Abstract
Prenatal methamphetamine exposure (PME) is a significant problem in several parts of the world and poses important health risks for the developing fetus. Research on the short- and long-term outcomes of PME is scarce, however. Here, we summarize present knowledge on the cognitive and behavioral outcomes of PME, based on a review of the neuroimaging, neuropsychology, and neuroscience literature published in the past 15 years. Several studies have reported that the behavioral and cognitive sequelae of PME include broad deficits in the domains of attention, memory, and visual-motor integration. Knowledge regarding brain-behavior relationships is poor, however, in large part because imaging studies are rare. Hence, the effects of PME on developing neurocircuitry and brain architecture remain speculative, and are largely deductive. Some studies have implicated the dopamine-rich fronto-striatal pathways; however, cognitive deficits (e.g., impaired visual-motor integration) that should be associated with damage to those pathways are not manifested consistently across studies. We conclude by discussing challenges endemic to research on prenatal drug exposure, and argue that they may account for some of the inconsistencies in the extant research on PME. Studies confirming predicted brain-behavior relationships in PME, and exploring possible mechanisms underlying those relationships, are needed if neuroscience is to address the urgency of this growing public health problem.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
With over 250 million users worldwide, methamphetamine (MA) has become a significant public health concern (United Nations Office on Drugs, & Crime 2010). The global rise in MA abuse during pregnancy has rendered a large number of infants and children at risk for the adverse consequences of prenatal methamphetamine exposure (PME; Meredith et al. 2005). Emerging evidence suggests that PME affects fetal growth and may lead to a variety of developmental, behavioral, and neurological abnormalities (Nguyen et al. 2010; Abar et al. 2013). However, outside of animal studies, very little is known about the extent of the medium- and long-term functional and structural effects of MA exposure in utero. Moreover, the effects of PME on the organization of developing neural circuitry, and whether/how subsequent recovery from those effects is possible, is not clear.
To our knowledge, there are no published literature reviews that focus exclusively on PME and on integrating knowledge from cognitive and neuroimaging studies of children and adolescents who have been exposed to MA prenatally. Here, we aim to bring light to the small but accumulating body of research delineating the fetal and longer-term clinical outcomes of PME. We first examine the postulated effects of PME on developing circuitry, and follow with a review of the structural, metabolic, and functional neuroimaging studies of prenatally MA-exposed populations. We then present a summary of affective, behavioral, and cognitive impairments identified by longitudinal studies. The findings discussed have growing implications for preventative interventions aimed at women of childbearing age who abuse MA, as well as for secondary interventions aimed at improving the condition of children with PME.
Methamphetamine abuse: epidemiology, risks, and consequences
MA abuse is the fastest growing illicit drug problem worldwide, and is reaching epidemic proportions (United Nations Office on Drugs, and Crime 2013; Wouldes et al. 2013). Levels of MA use seem to be increasing in high- and middle-income countries, and in countries near major drug production areas (Degenhardt and Hall 2012). For example, according to the Substance Abuse and Mental Health Services Administration in the United States (2010), 12.8 million individuals (aged 12 years and older) reported using MA at least once in their lifetime, with a 37 % increase in user prevalence rates reported between 2008 and 2009. Similar increases have been observed in the Asia-Pacific region, an area with several prolific drug trafficking syndicates (McKetin et al. 2008). Although such data suggest globally increasing trends, systematic data for MA use in low-income countries (particularly those in Africa) are largely unavailable.
Although MA use is cause for concern in both sexes, female users are more likely to engage in high-risk sexual behaviors (e.g., having unprotected sex with multiple partners, working in the sex trade), and are therefore at greater risk for unplanned pregnancy (Semple et al. 2005; Terplan et al. 2010). Negative consequences of maternal drug use extend beyond a woman’s own health; they threaten her developing fetus as well. Clinical studies have demonstrated that these negative consequences for the fetus can include decreased weight, length, and head circumference upon birth (Little et al. 1988; Smith et al. 2006; Nguyen et al. 2010), as well as compromised neonatal behavioral outcomes (e.g., poor feeding, abnormal sleep patterns, under-arousal, and reduced movement scores; Oro and Dixon 1987; Smith et al. 2008; LaGasse et al. 2011). These negative outcomes may be compounded by poor nutrition and increased likelihood of exposure to violence, factors frequently associated with maternal drug use (Behnke et al. 2013). Thus, the effects of PME on the fetus, and on ongoing development, are of increasing concern to health care professionals and to policymakers. Unfortunately, however, there is still widespread lack of awareness regarding the potential effects of PME on the developing fetus and on long-term behavior, cognition, and affect.
Methods
We searched the PubMed and EBSCOHost online databases for studies pertaining to the cognitive, structural, and functional outcomes of PME in humans and animals. The search terms were “prenatal methamphetamine exposure”, “neuroimaging in prenatal methamphetamine exposure”, “neurocognitive outcomes in prenatal methamphetamine exposure”, and similar combinations of those words. We limited our search to articles published in English between 1995 and 2013. The search located 132 potentially relevant articles; 41 of these were actually relevant and were available to us via our university’s library. These articles, and papers cited in their reference lists, were included in this review.
Effects of methamphetamine on developing neural circuitry
MA is one of a group of sympathomimetic drugs that stimulate the central nervous system (CNS). It passes readily through the placenta and the blood-brain barrier and can have significant vasoconstrictive effects on the developing fetus, resulting in decreased uteroplacental blood flow and fetal hypoxia (Won et al. 2001; Golub et al. 2005). Although the biochemical mechanisms of action and toxicity of MA for the adult brain have been well documented (see Scott et al. 2007; Kish 2008; Cruickshank and Dyer 2009), the effects of PME on the organization of developing neural circuitry, and whether/how subsequent recovery from those effects is possible, is unclear (Sowell et al. 2010). Furthermore, because of the multiple interactions among developing neuronal systems that determine the organization of brain circuitry, the effects of MA on neural connectivity in the immature CNS are likely to be different to those in adults.
