Abstract
Early life adversity has been associated with the development of various neuropsychiatric disorders in adulthood such as depression and anxiety. The aim of this study was to determine if stress during adulthood can exaggerate the depression-/anxiety-like behaviour observed in the widely accepted maternally separated (MS) Sprague–Dawley (SD) rat model of depression. A further aim was to determine whether the behavioural changes were accompanied by changes in hippocampal brain-derived neurotrophic factor (BDNF) and the protein profile of the prefrontal cortex (PFC). Depression-/anxiety-like behaviour was measured in the elevated plus maze, open field and forced swim test (FST) in the MS SD rats exposed to chronic restraint stress in adulthood. As expected, MS increased immobility of SD rats in the FST but restraint stress did not enhance this effect of MS on SD rats. A proteomic analysis of the PFC revealed a decrease in actin-related proteins in MS and non-separated rats subjected to restraint stress as well as a decrease in mitochondrial energy-related proteins in the stressed rat groups. Since MS during early development causes a disruption in the hypothalamic‐pituitary‐adrenal axis and long-term changes in the response to subsequent stress, it may have prevented restraint stress from exerting its effects on behaviour. Moreover, the decrease in proteins related to mitochondrial energy metabolism in MS rats with or without subsequent restraint stress may be related to stress per se and not depression-like behaviour, because rats subjected to restraint stress displayed similar decreases in energy-related proteins and spent less time immobile in the FST than control rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic exposure to stressful life events is considered a major risk factor for the development of various psychiatric disorders, including major depression (Kendler et al. 1998, 1999). Moreover, individuals with a history of childhood trauma are more vulnerable to developing depression than individuals with no childhood trauma (Harkness et al. 2006). In addition, the type of childhood trauma or recent stressful event has differential effects on depression symptomatology (van Veen et al. 2013). However, many other factors determine the outcome of childhood trauma including genetic influences (Rutter 2007, 2010).
Activation of the stress response via the hypothalamic‐pituitary‐adrenal (HPA) axis is required to maintain homeostasis and therefore minimize the impact of a threat (O’Connor et al. 2000). However, chronic stress may adversely impact brain function resulting in psychiatric disorders such as depression and anxiety (McEwen 2000, 2008). Limbic brain areas such as the hippocampus and prefrontal cortex (PFC) not only regulate the stress response system (Diorio et al. 1993; Herman and Cullinan 1997) but also play a role in depression therefore providing an interface between stress and neuropsychiatric disorders (McEwen 2000; Raison and Miller 2003). Various animal models, including chronic restraint stress, have been developed to study the effects of chronic stress on the neurobiology of depression. Restraint stress has proved to be a valid stress-inducing animal model of depression and anxiety (Lee et al. 2013; Chiba et al. 2012). Previous studies showed that restraint stress repeated for 5 to 14 days induced an increase in the duration of immobility in the forced swim test (FST) and decreased sucrose consumption in rats, indicative of depression-like behaviour (Lee et al. 2013; Cancela et al. 1991; Marais et al. 2008; Tabassum et al. 2010). These rats also displayed more anxiety-like behaviour as evidenced by decreased time spent in the open arms and increased time spent in the closed arms of the elevated plus maze (EPM) (Chiba et al. 2012; Tabassum et al. 2010). Some of these rats had also been subjected to maternal separation (MS) (Marais et al. 2008; Uchida et al. 2010).
Brain-derived nerve growth factor (BDNF) is densely expressed in the hippocampus (Yan et al. 1997) and suggested to be involved in the aetiopathology of depression (Castrén and Rantamäki 2010). It has been reported that chronic stress that results in depression-like behaviour in rodents, decreased BDNF levels in the hippocampus and PFC (Ray et al. 2011; Xu et al. 2004; Xu et al. 2006) although some studies have reported an increase (Larsen et al. 2010; Naert et al. 2011) or no effect on BDNF (Kuroda and McEwen 1998; Rosenbrock et al. 2005).
Depression is a multifaceted disorder with various causative factors involving multiple signalling pathways that regulate the release of many neurotransmitters and neurotrophins, as suggested by studies that investigated the neurobiology of animal models of depression (Gass and Riva 2007; Husum et al. 2008; Mathé et al. 2007; Popoli et al. 2001; Ryan et al. 2009; Wörtwein et al. 2006). In view of a number of studies that reported molecular changes in a gene-environment model of depression (Blaveri et al. 2010; Carboni et al. 2010; Mallei et al. 2011; Piubelli et al. 2011), it was considered necessary to further investigate differently regulated proteins also in an early environmental rat model subjected to stress in adulthood. This may provide further insight into the stress-induced pathology that gives rise to the depression-like behaviour in rats. Proteomic analysis with iTRAQ labeling allows separation and identification of many proteins and provides a sensitive method to identify the molecular changes associated with depression (Han et al. 2015; Wu et al. 2006).
The aim of the current study was therefore to measure the BDNF concentration in the ventral hippocampus and to follow with analysis of the proteomic profile of the PFC to identify differential protein expression related to depression-like behaviour in rats exposed to early-life MS and restraint stress in adulthood. This was the first proteomic study to assess protein regulation in the PFC of the early life MS model of depression that was also subjected to stress in adulthood.
Experimental procedures
Animals
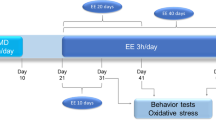
The study was approved by the University of Cape Town Faculty of Health Sciences Animal Ethics Committee (#010/036) and conducted in accordance with the guidelines of the South African National Standard: The care and use of animals for scientific purposes (2008). A total of 51 Sprague–Dawley (SD) rats that were bred in the Satellite Animal Facility at the University of Cape Town, were used for the study. Rats were housed in plexiglass cages with woodchip bedding in a temperature-controlled room (23 ± 1 °C) with food and water available ad libitum. The housing facility was maintained on a 12 h light/dark cycle (lights on from 06 h00 to 18 h00). This study consisted of 4 groups: control SD (Ctr, n = 13), maternally separated SD (MS, n = 12), restraint stressed SD (RS, n = 13) and maternally separated SD rats that were restraint stressed in adulthood (MS + RS, n = 13).
Maternal separation
The date of birth was designated postnatal day (P) 0. On P2, rats that were born in the Satellite Animal Facility were culled to 8 pups per litter to allow equal nourishment of litters. MS was carried out for 3 h per day between 09 h00 and 13 h00 from P2 to P14 (Daniels et al. 2004). Pups were transferred (together with some home cage bedding to avoid handling of the pups) to a clean cage and housed in a different room (31–33 °C, to prevent hypothermia) to prohibit communication between pups and dam by means of ultrasonic vocalizations. After 3 h of separation, pups were returned to the home cage with the dam. Care was taken to prevent handling of the pups. Non-maternally separated control rats were normally reared and left undisturbed during the MS procedure. Cages of MS and non-maternally separated rats were cleaned twice a week, half of the soiled bedding was replaced with fresh bedding to prevent disturbance of the pups. Pups were weaned at P21; males were separated from females and housed 2–4 in a cage.
