Abstract
Thyroid hormones (THs) exert a broad spectrum of effects on the central nervous system (CNS). Hypothyroidism, especially during CNS development, can lead to structural and functional changes (mostly resulting in mental retardation). The hippocampus is considered as one of the most important CNS structures, while the investigation and understanding of its direct and indirect interactions with the THs could provide crucial information on the neurobiological basis of the (frequently-faced in clinical practice) hypothyroidism-induced mental retardation and neurobehavioral dysfunction. THs-deficiency during the fetal and/or the neonatal period produces deleterious effects for neural growth and development (such as reduced synaptic connectivity, delayed myelination, disturbed neuronal migration, deranged axonal projections, decreased synaptogenesis and alterations in neurotransmitters’ levels). On the other hand, the adult-onset thyroid dysfunction is usually associated with neurological and behavioural abnormalities. In both cases, genomic and proteomic changes seem to occur. The aim of this review is to provide an up-to-date synopsis of the available knowledge regarding the aforementioned alterations that take place in the hippocampus due to fetal-, neonatal- or adult-onset hypothyroidism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid hormones (THs), thyroxine (T4) and 3,5,3'-triiodothyronine (T3), act on most tissues, including the kidneys, the heart and the adipose tissue, and exert important actions during development and maturation (Moreno et al. 2008; Peeters 2008). The central nervous system (CNS) is also an important target for the THs, not only during development but also in adulthood (Bernal 2007; Calzà et al. 1997). Fetal and postnatal TH-deficiency may cause irreversible mental retardation and neurological deficits (Delange 2000; Glinoer 2007), while adult-onset hypothyroidism is linked to impaired learning and memory, as well as to depression (Lass et al. 2008).
Both THs (T4 and T3) may enter the CNS through specific transporters; T4 is converted to the active TH (T3) in glial cells, astrocytes, and tanycytes, although the main target cells are found in neurons and maturing oligodendrocytes (Bernal 2005). Within the CNS, THs seem to influence a large number of effects related to neural-cell migration, growth, differentiation and signalling (Williams 2008). The nuclear TH receptors (TRs), that belong to the steroid/TH receptor superfamily of ligand-dependent transcription factors, are the mediators through which THs positively regulate a large number of genes (Huang et al. 2008b).
Hippocampus is considered as one of the most important CNS regions, involved in crucial neuronal networks responsible for cognitive, emotional and motivational functions (White et al. 2000). Since studies on the THs-induced effects on CNS are of crucial importance for both perinatal periods and adulthood, the aim of this review is to provide an up-to-date synopsis of our knowledge on the structural and functional changes that take place in the hippocampus due to neonatal- as well as adult-onset hypothyroidism.
Structural alterations in the hippocampus due to hypothyroidism
Alterations in the hippocampal cells’ morphology and migration during development
Hippocampus is a structure containing only a few major cell types and well-defined inputs and outputs. These characteristics offer the opportunity to study cell formation, migration and differentiation in this brain region. The granule cells nearest to the molecular layer are formed earlier than those towards the hilus, while their differentiation starts earlier in the suprapyramidal blade of the dentate gyrus than in the infrapyramidal blade (Schlessinger et al. 1978). On the other hand, the pyramidal cells are generated in an “inside-out” pattern: the deeper cells are formed before the more superficial cells in the pyramidal layer and during maturation the apical dendrites of the pyramidal cells are formed earlier than the basal dendrites (Stanfield and Cowan 1979).
Several studies on the development of the hippocampus during hypothyroidism have shown marked and irreversible changes in the growth of neurons (Rabié et al. 1979), although some brain regions are less vulnerable to early developmental insults and can recover (Farahvar et al. 2007). Moreover, most of the morphological alterations observed in the CNS of hypothyroid animals (Table 1) in parallel to those found in human studies performed in patients with TH-deficiency (Gilroy and Meyer 1975).
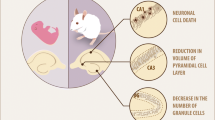
THs are important in the establishment of the CA1 to CA4 gradient of pyramidal cell differentiation and in the development of the spatiotemporal relationship between pyramidal and granule cells of the hippocampus. TH-deficiency leads to distortions rather than synchronized shifts in the relative development of different hippocampal regions (Bernal 2005). The reduction in number and maturation of granule cells, together with the asynchronous alteration in the maturation of pyramidal cells may result in a major distortion of the aforementioned relationships between pyramidal and granule cells in TH-deficient animals. Such distortions may be instrumental in causing the functional impairments that are often seen in congenital hypothyroidism.
In TH-deficient rats, the maturation of both granule cells and pyramidal cells is impaired. Hypothyroidism, starting either in the neonatal period or in adulthood, leads to a reduction in the total number of granule cells in the dentate gyrus, which is not restored by the normalization of TH-levels (Madeira et al. 1991a).
Additionally, the effect of hypothyroidism on the pyramidal cells depends on the position of the cells in the Ammon’s horn of the hippocampus. In general, pyramidal cells of hippocampal regions display a neurogenic pattern that is markedly different from that of granule cells, as it occurs predominantly during the prenatal period (Altman and Bayer 1990a,b). Hippocampal neurons are generated following a dentate-to-subicular sequence in opposition to the remaining neurons of the hippocampal formation (Bayer 1980). Although the pyramidal cells of the CA3 region are produced earlier than those addressed to the CA1 region, the later generated pyramidal cells of the CA1 region attain their final position at least one day prior to the CA3 pyramidal cells (Altman and Bayer 1990b). As a consequence of their prolonged neurogenesis, only at birth do they reach their final location within the pyramidal cell layer. Therefore, the differentiation of pyramidal cells, including the growth of their dendrites and the establishment of synaptic contacts, occurs mainly during the postnatal period, and only 3 to 4 weeks after birth does the structural organization of the CA1 and CA3 regions resemble their adult form.
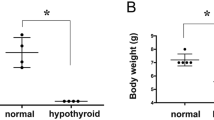
In neuronal populations that undergo a significant postnatal formation, apart from those alterations, a reduction in the number of neurons is likely to occur, since both neuronal proliferation and migration are also affected by the TH-deficiency and, thereby, interfere with the process of cell acquisition (Madeira et al. 1988). Madeira et al. (1992) have thoroughly described the morphological alterations that take place in the rat hippocampus during postnatal hypothyroidism. In 30-day-old animals, the weight of the hippocampal formations was significantly reduced in hypothyroid groups when compared to the respective controls. The morphology of the pyramidal cell layer of the hippocampal CA3 region was identical in all groups studied, in contrast to the CA1 pyramidal cell layer, where its characteristic palisade arrangement was not present. Furthermore, the volume of the CA1 and CA3 pyramidal cell layers was significantly smaller in hypothyroid than in control groups. The areal and numerical densities of the CA3 pyramidal cells were found to be significantly increased in both male and female hypothyroid groups when compared to the respective control ones. No significant hypothyroidism-induced effect was found in the areal density of CA1 pyramidal neurons of 30-day-old animals. The total number of CA1 pyramidal cells was significantly reduced (20.2% in males and 18.7% in females) in hypothyroid groups when compared with the respective control groups, while there was no significant effect of hypothyroidism in the total number of CA3 pyramidal cells (Madeira et al. 1992).