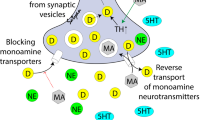
The development of neural networks is influenced by numerous morphogenetic events, including proliferation, migration, differentiation and apoptosis, neurite growth, synaptogenesis, and neuronal pruning. These events are modulated by, among other factors, pre- and post-synaptic electrical activity, neurotrophic factor signaling, and multiple neurotransmitter systems (Frost and Cadet 2000). With specific regard to the latter, the primary monoaminergic pathways (i.e., those using norepinephrine (NE), dopamine (DA), and serotonin (5HT)) project widely and develop early. This spatial and temporal arrangement allows these pathways to modulate the development of non-aminergic neural elements and the connections between them. Thus, damage to monoaminergic neurons in utero may have a secondary effect on a wide variety of neural circuits (see Fig. 1).
Schematic diagram indicating how the effect of MA on the serotonergic, dopaminergic, or glutaminergic systems can modulate the development of non-aminergic neural circuitry. The notation ‘±lesions’ indicates that MA-induced modulation of signaling in the GLU, 5HT, and DA systems is not necessarily accompanied by overt destruction of neurons or their processes in these systems. (Reproduced with permission from Frost and Cadet 2000)
Regarding the effects of the acute and chronic MA exposure on mature serotonergic, dopaminergic, and glutaminergic axons, a substantial body of literature, encompassing both animal and human studies, suggests multiple mechanisms of action (Nordahl et al. 2003; Sulzer et al. 2005; Barr et al. 2006; Scott et al. 2007). The principal mechanism by which MA stimulates the excessive release of monoamines (primarily, DA) includes the redistribution of catecholamines from synaptic vesicles to the cytosol (Brown et al. 2001) and the reverse transport of neurotransmitters through plasma membrane transporters (Khoshbouei et al. 2003). Additionally, there is also evidence that MA blocks the catabolism of catecholamines by inhibiting the activity of mitochondrial monoamine oxidase (MAO), and by increasing the activity and expression of the dopamine-synthesizing enzyme, tyrosine hydroxylase (TH) (Schmitz et al. 2001; Sulzer et al. 2005). As a result of these multiple mechanisms, MA acts as a highly potent releaser of monoamines, giving rise to MA-induced neurotoxicity. This neurotoxicity is evident in several neurotransmitter systems, but is most notable in the nigrostriatal dopaminergic pathways. Thus, it is possible that PME potentially alters the function of the dopamine-rich fronto-striatal-thalamocortical loops (Cass 1997).
Given these effects of MA on mature monoaminergic pathways, it is reasonable to suggest that developing monoaminergic neurons may also be susceptible to MA-induced neurotoxicity (Cadet and Krasnova 2009). In rat studies TH (responsible for the conversion of L-tyrosine to L-DOPA, a precursor for DA) has been identified as an effective marker in assessing potential effects of MA on developing DA systems (Gomes-da-Silva et al. 2002). The action of TH early in CNS development is associated with the differentiation of neuronal groups, and with neurochemical processes that control axonal guidance, neuronal recognition, and synaptogenesis (Flames and Hobert 2011). Because TH levels appears to be permanently reduced following repeated MA administrations in rat pups, it is possible that the action of MA on TH gene expression early in development might affect critical processes related to dopaminergic activity (Graham et al. 2011, 2013). Specifically, MA-induced depletion of TH has been found to alter the pattern of maturation of dopaminergic TH-containing neurons in the dorsal striatum, prefrontal cortex, nucleus accumbens, and substantia nigra (Kaewsuk et al. 2009). Such findings have been replicated in several rat studies, suggesting implication of the mesolimbic dopaminergic pathway in PME (Suzuki et al. 2003; Bubenikova-Valesova et al. 2009).
The evidence reviewed above is drawn from rat studies involving multiple high-dose exposure to MA throughout periods of fetal development. However, even single-dose MA exposure in utero may have significant developmental consequences. Jeng, Wong, Ting-A-Kee, and Wells (2005) found that a relatively low dose (20 or 40 mg/kg ip, resulting in similar concentrations observed in premature infants born to MA-abusing mothers) of MA administered once-off during the embryonic or fetal period in pregnant rats caused oxidative DNA damage in the brain, and long-standing (potentially permanent) postnatal functional deficits. Of note here is that these functional deficits arose without any concomitant alterations in dopaminergic nerve terminal density (evidenced by staining for TH). Taken together, these results suggest that even single-dose PME may have negative developmental consequences, and that the mechanisms underlying these effects might be distinct from those identified by models using multiple-dose schedules.
Given the plasticity of the developing brain (Andersen 2003; Johnston et al. 2009) one may infer that neurological insult associated with PME would be compensated for more effectively than that associated with adult exposure. However, the molecular and morphological changes that take place during development seem to bring about windows of selective vulnerability, in addition to allowing compensatory repair mechanisms to function (Stanwood and Levitt 2004; Vaccarino and Ment 2004; Jeng et al. 2005). For example, it is possible that striatal dopaminergic neurons may continue to develop even after PME, but that their development may occur aberrantly. This aberrant development might involve atypical processes of neural patterning and/or remodeling in addition to, or instead of, dopaminergic cell loss (Jeng et al. 2005). Although further research is needed to validate these claims, it seems clear that the mechanisms of action in PME are a combination of the interaction of MA with monoaminergic neurotransmitter systems in the developing fetal brain as well as changes in brain morphogenesis (Won et al. 2001; Cui et al. 2006).
Structural and metabolic neuroimaging studies
Because striatal structures have the highest densities of dopaminergic synapses, one might predict that the neurotoxic effects of MA would be pronounced in these regions (Chang et al. 2007). Using structural magnetic resonance imaging (sMRI), Chang et al. (2004) confirmed this prediction: they found that, compared to unexposed age-matched controls (n = 15), children exposed to MA in utero (n = 13; age range = 3-16 years, M = 6.9, SD = 3.5) showed significant regional volumetric reductions in the globus pallidus and putamen, and marginal decreases in caudate size. They also found significant volumetric reductions in the hippocampus bilaterally.