Restraint stress
Some rats were chronically stressed during adulthood according to the restraint procedure employed by Marais et al. (2008). Rats were restrained in a Perspex holder for 4 h on 5 consecutive days between 09 h00 and 13 h00 at P61-P67. Following 5 days of restraint stress, rats were left undisturbed for 7 days to overcome the short-term effects of stress before depression-like behaviour was measured in the FST.
Behaviour
Behavioural tests were conducted in the light phase (06 h00-09 h00). Adult male rats were transferred to the behavioural room on the morning of behavioural testing and allowed to habituate for 1 h to the room conditions. The 51 rats were tested in batches of 4–7 rats on different days according to their date of birth.
Forced swim test
Seven days after restraint stress (P72–P78), rats were placed in individual transparent cylinders (40 × 19 cm) containing 30 cm of water (23–25 °C). The FST was performed in 4 cylinders so that four rats could be tested at the same time. Black cardboard sheets were placed between the cylinders to prevent visual contact between rats during the test and potentially alter their behaviours. Each rat was allowed to swim for 15 min (habituation). After 24 h, the rats were exposed to a second 5-min test swim session. The behaviour of rats was recorded from the front with a Sony Handycam, DCR-SX83E video camera for subsequent scoring. The FST behaviours included time spent immobile (making only movements necessary for the rat to keep its head above the water), swimming (horizontal movement of the rat around the tank) and climbing (vertical movements with the forepaws along the side of the cylinder) (Cryan et al. 2002) and were measured manually and separately by an experienced observer blind to the experimental conditions.
Elevated plus maze
Four days after the FST (P77–P83), anxiety-like behaviour was measured using the EPM in a different room than the FST, with lighting at 40 lux. The EPM consisted of two opposing open arms 50 × 10 cm and two opposing closed arms with 40 cm high walls and elevated 50 cm above the floor. The arms were connected by a central 10 × 10 cm square. The rat was placed in the central square facing one of the open arms and allowed to freely explore the open and closed arms of the EPM for 5 min. Between recordings, the arena was cleaned with 70 % ethanol to prevent the scent of the previous rat in the apparatus from influencing the behavior of other animals. An aerial view of the EPM was recorded with a Sony Handycam, DCR-SX83E video camera for subsequent scoring of time spent in the open arms, closed arms and central square with Noldus Ethovision XT version 7 software (Noldus, Wageningen, Netherlands). Center-point detection was used with Noldus Ethovision to track the movement of the rat in order to determine time spent in the different zones of the EPM.
Open field test
The open field test (OFT) was performed immediately after the EPM in the same room. The open field consisted of a 100 × 100 cm black arena with 50 cm high walls. The arena was cleaned with 70 % ethanol after each behavioural recording. The OFT was performed by placing the rat in one of the corners of the open field facing the open arena. The rat was allowed to explore the arena for 5 min. Behaviour was recorded with a video camera (Sony Handycam, DCR-SX83E, Japan) and subsequently analyzed using Ethovision software (Noldus, Wageningen, Netherlands). Locomotor activity (total distance travelled) was scored.
Two days after behavioural assessment (P79–P85), rats were decapitated, brains removed and the PFC and ventral hippocampus rapidly dissected according to the rat brain atlas of Paxinos and Watson (1986). Following dissection, the brain areas were immediately snap frozen and stored in liquid nitrogen until later biochemical analysis. A total of 10 rats/group were selected for determination of hippocampal BDNF levels according to their immobility scores in the FST. The rats with scores closest to the mean value of their respective group (Ctr, MS, RS and MS+RS) were selected. The PFC of 6 of these rats/group was used for proteomic analysis.
Biochemistry
BDNF determination with enzyme-linked immunosorbent assay (ELISA)
The ventral hippocampus tissue samples of SD behavioural rat groups (Ctr (n = 10), MS (n = 10), RS (n = 10) and MS+RS (n = 10)) were used to determine BDNF concentration. Brain samples were weighed and sonicated for 15 s in 300 μl lysis buffer (137 mM NaCl, 20 mM Tris–HCl (pH 8), 1 % Tergitol-type nonyl phenoxypolyethoxylethanol (NP40), 10 % glycerol, 1 mM phenylmethanesulfonyl fluoride (PMSF), 10 μg/ml aprotinin, 1 μg/ml leupeptin and 0.5 mM sodium vanadate). Following sonication, samples were mixed on a vortex mixer and centrifuged at 12, 000×g at 4 °C for 30 min. A volume of 40 μl of each sample supernatant was used for ELISA. The BDNF concentration was determined according to the manufacturer’s instructions (Promega Corporation, Madison, WI, USA). Determinations were done in duplicate. Results are expressed as pg/mg wet weight (ww).
Proteomics
Samples of the PFC obtained from 3 SD rats per experimental group were pooled (3 SD rats/digest), sonicated in 1 M triethylammonium bicarbonate (TEAB) buffer and centrifuged at 17, 200×g for 30 min at 4 °C. Supernatants were collected and protein concentration, iTRAQ labeling and liquid chromatography and mass spectrometry (LC-MS) were carried out by the Centre for Proteomic and Genomic Research (CPGR) at the University of Cape Town.
Protein concentration of the supernatant was determined with a nanodrop at 280 nm. The samples were adjusted to 10 μg/μl with 50 mM TEAB and incubated for 1 h at 60 °C with 100 mM Tris (2-carboxyethyl) phosphine (TCEP) reducing agent. Methyl methanethiosulfonate (MMTS) was added to the samples to block reduced cysteines followed by incubation for 15 min at room temperature. Following incubation, samples were adjusted to 45 μl with 50 mM TEAB and 1 μg/μl trypsin added. The reaction was allowed to proceed at 37 °C for 18 h where after the samples were suspended in 0.1 % trifluoroacetic acid (TFA). The digested samples were reduced to a volume of 10 μl and made up to a final volume of 10 μl with 1 M TEAB to adjust the pH (>7.5). The iTRAQ labeling was carried out using an 8-plex labeling kit for Ctr, MS and RS rat groups (2 pooled PFC digests for each group) and a 4-plex labeling kit for the Ctr and MS + RS rat groups (2 pooled PFC digests for each group). Labeling was performed at room temperature for 2 h according to the manufacturer’s instructions (Applied Biosystems).
Label confirmation was performed by applying the sample mixture to a C-18 column conditioned with 20 μl of 100 % acetonitrile (ACN) and equilibrated with 20 μl of 0.1 % TFA. Volumes of 1 μl of each sample were pooled and desalted using a C18 ZipTip, washed with 20 μl of 0.1 % TFA and eluted with 10 μl of 50 % ACN and 0.1 % TFA. The desalted sample was mixed with α-cyano-4-hydroxycinnamic acid (CHCA, Sigma Aldrich) solution and spotted on a MALDI target plate. Labeling was confirmed using tandem mass spectroscopy (MS/MS). After label confirmation, 100 μl of Milli Q water was added to each sample and incubated at room temperature for 30 min. The samples were pooled and the volume reduced to 100 μl. The pooled sample was stored for further LC-MS analysis.
