Abstract
Tyrosine accumulates in inborn errors of tyrosine catabolism, especially in tyrosinemia type II, where tyrosine levels are highly elevated in tissues and physiological fluids of affected patients. Tyrosinemia type II is a disorder of autosomal recessive inheritance characterized by neurological symptoms similar to those observed in patients with creatine deficiency syndromes. Considering that the mechanisms of brain damage in these disorders are poorly known, in the present study our main objective was to investigate the in vivo and in vitro effects of different concentrations and preincubation times of tyrosine on cytosolic and mitochondrial creatine kinase activities of the cerebral cortex from 14-day-old Wistar rats. The cytosolic CK was reduced by 15% at 1 mM and 32% at 2 mM tyrosine. Similarly, the mitochondrial CK was inhibited by 15% at 1 mM and 22% at 2 mM tyrosine. We observed that the inhibition caused by tyrosine was concentration-dependent and was prevented by reduced glutathione. Results also indicated that mitochondrial, but not cytosolic creatine kinase activity was inhibited by tyrosine in a time-dependent way. Finally, a single injection of L-Tyrosine methyl ester administered i.p. decreased cytosolic (31%) and mitochondrial (18%) creatine kinase activities of brain cortex from rats. Considering that creatine kinase is an enzyme dependent of thiol residues for its function and tyrosine induces oxidative stress, the results suggest that the inhibition caused by tyrosine might occur by oxidation of essential sulfhydryl groups of the enzyme. In case this also occurs in patients with tyrosinemia, it is possible that creatine kinase inhibition may contribute to the neurological dysfunction characteristic of tyrosinemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tyrosinaemia type II, also known as Richner–Hanhart syndrome or oculocutaneous tyrosinaemia, is an autosomal recessive inherited disorder caused by deficiency of hepatic cytosolic tyrosine aminotransferase (TAT) (Ruetschi et al. 2000). This disease is often associated with consanguinity and the incidence is less than 1 in 250,000 (Macsai et al. 2001). It is characterized by bilateral pseudodendritic keratitis, painful palmoplantar hyperkeratotic lesions and mental retardation (Mitchell et al. 2001). The involvement of the central nervous system (CNS) is variable and ranges from severe mental retardation to slight decrease in intelligence and may be associated with microcephaly, tremor, ataxia, self-mutilating behavior, fine motor coordination disturbances, language deficits and convulsions (Rabinowitz et al. 1995; Mitchell et al. 2001).
Plasma concentrations of tyrosine are raised and the disorder is successfully treated with a low-phenylalanine and low-tyrosine diet (Mitchell et al. 2001). Moreover, plasma tyrosine levels are higher in TAT-deficiency comparing to the other causes of hypertyrosinemia and range from 370 to 3,420 μM (normal <90 μM) along with the accumulation of some tyrosine derivatives, namely 4-hydroxyphenylpyruvic acid, 4-hydroxy-phenyllactic acid and 4-hydroxyphenylacetic acid (Rabinowitz et al. 1995; Mitchell et al. 2001; Held 2006). Neuropathologic findings of tyrosinemic patients include metabolic astrocytosis and delay in myelination. Sener (2005) also reported intramyelinic edema attributable to status spongious in a 5-month-old boy with tyrosinemia type I and Proton MR spectroscopy confirmed the presence of tyrosine in the lesion sites. Although a causal link with hypertyrosinemia is not formally established, current data do not eliminate the possibility that elevated levels of tyrosine and/or its derivatives may have noxious effects on CNS development in these patients (Uylings 2000).
Creatine kinase (CK), EC 2.7.3.2, catalyzes the reversible transfer of the N-phosphoryl group from phosphocreatine to ADP regenerating ATP. This enzyme is part of an important system maintaining energy homeostasis of cells with high and fluctuating energy requirement (Wallimann et al., 1992). CK-deficient mice showed mutation-dependent abnormalities in brain morphology and behavior including impaired spatial learning, reduced nestbuilding activity, diminished acoustic startle reflex responses (Jost et al., 2002; Streijger et al., 2005) and reduced brain energy (Kekelidze et al, 2001; In ’t Zandt et al, 2004; Streijger et al, 2010). On the other hand, reduced brain creatine levels and CK activity are implicated in some neurodegenerative diseases partially treated with creatine supplementation (Béard and Braissant, 2010).
