and extract alone groups. Results indicated in Table 2. The injection of Aβ in the hippocampus significantly increased the AChE activity and MDA production in compared to control group. Treatment with S. bachtiarica extract after Aβ injection significantly attenuated the Aβ-induced AChE activity increase and lipid peroxidation. Also treatment with S. bachtiarica extract alone significantly inhibited the AChE activity in the hippocampus.
Discussion
In the present study the protective effect of S. bachtiarica on Aβ-induced toxicity in primary cell culture and in animal model of AD were investigated. In our previous study we had shown that S.bachtiarica methanolic extract protect PC12 cells against Aβ-induced cytotoxicity and then to confirm in vitro protective activity of this extract on Aβ-induced cytotoxicity, CGNs culture was used in the present study. CGNs culture has widely been used as a model for studying cellular and molecular mechanisms of neural cell apoptosis, survival, neurodegeneration and neuroprotection (Contestabile 2002). The Aβ-induced cytotoxicity in CGNs culture was used for studying of effectiveness of agents which are proposed for use in AD (Wei et al. 2000). Our result indicated that S. bachtiarica methanolic extract significantly protect cultured cerebellar granule neurons against Aβ-induced cytotoxicity and confirm the in vitro neuroprotective activity of this extract.
Then for further investigation of neuroprotective activity and usefulness of this extract in treatment of AD, in vivo animal model of AD was used. It has been shown that single injection of Aβ (25–35) in cerebroventricular or hippocampus induces learning and memory impairment in Morris Water Maze, radial arm maze and passive avoidance in rat (Sun and Alkon 2002, Yamaguchi and Kawashima 2001, Ghahremanitamadon et al. 2014) Also cholinergic deficit, oxidative stress and neuronal cell loss in the hippocampus have been reported as a result of Aβ (25–35) administration (Stepanichev et al. 2004). Then several studies have been used Aβ- injected animals as an in vivo model of AD. In our study administration of S. bachtiarica extract for 19 days could ameliorate Aβ-induced memory deficit in MWM. On the other hand increased level of lipid peroxidation and AChE activity were observed in Aβ-injected animals which were attenuated following S. bachtiarica extract administration. These results indicate that S. bachtiarica extract ameliorate Aβ-induced oxidative stress in the hippocampus. The oxidative stress plays important role in the pathogenesis of AD and Aβ-induced toxicity. It has been shown that cerebroventricular Aβ administration increases ROS production and decrease antioxidant enzyme level resulted in oxidative stress and leads to oxidative damage in neurons such as lipidperoxidation and protein oxidation (Parks et al. 2001). It is reported that natural antioxidant ameliorate Aβ-induced oxidative stress in both cell culture and brain tissue may be through free radical scavenging or metal ion chelating activity.
Our study indicated that S. bachtiarica methanolic extract shows antioxidant activity in FRAP assay and also contain polyphenolic compounds. It is reported that plant polyphenols have potent antioxidant activity and as a result, have a beneficial effect in various diseases such as cancers, cardiovascular diseases, diabetes, osteoporosis and neurodegenerative diseases (Pandey and Rizvi 2009). The neuroprotective activity of natural polyphenols was reported against various neuronal injury and neurological disorders. Several polyphenols such as resviratrol, quersetine and rosmarinic acid and also plants which contain polyphenols ameliorate Aβ-induced oxidative stress and neural cell death through scavenging of free radicals or up regulation of cellular antioxidant defenses. Beyond the antioxidant activity, polyphenols protect neural cells through activation of cell survival signaling pathways sach as MAP kinase and PI3K/Act (Vauzour 2012). Then it is suggested that polyphenols in S. bachtiarica methanolic extract is responsible for its antioxidant and neuroprotective activity also the role of the other compounds is not ignored and needs further investigation. The phytochemical investigation has