The LC was performed using a Dionex Ultimate 3000 nano-HPLC system. The HPLC fractionated peptides were dissolved in sample loading buffer (95 % water, 5 % Acetonitrile, 0.05 % TFA) and loaded on a C18 trap column (100 μm × 20 mm × 5 μm). Chromatographic separation was performed with an Acclaim Pepmap (Thermo Fisher Scientific, USA) C18 column (75 μm × 250 mm × 3 μm). The mobile phases consisted of solvent A (0.1 % formic acid in water) and solvent B (80 % ACN, 10 % water, and 0.1 % formic acid). The gradient was delivered at 250 nl/min and consisted of a linear gradient of mobile phase B initiating with solvent B: 6–30 % over 148 min.
Mass spectroscopy was performed using an electron spray ion source coupled to a Q-Exactive quadrupole-Orbitrap mass spectrometer (Thermo Fisher Scientific, USA). The MS was operated in positive electron spray ionized mode with a capillary temperature of 250 °C. The applied electrospray voltage was set at 1.95 kV. Data were collected in the full scan mode and data-dependent MS/MS modes with a maximum ion injection time of 50 ms. In full scan mode, ions were collected in the mass range of 350–2000 m/z and acquired at a resolution of 70,000 at m/z 200.
Data analysis was performed using Scaffold software (Proteome software Inc., Portland, OR) using the Rattus sequence database (Source: UniprotKB-www.uniprot.org, filtered by “Organism−‘Rattus’”, dated 22/03/2014) with the MASCOT (Matrix Science, London, U.K.; version 2.1) search algorithm. All identified peptides had an ion score above the Mascot peptide identity threshold (a high confidence score of 99 % and a low false discovery rate (FDR) of 1 %), and a target protein was considered identified if at least two such unique peptide matches were apparent for the protein. Acquired intensities in the experiment were globally normalized across all acquisition runs. Individual quantitative samples were normalized within each acquisition run. Intensities for each peptide were normalized within the assigned protein. The reference channels were normalized to produce a 1:1 fold change. All normalization calculations were performed using medians to multiplicatively normalize data. Values for duplicate rat groups were averaged and MS, RS and MS+RS rat groups expressed as fold difference relative to the Ctr group. Rat groups were considered to be increased or decreased if they were ± 0.2 fold different from Ctr rats (Serang et al. 2013).
Statistical analysis
Behavioural and BDNF data were normally distributed (Shapiro-Wilk test). The data were analyzed by means of analysis of variance (ANOVA), followed by Tukey’s post-hoc test with correction for multiple comparisons. Behavioural and BDNF data are presented as mean±SEM.
Results
Behaviour
Forced swim test
One-way ANOVA revealed a significant effect of stress on immobility (F(3, 46) = 19.56, p < 0.001) and swimming (F(3, 46) = 32.03, p < 0.001) in the FST. Post-hoc comparisons revealed that both MS (p < 0.01) and MS + RS rats (p < 0.05) spent significantly more time immobile than Ctr rats and the RS rats spent significantly less time immobile than Ctr (p < 0.05), MS (p < 0.001) and MS + RS rats (p < 0.001; Fig. 1a). Both MS (p < 0.001) and MS + RS rats (p < 0.05) spent significantly less time swimming than Ctr rats and RS rats spent significantly more time swimming than Ctr, MS and MS + RS rats (p < 0.001; Fig. 1b).
Immobility, swimming and climbing behaviours of control SD, MS SD, restraint stressed SD and restraint stressed MS SD rats in the FST. MS and MS + RS increased immobility (a) and decreased swimming (b) behaviours. Restraint stress decreased immobility (a) and increased swimming (b) behaviours in the FST. Restraint stress had no additional effect on MS rats. * MS and MS + RS rats significantly different from Ctr rats, p < 0.05; ** RS rats significantly different from Ctr, MS and MS + RS rats, p < 0.001; Tukey’s post-hoc test (n = 12-13/group). Data presented as mean ± SEM
Elevated plus maze
One-way ANOVA showed no effect of stress on the amount of time spent in the open and closed arms of the EPM (Supplementary Fig. 3).
Open field test
One-way ANOVA revealed a significant effect of stress on distance travelled (F(3, 47) = 8.03, p < 0.001) in the OFT. Post-hoc comparisons revealed that both MS (p < 0.001) and MS + RS rats (p < 0.05) travelled a significantly longer distance than Ctr rats (Fig. 2).
Distance travelled by control SD, MS SD, restraint stressed SD and restraint stressed MS SD rats in the OFT. MS and MS + RS increased activity in the OFT and restraint stress had no effect on distance travelled. *MS and MS + RS rats significantly different from Ctr rats, p < 0.05; Tukey’s post-hoc test (n = 12-13/group). Data presented as mean ± SEM
Biochemistry
BDNF concentration in the ventral hippocampus
One-way ANOVA showed no effect of stress on BDNF concentration in the ventral hippocampus (Supplementary Fig. 4).
Proteomics
Using the Rattus sequence database, 814 proteins were identified for the 8-plex (Supplementary table 3) and 869 proteins (Supplementary table 4) identified for the 4-plex with > 95 % confidence. Proteomic analysis identified qualitative differences in protein profiles of rats representing different experimental groups. In the 8-plex, 32 proteins were found to be increased or decreased in the PFC of MS and RS relative to Ctr rats (Table 1). In the 4-plex, 29 proteins were found to be increased or decreased in the PFC of MS+RS relative to Ctr rats (Table 2).
The 8-plex (Table 1) quantification revealed that MS and RS rats had decreased actin-associated structural proteins (Actin related protein 2/3 complex, subunit 3, Alpha II spectrin or ARP10 actin-related protein 10 homolog) compared to Ctr rats. The 8-plex and 4-plex (Tables 1 and 2) quantification revealed that MS, RS and MS+RS rats had decreased mitochondrial energy-related proteins (Trifunctional enzyme subunit alpha, NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 10, ATP synthase subunit O, Cytochrome b-c1 complex subunit 1, ATP synthase gamma chain, Mitochondrial 2-oxoglutarate/malate carrier protein) compared to Ctr rats. Neurotransmission/signalling proteins in MS, RS and MS+RS rats were increased or decreased compared to Ctr rats. However, proteins involved in glutamate and opioid signalling (Opioid-binding protein/cell adhesion molecule, Glutamate receptor 2) were decreased in the MS+RS compared to Ctr rats. Furthermore, the 4-plex revealed a decrease in proteins involved in protein synthesis (Protein Tardbp, 40S ribosomal protein S12, RCG45615, isoform CRA_a and Tyrosine–tRNA ligase) and increase in proteins involved in protein degradation (F-box only protein 2 and COP9 (Constitutive photomorphogenic) homolog, subunit 7a) in MS+RS compared to Ctr rats. In further support of the disruption in protein synthesis/degradation in MS+RS rats, carboxypeptidase E appeared to be decreased.