CK isoenzymes have been repeatedly considered sensitive targets of reactive species such as H2O2, and peroxynitrite (Yuan et al., 1992; Konorev et al., 1998). Recently, our research group reported that high concentrations of tyrosine provoke in vitro oxidative stress (decrease of enzymatic and non-enzymatic antioxidant defenses, change of the redox state and increase of DNA damage) in the cerebral cortex of young rats (Sgaravatti et al. 2008). Thus, considering that CK is a thiolic enzyme crucial for brain energy metabolism and function, CK deficiency is associated to neurodegenerative diseases and that the mechanisms underlying the neurological dysfunction in hypertyrosinemic patients are poorly known, in this work we investigated the in vitro and in vivo (ex-vivo) effects of tyrosine on CK activity in homogenates of cerebral cortex from 14-day-old rats in order to clarify its participation in the brain damage mechanisms responsible for the neurological impairment observed in hypertyrosinemic patients.
Materials and methods
Reagents and equipments
All chemicals were purchased from Sigma (St. Louis, MO, USA). L-Tyrosine was prepared on the day of the experiment in distilled water and added to homogenates at final concentrations of 0.1, 1.0 and 2.0 mM for the in vitro experiments. L-Tyrosine methyl ester was dissolved in 0.1% Tween 20 in saline solution for the in vivo experiments. A double-beam spectrophotometer with temperature control (Hitachi U-2001) was used for the measurements. Eppendorf 5417R was used for centrifugation procedures.
Animals
Fourteen-day-old Wistar rats bred in the Department of Biochemistry, ICBS, UFRGS, Porto Alegre, RS, Brazil were used in the experiments. Rats were kept with dams until they were sacrificed without anesthesia. The rats had free access to water and to a standard commercial chow (Germani, Porto Alegre, RS, Brazil) containing 20.5% protein (predominantly soybean supplemented with methionine), 54% carbohydrate, 4.5% fiber, 4% lipids, 7% ash and 10% moisture. Temperature was maintained at 24 ± 1°C, with a 12–12 h light–dark cycle. The “Principles of Laboratory Animal Care” (Guide for the Care and Use of Laboratory Animals, NIH publication no. 80–23, revised 1996; http://www.nap.edu/readingroom/books/labrats/) were followed in all the experiments, and the experimental protocol was approved by the Ethics Committee For Animal Research of the Federal University of Rio Grande do Sul.
In vitro experiment
First, assays were performed to determine the in vitro influence of tyrosine (0.1, 1.0 and 2.0 mM) in cerebral cortex homogenates. Next, the effect of 1.0 mM GSH alone or associated to tyrosine 2.0 mM was tested. The brain cortical fractions were incubated for 30–90 min at 37°C depending on the experimental design with the same reaction mixture described below with tyrosine and/or GSH, with the exception of control.
Acute administration of L-tyrosine methyl ester
L-Tyrosine methyl ester was dissolved in 0.1% Tween 20 in saline solution (pH was adjusted to 7.4) and the equivalent to 500 mg/Kg body weight of L-Tyrosine was administered intraperitoneally. This dose was chosen in order to obtain tyrosine concentrations about 10 times normal 1 h after administration (Morre et al. 1980; Bongiovanni et al. 2003), which are similar variations of plasma tyrosine concentration observed in patients affected by tyrosinemia type II (Mitchell et al. 2001). Controls received 0.1% Tween 20 in saline solution. One hour after injection rats were killed and the brain was rapidly removed.