Regarding the CA3 region, hypothyroidism, whatever its duration and settling time, does not clearly interfere with the total number of pyramidal cells, despite the reduction in the volume of the CA3 pyramidal cell layer and the parallel increase in the areal and numerical densities of its neurons. As no differences were found in the mean nuclear volume of pyramidal cells between hypothyroid and control groups, we could infer that the referred changes depend on the presence of a poorly developed neuropil in hypothyroid animals. This is in line with the observations that the lack of THs impairs the normal growth of axons and dendrites (Moskovkin and Marshak 1982; Legrand 1982–1983; Pérez-Delgado et al. 1987; Alva-Sánchez et al. 2002). Additionally, the neuritic regrowing capabilities are preserved following neonatal hypothyroidism, as in recovery groups, in which normal levels of THs are re-established, the volume of CA3 neuropil attains the values found in controls.
Furthermore, Rami et al. (1986a, b) also described a marked reduction in the development of the dendritic arborization of granule cells of the dentate gyrus due to the induction of experimental hypothyroidism. However, this feature of dendritic maturation was restored to normal after 2 days of T4 replacement. In normal rats, pyramidal cells of Ammon’s horn have numerous fine and branched basal dendrites. TH-deficiency resulted in less developed dendrites, with about a 30% reduction in the number of branching points on apical dendritic trees in all areas of the Ammon’s horn. The effect was much greater in the hippocampal CA3 region than in the CA1. The pyramidal cells from CA1-2 regions of normal hippocampus show a delay in maturation compared to the cells from CA3-4 regions. Experimental hypothyroidism did not markedly alter the qualitative shape of the dendritic field in the CAl-2 and CA3-4 areas from that already described in developing normal rats. However, a quantitatively significant reduction in branching points was observed at all levels, and in particular in the branching points on basal dendrites and the arborization of distal parts of the apical dendrites were found to be markedly affected. The administration of physiological doses of T4 confirmed that the areas where the pyramidal cell maturation was most impaired by TH-deficiency were also the most sensitive to the TH-treatment.
The stem cells of the hippocampal granule cells proliferate in the neuroepithelium limiting the lateral ventricle (Altman and Das 1966; Schlessinger et al. 1975). From day 18 of rat gestation, the cells formed by the neuroepithelium begin to immigrate through the fimbria and the stratum orients towards the exterior of the dentate gyrus. Some of the migrating cells establish a secondary germinal site, the “proliferative zone”. The cells then migrate through the “transit area” (polymorph layer) to reach their final position, forming the granular layer from day 20 of gestation. In the rat, the general features of the adult hippocampus are already present at birth. Accumulation of cells in the granular layer continues up to about three postnatal weeks, and it has been shown that approximately 80% of the granule cells and approximately 60% of the total cells of the adult hippocampus are formed after the rat birth (Alva-Sánchez et al. 2002).
Rami et al. (1986a, b) described extensively the growth and cell formation in the dentate gyrus of hypothyroid rats. The overall qualitative patterns of developmental increase of the area and of the cell number in the granular layer of TH-deficient rats were similar to those in the controls, but they differed quantitatively in a significant way. In the TH-deficient animals, the mitotic activity in the granule cell layer and the “transit area” (polymorph layer) was not altered, suggesting that the significant reduction in cell acquisition observed in the dentate gyrus is not due to changes in the replication of the proliferating granule cells.
Recent studies indicate that perinatal hypothyroidism induces the apoptosis of hippocampal neurons via the Bcl-2/Bax pathway (Huang et al. 2005a). In addition, as shown by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining, positive apoptotic neurons were increased in the CA3 region of the hippocampus in hypothyroid pups (Huang et al. 2008a), while ultrastructural morphological data of hypothyroid hippocampal pyramidal cells support the idea of apoptosis-induced neuronal death (Alva-Sánchez et al. 2007). The latency of memory was negatively correlated with the density of apoptotic neurons. Thus, alterations of hippocampal structure and ability may be one of the important reasons which cause brain impairment and mental disabilities.
Alterations in the hippocampal cells’ morphology during adulthood
The adult brain is less vulnerable to the deleterious effects of hypothyroidism. For a long period of time, no hypothyroid-induced changes in neuronal numbers had ever been reported in adults, apart from those observed in the granular layer of the dentate gyrus (Madeira et al. 1991b) in which neurons continue to be produced during adulthood (Altman and Bayer 1990a). More specifically, it was demonstrated that the volume of the granular layer was significantly smaller in male and female hypothyroid groups than in the respective controls and the number of granule cells per unit of surface area of the granular layer was significantly reduced in male and female hypothyroid groups when compared with the respective control group. No striking qualitative differences were found between controls and adult hypothyroid rats. It was also demonstrated that hypothyroidism, whatever its duration and time of onset, leads to a reduction of the total number of dentate granule cells, which is followed by a decrease in the volume of the granular layer and in the areal and numerical densities of its neurons. The damage inflicted by TH-deficiency, which seems to require the activation of N-methyl-D-aspartic acid (NMDA) channels (Alva-Sánchez et al. 2009), was found to vary according to the experimental model of hypothyroidism tested; i.e., in the total hypothyroid group (TH-deficient from day 0 until day 180) the values of all the parameters estimated were markedly decreased when compared with the corresponding controls, while in the adult-onset hypothyroid subgroup (TH-deficient from day 30 until day 180) such reductions were of less importance.
Regarding the effects of hypothyroidism settled in mature animals, Madeira et al. (1992) suggested that pyramidal cells were also affected. In spite of the number of pyramidal cells of the CA3 region in adult hypothyroid groups that remain unchanged, the neuropil seems to be rather vulnerable as the volume of CA3 pyramidal cell layer was reduced, thereby indicating that neuronal packing occurs; i.e., neurons are closer to each other due to an impoverishment of neuropil components, namely neuronal and glial processes. In the hippocampal CA1 region, the lack of THs leaded to an irreversible reduction in the total number of pyramidal cells in adult animals, since no recovery was noted once the euthyroid condition was re-established. It must be emphasized that adult-onset hypothyroidism also interfered with the normal morphology of the CA1 region, as may be inferred by the finding that in adult hypothyroid groups, the total number of pyramidal cells is smaller than in controls. This observation gives credence to the hypothesis that a lack of THs may induce cell death in the fully mature. Disturbances of neurogenesis, which have been described as underpinning the normal process of cell acquisition in several regions of the brain, cannot explain the reduction in neuronal numbers in adult-onset hypothyroidism. It is therefore likely that additional mechanisms, specific for the hippocampal CA1 region, might occur and explain the selective vulnerability of its neurons to TH manipulations. Such a hypothesis is further supported by the non-existence of neuronal death in the CA3 region of hypothyroid rats in spite of the peculiar and protracted postnatal synaptogenesis displayed by its neurons (Amaral and Dent 1981).