Discussion
The main findings of this study support MS-induced depression-like behaviour but do not find that restraint stress exacerbated the MS effect. Biochemical studies of the ventral hippocampus revealed no effect of MS and restraint stress on BDNF levels. Proteomic analysis of the PFC revealed a qualitative decrease in actin-associated proteins in MS SD rats (Actin related protein 2/3 complex, subunit 3 and Alpha II spectrin) and non-separated SD rats subjected to restraint stress (ARP10 actin-related protein 10 homolog) as well as a decrease in mitochondrial energy-related proteins in MS SD rats (Trifunctional enzyme subunit alpha, NADH dehydrogenase 1 alpha subcomplex and ADP/ATP translocase 1), non-separated SD rats subjected to restraint stress (Trifunctional enzyme subunit alpha and ATP synthase subunit O) and MS SD rats subjected to restraint stress (cytochrome subunits, ATP synthase and malate carrier protein). Furthermore, a qualitative decrease in proteins involved in protein synthesis (Protein Tardbp, RCG, 40s ribosomal protein S12, RCG45615 protein and tyrosine-tRNA ligase) and an increase in proteins involved in protein degradation (F-box protein and COP9) were found in MS SD rats subjected to restraint stress.
Rats subjected to MS or MS and restraint stress displayed depression-like behaviour as evidenced by increased immobility and decreased swimming behaviour in the FST. Restraint stress had no additive effect on MS rats. The depression-like behaviour of MS SD rats in the FST is in agreement with previous studies using the same MS protocol (Desbonnet et al. 2010; Dimatelis et al. 2012b; Lambás-Señas et al. 2009; Yoo et al. 2013). However, the current study showed that restraint stress decreased immobility and increased swimming behaviour in the FST. This is in contrast to studies that showed a stress-induced increase in immobility (Lee et al. 2013; Chiba et al. 2012; Ulloa et al. 2010). This paradoxical effect of restraint stress on immobility in the FST has been reported previously (Platt and Stone 1982; Swiergiel et al. 2007), an effect further supported by data demonstrating that chronically administered corticosterone decreased immobility in the FST (Brotto et al. 2001). There is no obvious reason for the decreased immobility following restraint stress but it has been suggested to be the result of adaptation to chronic stress and changes in the β-adrenergic receptor (Platt and Stone 1982; Swiergiel et al. 2007). The inconsistent effect of restraint stress on immobility between studies could possibly be ascribed to the different restraint stress protocols since different durations and intensity of restraint stress have different effects on immobility in the FST (Cancela et al. 1991; Suvrathan et al. 2010). For example, the study by Lee et al. (2013), Chiba et al. (2012) and Ulloa et al. (2010) found that rats displayed increased immobility in the FST after being restrained for 2 to 6 h for a much longer period (13 to 28 days) than in the current study (5 days). In contrast, 2 h of restraint stress for a shorter period (1 or 3 days) had no effect on immobility in the FST (Cancela et al. 1991). Also, different time points of behavioural testing may reveal different effects. The study by Marais et al. (2008) used a similar restraint stress protocol and measured depression-like behaviour in MS SD rats (P66) 24 h after the restraint stress procedure. Therefore, the fact that rats were left undisturbed for a period of 7 days and not tested immediately after the restraint stress procedure may have contributed to the lack of effect of restraint stress in the current study. We were interested in studying the long-term rather than the transient effects of stress, which is in contrast to studies that observed depression-like behaviour within 24 h after a similar restraint stress procedure (2–4 h for 5–7 consecutive days) (Cancela et al. 1991; Marais et al. 2008; Tabassum et al. 2010; Tamburella et al. 2010; Wang et al. 2015).
Maternal behaviour upon reunion with MS pups has been proposed to play a role in the behavioural and neuroendocrine responses to stress in adulthood (Meaney 2001; Zimmerberg and Sageser 2011). More specifically, the offspring of mothers that exhibited more licking and grooming of pups showed reduced plasma adrenocorticotropic hormone and corticosterone responses to acute stress and increased fearfulness in novel environments (Caldji et al. 1998; Francis and Meaney 1999; Liu et al. 1997). Therefore, differences between studies may be explained by differences in maternal care of the dam (licking and grooming). This may be important, since in the present study, litters were culled to 8 pups whereas several articles did not specify the litter size which may impact the amount of licking and grooming of the dam towards the pups and ultimately alter their depression-/anxiety-like behaviour in adulthood.
To exclude the possibility that the depression-like behaviour observed in the MS rats may be due to a change in motor activity, the distance travelled by the rat was determined in the OFT. Rats subjected to MS or MS and restraint stress displayed increased locomotor activity in the OFT compared to control rats. This is in line with results obtained by Lambás-Señas et al. (2009) who showed that MS SD rats displayed increased motor activity in a novel environment, using a similar MS protocol. Therefore, the immobility and swimming behaviour of the MS rats, with or without restraint stress, in the FST could not be attributed to a decrease in motor activity.
MS and restraint stress did not induce anxiety-like behaviour as measured by time spent in the closed arms of the EPM. This is consistent with various studies that found no anxiety-like behaviour in adult rats subjected to MS in early life (Dimatelis et al. 2012b; Rüedi-Bettschen et al. 2005; van Heerden et al. 2010; Yoo et al. 2013). However, when MS rats were restrained for 6 h for a period of 21 days, they displayed increased anxiety-like behaviour as evidenced by decreased time spent in the open arms of the EPM (Eiland and McEwen 2012). Anxiety-like behaviour may therefore be observed following more severe restraint stress in combination with the stress of MS.
BDNF is highly expressed in the hippocampus (Yan et al. 1997) and the results of its action include neuronal sprouting, synaptic reorganization and neurogenesis (Lindvall et al. 1994; McAllister et al. 1999). In the current study, MS and restraint stress had no effect on BDNF concentration in the ventral hippocampus. The results are in contrast to various studies that showed decreased BDNF expression in the hippocampus of MS or restraint stressed rats (Dimatelis et al. 2014; Xu et al. 2006). However, the neuronal mechanisms involved seem complex, since other studies found increased (Greisen et al. 2005) or even no effect of MS or restraint stress on BDNF expression in the hippocampus (Kuroda and McEwen 1998; Reagan et al. 2007; Roceri et al. 2004; Rosenbrock et al. 2005). Evidence suggests that the depression phenotype may exist without changes in BDNF expression and antidepressant drugs may exert an antidepressant effect without increasing BDNF expression (Hansson et al. 2011). It is also possible that the negative result in the current study is due to a different time point compared to other studies. The studies by Dimatelis et al. (2014) and Xu et al. (2006) showed decreased BDNF concentration in the hippocampus of rats subjected to MS or restraint stress 24 h after the FST, compared to 6 days in the current study. A transient decrease in hippocampal BDNF mRNA levels was observed after 60 min of exposure to footshock stress which returned to normal within 48 h (Rasmusson et al. 2002).
To further evaluate the effect of restraint stress and MS on rats, a proteomic analysis of the protein profile of the PFC was performed. Proteins that differed qualitatively in the PFC of rats subjected to MS, restraint stress or MS and restraint stress, fell into five broadly defined functional categories: (a) cytoskeletal, (b) energy metabolism, (c) neurotransmission, (d) protein synthesis and (e) protein degradation.