Sowell et al. (2010) replicated these findings in a sMRI study that compared the brain morphometry of children with prenatal MA exposure (n = 21; age range = 5–15 years, M = 9.66, SD = 1.85) to that of children with prenatal alcohol exposure (n = 13; age range = 5–15, M = 11.5, SD = 2.34). They found that, although both groups of children showed widespread volumetric reductions (including in striatal, thalamic, prefrontal, and occipitoparietal regions) and some volumetric increases in limbic structures, MA-exposed children showed more severe volume reductions in the striatum, and more pronounced increases in limbic structures such as the anterior and posterior cingulate and the inferior frontal gyrus. These findings are not only generally consistent with predictions derived from the rat studies reviewed above, but they are also consistent with research showing that striatal and limbic structures are sites of neurotoxicity in adult MA users (Sulzer et al. 2005).
To our knowledge, the two studies reviewed above are the only two published sMRI studies on the effects of PME. Despite some consistency in findings, the etiology of the volume reductions/increases is not entirely clear. For instance, it is possible that the volume increases in the cingulate and other associated limbic cortices may serve as a compensatory mechanism for dysfunction in the dopamine-rich striatal and thalamic structures. Alternatively, the volume increases may represent an aberrant process due to inadequate synaptic pruning and/or reduced myelination as a result of PME (Sowell et al. 2003, 2010).
To date, one study has examined microstructural brain changes as a result of PME in humans. Cloak et al. (2009), using diffusion tensor imaging (DTI), found that, compared to age-matched controls (n = 37), prenatally MA-exposed children (n = 29; age range = 3–5 years, M = 4.03, SD = 0.10) had lower diffusion in frontal and parietal white matter tracts. While the exact mechanism of how PME may lead to lower brain diffusivity is unknown, lower white matter diffusivity typically reflects, among other elements, more compact axonal fibers. Such findings of reduced myelination and higher dendritic density have also been reported in rat pups exposed to MA (Melo et al. 2006; Jedynak et al. 2007).
The findings of lower diffusivity in frontal and temporal white matter are consistent with those of two magnetic resonance spectroscopy (MRS) studies, the latter of which was conducted on the same sample (Smith et al. 2001; Chang et al. 2009). The first of these studies (n = 12; average age = 8.1 years, SD = 0.8) found elevated levels of creatine (Cr) in the striatum in children with a history of prenatal MA exposure. The latter, using a much larger sample (n = 52; average age = 3.91, SD = .06), found higher metabolite concentrations in total Cr, as well as N-acetyle (NA) and glutamate and glutamine (GLX) in the frontal white matter and thalamus. Since the three metabolites NA, Cr, and GLX are present in neurons, the higher concentrations in the white matter suggest increased axonal density or compactness in the MA-exposed children (as confirmed by the DTI results discussed earlier, Cloak et al. 2009).
As with the sMRI findings, the etiology for the increased metabolite concentrations in children with PME is not clear. For example, it is possible that the higher metabolite levels suggest accelerated growth patterns in these children. Previous MRS studies of normally developing children (Kreis et al. 1993), and of healthy mice (Weiss et al. 2009), found age-dependent increases in tCR, NA, and GLX, similar to the levels observed in the MRS studies discussed above. Alternatively, it is possible that the known vasoconstrictive effects of PME could result in an alteration in cell energy metabolism in children with PME (Won et al. 2001; Golub et al. 2005).
Functional neuroimaging studies
Again, this is a small body of literature, featuring only two published studies. Those studies did, however, report results that one might interpret as being broadly consistent with those from sMRI and animal studies: They also identified limbic and striatal regions as being particularly vulnerable to the effects of PME.
In a design similar to that of Sowell et al.’s (2010) sMRI study, Lu et al. (2009) compared functional activation in response to a verbal memory task in three groups of children: those with prenatal exposure to both MA + alcohol (n = 14; age range = 7–15 years, M = 9.5, SD = 1.9), those with prenatal exposure to alcohol alone (n = 9; M = 11.3, SD = 2.7), and age-matched controls (n = 20). Although both exposure groups showed similarly impaired performance on verbal tasks, children with PME recruited more diffuse areas in the bilateral medial temporal lobes. The authors interpreted these findings as suggesting that children with PME may need to allocate additional (and more diffuse) resources to achieve the same level of medial temporal lobe function and to compensate for a less efficient verbal memory network.
The other published fMRI study in this area of research (again, using the same study design as the one described above), found more severe functional alterations in children with prenatal MA and concomitant alcohol exposure (n = 19; age range = 7–13 years, M = 9.2, SD = 1.8) than those with alcohol exposure alone (n = 13; age range = 7–15, M = 11.5, SD = 2.44), compared to age-matched controls (n = 18) (Roussotte et al. 2011). Specifically, abnormal (decreased) activation was observed in several fronto-striatal circuits in children with prenatal MA + alcohol exposure as they completed a visuospatial working memory task, relative to the control group. This finding is consistent with the evidence of structural and metabolic abnormalities of the striatum and fronto-striatal connections discussed in the previous section of this review (i.e. Smith et al. 2001; Chang et al. 2004, 2009). However, in this study, instead of the hypothesized negative correlations between activation in the caudate nucleus (implicated in working memory) and task accuracy, no correlation between performance and activation in the caudate was observed. Unexpectedly, the statistical analyses revealed a negative correlation between task accuracy and activation in the putamen (implicated in the motor loop) bilaterally in the meth + alcohol-exposed group.
One possible interpretation for this finding may be offered by way of understanding the potential interactions between the basal ganglia-thalamocortical circuits and their regional connections. Each circuit is organized in parallel and underlies specific motor, cognitive or affective functions (Cummings 1993). For example, the dorsolateral prefrontal loop (implicated in working memory) engages the dorsolateral caudate (Lewis et al. 2004), whereas the putamen is involved in the motor loop (Cummings 1993). Because striatal dopamine depletion (such as that observed in rat studies on PME) has been found to result in changes in cortico-striatal network properties, it is possible that this depletion may lead to a remapping of cerebral connectivity (in particular, reduced spatial segregation) and results in increased interaction between the different cortico-striatal loops (Helmich et al. 2010).