Preparation of brain cortical fractions
Animals were sacrificed by decapitation without anesthesia. The brain was rapidly removed and dissected on a glass dish over ice for in vitro and in vivo experiments. Time elapsed from decapitation to place the brain on the ice was less than 1 min. After dissection, the cerebral cortex was washed in SET buffer (0.32 M sucrose/1 mM EGTA/10 mM Tris–HCl, pH 7.4), minced finely and homogenized in the same SET buffer (1:10, w/v with a Potter-Elvehjem glass homogenizer). At least two homogenizations of 30 s duration were performed at approximately 1000 rpm with an electrically driven Teflon pestle. The homogenate was collected for determination of cytosolic and mitochondrial fractions. A portion of the homogenate was centrifuged at 800 X g for 10 min at 4°C, the pellet was discarded and the supernatant was centrifuged at 10,000 X g for 15 min at 4°C. The supernatant of the second centrifugation, containing cytosol and other cellular components as endoplasmic reticulum, was collected for determination of cytosolic CK activity (Cy-CK). The pellet, containing mitochondria, myelin, synaptosomes, and membrane fragments, was washed twice with the same Tris–sucrose isotonic buffer, resuspended in 100 mM MgSO4–Trizma buffer, pH 7.5, for determination of mitochondrial CK activity (Mi-CK). Homogenate, cytosolic and mitochondrial fractions were stored for no longer than one week at −70°C when the assay was not carried out immediately.
Creatine kinase activity assay
The reaction mixture contained the following final concentrations: 65 mM Tris–HCl buffer, pH 7.5, 7.0 mM phosphocreatine, 9.0 mM MgSO4, and approximately 1 μg protein in a final volume of 0.1 ml. After 30–90 min of pre-incubation at 37°C, the reaction was started by the addition of 0.3 μmol of ADP. The reaction was stopped after 10 min by the addition of 1 μmol p-hydroxymercuribenzoic acid. The reagent concentrations and the incubation time were chosen to assure linearity of the enzymatic reaction. Appropriate controls were carried out to measure chemical hydrolysis of phosphocreatine. The creatine formed was estimated according to the colorimetric method of Hughes (1962). The color was developed by the addition of 0.1 ml of 2% α-naphtol and 0.1 ml of 0.05% diacetyl in a final volume of 1 mL and read after 20 min at 540 nm. None of the substances added to the assay medium interfered with the color development or spectrophotometric readings. Results were expressed as μmol of creatine formed per min per mg protein.
Protein determination
The protein content of cerebral cortex fractions was determined by the method of Lowry et al. (1951), using serum bovine albumin as the standard.
Statistical analysis
Data were analyzed by Student’s t-test or by ANOVA, depending on the experimental design. Data from concentration effect was performed by one-way ANOVA followed by the Tukey test when the F value was significant. Data from time effect were analyzed by three-way ANOVA with repeated measures (saline/ tyrosine, saline/GSH and time – 30, 60, 90 min - as factors). Comparison between means, when appropriate, was performed by one-way ANOVA followed by the Tukey test when the F value was significant. Linear regression was used to evaluate dose or time dependency. Data from the in vivo experiments were analyzed by the independent Student t test. All analyses were performed using the Statistical Package for the Social Sciences (SPSS) software in a PC compatible computer. Values of p < 0.05 were considered to be significant.
Results
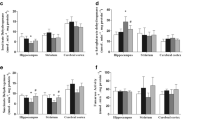
First, Cy-CK and Mi-CK from cerebral cortex cytosolic fraction were assayed in vitro in the presence of 0.1, 1.0 and 2.0 mM tyrosine concentration (Fig. 1). Cy-CK activity was markedly reduced by 2.0 mM tyrosine (32%) and linear regression showed that the amino acid inhibited this activity in a concentration-dependent way (F(1,26) = 22.39; β = −0.68; p < 0.001). Similarly, Mi-CK activity was inhibited at 1.0 (15%) and 2.0 (22%) mM in a concentration-dependent way (F(1,26) = 26.54; β = −0.71; p < 0.001).