Cytoskeletal proteins such as microfilaments, microtubules and intermediate filaments play a central role in neuronal function and the creation and maintenance of cell shape (Pigino et al. 2006). In the current study, actin microfilament-associated proteins such as actin related protein 2/3, alpha II spectrin and actin-related protein 10 appeared to be reduced in rats subjected to MS or restraint stress. On the other hand, actin binding proteins, such as the myosin regulatory light chain and tropomyosin alpha isoform appeared to be increased in rats subjected to MS or MS and restraint stress. It has previously been shown that MS reduced actin microfilament-associated proteins in the nucleus accumbens and ventral hippocampus suggesting altered neurotransmission and synaptic function (Daniels et al. 2012; Dimatelis et al. 2012a). Furthermore, it has also been shown that a combination of various stressors (restraint stress, forced swim stress and ether vapor) decreased structural proteins in the ventral hippocampus (Uys et al. 2008). In line with the current study, Daniels et al. (2012) found that MS increased the expression of tropomyosin-5 in the ventral hippocampus. Tropomyosin and myosin regulatory light chain not only bind to actin, but also increase axoplasmic transport, synaptic rearrangement, vesicle transport and neurotransmitter release (Lees-Miller et al. 1990; Mochida 1995). It therefore appeared that the stress of MS increased the expression of myosin regulatory light chain and tropomyosin alpha isoform in the rats subjected to MS or MS and restraint stress, to compensate for the loss of structural proteins. Thus, the stress of MS and restraint mostly decreased actin-associated proteins, which function to maintain the cytoskeletal network and may have altered neuronal function in the PFC.
The results of the current study suggest a decrease in proteins involved in mitochondrial energy metabolism in the PFC of rats subjected to MS, restraint stress or MS and restraint stress. These proteins included trifunctional enzyme subunit alpha, ATP synthase subunit O, ATP synthase gamma chain, cytochrome b-c1 complex subunit 1 and mitochondrial 2-oxoglutarate/malate carrier protein. Most of these proteins are involved in oxidative phosphorylation of ADP to produce ATP (ATP synthase subunit O, ATP synthase gamma chain, cytochrome b-c1 complex subunit 1 and mitochondrial 2-oxoglutarate/malate carrier protein). Dysfunction of the mitochondrial electron transport chain has been suggested to be an important factor in the pathogenesis of neuropsychiatric disorders such as depression (Tobe 2013) as well as being associated with cellular degeneration (Calabrese et al. 2001). The findings of the current study are in line with previous studies that showed decreased energy-related proteins in the hippocampus in response to chronic unpredictable stress (21 days), learned helplessness and MS stress (Dimatelis et al. 2012a; Mallei et al. 2011; Mu et al. 2007). Furthermore, chronic mild stress (40 days) or restraint stress (7–21 days) in Wistar rats has been previously shown to decrease energy-related proteins in the PFC associated with oxidative phosphorylation (Madrigal et al. 2001; Réus et al. 2014). Dysfunction of mitochondrial energy metabolism may compromise neuronal function as a possible consequence of mitochondrial damage and degeneration in rats subjected to MS, restraint stress or MS and restraint stress. The energy-related proteins that appeared to be reduced in MS rats with or without restraint stress also appeared to be reduced in non-maternally separated rats subjected to restraint stress, suggesting that this may be a common effect of stress on the PFC, unrelated to depression-like behaviour.
A number of proteins involved in neurotransmission/signalling were differentially expressed in MS, non-maternally separated rats and MS rats subjected to restraint stress. The AMPA glutamate receptor 2 (GluR2) appeared to be decreased in the rats subjected to MS and restraint stress. The involvement of AMPA receptors in animal models of depression and mechanism of antidepressant action has been well documented (Chourbaji et al. 2008; Li et al. 2001; Martinez-Turrillas et al. 2002; Martínez-Turrillas et al. 2007). AMPA receptor knockout mice displayed increased learned helplessness, decreased serotonin and noradrenaline levels and increased NMDA receptor expression in the hippocampus (Chourbaji et al. 2008). This was further supported by evidence showing decreased GluR2 mRNA expression in the hippocampus of Wistar rats previously exposed to MS (3 h daily for 21 days) (Pickering et al. 2006). Furthermore, AMPA receptor channel impermeability to calcium is dependent on the GluR2 subunit and cells that contain AMPA receptors lacking the GluR2 subunit show high calcium permeability and as a result, vulnerability to excitotoxicity (Liu and Zukin 2007). It is therefore evident that modifications of AMPA receptors alter synaptic function through various signalling proteins that underlie the neuropathology of psychiatric disorders such as depression.
Proteins involved in protein synthesis appeared to be decreased (Protein Tardbp, 40S ribosomal protein S12, RCG45615/ 60S ribosomal protein L12 and Tyrosine tRNA ligase) and proteins involved in protein degradation (F-box only protein 2 and COP9 homolog, subunit 7a) appeared to be increased in the PFC of rats subjected to both MS and restraint stress. Inhibition of protein synthesis is a common response of cells to severe forms of stress such as thermal, physical and metabolic stress and viral infection (Clemens et al. 2000; Clemens et al. 1998; Gale et al. 2000; Sheikh and Fornace 1999). Restraint stress for 1 h daily for 4 weeks produced increased protein degradation in the brains of SD rats (Hong et al. 2014). The apparent decreased protein synthesis and increased protein degradation in the current study may possibly result in atrophy and reduced function of PFC neurons in rats subjected to MS and restraint stress. In conclusion, rats exposed to early life MS with or without additional restraint stress, displayed depression-like behaviour in the FST. Since MS on its own produced depression-like behaviour, it may be suggested that restraint stress, that has similar effects on the HPA axis and acts on the same signalling pathways, was prevented from exerting its depression-like effects in MS rats. Furthermore, reduced mitochondrial energy-related, structural actin-associated proteins as well as disruption in proteins associated with protein synthesis and degradation, may be the result of the stress of MS and restraint stress on the PFC.