Such findings have been shown to occur as a result of reduced levels of striatal DA in Parkinson’s disease (Morrow et al. 2011). Therefore, it is possible that a mechanism similar to the one occurring in Parkinson’s disease may take place in children with PME. That is to say, damage to DA terminals in the striatum during ontogeny may affect the development of neural circuits and lead to a comparable remapping phenomenon, as suggested by Cadet and Krasnova (2009). Thus, in children with PME, it is possible that due to reduced spatial discrimination between the motor loop and dorsolateral prefrontal loop, the dorsolateral prefrontal loop activated during working memory tasks may engage the putamen, rather than the dorsolateral caudate (Roussotte et al. 2011).
Notably, due to limited research, the above studies and postulated explanations are largely speculative, with little supporting evidence. Moreover, although the functional connectivity data reveal specific task-related changes in BOLD response in the prenatally MA-exposed population, they do not examine the possibility of altered underlying global attentional modulation. The recent discovery of consistent regions that are more active during resting periods than during cognitive demand (see Greicius et al. 2003; Fox and Greicius 2010), suggests scope for examining resting state data in prenatally MA-exposed populations. However, up to date, there is no published data on resting sate connectivity in prenatally MA-exposed individuals, and only one study has examined global organization of functional brain connectivity in adult MA-abusers (Ahmadlou et al. 2013).
Affective, behavioral, and cognitive outcomes
There is a small literature on long- and short-term outcomes of PME in humans. The most extensive follow-up data on affective, behavioral, and cognitive outcomes following PME are provided by Swedish researchers who tracked a cohort of 65 MA-exposed children from birth to age 14. They have reported, in that cohort of children with continuous MA exposure throughout gestation, a variety of adverse physical, cognitive, emotional, and social effects, including increased prevalence of attention-deficit/hyperactivity disorder (ADHD), aggression, and learning difficulties attributed to deficits in attention, memory, and motivation (for a timeline of findings, see Eriksson et al. 1978, 1981, 1989, 1994; Eriksson and Zetterström 1994, 2000). In that cohort, the first few months of life were marked by signs of drowsiness and lethargy (Billing et al. 1980). By the age of 1 year, the children began showing affective characteristics of autism, speech impediments, and signs of wariness of strangers. At age 4, IQ was lower than that of population controls (Billing et al. 1988), and at age 8 prenatal exposure predicted aggressive behavior towards peers (Billing et al. 1994). By the age of 14, the children showed delays in math and language performance, and difficulties with physical fitness appeared to affect academic advancement (Cernerud et al. 1996). Despite the robust database of behavioral profiles generated by research on this Swedish cohort, issues around some key methodological aspects affect the reliability of possible interpretations. These issues include lack of a control group and uncontrolled for confounding drug exposures.
Given the presence of those methodological issues with the Swedish research, one might argue that the ongoing Infant Development, Environment, and Lifestyle (IDEAL) study (Della Grotta et al. 2010), which uses cohorts from New Zealand and America, is the first and largest systematically controlled study of neurobehavioral outcomes in prenatally MA-exposed children. The IDEAL study was the first to publish dose-response and trimester-related effects (validated by meconium testing) in prenatally MA-exposed children (LaGasse et al. 2011). From birth to 36 months, heavy PME was related to lower arousal, increased lethargy, and greater levels of physiological stress. In particular, first-trimester MA use was associated with greater physiological and CNS stress, and third-trimester use with more lethargy and hypotonicity.
Data from the IDEAL study have also been used to examine the effects of prenatal MA exposure on motor and cognitive development in children between the ages of 1 and 3 years (Smith et al. 2011). At 1 year, children with PME presented with subtly impaired fine motor performance, with the greatest disturbances observed in children with heavy PME. At age 3, however, both high- and low-dose groups presented with no PME-related motor impairment. So far, these findings yield inconsistent results in relation to some of the cognitive behavioral evidence from neuroimaging studies (discussed under Structure-Function Correlations below). For example, Chang et al. (2009) did find significantly impaired performance on tasks of visuomotor integration in children with PME at the age of 4. Notably, however, the IDEAL study is still in its infancy. Most of the structural and metabolic imaging studies reviewed in this paper have made use of samples older than the age of 3; thus, long term follow up the children in the IDEAL study may reveal comparable results.
One recent IDEAL publication (LaGasse et al. 2012) reported on the behavioral assessment of an older cohort of prenatally MA-exposed children (ages 3 and 5 years). Consistent with the data from the Swedish cohort, which reported aggressive behavior, attentional issues, and adjustment issues (Billing et al. 1988, 1994), similar affective and behavioral problems were identified. Controlling for normal developmental trajectories, PME was related to heightened emotional reactivity and more anxious/depressive symptoms at both ages, and with externalizing and ADHD problems evident at age 5. Both withdrawn behavior and attention problems were associated with heavy PME at age 3 and age 5. Further investigation by Twomey et al. (2013) found that home environments that were more stable and sensitive to the emotional and developmental needs of these children were associated with decreased risk of internalizing and externalizing behavioral problems. This finding, however, was independent of MA exposure (the control group displayed similar behavioral challenges), thus placing the child’s behavioral problems in the context of the larger family system. Therefore, consideration of the implications of a child’s home environment is particularly important in working with vulnerable, high-risk populations such as perinatal substance users.
Structure-function correlations
All the studies reviewed thus far have presented evidence of structural and functional deficits as well as clinical cognitive and behavioral phenotypes in PME. While such research contributes to our knowledge of the seemingly multifaceted manifestations of PME, correlational analyses investigating brain-behavior relationships provide opportunity for a clearer understanding of the neurobehavioral and neuroanatomical tenets that underlie developmental outcomes in PME. Among the studies described under Structural and Metabolic Neuroimaging Studies, three have reported correlations between structural or metabolic brain abnormalities and neurocognitive performance in children with PME.
Chang et al. (2004) found that the smaller size of the putamen and globus pallidus in children with PME was associated with poorer attentional task performance, suggesting involvement of the basal ganglia structures. In the same study, reduced hippocampal volumes were also associated with poor performance on a verbal memory task. These correlations of performance on sustained attention and delayed verbal memory with volumetric reductions in subcortical structures (putamen and hippocampus) are also consistent with reports linking smaller striatal brain volume (and deficits in the dopaminergic system) with impaired learning in children with PME (Thompson et al. 2009).