In vitro effects of different concentration of tyrosine on CK activity of cytosolic (Cy-CK) and mitochondrial (Mi-CK) fractions of the cerebral cortex from young Wistar rats. Results are expressed as μmol of creatine per min per mg of protein. Data are mean ± SD for 7 independent experiments performed in triplicate. * p < 0.05; **p < 0.01 compared to the controls (Tukey test)
Next, CK activity was measured in the presence of 2.0 mM tyrosine and/or 1.0 mM GSH. Tyrosine was added to the incubation medium containing the cytosolic or the mitochondrial cerebral cortex fractions obtained from non-treated rats and preincubated for 30, 60 or 90 min with or without GSH. Mi-CK showed a significant saline/Tyr by saline/GSH by time interaction (F(2,48) = 4.13; p < 0.05), indicating that Tyr inhibits CK activity along time and GSH prevents this inhibition. Furthermore, regression analysis showed that the inhibition of Mi-CK activity by tyrosine was time-dependent (F(1,19) = 33.66; β = −0.79; p < 0.0001) (Fig. 2).
In vitro effect of GSH on the inhibition of CK activity caused by tyrosine in mitochondrial fraction of the cerebral cortex from young Wistar rats. Results are expressed as μmol of creatine per min per mg of protein. Data are mean ± SD for 7 independent experiments performed in triplicate. *p < 0.05; ***p < 0.001compared to the other groups (Tukey test)
On the other hand, Cy-CK showed significant time effect (F(2,48) = 19.9; p < 0.05) but not saline/Tyr by saline/GSH by time interaction. Analyzing after colapsing time, saline/Tyr by saline/GSH interaction was significant (F(1,24) = 21.55; p < 0.05), indicating that Tyr and GSH had different effects on the enzyme activity, GSH preventing part of the inhibition caused by Tyr. Linear regression analysis showed that the inhibition caused by tyrosine was not time-dependent (F(1,19) = 0.16; β = −0.09; p > 0.05), reinforcing the observation that the effects of Tyr on Cy-CK and Mi-CK are different (Fig. 3).
In vitro effect of GSH on the inhibition of CK activity caused by tyrosine in cytosolic fraction of the cerebral cortex from young Wistar rats. Results are expressed as μmol of creatine per min per mg of protein. Data are mean ± SD for 7 independent experiments performed in triplicate. *p < 0.05; ***p < 0.001 compared to the other groups (Tukey test)
Finally, the effect of a single administration of L-tyrosine methyl ester on Cy-CK and Mi-CK (Fig. 4) of cerebral cortex were studied. Tyrosine administration significantly reduced Cy-CK (t(12) = 3.38; p < 0.05) and Mi-CK (t(10) = 2.38; p < 0.05) activities.
Effect of acute administration of L-tyrosine methyl ester on CK activity of cytosolic and mitochondrial fractions in cerebral cortex from young Wistar rats. Results are expressed as μmol of creatine per min per mg of protein. Data are mean ± SD for 7 independent experiments performed in triplicate. *p < 0.05; **p < 0.01 compared to control (Student’s t-test for unpaired samples)
Discussion
Inherited tyrosinemia is classified into type I (deficiency of fumarylacetoacetate hydrolase) with hepatorenal diseases, type II (deficiency of hepatic TAT) with oculocutaneous and CNS disturbances, and type III (deficiency of 4-hydroxyphenylpyruvate) with normal liver function and intermittent neurologic anomalies (Mitchell et al. 2001). Furthermore, all types demonstrate elevated tyrosine levels in tissues and physiological fluids of patients with tyrosinemia, but plasma tyrosine levels are higher in TAT-deficiency comparing to the other causes of hypertyrosinemias. Mental retardation and other neurological findings have been reported in patients affected by tyrosinemia type II (Mitchell et al. 2001). Although the molecular deficiency and the symptoms of TAT deficiency are well described, the mechanisms responsible for the neuropathophysiology of this metabolic disorder are largely unknown. Considering that CK is a target for other amino acids accumulating in some metabolic diseases of amino acid metabolism, CK deficiency has been observed in some neurodegenerative diseases and patients with creatine deficiency syndromes present symptoms similar to those observed in patients affected by tyrosinemia (Béard and Braissant, 2010), in the present work, we investigated the in vitro and in vivo effect of tyrosine on CK activity of cerebral cortex from 14-day-old Wistar rats.