Additionally, the dentate gyrus of the hippocampal formation undergoes neurogenesis in adult mammals, including humans. Adult neurogenesis takes place in two main neurogenic areas: the subventricular zone (SVZ) adjacent to the lateral ventricles (which generates olfactory bulb interneurons), and the subgranular zone (SGZ; which gives rise to granular neurons in the hippocampal dentate gyrus) (Gage 2002). Short-term adult-onset hypothyroidism significantly reduces SGZ proliferative capacity and impairs dendritic arborization of immature neurons (Montero-Pedrazuela et al. 2006). In particular, adut-onset hypothyroidism impaired normal neurogenesis in the SGZ of the rat dentate gyrus with a 30% reduction in the number of proliferating cells. The same reduction was observed in newborn neuroblasts. Treatment with TH increased the number of proliferating cells and proliferative clusters, even above euthyroid values, in hypothyroid rats. It is possible that cell proliferation in the SGZ of hypothyroid rats becomes more sensitive to TH treatment, so that even slightly increased TH levels following a TH-treatment of these animals could result in a significant upregulation of proliferation.
Functional alterations in the hippocampus due to hypothyroidism
The hypothyroid state appears to promote a series of functional neuronal defects during the developmental process of the hippocampal formation, which vary from cell survival and differentiation to neuronal signalling properties and subcellular responses (Table 2).
Hypothyroidism and hippocampal gene expression
In addition, according to the nuclear pathway of the THs’ action, their effects on developmental processes are carried out through the control of gene expression. The main period of brain sensitivity to THs extends through the postnatal state, in which most of the effects of hypothyroidism on neural tissue structure and gene expression have been described. During this period, several genes are down- or up-regulated by THs (Royland et al. 2008), and in some of these genes, TH-responsive elements have been detected in their regulatory regions (Table 3). The genes regulated during the postnatal period become refractory to the action of TH in juvenile and adult rats beyond postnatal days 25–30, with a few exceptions.
Myelination-related alterations in the hippocampus due to hypothyroidism
An important feature of the hypothyroid brain is the delayed myelination and poor deposition of myelin (Bernal 2005). The amount of myelin deposited in white matter areas is reduced, and the final number of myelinated axons is lower than in normal animals. The lower myelination is mainly a result of impaired oligodendrocyte differentiation, but other factors may also contribute. For example, the impaired axon maturation may lower the number of axons reaching the critical size to be myelinated. Thus, although lower in number, most of the myelinated axons present in hypothyroid animals appear to have a normal thickness of the myelin sheath (Guadaño Ferraz et al. 1994).
Most of the genes identified so far are expressed and regulated by THs during the postnatal period, towards the direction of accelerating the normal up- or down-regulation taking place after birth (Bernal 2005). A good example is provided by the myelin genes, which are induced a few days after birth accompanying oligodendrocyte differentiation and the myelination wave. In the absence of THs, accumulation of myelin gene products—mRNA and protein—proceeds at a slower rate, and normal concentrations are attained later in development compared to normal animals.
In vivo, THs influence to a similar extent, and with similar patterns, the expression of all myelin genes analyzed. Among them, the genes encoding the structural proteins; proteolipid protein (50% of total), myelin basic protein (30% of total), and myelin associated glycoprotein (1% of total) belong to the best characterized because of their relative abundance (Sutcliffe 1988). The period of TH-sensitivity for these genes in the rat brain initiates from approximately the end of the first postnatal week, but the timing of regulation has a strong regional component. THs, in particular, accelerate the myelinating process and in hypothyroid animals, myelination is delayed. The primary action of THs is exerted at the level of oligodendrocyte differentiation, and THs, in vivo, promote accumulation of differentiated oligodendrocytes (Schoonover et al. 2004). The myelination wave proceeds from caudal to anterior regions, and the influence of THs follows a similar pattern. Therefore, it is frequently determined that the differences in myelin gene expression between normal and hypothyroid rats become evident at different ages in different regions. In hippocampus, myelin mRNA and proteins (myelin basic protein, proteolipid protein, myelin-associated glycoprotein and 2’:3’-cyclic nucleotide 3’-phosphodiesterase) are reduced in hypothyroid rats compared to normal rats only around postnatal days 20–25 (Ibarrola and Rodríguez-Peña 1997). In all cases, expression of myelin genes becomes normalized with age, even in the absence of TH-treatment. Finally, myelin genes become refractory to THs in adult individuals.
Mitochondrial function in the hippocampus under hypothyroidism
THs have been reported to play an important role in the regulation of mitochondrial function in several tissues (such as liver, kidney, and skeletal muscle). Moreover, some biochemical analyses have suggested that neonatal hypothyroidism causes alterations in brain mitochondria (Battie and Verity 1979; Katyare et al. 1994). Furthermore, mitochondria seem to participate in hypothyroid-induced apoptotic phenomena that take place in hippocampal neurons of developing rats (Huang et al. 2005b).
Hippocampal cell migration deregulation due to hypothyroidism
Cell migration is an important process of the developing brain which is extremely sensitive to the lack of THs. Induction of transient maternal hypothyroidism in pregnant rats at embryonic days 12–15 led to significant misplacement of cells in the neocortex and hippocampus of the offspring when analyzed at 40 days of age (Ausó et al. 2004). The mechanisms by which THs influence migration have not been defined with certainty, although some molecules involved in migration have been found to be under TH-regulation. Alvarez-Dolado et al. (1999) described the influence of hypothyroidism on two proteins, Reelin and Disabled-1 (Dab1), which are related to cell migration in the hippocampus. These proteins are under TH-control and are critical for neuronal migration, leading to lamination and precise cellular localization during CNS development. Reelin’s main function is to signal the migrating neurons when to stop. Since Reelin regulates Dab1 phosphorylation, Dab1 may act within migrating neurons in response to a Reelin signal. As shown by Northern analysis, in situ hybridization and immunohistochemistry studies, hypothyroid rats express decreased levels of Reelin RNA and protein during the perinatal period (embryonic day 18 and postnatal day 0). At later ages, however, Reelin was more abundant in the hippocampus of hypothyroid rats (postnatal day 5), and no differences were detected at postnatal day 15. Conversely, Dab1 levels were higher at postnatal day 0, and lower at postnatal day 5 in hypothyroid animals. Interestingly, in contrast to Reelin, Dab1 RNA levels are not affected by hypothyroidism. However, Dab1 protein levels are modulated in TH-deficient rats. This effect may be caused by a direct effect of T3 on Dab1 mRNA translation or on Dab1 protein stability. Alternatively, changes in Dab1 protein content may be an indirect consequence of the reduction in Reelin expression due to the lack of THs.