In the second correlational study, investigating potential interactional effects of prenatal MA and alcohol exposure on cognitive outcomes, Sowell et al. (2010) found volumetric reductions in striatal and thalamic regions for both the prenatal MA + alcohol and the alcohol-exposed groups, compared to controls. For the prenatal MA + alcohol-exposed group, reduced volumes in the caudate nucleus (associated with memory, learning, motor control, and punishment and reward) were negatively correlated with IQ. This effect was not observed in the alcohol-exposed group, suggesting that children with PME may have more severe cognitive outcomes than those exposed to alcohol alone. Given the prevalence of concomitant alcohol exposure in PME research, as indicated by the several of the studies reviewed here (Lu et al. 2009; Roussotte et al. 2011), such findings warrant further replication.
In terms of metabolic correlates, one MRS study (Chang et al. 2009) found that decreased metabolic activity in the frontal white matter and thalamic regions was correlated with poor performance on tasks for visuomotor integration. In line with some of the behavioral findings in previous research (Chang et al. 2004), as well as animal studies (Šlamberová et al. 2006), it is possible that PME results in altered psychomotor development via the dopaminergic system in the fronto-striatal or thalamocortical pathways.
Challenges in prenatal drug exposure research
Research on prenatal drug exposure continues to be a controversial topic in terms of ethical responsibility and national policy. Regarding research ethics, views on what constitutes an appropriate response to drug-exposed infants vary due to the many complex issues endemic to perinatal substance use. For example, reporting pregnant illicit drug users to child welfare authorities is often argued to be an ethical obligation; however, research shows that pregnant women who fear prosecution and loss of custody as a result of their drug use are less likely to seek help or essential prenatal care (Poland et al. 1993; Roberts and Nuru-Jeter 2010). Although the scope of this review does not cover the aforementioned complexities, an appropriate response in terms of research on prenatal drug exposure and social policy is an important consideration, and we refer the reader elsewhere (see Ondersma et al. 2000; Lester et al. 2004; Thompson et al. 2009). Our focus here, instead, is on methodological constraints pertinent to research on PME.
From a methodological perspective, research on the effects of PME is problematic and has, to date, been limited. One challenging aspect is that prevalence statistics are difficult to establish and often fluctuate from site to site. Variations in prevalence rates may be attributed to differing sampling and drug detection methods (e.g., immunoassay vs. meconium testing), screening women in different settings (e.g., community samples vs. targeted samples, such as drug rehabilitation centers or prisons), and obtaining data at various points in time (Behnke et al. 2013). For example, many of the existing studies on PME feature retrospective designs, thus data pertaining to the quantity, frequency, and combinations of drugs used, and at what points they were used during pregnancy, is often inaccurate or unavailable (Maisto et al. 1990; Kaltenbach and Finnegan 1993).
In direct relation to the challenges outlined above, a frequently cited limitation in PME research is that documentation of MA use by women during pregnancy is typically based on self-report measures without verification by toxicological analysis. Moreover, information from the primary source is often inaccessible. This is because many child participants in PME studies have been removed from the custody of their biological mothers due the presence of drugs, violence, and neglect in the home (Smith et al. 2007). Therefore, information regarding the mother’s substance use history is often collected by way of medical and legal records, and/or reports from adoptive or foster care parents. Consequently, establishing whether there is a dose-response curve in terms of structural and functional outcome, and the shape of that curve, is problematic.
The issue of accuracy of maternal drug exposure histories is further compounded by the prevalence of polysubstance abuse. Children with PME are at high risk of in utero exposure to other substances, including alcohol, tobacco cocaine, marijuana, and opiates (Smith et al. 2006). Abuse of each of these substances has its own particular consequences for brain structure and function (Smith et al. 2006; Salisbury et al. 2009; Sowell et al. 2010); hence, in poly-substance abusing mothers, it is difficult to tease apart the effects of MA from that of (an)other drug(s). One way of resolving the confounding effects of polydrug abuse in PME research might be to recruit samples without concurrent polydrug exposure; however, such samples are rare. Therefore, research on children with PME might be more ecologically valid if, in fact, one did not try to recruit those with “pure” exposures to MA alone. Thus, a more viable solution might be to recruit larger samples in order to isolate the effects of a specific drug. With a large enough sample size, there may be enough statistical power and variability among various combinations of drugs to be able to covary or adjust the effects of the drug of interest for the effects of other drugs.
Conclusions and future directions
We have discussed, using findings from animal studies, some of the underlying neuroteratogenic mechanisms of PME. We have also summarized findings from the behavioral and brain imaging literature in infants and children exposed to MA prenatally.
The teratogenic effects of PME appear likely to result from interference with the neurotropic roles of monoaminergic transmitters (particularly DA) during brain development (Frost and Cadet 2000). This interference with the DA system is, in turn, postulated to have a significant effect on cortical neuronal development, and may lead to morphologic deviations in several brain structures (particularly the striatum and several limbic structures). Overall, there are too few published neuroimaging studies on the effects of PME in humans to draw any conclusive thoughts regarding the brain systems most affected by PME. However, despite the limited evidence, diverse methodology, and small sample sizes, some consistent patterns of abnormalities have emerged: It appears that the development of areas of the brain responsible for the regulation of attention, memory, visual-motor integration, and executive functioning are particularly vulnerable to PME. The striatum, specifically, seems to be susceptible to structural and metabolic alterations as a result of PME (Smith et al 2001; Chang et al. 2004).
Recently, the IDEAL study has begun systematic documentation of some of these functional and behavioral deficits in infants with PME. Findings include poor movement quality, decreased arousal, increased stress, and attention difficulties (LaGasse et al. 2011). Support for the clinical significance of these abnormalities is also beginning to surface: There are suggestions that PME may have significant neurocognitive effects even beyond that of frequently co-occurring alcohol exposure (Sowell et al. 2010). Unfortunately, long-term developmental data for older controlled cohorts (over the age of 3 years) is scarce; however, existing research suggests that children with PME may be more vulnerable to disorders of executive function manifested by externalizing behavioral problems and aggression (LaGasse et al. 2012).