indicated that the main polyphenols in the S. bachtiarica methanolic extract are luteolin, naringenin and rosmarinic acid (Khodabakhsh et al. 2010). The neuroprotective activity was reported for these compounds in several studies. Luteolin has the antioxidant, anti-inflammatory and radical scavenging activity and protect neural cells against H2O2-induces DNA damage (Cheng et al. 2010). Also luteolin reduce Aβ production an Aβ-induced apoptosis in SHSY5Y cell culture (Liu et al. 2011). In addition luteolin ameliorate Aβ-induced memory deficit in MWM performance and passive avoidance through activation of antioxidant defense and restoring the Aβ-induced oxidative stress (Tsai et al. 2010). Naringenin prevent neural cell death in cerebral ischemia with activation of anti inflammatory mechanism (Bai et al. 2014). Naringenin ameliorate iron-over load induced oxidative stress and neurotoxicity (Chtourou et al. 2014) also, it improve cognition function and reduce Aβ production in streptozocine- induced dementia model of rat (Yang et al. 2014). Rosmarinic acid is a natural polyphenol with neuroprotective activity which was mostly found in lamiaceae family. Protective effect of Rosmarinic acid against chromium VI induced neurotoxicity has been reported (Dashti et al. 2014). It protects PC12 cells against Aβ-induced toxicity and oxidative stress (Iuvone et al. 2006). Rosmarinic acid ameliorates Aβ-induced memory impairment by proxynitrit free radical scavenging activity (Alkam et al. 2007). It is a potent mito-protectant and improves mitochondrial dysfunction in neurodegenerative diseases (Camilleri et al. 2013). Also Rosmarinic acid and luteolin inhibit beta-secretase activity an enzyme involves in Aβ production (Choi et al. 2008).Then it is suggested that these polyphenols may be have a role in the neuroprotective activity of S. bachtiarica methanolic extract.
Degeneration of the cholinergic innervations in the septo-hippocampal pathway is the common pathological feature in AD (Schliebs and Arendt 2006, Kar et al. 2004). It is reported that impaired memory function are associated with reduction of ChAT activity and immunorectivity in the animal model of AD (Yamaguchi et al. 2002). In our study, Aβ-injected directly into the hippocampus resulted in learning and memory impairment which associated with reduced ChAT immunostaining in the hippocampus, indicate cholinergic neuron loss. Treatment with extract in concentration which improves memory impairment could increase ChAT immunostaining revealed increase cholinergic neuron survival. In addition AChE activity, which was increased in Aβ-injected group, decreased in extract treated group. Also AChE activity was decreased in extract alone treated animals. Then it is deduced that S. bachtiarica methanolic extract has inhibitory effect on brain AChE activity, but interestingly extract did not show any direct inhibitory effect on pure enzyme activity. Then it is suggested that extract may be reduce the AChE protein expression in the hippocampus. The AChE is a key enzyme in the cholinergic nervous system which hydrolysis acetylcholine and terminates its action. Current pharmacotherapy for AD is using AChE inhibitors which increase Achetylcholine level through inhibition of the cholinesterases and improve AD symptoms by facilitating cholinergic neurotransmission (Lleo et al. 2006). but alternative functions of AChE unrelated with the hydrolysis of acetylcholine are supposed in AD. It is reported that AChE activity was increase around the amyloid plaques and the expression of AChE protein is increased in AD brain. AChE protein interacts with Aβ peptide and increases its toxicity and deposition to form plaque. Then AChE is the target of several study for finding new therapeutic agent for treatment of AD (Campanari et al. 2014, Garcia-Ayllon et al. 2011).
In conclusion our results indicate that S. bachtiarica methanolic extract improve Aβ-induced memory impairment, oxidative stress and cholinergic dysfunction. These effect of extract at least as part could be attributed to the polyphenolic compounds. According to this finding it is suggested that S. bachtiarica methanolic extract can be a candidate for further investigation as therapeutic agent in AD.