References
Blaveri E, Kelly F, Mallei A, Harris K, Taylor A, Reid J, Razzoli M, Carboni L, Piubelli C, Musazzi L, Racagni G, Mathé A, Popoli M, Domenici E, Bates S (2010) Expression profiling of a genetic animal model of depression reveals novel molecular pathways underlying depressive-like behaviours. PLoS ONE 5:1–10
Brotto LA, Gorzalka BB, Barr AM (2001) Paradoxical effects of chronic corticosterone on forced swim behaviours in aged male and female rats. Eur J Pharmacol 424:203–209
Calabrese V, Scapagnini G, Giuffrida Stella AM, Bates TE, Clark JB (2001) Mitochondrial involvement in brain function and dysfunction: relevance to aging, neurodegenerative disorders and longevity. Neurochem Res 26:739–764
Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ (1998) Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci USA 95:5335–5340
Cancela LM, Rossi S, Molina VA (1991) Effect of different restraint schedules on the immobility in the forced swim test: modulation by an opiate mechanism. Brain Res Bull 26:671–675
Carboni L, Becchi S, Piubelli C, Mallei A, Giambelli R, Razzoli M, Mathé AA, Popoli M, Domenici E (2010) Early-life stress and antidepressants modulate peripheral biomarkers in a gene–environment rat model of depression. Prog Neuro-Psychopharmacol Biol Psychiatry 34:1037–1048
Castrén E, Rantamäki T (2010) Role of brain-derived neurotrophic factor in the aetiology of depression. CNS Drugs 24:1–7
Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H (2012) Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuro-Psychopharmacol Biol Psychiatry 39:112–119
Chourbaji S, Vogt MA, Fumagalli F, Sohr R, Frasca A, Brandwein C, Hörtnagl H, Riva MA, Sprengel R, Gass P (2008) AMPA receptor subunit 1 (GluR-A) knockout mice model the glutamate hypothesis of depression. FASEB J Off Publ Fed Am Soc Exp Biol 22:3129–3134
Clemens MJ, Bushell M, Morley SJ (1998) Degradation of eukaryotic polypeptide chain initiation factor (eIF) 4G in response to induction of apoptosis in human lymphoma cell lines. Oncogene 17:2921–2931
Clemens MJ, Bushell M, Jeffrey IW, Pain VM, Morley SJ (2000) Translation initiation factor modifications and the regulation of protein synthesis in apoptotic cells. Cell Death Differ 7:603–615
Cryan JF, Markou A, Lucki I (2002) Assessing antidepressant activity in rodents: recent developments and future needs. TRENDS Pharmacol Sci 23:238–245
Daniels WMU, Pietersen CY, Carstens ME, Stein DJ (2004) Maternal separation in rats leads to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metab Brain Dis 19:3–14
Daniels WMU, Marais L, Stein DJ, Russell VA (2012) Exercise normalizes altered expression of proteins in the ventral hippocampus of rats subjected to maternal separation. Exp Physiol 97:239–247
Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG (2010) Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 170:1179–1188
Dimatelis JJ, Russell VA, Stein DJ, Daniels WM (2012a) Effects of maternal separation and methamphetamine exposure on protein expression in the nucleus accumbens shell and core. Metab Brain Dis 27:363–375
Dimatelis JJ, Stein DJ, Russell VA (2012b) Behavioral changes after maternal separation are reversed by chronic constant light treatment. Brain Res 1480:61–71
Dimatelis JJ, Russell VA, Stein DJ, Daniels WM (2014) Methamphetamine reversed maternal separation-induced decrease in nerve growth factor in the ventral hippocampus. Metab Brain Dis 29:433–439
Diorio D, Viau V, Meaney MJ (1993) The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci 13:3839–3847
Eiland L, McEwen BS (2012) Early life stress followed by subsequent adult chronic stress potentiates anxiety and blunts hippocampal structural remodeling. Hippocampus 22:82–91
Francis DD, Meaney MJ (1999) Maternal care and the development of stress responses. Curr Opin Neurobiol 9:128–134
Gale M Jr, Tan SL, Katze MG (2000) Translational control of viral gene expression in eukaryotes. Microbiol Mol Biol Rev: MMBR 64:239–280
Gass P, Riva MA (2007) CREB, neurogenesis and depression. Bioessays: News Rev Mol Cell Dev Biol 29:957–961
Greisen MH, Altar CA, Bolwig TG, Whitehead R, Wörtwein G (2005) Increased adult hippocampal brain-derived neurotrophic factor and normal levels of neurogenesis in maternal separation rats. J Neurosci Res 79:772–778
Han X, Shao W, Liu Z, Fan S, Yu J, Chen J, Qiao R, Zhou J, Xie P (2015) iTRAQ-based quantitative analysis of hippocampal postsynaptic density-associated proteins in a rat chronic mild stress model of depression. Neuroscience 298:220–292
Hansson AC, Rimondini R, Heilig M, Mathé AA, Sommer WH (2011) Dissociation of antidepressant-like activity of escitalopram and nortriptyline on behaviour and hippocampal BDNF expression in female rats. J Psychopharmacol 25:1378–1387
Harkness KL, Bruce AE, Lumley MN (2006) The role of childhood abuse and neglect in the sensitization to stressful life events in adolescent depression. J Abnorm Psychol 115:730–741
Herman JP, Cullinan WE (1997) Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci 20:78–84
Hong IS, Lee HY, Kim HP (2014) Anti-oxidative effects of rooibos tea (Aspalathus linearis) on immobilization-induced oxidative stress in rat brain. PLoS ONE 9:1–9
Husum H, Wörtwein G, Andersson W, Bolwig TG, Mathé AA (2008) Gene-environment interaction affects substance P and neurokinin A in the entorhinal cortex and periaqueductal grey in a genetic animal model of depression: implications for the pathophysiology of depression. Int J Neuropsychopharmacol 11:93–101
Kendler KS, Karkowski LM, Prescott CA (1998) Stressful life events and major depression: risk period, long-term contextual threat, and diagnostic specificity. J Nerv Mental Dis 186:661–669
Kendler KS, Karkowski LM, Prescott CA (1999) Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry 156:837–841
Kuroda Y, McEwen BS (1998) Effect of chronic restraint stress and tianeptine on growth factors, growth-associated protein-43 and microtubule-associated protein 2 mRNA expression in the rat hippocampus. Brain Res Mol Brain Res 59:35–39
Lambás-Señas L, Mnie-Filali O, Certin V, Faure C, Lemoine L, Zimmer L, Haddjeri N (2009) Functional correlates for 5-HT1A receptors in maternally deprived rats displaying anxiety and depression-like behaviors. Prog Neuro-Psychopharmacol Biol Psychiatry 33:262–268
Larsen MH, Mikkelsen JD, Hay-Schmidt A, Sandi C (2010) Regulation of brain-derived neurotrophic factor (BDNF) in the chronic unpredictable stress rat model and the effects of chronic antidepressant treatment. J Psychiatr Res 44:808–816
Lee B, Sur B, Park J, Kim SH, Kwon S, Yeom M, Shim I, Lee H, Hahm DH (2013) Chronic administration of baicalein decreases depression-like behavior induced by repeated restraint stress in rats. Korean J Physiol Pharmacol 17:393–403