In conclusion, the study of the effects of PME is still evolving and further investigations are needed to confirm existing findings. The reviewed literature suggests that children exposed to MA in utero may experience a range of neurotransmitter and neurostructural alterations, with potential long-term cognitive and behavioral sequelae. Currently, we do not know how data from different imaging modalities relate to each other in this literature, and we know little about how imaging findings correspond to child behavioral and cognitive functioning. Future research might investigate PME-related changes in brain function, structure, and connectivity in longitudinal cohorts to determine how these changes translate to neurocognitive and behavioral effects over time. To address some of the current methodological concerns, future studies should, as far as (ethically and otherwise) possible, rely on prospectively enrolled subjects, for whom more accurate exposure histories and dosages can be obtained. More accurate exposure histories will provide important insights into dose-response relationships, thus helping clinicians devise interventions that address the specific needs of children with PME.
References
Abar B, LaGasse LL, Wouldes T, Derauf C, Newman E, Shah R et al (2013) Cross-national comparison of prenatal methamphetamine exposure on infant and early child physical growth: a natural experiment. Prev Sci 1:1–10
Ahmadlou M, Ahmadi K, Rezazade M, Azad-Marzabadi E (2013) Global organization of functional brain connectivity in methamphetamine abusers. Clin Neurophysiol 124:1122–1131
Andersen SL (2003) Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev 27:3–18
Barr AM, Panenka WJ, MacEwan GW, Thornton AE, Lang DJ, Honer WG, Lecomte T (2006) The need for speed: an update on methamphetamine addiction. J Psychiatry Neurosci 31:301–313
Behnke M, Smith VC, Levy S, Ammerman SD, Gonzalez PK, Ryan SA et al (2013) Prenatal substance abuse: short-and long-term effects on the exposed fetus. Pediatrics 131:1009–1024
Billing L, Eriksson M, Larsson G, Zetterström R (1980) Amphetamine addiction and pregnancy. Acta Paediatr 69:675–680
Billing L, Eriksson M, Steneroth G, Zetterström R (1988) Predictive indicators for adjustment in 4-year-old children whose mothers used amphetamine during pregnancy. Child Abuse Negl 12:503–507
Billing L, Eriksson M, Jonsson B, Steneroth G, Zetterström R (1994) The influence of environmental factors on behavioural problems in 8-year-old children exposed to amphetamine during fetal life. Child Abuse Negl 18:3–9
Brown JM, Hanson GR, Fleckenstein AE (2001) Regulation of the vesicular monoamine transporter-2: a novel mechanism for cocaine and other psychostimulants. J Pharmacol Exp Ther 296:762–767
Bubenikova-Valesova V, Kacer P, Syslova K, Rambousek L, Janovsky M, Schutova B et al (2009) Prenatal methamphetamine exposure affects the mesolimbic dopaminergic system and behavior in adult offspring. Int J Dev Neurosci 27:525–530
Cadet JL, Krasnova IN (2009) Molecular bases of methamphetamine-induced neurodegeneration. Int Rev Neurobiol 88:101–119
Cass WA (1997) Decreases in evoked overflow of dopamine in rat striatum after neurotoxic doses of methamphetamine. J Exp Ther 280:105–113
Cernerud L, Eriksson M, Jonsson B, Steneroth G, Zetterstrom R (1996) Amphetamine addiction during pregnancy: 14-year follow-up of growth and school performance. Acta paediatrica 85:204–208
Chang L, Smith LM, LoPresti C, Yonekura ML, Kuo J, Walot I, Ernst T (2004) Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Res Neuroimaging 132:95–106
Chang L, Alicata D, Ernst T, Volkow N (2007) Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction 102:16–32
Chang L, Cloak C, Jiang CS, Farnham S, Tokeshi B, Buchthal S et al (2009) Altered neurometabolites and motor integration in children exposed to methamphetamine in utero. Neuroimage 48:391–397
Cloak CC, Ernst T, Fujii L, Hedemark B, Chang L (2009) Lower diffusion in white matter of children with prenatal methamphetamine exposure. Neurology 72:2068–2075
Cruickshank CC, Dyer KR (2009) A review of the clinical pharmacology of methamphetamine. Addiction 104:1085–1099
Cui C, Sakata Haga H, Ohta KI, Nishida M, Yashiki M, Sawada K, Fukui Y (2006) Histological brain alterations following prenatal methamphetamine exposure in rats. Congenit Anom 46:180–187
Cummings JL (1993) Frontal-subcortical circuits and human behavior. Arch Neurol 50:873
Degenhardt L, Hall W (2012) Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet 375:55–70
Della Grotta S, LaGasse LL, Arria AM, Derauf C, Grant P, Smith LM et al (2010) Patterns of methamphetamine use during pregnancy: results from the Infant Development, Environment, and Lifestyle (IDEAL) Study. Matern Child Health J 14:519–527
Eriksson M, Zetterström R (1994) Amphetamine addiction during pregnancy: 10-year follow-up. Acta Paediatr 83:27–31
Eriksson M, Larsson G, Winbladh B, Zetterström R (1978) The influence of amphetamine addiction on pregnancy and the newborn infant. Acta paediatrica Scandinavica 67:95
Eriksson M, Larsson G, Zetterström R (1981) Amphetamine addiction and pregnancy: ii. pregnancy, delivery and the neonatal period. Socio-medical aspects. Acta Obstet Gynecol Scand 60:253–259
Eriksson M, Billing L, Steneroth G, Zetterstrom R (1989) Health and development of 8-Year-old children whose mothers abused amphetamine during pregnancy. Acta Paediatr 78:944–949
Eriksson M, Jonsson B, Steneroth G, Zetterström R (1994) Cross-sectional growth of children whose mothers abused amphetamines during pregnancy. Acta Paediatr 83:612–617