Neural cell adhesion molecule (NCAM) has an important role in the control of cell-to-cell interactions taking place during the migration and differentiation of neuronal cell populations during development (Iglesias et al. 1996). In euthyroid rats, NCAM was detected in neurons of all hippocampal regions. Levels of hybridization were higher on the CA1 and CA3 areas, as well as in the dentate gyrus. A similar distribution was found in the hypothyroid rats. However, whereas NCAM expression was reduced at postnatal day 15 and further reduced at postnatal day 20 in control animals, it remained at higher levels in hypothyroid animals. TH-deficiency did not seem to alter the pattern of NCAM expression, but to inhibit or delay the developmental reduction in NCAM expression.
Tenascin-C, a secreted protein mainly expressed by astrocytic cells in the developing brain, is also involved in the regulation of cell adhesion and migration, neuron-neuron and neuron-glia interaction, neurite outgrowth and perhaps neuronal function (Chiquet-Ehrismann et al. 1995). Alvarez-Dolado et al. (1998) investigated the effect of congenital hypothyroidism on tenascin-C expression. At birth, in situ hybridization studies in hypothyroid rats showed an abnormal up-regulation of tenascin-C in the hippocampus. Significantly, T4-treatment of hypothyroid rats led to normalization of tenascin-C levels in most areas. It was also shown that T3 is regulating tenascin-C expression directly at the transcriptional level. Furthermore, hypothyroidism caused an increase in the number of cells expressing tenascin-C in the stratum lacunosum moleculare and the molecular layer of the dentate gyrus on postnatal day 5 and postnatal day 15. Finally, T3 responsiveness of tenascin-C gene expression in the brain is restricted to the developmental period. These defects in cell locations may lead to alterations in the pattern of connectivity.
Hippocampal cell differentiation under hypothyroidism
It is important to consider, in relation to the effect of THs, that maturation of radial glia is altered by maternal hypothyroidism. In particular, Martínez-Galán et al. (1997) described the prenatal effect of goitrogen treatment with methimazole and the prenatal effect of a very low iodine content diet on the maturation of radial glial cells of the CA1 hippocampal region of 21-day-old fetuses.
Some of the effects of THs on differentiation might also be mediated through control of the expression of neurotrophins, the presence of which is necessary for the survival of defined neuronal populations during CNS development and for the formation of the complex cellular networks that define brain structure and function (Wagner and Kostyk 1990). A few neurotrophins have been characterized and cloned including nerve growth factor (NGF), which seems to regulate the ontogeny of a number of neuronal structures in CNS in cooperation to T4 (Clos and Legrand 1990), and neurotrophin-3 (NT-3). Alvarez-Dolado et al. (1994) investigated the effect of neonatal and adult-onset hypothyroidism on the expression of many neurotrophin genes in different rat brain regions. In the hippocampus of neonatal hypothyroid animals, the NGF mRNA levels were diminished on postnatal day 15 as compared to control animals (20–50%). This reduction was also found in adult-onset hypothyroidism in the same area. Furthermore, neonatal hypothyroidism caused an increase in NT-3 mRNA concentration, but this difference was not observed in adult-onset hypothyroidism. These changes in the expression of some neurotrophin genes may be the basis for some of the alterations observed in the hypothyroid brain.
Using bromodeoxyuridine (BrdU), Desouza et al. (2005) demonstrated that adult-onset hypothyroidism significantly decreases hippocampal neurogenesis. This decline is predominantly the consequence of a significant decrease in the survival and neuronal differentiation of BrdU-positive cells. Both the decreased survival and neuronal differentiation of hippocampal progenitors could be rescued by restored euthyroid status.
Moreover, the effects of adult-onset pharmacologically-induced TH-deficiency on neural precursor proliferation and on newborn cell survival in the granule cell layer of adult rat dentate gyrus have been investigated (Ambrogini et al. 2005). In this study, no change in cell proliferation was found in the adult granule cell layer under TH-deficiency conditions, suggesting that mitotic activity of the neural precursors was not affected. On the contrary, newborn cell survival dramatically decreased under these conditions when compared with controls, leading to a lower number of immature neurons being added to the granule cell layer. It was also mentioned that in conditions of hypothyroidism, new neurons exhibit a delay in neuronal differentiation and a very immature morphology. Moreover, the expression of TUC-4 protein, a protein that is associated with neurite outgrowth and whose expression decreases with cell maturation, was prolonged under hypothyroidism conditions. Additionally, the expression of NeuN protein, a protein whose expression increases with cell maturation, did not change. This effect might be related to the persistent morphological immaturity of newborn neurons, when compared to controls, which makes the prolonged expression of the growth-associated protein TUC-4 necessary, and/or to the direct action of THs on TUC-4 gene transcription.
Hippocampal cell signaling modification due to hypothyroidism
Several proteins directly involved in intracellular signalling are under direct control of THs. A TH-dependent gene that is involved in cell signalling in the hippocampus is RC3 (or neurogranin). RC3 is a 78-amino acid protein kinase C substrate, located in the dendritic spines and the soma of neurons (Baudier et al. 1991). The non-phosphorylated form of RC3, in the presence of low calcium (Ca2+) concentrations, binds calmodulin. In that way, RC3 regulates its availability and, therefore, depending on its state of phosphorylation, the activation of calmodulin targets (calmodulin kinase II, nitric oxide synthase, and other important targets). These properties suggest that RC3 participates in postsynaptic events involving Ca2+ as a second messenger, including long-term potentiation (LTP), a prevalent model of memory in vertebrates, and other forms of synaptic plasticity. RC3 is also thought to play a role in the cascade of events triggered by the binding of glutamate to NMDA receptors in the postsynaptic neurons.
RC3 shows a region-specific dependence of T3. Although THs are essential for its normal expression in the dentate gyrus, the pyramidal cells of hippocampus do not exhibit the same T3-dependent RC3 expression, despite the presence of T3 receptors. Iniguez et al. (1996) analyzed the expression of RC3 mRNA by in situ hybridization in the brains of rats of different thyroidal status. RC3 expression in normally developing rats occurs in two phases: one, relatively independent of THs before postnatal day 10, and another beyond day 10, which is sensitive to THs.
Transcription factors’ deregulation in the hypothyroid hippocampus
THs regulate the expression of proteins involved in transcription, stability of mRNA and splicing. Among the transcription factors, the mRNA for nerve growth factor-induced gene A (NGFI-A) is decreased in the hippocampus of hypothyroid rats, associated with a subsequent reduction in its protein content. Mellström et al. (1994) described the effect of hypothyroidism on NGFI-A gene expression in the hippocampus thoroughly. In the dentate gyrus, there wasn’t any significant reduction of the NGFI-A mRNA levels at any developmental stage. This may indicate that the effects of THs on the development of the dentate gyrus are not mediated through NGFI-A. On postnatal day 0, hypothyroid rats showed reduced NGFI-A mRNA levels in hippocampal regions CA1 and CA2, with no decrease in CA3. Later, on postnatal day 5, the reduction was also noticeable in area CA3. Finally, on postnatal day 15 there were no differences in the NGFI-A mRNA levels in any region of the hippocampus between the control and hypothyroid rats, which implies a normalization with age of the NGFI-A mRNA levels.