Cerebral cortex was used to investigate the effect of tyrosine on CK activity because tyrosine concentration into this brain region showed the highest relative increase compared to other regions when tyrosine is administered to rats (Morre et al. 1980; Bongiovanni et al. 2003). Furthermore, as the development of CNS occurs in phases, which follow a precise sequence (Morgane et al. 2002), we have chosen to study 14-day-old rats because during the second postnatal week, Slotkin et al. (2005) identified a crucial phase of vulnerability of neuronal cells, corresponding to peak periods of differentiation when metabolic demands are especially high. In addition, the period of fastest dendritic outgrowth in rat cerebral cortex is between 8 and 14 postnatal days, whereas in the human is during the first 2–3 years (Uylings 2000). In this regard, tyrosinemic patients, like many other inherited metabolic diseases, are subjected to high levels of accumulated metabolites (in this case, tyrosine) in postnatal period presenting symptoms during critical stages of CNS development (Mitchell et al. 2001).
In the present study, we demonstrated that high concentrations of tyrosine, similar to those found in the brain cortex of tyrosinemia type II patients, inhibited the in vitro CK activity of cytosolic and mitochondrial fractions of cerebral cortex homogenates from rats in concentration-dependent way. We observed that tyrosine inhibited in vitro the Cy-CK and that this inhibition was prevented by reduced glutathione (GSH) at all tested preincubation times, but the inhibition was not time-dependent. On the other hand, Mi-CK also was inhibited by tyrosine and the inhibition was prevented by GSH at all tested preincubation times in a time-dependent pattern. These results suggest that oxidative stress is a possible mechanism of the enzyme activity inhibition, altering crucial thiol residues for the enzyme activity. The time-dependent inhibition of Mi-CK and not in Cy-CK is in agreement with this hypothesis, since mitochondria is the main source of free radicals generation (Halliwell 2001; Halliwell 2006; Sas et al. 2007). So, the time-dependent inhibition of Mi-CK could at least in part be caused by generation of oxidative stress induced by tyrosine, because it is well known that CK activity decreases after exposure to agents promoting generation of free radicals, probably by oxidation of the sulfhydryl residues of the enzyme (Burmistrov et al. 1992; Yuan et al. 1992; Mekhfi et al. 1996; Wolosker et al. 1996; Arstall et al. 1998; Konorev et al. 1998; Stachowiak et al. 1998; Wallimann et al. 1998; Koufen and Stark 2000) and reagents reacting with thiols (Gross et al. 1996; Wolosker et al. 1996). This may explain our present results showing that GSH prevented the inhibitory effect of tyrosine on CK activity.
Previous findings from our laboratory demonstrated that rats subjected to L-tyrosine acute administration increases oxidative stress, observed by increase of lipoperoxidation, carbonylation of proteins, decrease of GSH and thiol-disulfide redox state (SH/SS ratio) (Sgaravatti et al. 2009). It is known that numerous processes are dependent upon cellular redox state, which may be related to the reduction of CK activity in neurodegenerative diseases such as Alzheimer and Pick (Wolosker et al. 1996; Aksenov et al. 1997).
The influence of acute administration of L-tyrosine methyl ester on CK activity in cerebral cortex of rats was also investigated. We demonstrated that tyrosine reduced Cy-CK and Mi-CK activities, probably by a direct action of tyrosine on CK, or by reactive species (Sgaravatti et al. 2008). However, we cannot rule out that tyrosine derivatives might contribute for the present in vivo effects, since increased urinary excretion of 4-hydroxyphenylpyruvic acid, 4-hydroxyphenyllatic acid, 4-hydroxyphenylacetic acid, N-acetyltyrosine, and 4-tyramine were observed in hypertyrosinemic patients (Rabinowitz et al. 1995; Mitchell et al. 2001; Held 2006).
