Abstract
Neurocognitive dysfunction of varying degrees is common in patients with hepatitis B virus-related cirrhosis (HBV-RC) without overt hepatic encephalopathy (OHE). However, the neurobiological mechanisms underlying these dysfunctions are not well understood. We sought to identify changes in the neural activity of patients with HBV-RC without OHE in the resting state by using the amplitude of low-frequency fluctuation (ALFF) method and to determine whether these changes were related to impaired cognition. Resting-state functional MRI data from 30 patients with HBV-RC and 30 healthy controls matched for age, sex, and years of education were compared to determine any differences in the ALFF between the two groups. Cognition was measured with the psychometric hepatic encephalopathy score (PHES), and the relationship between these scores and ALFF variation was assessed. Compared with controls, patients showed widespread lower standardized ALFF (mALFF) values in visual association areas (bilateral lingual gyrus, middle occipital gyrus, and left inferior temporal gyrus), motor-related areas (bilateral precentral gyrus, paracentral lobule, and right postcentral gyrus), and the default mode network (bilateral cuneus/precuneus and inferior parietal lobule). Higher mALFF values were found in the bilateral orbital gyrus/rectal gyrus. In patients, mALFF values were significantly positive correlated with the PHES in the right middle occipital gyrus and bilateral precentral gyrus. Our findings of resting-state abnormalities in patients with HBV-RC without OHE suggest that neurocognitive dysfunction in patients with HBV-RC without OHE may be caused by abnormal neural activity in multiple brain regions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hepatitis B virus-related cirrhosis (HBV-RC) is a serious health problem in Asia with high infection, morbidity, and mortality rates (Moriwaki et al. 2010). Hepatic encephalopathy (HE) is the most common neuropsychiatric complication in end-stage HBV-RC. It is characterized by a wide spectrum of mental status, ranging from subtle cognitive dysfunction to severe coma. Clinically, overt hepatic encephalopathy (OHE) is easily identified in patients with cirrhosis by the appearance of neuropsychiatric symptoms and is usually staged according to the West Haven criteria. In contrast, patients with cirrhosis without OHE always present as essentially normal on clinical neuropsychiatric examination. However, increasing data (Dhiman and Chawla 2009; Li et al. 2004; Moriwaki et al. 2010) indicate that neurocognitive dysfunction of varying degrees is common in patients without any signs of OHE.
Neurocognitive dysfunction in cirrhosis without OHE has recently become more relevant because it has been associated with OHE, mortality, poorer quality of life, and deterioration in daily functioning (Cordoba 2011). As a result, several approaches have been used to explore cerebral function in these patients, including neuropsychological tests, electroencephalography, critical flicker frequency, and so on (Cordoba 2011; Weissenborn 2008). These studies reported cognitive deficits mainly in visual perception, visuoconstructive abilities, impaired fine motor skills and attention, whereas general intelligence and speaking are preserved (Dhiman and Chawla 2009; Li et al. 2004; Weissenborn 2008). However, most of these tests have been developed to diagnose minimal HE and do not reveal the underlying pathophysiology mechanisms.
MRI is a non-invasive technique that can be used to assess metabolic changes in the brain, impaired brain function, and brain biochemistry. Magnetic resonance spectroscopy studies revealed that myoinositol and choline signals are reduced and that glutamine-glutamate signals are increased in several brain regions in patients with cirrhosis (Huda et al. 2008; McPhail and Taylor-Robinson 2010). Magnetization transfer imaging and diffusion tensor imaging studies, which reflect the integrity of the white mater tract, also reveal mild and diffuse edema in cirrhotic brains (Huda et al. 2008; McPhail and Taylor-Robinson 2010). A recent study (Chen et al. 2012a) combining voxel-based morphometry with voxel-based diffusion tensor imaging found that patients with HBV-RC without OHE exhibited diffuse white matter abnormalities, including decreased white matter volume and fractional anisotropy values. However, the underlying functional changes in brains of these patients are still not fully understood.