References
Ahanjan M, Ghaffari J, Mohammadpour G, Nasrolahie M, Haghshenas MR, Mirabi AM (2011) Antibacterial activity of Satureja bakhtiarica bung essential oil against some human pathogenic bacteria. Afr J Microbiol Res 5:4764–4768
Alkam T, Nitta A, Mizoguchi H, Itoh A, Nabeshima T (2007) A natural scavenger of peroxynitrites, rosmarinic acid, protects against impairment of memory induced by abeta(25–35). Behav Brain Res 180:139–145
Azami K, Etminani M, Tabrizian K, Salar F, Belaran M, Hosseini A, Hosseini-Sharifabad A, Sharifzadeh M (2010) The quantitative evaluation of cholinergic markers in spatial memory improvement induced by nicotine–bucladesine combination in rats. Eur J Pharmacol 636:102–107
Bai X, Zhang X, Chen L, Zhang J, Zhang L, Zhao X, Zhao T, Zhao Y (2014) Protective effect of naringenin in experimental ischemic stroke: down-regulated NOD2, RIP2, NF-kappaB, MMP-9 and up-regulated claudin-5 expression. Neurochem Res 39:1405–1415
Balali P, Soodi M, Saeidnia S (2012) Protective effects of some medicinal plants from Lamiaceae family against beta-amyloid induced toxicity in PC12 cell. Tehran Univ Med J (TUMJ) 70:402–409
Birks J (2006) Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev 25
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Butterfield DA (1997) Beta-Amyloid-associated free radical oxidative stress and neurotoxicity: implications for Alzheimer’s disease. Chem Res Toxicol 10:495–506
Camilleri A, Zarb C, Caruana M, Ostermeier U, Ghio S, Hogen T, Schmidt F, Giese A, Vassallo N (2013) Mitochondrial membrane permeabilisation by amyloid aggregates and protection by polyphenols. Biochim Biophys Acta 11:28
Campanari ML, Garcia-Ayllon MS, Blazquez-Llorca L, Luk WK, Tsim K, Saez-Valero J (2014) Acetylcholinesterase protein level is preserved in the Alzheimer’s brain. J Mol Neurosci 53:446–453
Carter J, Lippa CF (2001) Beta-amyloid, neuronal death and Alzheimer’s disease. Curr Mol Med 1:733–737
Cavallucci V, D’amelio M, Cecconi F (2012) Abeta toxicity in Alzheimer’s disease. Mol Neurobiol 45:366–378
Chen Z, Zhong C (2014) Oxidative stress in Alzheimer’s disease. Neurosci Bull 30:271–281
Cheng HY, Hsieh MT, Tsai FS, Wu CR, Chiu CS, Lee MM, Xu HX, Zhao ZZ, Peng WH (2010) Neuroprotective effect of luteolin on amyloid beta protein (25–35)-induced toxicity in cultured rat cortical neurons. Phytother Res 24:S102–S108
Choi SH, Hur JM, Yang EJ, Jun M, Park HJ, Lee KB, Moon E, Song KS (2008) Beta-secretase (BACE1) inhibitors from Perilla frutescens var. acuta. Arch Pharm Res 31:183–187
Chtourou Y, Fetoui H, Gdoura R (2014) Protective effects of naringenin on iron-overload-induced cerebral cortex neurotoxicity correlated with oxidative stress. Biol Trace Elem Res 158:376–383
Contestabile A (2002) Cerebellar granule cells as a model to study mechanisms of neuronal apoptosis or survival in vivo and in vitro. Cerebellum 1:41–55
Dashti A, Soodi M, Amani N (2014) Cr (VI) induced oxidative stress and toxicity in cultured cerebellar granule neurons at different stages of development and protective effect of Rosmarinic acid. Environ Toxicol 12:22041
Ellman GL, Courtney KD, Andres Jr V, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Garcia-Ayllon MS, Small DH, Avila J, Saez-Valero J (2011) Revisiting the role of acetylcholinesterase in Alzheimer’s disease: cross-talk with P-tau and beta-amyloid. Front Mol Neurosci 4:1–9
Ghahremanitamadon F, Shahidi S, Zargooshnia S, Nikkhah A, Ranjbar A, Soleimani Asl S (2014) Protective effects of Borago officinalis extract on amyloid beta-peptide(25–35)-induced memory impairment in male rats: a behavioral study. Biomed Res Int 798535:11