Eriksson M, Jonsson B, Steneroth G, Zetterström R (2000) Amphetamine abuse during pregnancy: Environmental factors and outcome after 14-15 years. Scand J Public Health 28:154–157
Flames N, Hobert O (2011) Transcriptional control of the terminal fate of monoaminergic neurons. Annu Rev Neurosci 34:153–184
Fox MD, Greicius M (2010) Clinical applications of resting state functional connectivity. Front Syst Neurosci 4:19
Frost DO, Cadet JL (2000) Effects of methamphetamine-induced neurotoxicity on the development of neural circuitry: a hypothesis. Brain Res Rev 34:103–118
Golub M, Costa L, Crofton K, Frank D, Fried P, Gladen B et al (2005) NTP-CERHR Expert Panel Report on the reproductive and developmental toxicity of amphetamine and methamphetamine. Birth Defects Res B Dev Reprod Toxicol 74:471–584
Gomes-da-Silva J, Pérez-Rosado A, Miguel R, Fernández-Ruiz J, Silva M, Tavares MA (2002) Prenatal Exposure to Methamphetamine in the Rat. Ann N Y Acad Sci 965:68–77
Graham D, Amos-Kroohs R, Braun A, Grace C, Schaefer T, Skelton M et al (2011) Long-term receptor alterations from developmental methamphetamine exposure in rats by use of selective pharmacological agonists and antagonists. Neurotoxicol Teratol 33:497–498
Graham DL, Amos-Kroohs RM, Braun AA, Grace CE, Schaefer TL, Skelton MR et al (2013) Neonatal (+)-methamphetamine exposure in rats alters adult locomotor responses to dopamine D1 and D2 agonists and to a glutamate NMDA receptor antagonist, but not to serotonin agonists. Int J Neuropsychopharmacol 16:377–391
Greicius MD, Krasnow B, Reiss AL, Menon V (2003) Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 100:253–258
Helmich RC, Derikx LC, Bakker M, Scheeringa R, Bloem BR, Toni I (2010) Spatial remapping of cortico-striatal connectivity in Parkinson's disease. Cereb Cortex 20:1175–1186
Jedynak JP, Uslaner JM, Esteban JA, Robinson TE (2007) Methamphetamine-induced structural plasticity in the dorsal striatum. Eur J Neurosci 25:847–853
Jeng W, Wong AW, Ting-A-Kee R, Wells PG (2005) Methamphetamine-enhanced embryonic oxidative DNA damage and neurodevelopmental deficits. Free Radic Biol Med 39:317–326
Johnston MV, Ishida A, Ishida WN, Matsushita HB, Nishimura A, Tsuji M (2009) Plasticity and injury in the developing brain. Brain Dev 31:1–10
Kaewsuk S, Sae-ung K, Phansuwan-Pujito P, Govitrapong P (2009) Melatonin attenuates methamphetamine-induced reduction of tyrosine hydroxylase, synaptophysin and growth-associated protein-43 levels in the neonatal rat brain. Neurochem Int 55:397–405
Kaltenbach KA, Finnegan LP (1993) Studies of prenatal drug exposure and environmental research issues: the benefits of integrating research within a treatment program. NIDA Res Monogr 117:259–259
Khoshbouei H, Wang H, Lechleiter JD, Javitch JA, Galli A (2003) Amphetamine-induced dopamine efflux. A voltage-sensitive and intracellular Na + -dependent mechanism. J Biol Chem 278:12070
Kish SJ (2008) Pharmacologic mechanisms of crystal meth. CMAJ 178:1679–1682
Kreis R, Ernst T, Ross BD (1993) Absolute quantitation of water and metabolites in the human brain. II: metabolite concentrations. J Magn Reson 102:9–19
LaGasse LL, Wouldes T, Newman E, Smith LM, Shah RZ, Derauf C et al (2011) Prenatal methamphetamine exposure and neonatal neurobehavioral outcome in the USA and New Zealand. Neurotoxicol Teratol 33:166–175
LaGasse LL, Derauf C, Smith LM, Newman E, Shah R, Neal C et al (2012) Prenatal methamphetamine exposure and childhood behavior problems at 3 and 5 years of age. Pediatrics 129:681–688
Lester BM, Andreozzi L, Appiah L (2004) Substance use during pregnancy: time for policy to catch up with research. Harm Reduct J 1:5
Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM (2004) Striatal contributions to working memory: a functional magnetic resonance imaging study in humans. Eur J Neurosci 19:755–760
Little BB, Snell LM, Gilstrap LC (1988) Methamphetamine abuse during pregnancy: outcome and fetal effects. Obstet Gynecol 72:541–544
Lu LH, Johnson A, O’Hare ED, Bookheimer SY, Smith LM, O’Connor MJ, Sowell ER (2009) Effects of prenatal methamphetamine exposure on verbal memory revealed with fMRI. J Dev Behav Pediatr 30:185
Maisto SA, McKay JR, Connors GJ (1990) Self-report issues in substance abuse: State of the art and future directions. Behavioral Assessment 12:117–134
McKetin R, Kozel N, Douglas J et al (2008) The rise of methamphetamine in Southeast and East Asia. Drug and Alcohol Review 27:220–228
Melo P, Moreno VZ, Vázquez SP, Pinazo-Durán MD, Tavares MA (2006) Myelination changes in the rat optic nerve after prenatal exposure to methamphetamine. Brain Res 1106:21–29
Substance Abuse and Mental Health Services Administration (2010) Treatment episode data set (TEDS): 1998–2008. State admissions to substance abuse treatment services. DASIS Series: S-55 (HHS Publication No. SMA 10-4613). Rockville, MD: Author
Meredith CW, Jaffe C, Ang-Lee K, Saxon AJ (2005) Implications of chronic methamphetamine use: A literature review. Harv Rev Psychiatry 13:141–154
Morrow BA, Roth RH, Redmond DE, Elsworth JD (2011) Impact of methamphetamine on dopamine neurons in primates is dependent on age: implications for development of Parkinson’s disease. Neurosci 189:277–285
Nguyen D, Smith LM, LaGasse LL, Derauf C, Grant P, Shah R et al (2010) Intrauterine growth of infants exposed to prenatal methamphetamine: results from the infant development, environment, and lifestyle study. J Pediatr 157:337–339
Nordahl TE, Salo R, Leamon M (2003) Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: A review. J Neuropsychiatry Clin Neurosci 15:317–325