Functional MRI (fMRI) has been extensively used to investigate various brain functions. As a new branch of fMRI, resting-state fMRI can reflect the baseline brain activity. Biswal et al. (Biswal et al. 1995) reported that the low-frequency (0.01–0.08 Hz) fluctuations of the resting-state fMRI signal are physiologically important and suggested that they reflect spontaneous neuronal activity (Biswal et al. 1995). Evidence increasingly indicates that the pathophysiology of many neurocognitive dysfunctions may be associated with the spontaneous changes in brain activity fluctuations as measured with resting-state fMRI (Fox et al. 2005; Mantini et al. 2007). More recently, amplitude of low-frequency fluctuation (ALFF) (Zang et al. 2007), a method developed to analyze these low-frequency fluctuations, has also been used as an effective fMRI algorithm to detect a wide range of brain disorders, such as the Alzheimer’s disease (Dai et al. 2012), depressive disorder (Zhu et al. 2012), and hepatic encephalopathy (HE) (Chen et al. 2012b; Qi et al. 2012). Several recent fMRI studies (Chen et al. 2012b; Qi et al. 2012) with ALFF have found that cirrhotic patients had wide-spreadly abnormal spontaneous brain activity. Nevertheless, the results of these studies were not entirely consistent, perhaps because of differences in the causes of cirrhosis, which may have different effects on cerebral alterations (Burra et al. 2004). Additionally, most of these studies were focused on abnormal baseline brain activity in cirrhotic patients with low-grade HE or OHE.
Abnormalities in ALFF in patients with HBV-RC have not been studied intensively. Moreover, little is known about the relationships between these neurocognitive impairments and any functional abnormalities in those patients. The purpose of this study was to identify modulations in neural activity in patients with HBV-RC without OHE in the resting state with the ALFF method. More importantly, we also sought to determine whether changes of neural activity were related to the psychometric hepatic encephalopathy score (PHES), a validated battery of psychometric tests used to assess the peculiar cognitive impairments in patients with cirrhosis (Duarte-Rojo et al. 2011; Weissenborn 2008).
Patients and methods
Patients
Thirty patients (25 male; mean age, 45.6 ± 9.4 years; age range, 32 ~ 66 years) with chronic liver cirrhosis caused by HBV infection were included in this prospective study. All patients had HBV-RC diagnosed by biopsy or on the basis of case history, clinical examination, biochemical and imaging findings. Overt hepatic encephalopathy was diagnosed when the West Haven criteria indicated stage I disease or higher. Patients were excluded if they had current symptoms of OHE at the time of recruitment, any history of OHE, other types of viral hepatitis, gastrointestinal hemorrhage or bacterial infection (within 1 month before the study), a transjugular intrahepatic portosystemic or a surgical portocaval shunt, diffuse hepatocellular carcinoma, or were taking drugs that could alter cerebral function. All patients underwent a detailed clinical examination. The severity of liver disease was determined according to the Child-Pugh score.
For comparison, 30 healthy controls (25 male; mean age, 45.2 ± 9.1 years; age range, 32 ~ 63 years) matched for age (within 3 years), sex and years of education (within 3 years), without disease of liver and other systems, were recruited through advertising within the hospital. All controls received detailed clinical and neurological examinations on the same day as the fMRI scans.
Exclusion criteria for all patients and controls included age lower than 18 or greater than 70 years, alcoholism, neurological or psychiatric diseases, a history of substantial head trauma, infection with human immunodeficiency virus, hypertension, diabetes, poor vision, other major medical illness, left-handedness, and any focal abnormality detected on routine brain MRI examinations.
This study was approved by the Medical Research Ethics Committee of Nanfang Hospital, Southern Medical University. Written informed consent was obtained from all the participants before the study. The clinical and demographic data of patients with HBV-RC without OHE and of healthy controls are shown in Table 1.
Neuropsychological tests
The PHES is calculated from a standardized battery of psychometric tests developed to assess the degree of cognitive impairment in patients with cirrhosis (Weissenborn 2008). All 30 patients and 30 controls completed the five paper-and-pencil psychometric tests that comprise the PHES—number connection tests A and B, the digit symbol test, the serial dotting test, and the line-tracing test—after an appropriate explanation and demonstration. The physicians participating in the study were trained in conducting and evaluating these psychometric tests. The PHES was generated from the sum of the scores of the five tests results from the five psychometric tests, with a range from +5 to −15 points. The method of calculating the PHES is reported in detail elsewhere (Lv et al. 2013).