Hajimehdipoor H, Ajani AGY, Saeidnia S (2014a) Comparative study of the total phenol content and antioxidant activity of some medicinal herbal extracts. Res J Pharmacognosy 1:21–25
Hajimehdipoor H, Mosaddegh M, Naghibi F, Haeri A, Hamzeloo-Moghadam M (2014b) Natural sesquiterpen lactones as acetylcholinesterase inhibitors. An Acad Bras Cienc 86(2):801–805
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353–356
Heo JH, Hyon L, Lee KM (2013) The possible role of antioxidant vitamin C in Alzheimer’s disease treatment and prevention. Am J Alzheimers Dis Other Demen 28:120–125
Hosseini-Sharifabad A, Mohammadi-Eraghi S, Tabrizian K, Soodi M, Khorshidahmad T, Naghdi N, Abdollahi M, Beyer C, Roghani A, Sharifzadeh M (2011) Effects of training in the Morris water maze on the spatial learning acquisition and VAChT expression in male rats. DARU, J Pharm Sci 19:166–172
Howes MJ, Houghton PJ (2012) Ethnobotanical treatment strategies against Alzheimer’s disease. Curr Alzheimer Res 9:67–85
Iuvone T, De Filippis D, Esposito G, D’amico A, Izzo AA (2006) The spice sage and its active ingredient rosmarinic acid protect PC12 cells from amyloid-beta peptide-induced neurotoxicity. J Pharmacol Exp Ther 317:1143–1149
Kar S, Slowikowski SP, Westaway D, Mount HT (2004) Interactions between beta-amyloid and central cholinergic neurons: implications for Alzheimer’s disease. J Psychiatry Neurosci 29:427–441
Khodabakhsh A, Gohari A, Davood A, Saeidnia S, Thesis, 1388–1389 (2010). Phytochemical Investigation of Satureja bachtiarica. Pharm.D, Islamic Azad university of Tehran
Kramer D, Minichiello L (2010) Cell culture of primary cerebellar granule cells. Methods Mol Biol 633:233–239
Liu R, Meng F, Zhang L, Liu A, Qin H, Lan X, Li L, Du G (2011) Luteolin isolated from the medicinal plant Elsholtzia rugulosa (Labiatae) prevents copper-mediated toxicity in beta-amyloid precursor protein Swedish mutation overexpressing SH-SY5Y cells. Molecules 16:2084–2096
Lleo A, Greenberg SM, Growdon JH (2006) Current pharmacotherapy for Alzheimer’s disease. Annu Rev Med 57:513–533
Luquin MR, Martinez-Vila E, Saldise L (1997) Mechanisms of cell death in Alzheimer’s disease. Rev Med Univ Navarra 41:34–45
Manayi A, Mirnezami T, Saeidnia S, Ajani Y (2012) Pharmacognostical evaluation, phytochemical analysis and antioxidant activity of the roots of Achillea tenuifolia LAM. Pharmacogn J 4:14–19
Mohammadpour G, Marzony ET, Farahmand M (2012) Evaluation of the anti-Leishmania major activity of Satureja bakhtiarica essential oil in vitro. Nat Prod Commun 7:133–136
Naghibi F, Mosaddegh M, Motamed SM, Ghorbani A (2005) Labiatae family in folk medicine in Iran: from ethnobotany to pharmacology. IJPR 2:63–79
Orhan IE, Senol FS, Sener B (2012) Recent approaches towards selected lamiaceae plants for their prospective use in neuroprotection. Stud Nat Prod Chem 38:397–415
Pandey KB, Rizvi SI (2009) Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med Cell Longev 2:270–278
Parks JK, Smith TS, Trimmer PA, Bennett Jr JP, Parker Jr WD (2001) Neurotoxic Abeta peptides increase oxidative stress in vivo through NMDA-receptor and nitric-oxide-synthase mechanisms, and inhibit complex IV activity and induce a mitochondrial permeability transition in vitro. J Neurochem 76:1050–1056
Paxinos G, Watson C (eds) (1997) The rat brain in stereotaxic coordinates. Academic Press, San Diego