The CK/creatine phosphate system exerts three integrated functions in brain cells: temporary energy buffering, metabolic capacity, energy transfer and metabolic control (Saks et al. 1996; Wallimann et al. 1992). This system is now recognized as an important metabolic regulator during health and disease (Wyss et al. 1992; Walliman et al. 1998). A decrease in CK activity is one of the biochemical markers of brain cell damage in age-related neurodegenerative diseases, including Alzheimer’s disease (Aksenov et al. 1997). The decrease of CK activity in the brain correlates well with the neurodegeneration parameters in severely affected regions in Alzheimer’s disease (Hensley et al. 1995). Therefore, damage of CK function may be an important part of a neurodegenerative pathway that leads to neuronal loss in the brain (Tomimoto et al. 1993). These findings are reinforced by the observation that creatine and phosphocreatine have neuroprotective effects against energy deprivation and glutamate excitoxicity, attributable to an enhancement of cytosolic high-energy phosphate stores (Brustovetsky et al. 2001). Besides, CK is also important to inhibit the Ca+2-induced opening of the mitochondrial permeability transition pore, which leads to apoptosis (O’Gorman et al. 1997).
Some of the in vitro effects of tyrosine on CK activity were elicited only at the highest concentration studied (2.0 mM). To our knowledge, the actual concentration that is reached into the brain of type II tyrosinemic patients is not known. It was reported that plasma tyrosine levels may exceed 1 mM in untreated type II patients (Scott 2006) and achieve up to 3.4 mM (Goldsmith et al. 1973; Lemonnier et al. 1979; Rabinowitz et al. 1995; Mitchell et al. 2001; Macsai et al. 2001; Viglizzo et al. 2006). So, it is quite possible that the effects presented here may have pathophysiological relevance to tyrosinemia type II
Taken together, the results show that tyrosine inhibits CK activity in the brain of young rats. Considering that CK is a key enzyme for energy homeostasis, if this enzyme inhibition also occurs in the brain of tyrosinemia type II patients, it is possible that the decrease of this enzyme activity may alter energy metabolism and function in the brain of the patients and contribute to the brain damage characteristic of this disease.
References
Aksenov MY, Aksenova MV, Payne RM, Smith CD, Markesbery WR, Carney JM (1997) The expression of creatine kinase isoenzymes in neocortex of patients with neurodegenerative disorders: Alzheimer’s and Pick’s disease. Experim Neurol 146:458–465
Arstall MA, Bailey C, GrossWL BM, Balligand JL, Kelly RA (1998) Reversible S-nitrosation of creatine kinase by nitric oxide in adult rat ventricular myocytes. J Mol Cell Cardiol 30:979–988
Béard E, Braissant O (2010) Synthesis and transport of creatine in the CNS: importance for cerebral functions. J Neurochem 115:297–313
Bongiovanni R, Yamamoto BK, Simpson C, Jaskiw GE (2003) Pharmacokinetics of systemically administered tyrosine: a comparison of serum, brain tissue and microdialysate levels in the rat. J Neurochem 87:310–317
Brustovetsky N, Brustovetsky T, Dubinsky JM (2001) On the mechanisms of neuroprotection by creatine and phosphocreatine. J Neurochem 76:425–434
Burmistrov SO, Mashek OP, Kotin AM (1992) The action of acute alcoholic intoxication on antioxidant system and creatine kinase activity in the brain of rat embryos. Eksp Klin Farmakol 55:54–56
Goldsmith LA, Kang E, Bienfang DC, Jimbow K, Gerald P, Baden HP (1973) Tyrosinemia with plantar and palmar keratosis and keratitis. J Pediatr 83:798–805
Gross WL, Bak MI, Ingwall JS, Arstall MA, Smith TW, Balligand JL, Kelly R (1996) Nitric oxide inhibits creatine kinase and regulates heart contractile reserve. Proc Natl Acad Sci USA 93:5604–5609