Imaging protocol
All MRI data were obtained with a 1.5-T MR imager (Achieva Nova-Dual; Philips, Best, the Netherlands) in the Department of Medical Imaging, Guangdong No. 2 Provincial People’s Hospital. Each participant was placed in a standard head coil fitted with foam padding to reduce head motion. Each was instructed to close both eyes, to keep as still as possible, and not to think of anything in particular or to fall asleep in the scanner. Routine imaging studies consisted of T1-weighted images and T2-FLAIR images acquired to detect clinically silent lesions. FMRI was conducted using an echo-planar imaging sequence with the following settings: repetition time, 3,000 ms; echo time, 50 ms; flip angle, 90°; field of view, 230 × 230 mm; matrix, 64 × 64; and total volumes, 160. Axial slices (n = 33) were collected at a thickness of 4.5 mm and no interslice gap. Resolution was 3.59 × 3.59 mm in-plane. Each fMRI scan lasted 8 min. After the examination, all participants were asked some questions to verify the degree of their adherence to instructions.
Image analysis
Imaging data were mainly preprocessed with Statistical Parametric Mapping (SPM8, http://www.fil.ion.ucl.ac.uk/spm). The first 10 time points for each participant were discarded to avoid transient signal changes before magnetization reached steady-state and to allow the participant to get used to the fMRI scanning noise. Raw data were corrected for slice-timing and realigned for head movement correction (data from participants with movement more than 1.5 mm of translation or more than 1.5° of rotation in any direction were excluded). Afterwards, all of the realigned images were spatially normalized into the Montreal Neurological Institute template, and each voxel was resampled to isotropic 3 × 3 × 3 mm3. The resting-state images were then spatially smoothed with an isotropic Gaussian kernel (full-width at half-maximum, 8 mm).
Further data preprocessing and ALFF analysis were performed with the REST software (http://resting-fmri.sourceforge.net). Preprocessing also included removing linear trends and temporally filtered (band pass, 0.01–0.08 Hz) to remove the effects of very-low-frequency drift and high-frequency noise. The procedure for calculating ALFF is described elsewhere (Chen et al. 2012b; Qi et al. 2012; Zang et al. 2007). The filtered time series was transformed to a frequency domain, and the power spectrum was obtained. The square root was thus calculated at each frequency of the power spectrum and the averaged square root was obtained across 0.01–0.08 Hz at each voxel. This averaged square root was taken as the ALFF. For standardization purposes, the ALFF of each voxel was divided by the global mean ALFF value. The standardized ALFF (mALFF) of each voxel should have a value of about 1. This standardization procedure is analogous to that used in positron emission tomography studies (Raichle et al. 2001).
Statistical methods
Wilcoxon signed-rank tests were used to analyze the differences in the PHES between two groups. Analyses were conducted using software (SPSS, version 13.0; Chicago, III), and a P value less than 0.05 was deemed significant.
To investigate differences in ALFF between the patients and controls, a two-sample t-test was executed on the individual mALFF maps in a voxel-by-voxel manner using age and years of education as covariates. Multiple comparisons were corrected using the AlphaSim program in the AFNI software determined by Monte Carlo simulations. Statistical maps of the two-sample t-test were created using a combined threshold of P < 0.001 and a minimum cluster size of 22 voxels, yielding a corrected threshold of P < 0.05.
We also performed a whole-brain voxel-based correlation analysis between mALFF values and the PHES using age and years of education as covariates. Given the exploratory nature of the study, we adopted a relatively liberal statistical threshold (A corrected significance level of P < 0.05 was obtained by clusters with a minimum cluster size of 74 voxels at an uncorrected individual voxel height threshold of P < 0.01). Then, mean mALFF values of all significantly different clusters revealed by voxel-based correlation analysis were extracted separately using the extract time series in REST and were input into SPSS. Finally, using age and years of education as covariates, the relationship between mean mALFF values in significant different areas and the PHES in patients was assessed with Spearman’s correlation coefficient. P < 0.05 (two-tailed) was used to determine significant correlations.
Results
Differences in PHES
Compared with healthy controls, cirrhotic patients performed significantly worse on the PHES test (−3 [−9 to 1] vs. [−2 to 3] respectively; P < 0.001).
ALFF changes in patients with HBV-RC without OHE
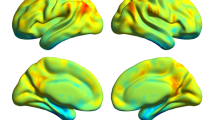
Differences in mALFF values between the patients and controls were widespread (Fig. 1 and Table 2). Compared with controls, patients had significantly lower mALFF values in the bilateral lingual gyrus, cuneus/precuneus/middle occipital gyrus, precentral gyrus, inferior parietal lobule and paracentral lobule, left inferior temporal gyrus and right postcentral gyrus. The values of mALFF were higher in the bilateral orbital gyrus/rectal gyrus.