Lees-Miller JP, Goodwin LO, Helfman DM (1990) Three novel brain tropomyosin isoforms are expressed from the rat alpha-tropomyosin gene through the use of alternative promoters and alternative RNA processing. Mol Cell Biol 10:1729–1742
Li X, Tizzano JP, Griffey K, Clay M, Lindstrom T, Skolnick P (2001) Antidepressant-like actions of an AMPA receptor potentiator (LY392098). Neuropharmacology 40:1028–1033
Lindvall O, Kokaia Z, Bengzon J, Elmér E, Kokaia M (1994) Neurotrophins and brain insults. Trends Neurosci 17:490–496
Liu SJ, Zukin RS (2007) Ca2+−permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci 30:126–134
Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ (1997) Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science (New York, NY) 277:1659–1662
Madrigal JL, Olivenza R, Moro MA, Lizasoain I, Lorenzo P, Rodrigo J, Leza JC (2001) Glutathione depletion, lipid peroxidation and mitochondrial dysfunction are induced by chronic stress in rat brain. Neuropsychopharmacol: Off Publ Am Coll Neuropsychopharmacol 24:420–429
Mallei A, Giambelli R, Gass P, Racagni G, Mathé AA, Vollmayr B, Popoli M (2011) Synaptoproteomics of learned helpless rats involve energy metabolism and cellular remodeling pathways in depressive-like behavior and antidepressant response. Neuropharmacology 60:1243–1253
Marais L, van Rensburg SJ, van Zyl JM, Stein DJ, Daniels WMU (2008) Maternal separation of rat pups increases the risk of developing depressive-like behavior after subsequent chronic stress by altering corticosterone and neurotrophin levels in the hippocampus. Neurosci Res 61:106–112
Martinez-Turrillas R, Frechilla D, Del Río J (2002) Chronic antidepressant treatment increases the membrane expression of AMPA receptors in rat hippocampus. Neuropharmacology 43:1230–1237
Martínez-Turrillas R, Del Río J, Frechilla D (2007) Neuronal proteins involved in synaptic targeting of AMPA receptors in rat hippocampus by antidepressant drugs. Biochem Biophys Res Commun 353:750–755
Mathé AA, Husum H, Khoury AE, Jiménez-Vasquez P, Gruber SHM, Wörtwein G, Nikisch G, Baumann P, Ågren H, Andersson W, Södergren Å, Angelucci F (2007) Search for biological correlates of depression and mechanisms of action of antidepressant treatment modalities. Do neuropeptides play a role. Physiol Behav 92:226–231
McAllister AK, Katz LC, Lo DC (1999) Neurotrophins and synaptic plasticity. Annu Rev Neurosci 22:295–318
McEwen BS (2000) The neurobiology of stress: from serendipity to clinical relevance. Brain Res 886:172–189
McEwen BS (2008) Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol 583:174–185
Meaney MJ (2001) Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Ann Rev Neurosci 24:1161–1192
Mochida S (1995) Role of myosin in neurotransmitter release: functional studies at synapses formed in culture. J Physiol, Paris 89:83–94
Mu J, Xie P, Yang ZS, Yang DL, Lv FJ, Luo TY, Li Y (2007) Neurogenesis and major depression: implications from proteomic analyses of hippocampal proteins in a rat depression model. Neurosci Lett 416:252–256
Naert G, Ixart G, Maurice T, Tapia-Arancibia L, Givalois L (2011) Brain-derived neurotrophic factor and hypothalamic-pituitary-adrenal axis adaptation processes in a depressive-like state induced by chronic restraint stress. MCN: Mol Cell Neurosci 46:55–66
O’Connor TM, O’Halloran DJ, Shanahan F (2000) The stress response and the hypothalamic-pituitary-adrenal axis: from molecule to melancholia. QJM: Mon J Assoc Phys 93:323–333
Paxinos G, Watson C (1986) The rat brain on stereotaxic co-ordinates, 2nd edn. Academic, San Diego
Pickering C, Gustafsson L, Cebere A, Nylander I, Liljequist S (2006) Repeated maternal separation of male Wistar rats alters glutamate receptor expression in the hippocampus but not the prefrontal cortex. Brain Res 1099:101–108
Pigino G, Kirkpatrick LL, Brady ST (2006) The cytoskeleton of neurons and glia. In: Siegel G, Albers RW, Brady S, Price D (eds) Basic neurochemistry: molecular, cellular, and medical aspects. Elsevier Academic Press, USA, pp 123–137
Piubelli C, Vighini M, Mathé AA, Domenici E, Carboni L (2011) Escitalopram modulates neuron-remodelling proteins in a rat gene-environment interaction model of depression as revealed by proteomics. Part I: genetic background. Int J Neuropsychopharmacol 14:796–833
Platt JE, Stone EA (1982) Chronic restraint stress elicits a positive antidepressant response on the forced swim test. Eur J Pharmacol 82:179–181
Popoli M, Mori S, Brunello N, Perez J, Gennarelli M, Racagni G (2001) Serine/threonine kinases as molecular targets of antidepressants: implications for pharmacological treatment and pathophysiology of affective disorders. Pharmacol Ther 89:149–170
Raison CL, Miller AH (2003) When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry 160:1554–1565
Rasmusson AM, Shi L, Duman R (2002) Downregulation of BDNF mRNA in the hippocampal dentate gyrus after re-exposure to cues previously associated with footshock. Neuropsychopharmacology 27:133–142
Ray B, Gaskins DL, Sajdyk TJ, Spence JP, Fitz SD, Shekhar A, Lahiri DK (2011) Restraint stress and repeated corticotrophin-releasing factor receptor activation in the amygdala both increase amyloid-b precursor protein and amyloid-b peptide but have divergent effects on brain-derived neurotrophic factor and pre-synaptic proteins in the prefrontal cortex of rats. Neuroscience 184:139–150
Reagan LP, Hendry RM, Reznikov LR, Piroli GG, Wood GE, McEwen BS, Grillo CA (2007) Tianeptine increases brain-derived neurotrophic factor expression in the rat amygdala. Eur J Pharmacol 565:68–75
Réus GZ, Abelaira HM, Maciel AL, dos Santos MA, Carlessi AS, Steckert AV, Ferreira GK, De Prá SD, Streck EL, Macêdo DS, Quevedo J (2014) Minocycline protects against oxidative damage and alters energy metabolism parameters in the brain of rats subjected to chronic mild stress. Metab Brain Dis 30:545–553
Roceri M, Cirulli F, Pessina C, Peretto P, Racagni G, Riva MA (2004) Postnatal repeated maternal deprivation produces age-dependent changes of brain-derived neurotrophic factor expression in selected rat brain regions. Biol Psychiatry 55:708–714
Rosenbrock H, Koros E, Bloching A, Podhorna J, Borsini F (2005) Effect of chronic intermittent restraint stress on hippocampal expression of marker proteins for synaptic plasticity and progenitor cell proliferation in rats. Brain Res 1040:55–63
Rüedi-Bettschen D, Pedersen EM, Feldon J, Pryce CR (2005) Early deprivation under specific conditions leads to reduced interest in reward in adulthood in Wistar rats. Behav Brain Res 156:297–310
Rutter M (2007) Psychopathological development across adolescence. J Youth Adolesc 36:101–110
Rutter M (2010) Gene–environment interplay. Depress Anxiety 27:1–4
Ryan B, Musazzi L, Mallei A, Tardito D, Gruber SHM, El Khoury A, Anwyl R, Racagni G, Mathé AA, Rowan MJ, Popoli M (2009) Remodelling by early-life stress of NMDA receptor-dependent synaptic plasticity in a gene-environment rat model of depression. Int J Neuropsychopharmacol 12:553–559