Furthermore, two other transcription factors, c-fos and c-jun, are immediate early genes (IEGs) whose expression is regulated by synaptic activity, and play an important role in the neuroplastic mechanisms, which are critical to memory consolidation. Both c-fos and c-jun are expressed in neuronal nuclei and play an essential role in neuronal differentiation (Herdegen et al. 1997). Furthermore, they enable neurons to translate extracellular stimulation into long-term adaptive cellular responses by activating genetic programmes (Karin et al. 1997). Therefore, it is likely that c-fos and c-jun are also important components for the induction of LTP in hippocampus. Moreover, since c-fos and c-jun proteins are sensitive indicators of early neuronal damage (Broude et al. 1997), the expression of c-jun and c-fos may be altered in hippocampus of hypothyroid rats. Indeed, Dong et al. (2005) demonstrated that in iodine-deficient and hypothyroid rats, congenital hypothyroidism decreases the protein level of c-fos and c-jun in hippocampus. The staining intensity of c-fos and c-jun proteins was lower in CA1 and CA3 regions and in the dentate gyrus (postnatal day 60), while the integrated optical density (IOD) in area CA1 was also significantly reduced compared to the control group (postnatal days 20, 30 and 60).
Hippocampal local blood flow under hypothyroid conditions
Some experimental studies have shown that along with hypothyroidism of any etiological background, the CNS demonstrates global impairments in blood supply (Constant et al. 2001). Gabrichidze et al. (2007) reported studies of postnatal changes in the blood supply of hippocampus in the offspring of rats kept in conditions of different levels of iodine deficiency, before conception and throughout gestation. In particular, local blood flow in the dorsal hippocampus in the offspring of rats kept on the iodine-deficient basal diet before and during gestation was 19% less than in controls. The authors, based on the well-known metabolism - blood flow relationship, which appears to be the major principle for the regulation of local blood flow in CNS tissue during changes in functional brain activity (Demchenko 1983), suggested that metabolic activity is decreased in the hippocampus of iodine-deficient groups. In other words, hypothyroidism evoked by iodine deficiency was the primary cause of the decrease in metabolic activity, which was followed by a reduction in local blood flow. In addition, the decreased blood flow might lead to a further decrease in TH-delivery, thus exacerbating the hypothyroid status. Nevertheless, hypothyroidism can have neuroprotective effects against ischemia-induced hippocampal damage by favoring inhibitory input and limiting excitotoxic input by catecholamines (Hemmings and Shuaib 1998).
Hypothyroidism-induced effects on hippocampal cellular axon elongation, guidance and cell-connectivity
In order to maintain the normal neuronal development and differentiation of neurons, THs probably alter the expression of various growth factors and work as a signal to synchronize axonal and dendritic outgrowth, which are important for the construction of neuronal networks. There has been an attempt to study the effect of neonatal hypothyroidism on the expressions of genes important in the regulation of axon elongation and guidance in the hippocampus (Wong and Leung 2001). Such genes are the growth cone protein genes: growth associated protein of 43 kDa (GAP-43) and Gαo1 and 2, as well as the axon guidance molecules related genes: semaphoring-3F (Sema3F) and collapsin response mediator protein (CRMP) genes. Growth cone proteins such as GAP-43 and Gαo isoforms are neuronal proteins necessary for the maintenance of the growth cones, which are responsible for the sensation of attractive and repellent guidance cues in their immediate environment (Kobayashi et al. 1997). Under the regulation of semaphorins (i.e. semaphorin-3A/collapsin-1, a secreted repellent axon guidance molecule) growth cones collapse leading to a programmed cell death of the respective axon. Sema3F is a transmembrane protein of the semaphorin family of the vertebrate, considered to be involved in developing neuronal networks.
Wong and Leung (2001) reported a significant up-regulation of β-actin, Gαo1, CRMP1 to 4 and GAP-43 genes in the hippocampus of neonatal hypothyroid rats at the age of day 16. Conversely, expressions of the Gαo2 and Sema3F genes were not affected. When studying CRMP-2 and CRMP-4 proteins by peptide specific polyclonal antibodies, a significant enhancing effect of neonatal hypothyroidism on CRMP-2 but not on CRMP-4 protein was indicated. In the same study, an up-regulation of β-actin transcription was reported, but no significant effects of the neonatal hypothyroidism on the protein amount was observed. Moreover, no statistical significance on the effects of treatment on Gαo and Gαo1 proteins were evidenced. The up-regulation of CRMP1 to 4 gene’s mRNA as well as of the CRMP-2 protein in the neonatal hypothyroid rats may represent part of the regulatory mechanism involved in the control of growth cone collapse in this animal model. Other results on the mechanism of CRMP-2-induced growth cone collapse suggest that phosphorylation of CRMP-2 may destabilize microtubule bundle in the growth cone and induce growth cone collapse (Gu and Ihara 2000).
The neural cell adhesion molecule (CAM) TAG-1, a member of the immunoglobulin superfamily is thought to play an important role in axonal growth and guidance, neurite outgrowth and the formation of highly ordered neural connections (Furley et al. 1990). TAG-1 RNA and protein levels are upregulated in the hypothyroid CNS (Alvarez-Dolado et al. 2001) in several areas including the hippocampus. In agreement with this, TAG-1 protein was overexpressed in the major fibre tracts arising from hippocampal commissures. In all cases, elevation of TAG-1 RNA and protein expression could be reversed by TH-treatment. Changes in TAG-1 protein levels may contribute to explain at least partially the alterations in neuronal maturation and connectivity found in the hypothyroid brain.
Effects of hypothyroidism on the hippocampal cholinergic system
The cholinergic system is well established to be involved in higher brain functions such as learning and memory (Bartus et al. 1982). Among five subtypes of muscarinic acetylcholine receptors, the muscarinic receptor 1 (M1) is one of those predominant in the hippocampus and the cerebral cortex (Mash and Potter 1986). Muscarinic activation facilitates the induction of LTP (Burgard and Sarvey 1990), so that changes of M1 expression may influence neural activity by modulating excitatory synaptic transmission. M1 has also been reported to activate a mitogen-activated protein kinase (MAPK) like M3 and M5 (Wotta et al. 1998). Furthermore, extracellular stimuli such as neurotransmitters and neurotrophins causing signal transduction via MAPK have been implicated in several cellular events during development (including morphological growth and differentiation and cell survival of neurons, along with synaptic plasticity) (Fukunaga and Miyamoto 1998).