Regions showing different mALFF values between patients with HBV-related cirrhosis and healthy controls. Regions showing higher mALFF values in the bilateral orbital gyrus in patients with HBV-related cirrhosis and lower mALFF values in the bilateral lingual gyrus, cuneus/precuneus/middle occipital gyrus, precentral gyrus, inferior parietal lobule and paracentral lobule, left inferior temporal gyrus cuneus and right postcentral gyrus. Threshold was set P < 0.05 (AlphaSim corrected). mALFF standardized amplitude of low-frequency fluctuation
Correlations between ALFF and PHES
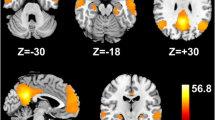
The regions in which mALFF values were significantly and positively correlated with the PHES were the right middle occipital gyrus (r = 0.66, P < 0.001) and bilateral precentral gyrus (left: r = 0.73, P < 0.001; right: r = 0.64, P < 0.001; Fig. 2 and Table 3).
The results of correlation analysis in patients with HBV-related cirrhosis (P < 0.05, AlphaSim corrected): regions in which mALFF values were significantly correlated with the PHES (a). Red means a positive correlation. The PHES positively correlated with ALFF values in the right MOG (b), bilateral PCG (c, d). mALFF standardized amplitude of low-frequency fluctuation, PHES psychometric hepatic encephalopathy score, MOG middle occipital gyrus, PCG precentral gyrus
Discussion
Compared with controls, the lower mALFF values in HBV-RC patients without OHE were widely distributed, mainly in the visual association areas (the bilateral lingual gyrus and the middle occipital gyrus and the left inferior temporal gyrus), motor-related areas (the bilateral precentral gyrus and the paracentral lobule, and the right postcentral gyrus), and the default-mode network (the bilateral cuneus/precuneus and inferior temporal gyrus). Higher mALFF values were also found mainly in the prefrontal cortex (the bilateral orbital gyrus/rectal gyrus). More importantly, mALFF values in right middle occipital gyrus and bilateral precentral gyrus correlated with the PHES.
The blood oxygenation level-dependent (BOLD) signal indirectly reflects neural activity and low-frequency fluctuations in the resting state. Although the exact biologic mechanisms of ALFF remain unclear, many studies have reported that the ALFF changes in the BOLD signal are associated with local neuronal spontaneous activity (Biswal et al. 1995; Zang et al. 2007). Different fMRI algorithms may reflect different aspects of comprehensive brain functions. Compared with other methods, such as functional connectivity analysis, ALFF has the advantage of directly reflecting the amplitude or intensity of spontaneous activity (Zang et al. 2007). Thus, to explore the alterations of brain activity by measuring the resting-state ALFF across the whole cerebral gray matter may provide more information on the dysfunction of cirrhotic brains.
The lingual gyrus, as the key part of visual cortex, is the visual processing center in the brain. This area is important in color perception, visual discrimination, and visual attention (Lee et al. 2000). The middle occipital gyrus is considered to be a part of the visual dorsal stream in sighted subjects (Wandell et al. 2007). The inferior temporal cortex is part of the ventral pathway, also called the “what” pathway, for visual object processing (Hitomi et al. 2012). The precentral gyrus cortex (M1, BA4) is intimately related to motor control. The paracentral lobule has been reported to be involved with lower extremity movements and attention to somatosensory stimulation (Forss et al. 1996; Lim et al. 1994). The postcentral gyrus is the location of the primary somatosensory cortex (SI, BA3, 1, 2), which is initially involved in processing information related to tactile awareness.
Our finding of markedly lower mALFF values in these visual-association and motor-related areas in cirrhotic brains is consistent with the findings from a recent study (Lv et al. 2013) in which a regional homogeneity method in another group of patients with HBV-RC without OHE detected decreased coherence of spontaneous neuronal activity in visual association (in the left lingual gyrus, middle temporal gyrus, and right middle occipital gyrus) and motor-related areas (the bilateral precentral gyrus and paracentral lobule). Positron emission tomography also reveals reduced bilateral cerebral glucose utilization in a medial portion of the primary and visual association cortices (Lockwood et al. 2002), as well as motor cortices (Weissenborn et al. 2007) in patients with cirrhosis.