Serang O, Cansizoglu AE, Käll L, Steen H, Steen JA (2013) Nonparametric Bayesian evaluation of differential protein quantification. J Proteome Res 12:4556–4565
Sheikh MS, Fornace AJ (1999) Regulation of translation initiation following stress. Oncogene 18:6121–6128
Suvrathan A, Tomar A, Chattarji S (2010) Effects of chronic and acute stress on rat behaviour in the forced-swim test. Stress: Int J Biol Stress 13:533–540
Swiergiel AH, Zhou Y, Dunn AJ (2007) Effects of chronic footshock, restraint and corticotropin-releasing factor on freezing, ultrasonic vocalization and forced swim behavior in rats. Behav Brain Res 183:178–187
Tabassum I, Siddiqui ZN, Rizvi SJ (2010) Effects of Ocimum sanctum and Camellia sinensis on stress-induced anxiety and depression in male albino Rattus norvegicus. Indian J Pharm 42:283–288
Tamburella A, Micale V, Leggio GM, Drago F (2010) The beta3 adrenoceptor agonist, amibegron (SR58611A) counteracts stress-induced behavioral and neurochemical changes. Eur Neuropsychopharmacol: J Eur Coll Neuropsychopharmacol 20:704–713
Tobe EH (2013) Mitochondrial dysfunction, oxidative stress, and major depressive disorder. Neuropsychiatr Dis Treat 9:567–573
Uchida S, Hara K, Kobayashi A, Funato H, Hobara T, Otsuki K, Yamagata H, McEwen BS, Watanabe Y (2010) Early life stress enhances behavioral vulnerability to stress through the activation of REST4-mediated gene transcription in the medial prefrontal cortex of rodents. J Neurosci 30:15007–15018
Ulloa JL, Castañeda P, Berríos C, Díaz-Veliz G, Mora S, Bravo JA, Araneda K, Menares C, Morales P, Fiedler JL (2010) Comparison of the antidepressant sertraline on differential depression-like behaviors elicited by restraint stress and repeated corticosterone administration. Pharmacol Biochem Behav 97:213–221
Uys JDK, Hattingh S, Stein DJ, Daniels WMU (2008) Large scale hippocampal cellular distress may explain the behavioral consequences of repetitive traumatic experiences: a proteomic approach. Neurochem Res 33:1724–1734
van Heerden JH, Russell V, Korff A, Stein DJ, Illing N (2010) Evaluating the behavioural consequences of early maternal separation in adult C57BL/6 mice; the importance of time. Behav Brain Res 207:332–342
van Veen T, Wardenaar KJ, Carlier IVE, Spinhoven P, Penninx BWJH, Zitman FG (2013) Are childhood and adult life adversities differentially associated with specific symptom dimensions of depression and anxiety? Testing the tripartite model. J Affect Disord 146:238–245
Wang F, Qiao M, Xue L, Wei S (2015) Possible involvement of μ opioid receptor in the antidepressant-like effect of Shuyu formula in restraint stress-induced depression-like rats. Evid-based Complement Alternat Med (eCAM) 2015:1–11
Wörtwein G, Husum H, Andersson W, Bolwig TG, Mathé AA (2006) Effects of maternal separation on neuropetide Y and calcitonin gene-related peptide in ‘depressed’ Flinders sensitive line rats: a study of gene-environment interactions. Prog Neuro-Psychopharmacol Biol Psychiatry 30:684–693
Wu WW, Wang G, Baek SJ, Shen RF (2006) Comparative study of three proteomic quantitative methods, DIGE, cICAT, and iTRAQ, using 2D gel- or LC-MALDI TOF/TOF. J Proteome Res 5:651–658
Xu H, Luo C, Richardson JS, Li XM (2004) Recovery of hippocampal cell proliferation and BDNF levels, both of which are reduced by repeated restraint stress, is accelerated by chronic venlafaxine. Pharmacogenomics J 4:322–331
Xu H, Chen Z, He J, Haimanot S, Li X, Dyck L, Li XM (2006) Synergetic effects of quetiapine and venlafaxine in preventing the chronic restraint stress-induced decrease in cell proliferation and BDNF expression in rat hippocampus. Hippocampus 16:551–559
Yan Q, Rosenfeld RD, Matheson CR, Hawkins N, Lopez OT, Bennett L, Welcher AA (1997) Expression of brain-derived neurotrophic factor protein in the adult rat central nervous system. Neuroscience 78:431–448
Yoo SB, Kim BT, Kim JY, Ryu V, Kang DW, Lee JH, Jahng JW (2013) Adolescence fluoxetine increases serotonergic activity in the raphe-hippocampus axis and improves depression-like behaviors in female rats that experienced neonatal maternal separation. Psychoneuroendocrinology 38:777–788
Zimmerberg B, Sageser KA (2011) Comparison of two rodent models of maternal separation on juvenile social behavior. Front Psychiatry 2:39–49
Acknowledgments
The authors thank the Institute for the Study of Affective Neuroscience (ISAN) and the National Research Foundation (NRF) for financial support. The authors would like to acknowledge the contributions of the Centre for Proteomic and Genomic Research (CPGR), University of Cape Town (UCT) who performed the proteomic analysis. We would also like to thank Ms Estella Minnaar for breeding the rats and performing the MS as well as the Animal Unit and Nuraan Ismail for taking care of the animals. Any opinion, finding and conclusion or recommendation expressed in this material is that of the authors and the NRF does not accept any liability in this regard.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest or other disclosures to report.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 3
Time spent in the open and closed arms of the EPM by control SD, MS SD, restraint stressed SD and restraint stressed MS SD rats. The EPM revealed no difference between Ctr, MS, RS, and MS + RS rats in time spent in the open arms (a) and closed arms (b) (n = 12-13/group). Data presented as mean ± SEM (GIF 35 kb)
Supplementary Fig. 4
BDNF levels in the ventral hippocampus of control SD, MS SD, restraint stressed SD and restraint stressed MS SD rats. No significant difference found in BDNF levels in the ventral hippocampus between Ctr, MS, RS and MS + RS rats (n = 10/group). Data presented as mean ± SEM (GIF 41 kb)
Supplementary Table 3
Proteomic (8-plex) profile of the PFC of Ctr, MS and restraint stressed (RS) rats. Data presented as a ratio to Ctr 1. The average Ctr ratio (Avg) was calculated and normalized to 1.0 (grey). * Data differed from the normalized Ctr/Ctr by more than 20 % (1.2-fold increase or decrease) (DOCX 278 kb)
Supplementary Table 4
Proteomic (4-plex) profile of the PFC of Ctr and MS + RS rats. Data presented as ratio to Ctr 1. The average Ctr ratio (Avg) was calculated and normalized to 1.0 (grey). * Data differed from the normalized Ctr/Ctr by more than 20 % (1.2-fold increase or decrease) (DOCX 224 kb)
Rights and permissions
About this article
Cite this article
van Zyl, P.J., Dimatelis, J.J. & Russell, V.A. Behavioural and biochemical changes in maternally separated Sprague–Dawley rats exposed to restraint stress. Metab Brain Dis 31, 121–133 (2016). https://doi.org/10.1007/s11011-015-9757-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-015-9757-y