Halliwell B (2001) Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging 18:685–716
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97:1634–1658
Held PK (2006) Disorders of tyrosine catabolism. Mol Genet Metab 88:103–106
Hensley K, Hall N, Subramaniam R, Cole P, Harris M, Aksenova MV, Aksenov MY, Gabbita SP, Carney JM, Lowell M, Markesbery WR, Butterfield DA (1995) Brain regional correspondence between Alzheimer’s disease histopathology biomarkers of protein oxidation. J Neurochem 65:2146–2156
Hughes BP (1962) A method for estimation of serum creatine kinase and its use in comparing creatine kinase. Clin Chim Acta 7:597–603
In ’t Zandt HJ, Renema WK, Streijger F, Jost C, Klomp DW, Oerlemans F, Van der Zee CE, Wieringa B, Heerschap A (2004) Cerebral creatine kinase deficiency influences metabolite levels and morphology in the mouse brain: a quantitative in vivo 1H and 31P magnetic resonance study. J Neurochem 90:1321–1330
Jost CR, Van der Zee CE, In ’t Zandt HJA, Oerlemans F, Verheij M, Streijger F, Fransen J, Heerschap A, Cools AR, Wieringa B (2002) Creatine kinase B-driven energy transfer in the brain is important for habituation and spatial learning behaviour, mossy fibre field size and determination of seizure susceptibility. Eur J Neurosci 15:1692–1706
Kekelidze T, Khait I, Togliatti A, Benzecry JM, Wieringa B, Holtzman D (2001) Altered brain phosphocreatine and ATP regulation when mitochondrial creatine kinase is absent. J Neurosci Res 1:66(5):866–872
Konorev E, Hogg N, Kalyanaraman B (1998) Rapid and irreversible inhibition of creatine kinase by peroxynitrite. FEBS Lett 427:171–174
Koufen P, Stark G (2000) Free radical induced inactivation of creatine kinase: sites of interaction, protection, and recovery. Biochim Biophys Acta 1501:44–50
Lemonnier F, Charpentier C, Odievre M, Larregue M, Lemonnier A (1979) Tyrosine aminotransferase isoenzyme deficiency. J Pediatr 94:931–932
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–267
Macsai MS, Schwartz TL, HinkleD HummelMB, MulhernMG Root-man D (2001) Tyrosinemia type II: nine cases of ocular signs and symptoms. Am J Ophthalmol 132:522–527
Mekhfi H, Veksler V, Mateo PH, Maupoil V, Rochette L, Ventura-Clapier R (1996) Creatine kinase in the main target of reactive oxygen species in cardiac myofibrils. Circ Res 17:1016–1027
Mitchell GA, Grompe M, Lambert M, Tanguay RM (2001) Hyper-tyrosinemia. In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds; Childs B, Kinzler KW, Vogelstein B, assoc eds. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill, pp 1777–1805
Morgane PJ, Mokler DJ, Galler JR (2002) Effects of prenatal protein malnutrition on the hippocampal formation. Neurosci Biobehav Rev 26:471–483
Morre MC, Hefti F, Wurtman RJ (1980) Regional tyrosine levels in rat brain after tyrosine administration. J Neural Transm 49:45–50
O’Gorman E, Beutner G, Dolder M, Korestsky AP, Brdiczka D, Wallimann T (1997) The role of creatine kinase in inhibition of mitochondrial permeability transition. FEBS Lett 414:253–257
Rabinowitz LG, Williams LR, Anderson CE, Mazur A, Kaplan P (1995) Painful keratoderma and photophobia: hallmarks of tyrosinemia type II. J Pediatr 126:266–269
Ruetschi U, Cerone R, Perez-Cerda C, Schiaffino MC, Standing S, Ugarte M, Holme E (2000) Mutations in the 4-hydroxyphenylpyruvate dioxygenase gene (HPD) in patients with tyrosinaemia type III. HumGenet 106(6):654–662
Saks VA, Ventura-Clapier R, Aliev MK (1996) Metabolic control and metabolic capacity: two aspects of creatine kinase functioning in the cells. Biochim Biophys Acta 1274:81–88
Sas K, Robotka H, Toldi J, Vécsei L (2007) Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. J Neurol Sci 257:221–239
Scott CR (2006) The genetic t yrosinemias. J Med Genet 142:121–126.