Recently, resting-state studies (Chen et al. 2012b; Qi et al. 2012) with the ALFF analysis algorithm also revealed decreased mALFF values in the visual (such as the lingual gyrus, middle occipital gyrus, and middle temporal gyrus) and motor associated areas (such as the supplemental motor area and right postcentral gyrus) in patients with low-grade or minimal HE. Given the findings of these neuroimaging studies, the reduced mALFF values in the visual association cortex and motor-related areas in patients with HBV-RC in the present study suggest dysfunction in those regions.
An important finding in this study was the positive correlation between the PHES and mean mALFF values in the visual-association area (the right middle occipital gyrus) and motor-related areas (the bilateral precentral gyrus). The PHES reliably reflects most minimal HE-related neuropsychological impairments because it assesses visual perception, construction, visual/spatial orientation, motor speed and accuracy, concentration, and attention in patients with cirrhosis (Weissenborn 2008; Weissenborn et al. 2001). The lower the cognitive abilities, the lower the mALFF values in those brain regions in patients with HBV-RC without OHE. Thus, we speculate that the decreased neuronal activity at baseline in the visual association and motor-related areas may contribute to the deficits in processing visual information and motor control, leading to some typical cognitive impairments in these patients, such as deficits in visual perception, visuoconstructive abilities, and impaired fine motor performance (Amodio et al. 2008; Weissenborn 2008). In turn, the significant correlation between the mALFF values and the PHES in spontaneous activity in the right middle occipital gyrus and the bilateral precentral gyrus indicate that differences in these regions could provide a new and objective biomarker of the progression of the cognitive impairment in these patients.
In the present study, mALFF values were lower in the bilateral cuneus/precuneus and inferior temporal gyrus, which are part of the default mode network (Raichle et al. 2001). This network helps maintain baseline brain activities related to self-awareness, episodic memory, and interactive modulation between internal mind activities and external tasks (Raichle et al. 2001). Neuroimaging studies (Chen et al. 2012b, c; Lv et al. 2013; Qi et al. 2012) consistently find that patients with cirrhosis have abnormal resting-state brain activity in regions within the default mode network, such as the precuneus and inferior parietal lobule. Thus, our study provides more evidence that the default mode network is involved in the cognitive deficits in these patients.
Interestingly, compared with healthy controls, patients with HBV-RC without OHE showed higher mALFF values in the bilateral orbital gyrus/rectal gyrus and those regions belong to a part of prefrontal cortex, which is the most complex and highly evolved neurocortex region. Abnormities of the prefrontal cortex have been reported in many neuroimaging studies (Huda et al. 2008; Weissenborn et al. 2007). However, as noted before (Chen et al. 2012c; Lv et al. 2013; Ni et al. 2012), performance on neuropsychological tests was not significantly correlated with indices of abnormal brain activity in this critical area in cirrhotic patients without OHE. Decreased functional activity in the resting state could be related to functional impairment, whereas an increase could be interpreted as compensatory reallocation or recruitment of cognitive resources. Recently, a compensatory neural mechanism during the visual judgment (Zafiris et al. 2004) and in the resting state (Qi et al. 2012; Chen et al. 2012b, c) has also been reported in cirrhotic patients without OHE. Thus, the higher mALFF values of the prefrontal cortex (the bilateral orbital gyrus/rectal gyrus) may compensate for the deficits in visual processing and impaired motor function in these patients. However, this concept needs to be confirmed.
Some limitations in our study are worth mentioning. First, as a preliminary study, our results are limited to a small patient cohort. A large-cohort study is needed. Also, a proper cutoff PHES for diagnosing minimal HE has not been established in our country. Thus, further group analysis is needed. Third, the fMRI of the BOLD signal relies on susceptibility-sensitive gradient-echo sequences, such as the echo-planar imaging sequence (Weiskopf et al. 2007). Thus, certain areas of the brain, especially the inferior brain regions, such as the orbital gyrus/rectal gyrus, are subject to signal loss and distortion from susceptibility artifacts. This loss is difficult to avoid, and an optimal echo-planar imaging settings are needed (Weiskopf et al. 2007). Finally, the slow sampling rate, which is frequently used in resting-state fMRI studies because it permits scanning the entire brain, could not be avoided. At a repetition time of slow sampling rates (as in this study TR = 3,000 ms) for multisection acquisitions, the cardiac and respiratory fluctuation effects might be aliased into the low-frequency BOLD MR signal fluctuations (Lowe et al. 1998). In future studies, simultaneous cardiac recording may provide a more direct correction.