Perry E, Howes MJ (2011) Medicinal plants and dementia therapy: herbal hopes for brain aging? CNS Neurosci Ther 17:683–698
Schliebs R, Arendt T (2006) The significance of the cholinergic system in the brain during aging and in Alzheimer’s disease. J Neural Transm 113:1625–1644
Sepand MR, Soodi M, Hajimehdipoor H, Sahraei E (2013) Comparison of neuroprotective effects of melissa officinalis total extract and its acidic and non-acidic fractions against A β-induced toxicity. Iran J Pharm Res 12:415–423
Singhal AK, Naithani V, Bangar OP (2012) Medicinal plants with a potential to treat Alzheimer and associated symptoms. Int J Nutr Pharmacol Neurol Dis 2:84–91
Soodi M, Sharifzadeh M, Naghdi N, Ostad N, Abdollahi M, Roghani A (2007) Systemic and developmental exposure to lead causes spatial memory deficits and a reduction in COX-2 immunoreactivity in the hippocampus of male rats. J Neurosci Res 85:3183–3192
Soodi M, Naghdi N, Hajimehdipoor H, Choopani S, Sahraei E (2014) Memory-improving activity of Melissa officinalis extract in naïve and scopolamine-treated rats. Res Pharm Sci 9:107–114
Stepanichev MY, Zdobnova IM, Zarubenko II, Moiseeva YV, Lazareva NA, Onufriev MV, Gulyaeva NV (2004) Amyloid-beta(25–35)-induced memory impairments correlate with cell loss in rat hippocampus. Physiol Behav 80:647–655
Sun MK, Alkon DL (2002) Impairment of hippocampal CA1 heterosynaptic transformation and spatial memory by beta-amyloid(25–35). J Neurophysiol 87:2441–2449
Tayeb HO, Yang HD, Price BH, Tarazi FI (2012) Pharmacotherapies for Alzheimer’s disease: beyond cholinesterase inhibitors. Pharmacol Ther 134:8–25
Tepe B (2015) Inhibitory effect of Satureja on certain types of organisms. Rec Nat Prod 9:1–18
Topcu G, Kusman T (2014) Lamiaceae family plants as a potential anticholinesterase source in the treatment ofAlzheimer’s disease. Bezmialem Science 1:1–25
Tsai FS, Cheng HY, Hsieh MT, Wu CR, Lin YC, Peng WH (2010) The ameliorating effects of luteolin on beta-amyloid-induced impairment of water maze performance and passive avoidance in rats. Am J Chin Med 38:279–291
Vauzour D (2012) Dietary polyphenols as modulators of brain functions: biological actions and molecular mechanisms underpinning their beneficial effects. Oxidative Med Cell Longev 914273:3
Wei H, Leeds PR, Qian Y, Wei W, Chen R, Chuang D (2000) Beta-amyloid peptide-induced death of PC 12 cells and cerebellar granule cell neurons is inhibited by long-term lithium treatment. Eur J Pharmacol 392:117–123
Yamaguchi Y, Kawashima S (2001) Effects of amyloid-beta-(25–35) on passive avoidance, radial-arm maze learning and choline acetyltransferase activity in the rat. Eur J Pharmacol 412:265–272
Yamaguchi Y, Matsuno T, Kawashima S (2002) Antiamnesic effects of azaindolizinone derivative ZSET845 on impaired learning and decreased ChAT activity induced by amyloid-beta 25–35 in the rat. Brain Res 945:259–265
Yang W, Ma J, Liu Z, Lu Y, Hu B, Yu H (2014) Effect of naringenin on brain insulin signaling and cognitive functions in ICV-STZ induced dementia model of rats. Neurol Sci 35:741–751
Acknowledgments
The Author wish to thank Tehran University of Medical Sciences and Health Services Grant No. 9108.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Soodi, M., Saeidnia, S., Sharifzadeh, M. et al. Satureja bachtiarica ameliorate beta-amyloid induced memory impairment, oxidative stress and cholinergic deficit in animal model of Alzheimer’s disease. Metab Brain Dis 31, 395–404 (2016). https://doi.org/10.1007/s11011-015-9773-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-015-9773-y