Ondersma SJ, Simpson SM, Brestan EV, Ward M (2000) Prenatal drug exposure and social policy: the search for an appropriate response. Child Maltreat 5:93–108
Oro AS, Dixon SD (1987) Perinatal cocaine and methamphetamine exposure: maternal and neonatal correlates. J Pediatr 111:571–578
Poland ML, Dombrowski MP, Ager JW, Sokol RJ (1993) Punishing pregnant drug users: enhancing the flight from care. Drug Alcohol Depend 31:199–203
Roberts S, Nuru-Jeter A (2010) Women’s perspectives on screening for alcohol and drug use in prenatal care. Women Health Iss 20:193–200
Roussotte FF, Bramen JE, Nunez SC, Quandt LC, Smith L, O’Connor MJ et al (2011) Abnormal brain activation during working memory in children with prenatal exposure to drugs of abuse: the effects of methamphetamine, alcohol, and polydrug exposure. NeuroImage 54:3067–3075
Salisbury AL, Ponder KL, Padbury JF, Lester BM (2009) Fetal effects of psychoactive drugs. Clin Perinatol 36:595
Schmitz Y, Lee CJ, Schmauss C, Gonon F, Sulzer D (2001) Amphetamine distorts stimulation-dependent dopamine overflow: effects on D2 autoreceptors, transporters, and synaptic vesicle stores. J Neurosci 21:5916–5924
Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I (2007) Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev 17:275–297
Semple SJ, Grant I, Patterson TL (2005) Female methamphetamine users: social characteristics and sexual risk behavior. Women & Health 40:35–50
Šlamberová R, Pometlová M, Charousová P (2006) Postnatal development of rat pups is altered by prenatal methamphetamine exposure. Prog Neuropsychopharmacol Biol Psychiatry 30:82–88
Smith LM, Chang L, Yonekura ML, Grob C, Osborn D, Ernst T (2001) Brain proton magnetic resonance spectroscopy in children exposed to methamphetamine in utero. Neurol 57:255–260
Smith LM, LaGasse LL, Derauf C, Grant P, Shah R, Arria A et al (2006) The infant development, environment, and lifestyle study: effects of prenatal methamphetamine exposure, polydrug exposure, and poverty on intrauterine growth. Pediatrics 118:1149–1156
Smith DK, Johnson AB, Pears KC, Fisher PA, DeGarmo DS (2007) Child maltreatment and foster care: unpacking the effects of prenatal and postnatal parental substance use. Child Maltreat 12:150–160
Smith LM, LaGasse LL, Derauf C, Grant P, Shah R, Arria A et al (2008) Prenatal methamphetamine use and neonatal neurobehavioral outcome. Neurotoxicol Teratol 30:20–28
Smith LM, LaGasse LL, Derauf C, Newman E, Shah R, Haning W et al (2011) Motor and cognitive outcomes through three years of age in children exposed to prenatal methamphetamine. Neurotoxicol Teratol 33:176–184
Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW (2003) Mapping cortical change across the human life span. Nat Neurosci 6:309–315
Sowell ER, Leow AD, Bookheimer SY, Smith LM, O’Connor MJ, Kan E et al (2010) Differentiating prenatal exposure to methamphetamine and alcohol versus alcohol and not methamphetamine using tensor-based brain morphometry and discriminant analysis. J Neurosci 30:3876–3885
Stanwood GD, Levitt P (2004) Drug exposure early in life: functional repercussions of changing neuropharmacology during sensitive periods of brain development. Curr Opin Pharmacol 4:65–71
Sulzer D, Sonders MS, Poulsen NW, Galli A (2005) Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol 75:406–433
Suzuki T, Mizuo K, Nakazawa H, Funae Y, Fushiki S, Fukushima S et al (2003) Prenatal and neonatal exposure to bisphenol-A enhances the central dopamine D1 receptor-mediated action in mice: enhancement of the methamphetamine-induced abuse state. Neurosci 117:639–644
Terplan M, Smith EJ, Glavin SH (2010) Trends in injection drug use among pregnant women admitted into drug treatment: 1994–2006. J Womens Health 19:499–505
Thompson BL, Levitt P, Stanwood GD (2009) Prenatal exposure to drugs: effects on brain development and implications for policy and education. Nat Rev Neurosci 10:303–312
Twomey J, LaGasse L, Derauf C, Newman E, Shah R, Smith L et al (2013) Prenatal methamphetamine exposure, home environment, and primary caregiver risk factors predict child behavioral problems at 5 Years. Am J Orthopsychiatry 83:64–72
United Nations Office on Drugs, & Crime (2010) World Drug Report 2010. United Nations Publications
United Nations Office on Drugs, and Crime (2013) World Drug Report 2013. United Nations Publications
Vaccarino FM, Ment LR (2004) Injury and repair in developing brain. Arch Dis Child Fetal Neonatal Ed 89:190–192
Weiss K, Melkus G, Jakob PM, Faber C (2009) Quantitative in vivo (1)H spectroscopic imaging of metabolites in the early postnatal mouse brain at 17.6 T. MAGMA 22:53–62
Won L, Bubula N, McCoy H, Heller A (2001) Methamphetamine concentrations in fetal and maternal brain following prenatal exposure. Neurotoxicol Teratol 23:349–354
Wouldes TA, LaGasse LL, Derauf C, Newman E, Shah R, Smith LM et al (2013) Co-morbidity of substance use disorder and psychopathology in women who use methamphetamine during pregnancy in the US and New Zealand. Drug Alcohol Depend 127:101–107
Acknowledgments
The South African National Research Foundation (NRF) and the Medical Research Council (MRC) of South Africa supported this research.
Conflict of interest statement
We declare no conflict of interest. The material contained herein is original; it is not currently submitted elsewhere, and has not been published previously.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kwiatkowski, M.A., Roos, A., Stein, D.J. et al. Effects of prenatal methamphetamine exposure: a review of cognitive and neuroimaging studies. Metab Brain Dis 29, 245–254 (2014). https://doi.org/10.1007/s11011-013-9470-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-013-9470-7