Conclusions
In summary, we found that patients with HBV-RC but without OHE have diffuse abnormalities in intrinsic brain activity and lower mALFF values mainly distributed in the visual-association areas, motor-related areas, and the default mode network, as well as higher mALFF values in the prefrontal cortex. In addition, the right middle occipital gyrus and bilateral precentral gyrus intrinsic activity were related to the PHES. Resting-state fMRI with ALFF analysis provided more evidence that the pathogenesis of neurocognitive dysfunction in patients with HBV-RC without OHE may be attributed to abnormal neural activity in multiple brain regions.
Abbreviations
- HBV-RC:
-
HBV-related cirrhosis
- OHE:
-
Overt hepatic encephalopathy
- ALFF:
-
Amplitude of low-frequency fluctuation
- mALFF:
-
Standardized amplitude of low-frequency fluctuation
- PHES:
-
Psychometric hepatic encephalopathy score
- HE:
-
Hepatic encephalopathy
- fMRI:
-
Functional MRI
- BOLD:
-
Blood oxygenation level-dependent
References
Amodio P, Campagna F, Olianas S, Iannizzi P, Mapelli D, Penzo M, Angeli P, Gatta A (2008) Detection of minimal hepatic encephalopathy: normalization and optimization of the Psychometric Hepatic Encephalopathy Score. A neuropsychological and quantified EEG study. J Hepatol 49:346–353
Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34:537–541
Burra P, Senzolo M, Pizzolato G, Ermani M, Chierichetti F, Bassanello M, Naccarato R, Dam M (2004) Does liver-disease aetiology have a role in cerebral blood-flow alterations in liver cirrhosis? Eur J Gastroenterol Hepatol 16:885–890
Chen HJ, Wang Y, Zhu XQ, Cui Y, Chen YC, Teng GJ (2012a) White matter abnormalities correlate with neurocognitive performance in patients with HBV-related cirrhosis. J Neurol Sci 321:65–72
Chen HJ, Zhu XQ, Jiao Y, Li PC, Wang Y, Teng GJ (2012b) Abnormal baseline brain activity in low-grade hepatic encephalopathy: a resting-state fMRI study. J Neurol Sci 318:140–145
Chen HJ, Zhu XQ, Yang M, Liu B, Zhang Y, Wang Y, Teng GJ (2012c) Changes in the regional homogeneity of resting-state brain activity in minimal hepatic encephalopathy. Neurosci Lett 507:5–9
Cordoba J (2011) New assessment of hepatic encephalopathy. J Hepatol 54:1030–1040
Dai Z, Yan C, Wang Z, Wang J, Xia M, Li K, He Y (2012) Discriminative analysis of early Alzheimer’s disease using multi-modal imaging and multi-level characterization with multi-classifier (M3). NeuroImage 59:2187–2195
Dhiman RK, Chawla YK (2009) Minimal hepatic encephalopathy. Indian J Gastroenterol 28:5–16
Duarte-Rojo A, Estradas J, Hernandez-Ramos R, Ponce-de-Leon S, Cordoba J, Torre A (2011) Validation of the psychometric hepatic encephalopathy score (PHES) for identifying patients with minimal hepatic encephalopathy. Dig Dis Sci 56:3014–3023
Forss N, Merlet I, Vanni S, Hamalainen M, Mauguiere F, Hari R (1996) Activation of human mesial cortex during somatosensory target detection task. Brain Res 734:229–235
Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005) The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 102:9673–9678
Hitomi T, Koubeissi MZ, Kaffashi F, Turnbull J, Luders HO (2012) Visual processing in the inferior temporal cortex: an intracranial event related potential study. Clin Neurophysiol
Huda A, Gupta RK, Rajakumar N, Thomas MA (2008) Role of magnetic resonance in understanding the pathogenesis of hepatic encephalopathy. Magn Reson Insights 2:109–122
Lee HW, Hong SB, Seo DW, Tae WS, Hong SC (2000) Mapping of functional organization in human visual cortex: electrical cortical stimulation. Neurology 54:849–854
Li YY, Nie YQ, Sha WH, Zeng Z, Yang FY, Ping L, Jia L (2004) Prevalence of subclinical hepatic encephalopathy in cirrhotic patients in China. World J Gastroenterol 10:2397–2401
Lim SH, Dinner DS, Pillay PK, Luders H, Morris HH, Klem G, Wyllie E, Awad IA (1994) Functional anatomy of the human supplementary sensorimotor area: results of extraoperative electrical stimulation. Electroencephalogr Clin Neurophysiol 91:179–193