Recently, Kobayashi et al. (2005) have demonstrated that the expression level of M1 mRNA in the hippocampus of propylthiouracil (PTU)-treated (hypothyroid) rats is reduced during the early stage of development, although this does not seem to persist into adulthood. Thus, based on the actions of M1 mentioned above, the PTU-induced reduction of M1 mRNA expression during the early stages of development may result in a disturbance of the neuronal growth, synapse formation and neuronal wiring during development, and cause the subsequent impairment of learning and memory after maturation.
Additionally, the activity of choline acetyltransferase (ChAT), an enzyme marker for cholinergic nerve terminals, is exquisitely sensitive to thyroid status. Indeed, perinatal exposure to various doses of PTU, beginning on gestational day 18 and terminating on postnatal day 21, evinced a graded reduction of ChAT activity in rat hippocampus (Sawin et al. 1998). Concomitant with the enzyme deficits, hemicholinium-3 binding was elevated, suggesting an increase in neuronal impulse activity. Since ChAT is a marker for the cholinergic nerve terminals, these subnormal enzyme activities suggested a proportional decrease of neuronal density. Nonetheless, these neurochemical alterations appeared to be recoverable by euthyroidism, and no further changes were observed as the rats reached adulthood. Similar results were obtained, when the experimental protocol used polychlorinated biphenyl (PCB) for the chemical induction of hypothyroidism in neonatal rats (Juárez de Ku et al. 1994).
As far as the adult-onset hypothyroidism is concerned, a significant increase of the acetylcholinesterase (AChE) activity (by approximately 22%) and a significant decrease of the sodium/potassium-adenosine-triphosphatase (Na+,K+-ATPase) activity (by approximately 45%) were observed when measured in the rat hippocampus on the 21st day of the PTU-induced hypothyroidism (Carageorgiou et al. 2007). Acetylcholine (ACh) is a very important neurotransmitter for CNS function. Its action is dependent on its metabolizing enzyme, AChE, which was found to be involved in the release of ACh (Kouniniotou-Krontiri and Tsakiris 1989; Rami et al. 1989). AChE also induces LTP in hippocampal pyramidal neurons (Appleyard 1995). The synaptic plasma membrane Na+,K+-ATPase is an enzyme that regulates the action potential that in turn is responsible for the regulation of synaptic transmission by other neurotransmitters (Sastry and Phillis 1977). As mentioned above, hypothyroidism significantly affected Na+,K+-ATPase and AChE activity on rat hippocampus.
Effects of hypothyroidism on the hippocampal adenine nucleotide hydrolysis
Adenine nucleotides represent an important class of extracellular molecules involved in the modulation of signalling pathways that are crucial for normal CNS functioning. In particular, adenosine triphosphate (ATP) is an excitatory neurotransmitter, and adenosine has inhibitory effects on neurotransmission. Adenosine is an endogenous purine associated with an important modulatory role in neuronal activity (Latini and Pedata 2001). Adenosine molecules activate the presynaptic adenosine A1 receptors, which reduce neurotransmitter release, depressing the neuronal activity in the CNS. Previous studies have demonstrated that the ATP, released as a neurotransmitter, is hydrolyzed to adenosine by the conjugated action of an ATP diphosphohydrolase (ecto-nucleoside triphosphate diphosphohydrolase 3; NTPDase3) and a 5'-nucleotidase in brain synaptosomes (Battastini et al. 1991).
Bruno et al. (2005) aimed at evaluating the effects of hypothyroidism on the hydrolysis of ATP to adenosine in hippocampal synaptosomes of rats during different phases of development. Hypothyroidism was induced in these rats by thyroidectomy and methimazole (0.05%) added to their drinking water for 14 days. Hypothyroidism increased the adenosine monophosphate (AMP) (but not the ATP or the adenosine diphosphate; ADP) hydrolysis in 14-day-old rats, increased the AMP (but not the ATP or the ADP) hydrolysis in 60-day-old rats and increased both the ATP and the AMP (but not ADP) hydrolysis in 420-day-old rats. The increased hydrolysis of AMP in the hippocampus of rats submitted to neonatal or adult hypothyroidism shows an activation of the 5'-nucleotidase activity. This fact may result in a substantial increase in the brain adenosine levels, disturbance of the hippocampal neurotransmission and finally, in hypothyroidism-related memory impairment, which is frequently associated with decreased excitatory transmission. Furthermore, the additional increase in the ATP hydrolysis in synaptosomes from 420-day-old hypothyroid rats shows a lower availability of ATP as an excitatory neurotransmitter. The low ATP levels as well as the potential increase in the adenosine levels could contribute to the severity of hypothyroidism during ageing.
Astrocytes are the major cellular population in the CNS and are indispensable partners of neurons, both in physiological and pathological conditions. Changes in the functional characteristics of these cells induced by TH-deficiency, during brain development, result in severe neurological alterations. Recently, Braganhol et al. (2006) demonstrated the effects of neonatal hypothyroidism on the metabolism of ATP to adenosine in cultured astrocytes from different brain regions. A significant increase in AMP hydrolysis by 47% was observed in hippocampal astrocytes from rats submitted to neonatal hypothyroidism in relation to astrocytes of control animals. Treatment with T3 significantly reverted the increase in AMP hydrolysis, indicating the presence of a rapid effect of T3 on astrocytes in this brain structure.
Effects of hypothyroidism on the hippocampal neurotransmission, neuromodulation and synaptic plasticity
TH-deficiency has been reported to affect the expression and/or activity of the enzymes that regulate the action of several neurotransmitter and neuromodulators. One of these enzymes, prostaglandin D2 (PGD2) synthase is the enzyme responsible for the synthesis of PGD2 from its precursor PGH2 (prostaglandin H2) in the CNS (Ujihara et al. 1988). PGD2 is a major prostaglandin in the brain of humans and rats and functions as a neuromodulator of several central actions such as the sleep-wake cycle, body temperature, neurotransmitter release and odour responses. Remarkably, PGD2 and some of its derivatives act as ligands for the peroxisome proliferator-activated receptors, which regulate genes involved in lipid metabolism. Also, PGD2 synthase has been identified as the p-trace protein, and sequence analysis data conclude that it belongs to the lipocalin family of small lipid carriers. Thus, PGD2 synthase has a dual action both as an enzyme and as a transporter of its own product throughout the brain. García-Fernández et al. (1997) described a complex pattern of PGD2 synthase immunoreactivity during the early postnatal development of the rat brain and a number of clear differences between control and hypothyroid animals. In particular in the hippocampus, PGD2 synthase protein levels were higher in neurons of the CA1 and CA3 regions and the dentate gyrus of hypothyroid animals on postnatal days 5, 15 and 25. Furthermore, by affecting the expression and/or location of the PGD2 synthase, and hence putatively the synthesis of PGD2 and its derivatives, hypothyroidism may alter key brain processes, such as myelination and others involving active lipid synthesis and metabolism.