Sener RN (2005) Tyrosinemia-computed tomography, magnetic resonance imaging, diffusion magnetic resonance imaging, and proton spectroscopy findings in the brain. J Comput Assist Tomogr 29:323–325
Sgaravatti AM, Vargas BA, Zandoná BR, Deckmann KB, Rockenbach FJ, Moraes TB, Monserrat JM, Sgarbi MB, Pederzolli CD, Wyse AT, Wannmacher CM, Wajner M, Dutra-Filho CS (2008) Tyrosine promotes oxidative stress in cerebral córtex of Young rats. Int J Dev Neurosci 26:551–559
Sgaravatti AM, Magnusson AS, De Oliveira AS, Rosa AP, Mescka CP, Zanin FR, Pederzolli CD, Wyse AT, Wannmacher CM, Wajner M, Dutra-Filho CS (2009) Tyrosine administration decreases glutathione and stimulates lipid and protein oxidation in rat cerebral cortex. Metab Brain Dis 24:415–425
Slotkin TA, Oliver CA, Seidler FJ (2005) Critical periods for the role of oxidative stress in the developmental neurotoxicity of chlorpyrifos and terbutaline, alone or in combination. Brain Res Dev Brain Res 157:172–180
Stachowiak O, Dolder M, Wallimann T, Richter C (1998) Mitochondrial creatine kinase is prime target of peroxynitrite-induced modification and inactivation. J Biol Chem 273:16694–16699
Streijger F, Oerlemans F, Ellenbroek BA, Jost CR, Wieringa B, Van der Zee CE (2005) Structural and behavioural consequences of double deficiency for creatine kinases BCK and UbCKmit. Behav Brain Res 157:219–234
Streijger F, Scheenen WJ, van Luijtelaar G, Oerlemans F, Wieringa B, Van der Zee CE (2010) Complete brain-type creatine kinase deficiency in mice blocks seizure activity and affects intracellular calcium kinetics. Epilepsia 51(1):79–88
Tomimoto H, Yamamoto K, Homburger HA, Yanagihara T (1993) Immunoelectron microscopic investigation of creatine kinase BB- isoenzyme after cerebral ischemia in gerbils. Acta Neuropathol 86:447–455
Uylings HB (2000) Development of the cerebral cortex in rodents and man. Eur J Morphol 38:309–312
Viglizzo GM, Occella C, Bleidl D, Rongioletti F (2006) Richner-Hanhart syndrome (tyrosinemia II): early diagnosis of an incomplete presentation with unusual findings. Pediatr Dermatol 23:259–261
Wallimann T, Wyss M, Brdiczka D, Nicolay K (1992) Intracellular compartmentation, structure and function of creatine kinase in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochemical Journal 281:21–40
Walliman T, Dolder M, Schlattner U, Eder M, Hornemann T, Kraft T, Stolz M (1998) Creatine kinase: an enzyme with a central role in cellular energy metabolism. MAGMA 6:116–119
Wolosker H, Panizzutti R, Englender S (1996) Inhibition of creatine kinase with S-nitrosoglutathione. FEBS Lett 392:274–276
Wyss M, Smeitink J, Wevers RA, Wallimann T (1992) Mitochondrial creatine kinase: a key enzyme of aerobic energy metabolism. Biochim Biophys Acta 1102:119–166
Yuan G, Kaneko M, Masuda H, Hon RG, Kobayashi A, Yamazak N (1992) Decrease in heart mitochondrial creatine kinase activity due to oxygen free radical. Biochim Biophys Acta 1140:78–84
Acknowledgements
This work was supported by the research grants from Programa de Núcleos de Excelência (PRONEX), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS) and FINEP Rede Instituto Brasileiro de Neurociência (IBN net).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Andrade, R.B., Gemelli, T., Rojas, D.B. et al. Tyrosine inhibits creatine kinase activity in cerebral cortex of young rats. Metab Brain Dis 26, 221–227 (2011). https://doi.org/10.1007/s11011-011-9255-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-011-9255-9