Lockwood AH, Weissenborn K, Bokemeyer M, Tietge U, Burchert W (2002) Correlations between cerebral glucose metabolism and neuropsychological test performance in nonalcoholic cirrhotics. Metab Brain Dis 17:29–40
Lowe MJ, Mock BJ, Sorenson JA (1998) Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. NeuroImage 7:119–132
Lv XF, Qiu YW, Tian JZ, Xie CM, Han LJ, Su HH, Liu ZY, Peng JP, Lin CL, Wu MS, Jiang GH, Zhang XL (2013) Abnormal regional homogeneity of resting-state brain activity in patients with HBV-related cirrhosis without overt hepatic encephalopathy. Liver Int 33:375–383
Mantini D, Perrucci MG, Del GC, Romani GL, Corbetta M (2007) Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci U S A 104:13170–13175
McPhail MJ, Taylor-Robinson SD (2010) The role of magnetic resonance imaging and spectroscopy in hepatic encephalopathy. Metab Brain Dis 25:65–72
Moriwaki H, Shiraki M, Iwasa J, Terakura Y (2010) Hepatic encephalopathy as a complication of liver cirrhosis: an Asian perspective. J Gastroenterol Hepatol 25:858–863
Ni L, Qi R, Zhang LJ, Zhong J, Zheng G, Zhang Z, Zhong Y, Xu Q, Liao W, Jiao Q, Wu X, Fan X, Lu GM (2012) Altered regional homogeneity in the development of minimal hepatic encephalopathy: a resting-state functional MRI study. PLoS One 7:e42016
Qi R, Zhang L, Wu S, Zhong J, Zhang Z, Zhong Y, Ni L, Zhang Z, Li K, Jiao Q, Wu X, Fan X, Liu Y, Lu G (2012) Altered resting-state brain activity at functional MR imaging during the progression of hepatic encephalopathy. Radiology 264:187–195
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001) A default mode of brain function. Proc Natl Acad Sci U S A 98:676–682
Wandell BA, Dumoulin SO, Brewer AA (2007) Visual field maps in human cortex. Neuron 56:366–383
Weiskopf N, Hutton C, Josephs O, Turner R, Deichmann R (2007) Optimized EPI for fMRI studies of the orbitofrontal cortex: compensation of susceptibility-induced gradients in the readout direction. MAGMA 20:39–49
Weissenborn K (2008) PHES: one label, different goods?! J Hepatol 49:308–312
Weissenborn K, Ennen JC, Schomerus H, Ruckert N, Hecker H (2001) Neuropsychological characterization of hepatic encephalopathy. J Hepatol 34:768–773
Weissenborn K, Ahl B, Fischer-Wasels D, van den Hoff J, Hecker H, Burchert W, Kostler H (2007) Correlations between magnetic resonance spectroscopy alterations and cerebral ammonia and glucose metabolism in cirrhotic patients with and without hepatic encephalopathy. Gut 56:1736–1742
Zafiris O, Kircheis G, Rood HA, Boers F, Haussinger D, Zilles K (2004) Neural mechanism underlying impaired visual judgement in the dysmetabolic brain: an fMRI study. NeuroImage 22:541–552
Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ, Wang YF (2007) Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev 29:83–91
Zhu Z, Lu Q, Meng X, Jiang Q, Peng L, Wang Q (2012) Spatial patterns of intrinsic neural activity in depressed patients with vascular risk factors as revealed by the amplitude of low-frequency fluctuation. Brain Res 1483:82–88
Acknowledgments
We thank the participants who magnanimously donate their time to participate in this study. Also, the authors are highly grateful to the anonymous reviewers for their significant and constructive comments and suggestions, which greatly improve the article.
Disclosures
The authors declare that no conflict of interest exists concerning this paper.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Drs. Xiao-Fei Lv, Min Ye and Lu-Jun Han contributed to the work equally.
Rights and permissions
About this article
Cite this article
Lv, XF., Ye, M., Han, LJ. et al. Abnormal baseline brain activity in patients with HBV-related cirrhosis without overt hepatic encephalopathy revealed by resting-state functional MRI. Metab Brain Dis 28, 485–492 (2013). https://doi.org/10.1007/s11011-013-9420-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-013-9420-4