Reductions in the TH-levels during critical periods of CNS development can have permanent, devastating effects on neurological function and particularly in terms of cognitive ability (Correia et al. 2009). Gilbert and Paczkowski (2003) examined field potentials evoked in the dentate gyrus of the hippocampal formation in vivo in adult offspring of dams administered PTU and rendered hypothyroid just prior to parturition. Developing rats were exposed to the thyrotoxicant PTU, through the drinking water of pregnant dams beginning on gestational day 18 and extending throughout the lactational period. Hippocampal field potentials reflect the summated excitatory and inhibitory output of a population of neurons. Intracellular recordings reveal that an initial excitatory postsynaptic potential (EPSP) is presumably the positive component of the field potential.
Accumulating evidence indicate that the effects of THs on synaptic plasticity may be related with the control of gene expression. Among the neuron-specific gene products dependent upon TH, RC3, as mentioned above, represents a potential target whose dysfunction may underlie impairment in hippocampal synaptic plasticity. RC3 is phosphorylated in response to LTP stimulation in the hippocampus. TH-deficiency during development reduces the final levels of neurogranin mRNA and protein expressed in the dentate gyrus. A permanent reduction in expression of neurogranin in dentate granule cells following developmental hypothyroidism may underlie the observed impairments in synaptic plasticity (Gerges et al. 2001). Under the same conditions as in the dentate gyrus described above, Gilbert (2004) studied the influence of hypothyroidism in synaptic transmission and plasticity in area CA1 of adult hippocampus. Synaptic transmission, short-term, and long-term synaptic plasticity were assessed. Consistent with observations in the dentate gyrus, somatic population spike amplitudes were reduced in assessments of baseline synaptic transmission of slices from PTU-exposed animals. No differences were identified in EPSP. Short-term plasticity of the EPSP as indexed by paired pulse facilitation was markedly impaired by PTU exposure. The LTP of the population spike was enhanced, consistent with findings in dentate gyrus, but no change in EPSP LTP was detected. Enhancements in population spike LTP in area CA1 and dentate gyrus may culminate from hypothyroid-induced changes in synaptic structure, cellular excitability, presynaptic Ca2+ function, or gamma-aminobutyric acid (GABA)-mediated inhibition. A number of TH-responsive genes that are critical for cell migration, dendritic arborization, synapse formation, myelination and structural changes within CA1 pyramidal cells have already been referred. Alterations in the expression of these genes due to hypothyroidism may lead to reversible changes in synaptic transmission and plasticity of CA1 area (Gerges et al. 2001; Sui and Gilbert 2003; Gerges and Alkadhi 2004; Alzoubi et al. 2005). Permanent disruption of synaptic function and/or plasticity may contribute to the neurological deficits associated with hormone deprivation during critical periods of CNS development, and to learning and memory deficits in adult-onset hypothyroidism (Sui et al. 2006).
The MAPKs family comprehend the extracellular signal-regulated kinases (ERKs), the Jun N-terminal kinases (JNKs) and p38 mitogen-activated protein kinases (p38MAPKs) (Chang and Karin 2001). Extracellular signals like growth factors and hormones activate the ERK pathway, while JNKs and p38MAPKs are primarily activated by stressful stimulus and are largely associated with programmed cell death in the brain. In the hippocampus, ERKs have been involved in neural plasticity such as LTP and memory formation, and recently it has been evidenced that p38MAPKs and JNKs are also involved in modulation of hippocampal plasticity (Thomas and Huganir 2004). Hippocampus of hypothyroid rats presented a significant increase (+50%) in ERK1/2 phosphorylation and a reduction (-50%) in p38MAPK phosphorylation compared to the control animals (Calloni et al. 2005; Sui et al. 2005). No differences were observed in JNK phosphorylation.
The GABAergic interneurons comprise the bulk of local inhibitory circuitry in brain, many of which contain the calcium binding protein, parvalbumin (PV) (Kosaka et al. 1987). PV confers on the interneuron specific electrical and metabolic properties that can impact their function. PV-expressing interneurons are basket and chandelier cells that synapse directly on the soma or initial axonal segment of principal cells of hippocampus. Activation of these local circuit neurons effectively limits the firing of action potentials by pyramidal cells of the hippocampus and granule cells of the dentate gyrus.
It should be noted that a reduction in PV immunoreactivity (PV-IR) in cortex and hippocampus of animals with moderate degrees of (PTU-induced) TH-insufficiency has been described (Gilbert et al. 2007). The cross-fostering study revealed that hormone insufficiency in the early postnatal period is both necessary and sufficient for altered expression of PV-IR in inhibitory neurons. Hormone reductions restricted to the prenatal period or initiated in adulthood were without effect on PV-IR. However, hormone insufficiency that spanned the prenatal and postnatal period produced more profound deficits in PV-IR than postnatal exposure alone. Of the three hippocampal subregions, the dentate gyrus appeared to be the most severely impacted. Although some recovery ensued on termination of exposure and return to euthyroid status in adulthood, significant suppression of PV-IR remained. These persistent alterations in PV-IR in hippocampal interneurons were associated with functional deficits in inhibitory synaptic transmission in the dentate gyrus. Synaptic inhibition of the perforant path-dentate gyrus synapse evaluated in adult offspring, in vivo, revealed dose-dependent reductions in paired pulse depression indicative of a suppression of GABA-mediated inhibition. In summary, all phases of synaptic inhibition were disrupted to varying degrees in animals exposed to PTU and a general pattern of disinhibition was apparent. Additionally, results of immunostaining for a marker of GABA neurons indicate that the total complement of GABA-containing neurons remains intact in hypothyroid animals (Berbel et al. 1996). Additional experimental data concluded that TH deficiency leads to disturbed chlorine ion (Cl-) homeostasis, delaying the onset of synaptic inhibition (Friauf et al. 2008).
Epilogue
THs have a broad spectrum of effects on the CNS. A decrease of them, especially during development leads to structural and functional dysfunction of the brain in humans and experimental animals. Additionally, severe hormone deprivation leads to reductions in physical growth and mental retardation, particularly if insufficiency occurs in the early postnatal period.
TH-deficiency during fetal and neonatal periods in rats produces deleterious effects in the hippocampus, such as reduced synaptic connectivity, delayed myelination, disturbed neuronal migration, deranged axonal projections, decreased synaptogenesis and alterations in the levels of neurotransmitters. Furthermore, adult thyroid dysfunction is also associated with neurological alterations and behavioural abnormalities. However, the mechanisms of actions of THs in the adult hippocampus are poorly understood.
In the past, hippocampus was considered as a non-target region of THs, despite the evidence that TH-deficiency in developing mammals is associated with severe morphological and functional alterations. Today, it is clear that THs have direct actions on the hippocampus, and that these actions are exerted through the nuclear receptors and regulation of gene expression. In particular, TH-deficiency in early life leads to lasting changes in the hippocampus, including a reduction in the number of granule cells in the dentate gyrus, as well as alterations in the chemical composition of cells and the retardation in the development of pyramidal and granule cells. These changes can affect the organization of neuronal connections in the hippocampus and thus contribute to some of the behavioural abnormalities seen in congenital cretinism.
Structural changes within pyramidal cells and dentate gyrus following perinatal hypothyroidism are associated with functional deficits in hippocampal synaptic circuitry and both pyramidal cells and dentate gyrus are implicated in this dysfunction. Also, deficits in short-term and long-term synaptic plasticity that take place in these regions have been associated with impairments on a variety of cognitive tasks. As the hippocampus represents a critical neural substrate for some forms of learning, compromises in hippocampal synaptic function and plasticity may contribute to cognitive impairments that accompany early TH-insufficiency
The reported different effects of THs on brain gene expression depending on the area studied and the age of the animal (Mellström et al. 1994) point to a very complex effect of hypothyroidism on brain development, where different kinds of neurons respond to this hormone in very different fashions. The surprisingly low number of genes found to be altered by hypothyroidism contrasts with the abundance of TH-receptors in the brain and the wide phenotypic alterations caused by TH-deficiency. Most of the TH-regulated genes in the rat brain are sensitive to the hormone only during a narrow window of the postnatal period. The critical period of TH sensitivity in the brain is limited to the first 2-3 postnatal weeks in the rat. In the human, the sensitive period would correspondingly start after midpregnancy.
Additionally, maternal hormones have recently been demonstrated to play an important role in cell migration in the fetal neocortex (Ausó et al. 2004), much before the onset of fetal thyroid gland function. Genes regulated in the fetal brain by maternal T4 have yet to be identified, and application of global analysis of gene expression using suitable models of fetal hypothyroidism may help to identify TH-regulated genes during fetal brain development.
Abbreviations
- THs:
-
thyroid hormones
- CNS:
-
central nervous system
- T4 :
-
thyroxine
- T3 :
-
3,5,3'-triiodothyronine
- TH:
-
thyroid hormone
- TRs:
-
TH receptors
- TUNEL:
-
terminal deoxynucleotidyl transferase dUTP nick end labeling
- NMDA:
-
N-methyl-D-aspartic acid
- SVZ:
-
subventricular zone
- SGZ:
-
subgranular zone
- Dab1:
-
Disabled-1 (gene)
- NCAM:
-
neural cell adhesion molecule
- NGF:
-
nerve growth factor
- NT-3:
-
neurotrophin-3
- BrdU:
-
bromodeoxyuridine
- RC3:
-
neurogranin
- Ca2+ :
-
calcium ion
- LTP:
-
long-term potentiation
- NGFI-A:
-
nerve growth factor-induced gene A
- IEGs:
-
immediate early genes
- IOD:
-
integrated optical density
- GAP-43:
-
growth associated protein of 43 kDa
- Sema3F:
-
semaphorin-3F
- CRMP:
-
collapsin response mediated protein
- CAM:
-
cell adhesion molecule
- M1:
-
muscarinic receptor 1
- MAPK:
-
mitogen-activated protein kinase
- PTU:
-
propylthiouracil
- ChAT:
-
choline acetyltransferase
- PCB:
-
polychlorinated biphenyl
- AChE:
-
acetylcholinesterase
- Na+,K+-ATPase:
-
sodium/potassium adenosine-triphosphatase
- ACh:
-
acetylcholine
- ATP:
-
adenosine triphosphate
- A1:
-
adenosine receptor 1
- NTPDase3:
-
ecto-nucleoside triphosphate diphosphohydrolase 3
- AMP:
-
adenosine monophosphate
- ADP:
-
adenosine diphosphate
- PGD2 :
-
prostaglandin D2
- PGH2 :
-
prostaglandin H2
- EPSP:
-
excitatory postsynaptic potential
- GABA:
-
gamma-aminobutyric acid
- ERKs:
-
extracellular signal-regulated kinases
- JNKs:
-
c-Jun N-terminal kinases
- p38MAPKs:
-
p38 mitogen-activated protein kinases
- PV:
-
parvalbumin
- PV-IR:
-
PV-immunoreactivity
- Cl- :
-
chlorine ion
References
Altman J, Das GD (1966) Autoradiographic and histological studies of postnatal neurogenesis. I. A longitudinal investigation of the kinetics, migration and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. J Comp Neurol 126:337–389
Altman J, Bayer SA (1990a) Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol 301:365–381
Altman J, Bayer SA (1990b) Prolonged sojourn of developing pyramidal cells in the intermediate zone of the hippocampus and their settling in the stratum pyramidale. J Comp Neurol 301:343–364
Alvarez-Dolado M, Iglesias T, Rodríguez-Peña A, Bernal J, Muñoz A (1994) Expression of neurotrophins and the trk family of neurotrophin receptors in normal and hypothyroid rat brain. Brain Res Mol Brain Res 27:249–257
Alvarez-Dolado M, González-Sancho JM, Bernal J, Muñoz A (1998) Developmental expression of the tenascin-C is altered by hypothyroidism in the rat brain. Neuroscience 84:309–322
Alvarez-Dolado M, Ruiz M, Del Río JA, Alcántara S, Burgaya F, Sheldon M, Nakajima K, Bernal J, Howell BW, Curran T, Soriano E, Muñoz A (1999) Thyroid hormone regulates reelin and dab1 expression during brain development. J Neurosci 19:6979–6993
Alvarez-Dolado M, Figueroa A, Kozlov S, Sonderegger P, Furley AJ, Muñoz A (2001) Thyroid hormone regulates TAG-1 expression in the developing rat brain. Eur J Neurosci 14:1209–1218
Alva-Sánchez C, Ortiz-Butrón R, Cuéllar-García M, Hernández-García A, Pacheco-Rosado J (2002) Anatomical changes in CA3 hippocampal region by hypothyroidism in rats. Proc West Pharmacol Soc 45:125–126
Alva-Sánchez C, Medina-Canales MG, Ramos-Godínez MP, Sánchez-Espíndola ME, Becerril-Montes A (2007) Ultrastructural morphology of hypothyroid hippocampal pyramidal cells reveals apoptotic neuronal death. Acta Microscopica 16:325–326
Alva-Sánchez C, Becerril A, Anguiano B, Aceves C, Pacheco-Rosado J (2009) Participation of NMDA-glutamatergic receptors in hippocampal neuronal damage caused by adult-onset hypothyroidism. Neurosci Lett 453:178–181
Alzoubi KH, Gerges NZ, Alkadhi KA (2005) Levothyroxin restores hypothyroidism-induced impairment of LTP of hippocampal CA1: electrophysiological and molecular studies. Exp Neurol 195:330–341
Amaral DG, Dent JA (1981) Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol 195:51–86
Ambrogini P, Cuppini R, Ferri P, Mancini C, Ciaroni S, Voci A, Gerdoni E, Gallo G (2005) Thyroid hormones affect neurogenesis in the dentate gyrus of adult rat. Neuroendocrinology 81:244–253
Appleyard ME (1995) Acetylcholinesterase induces long-term potentiation in CA1 pyramidal cells by a mechanism dependent on metabotropic glutamate receptors. Neurosci Lett 190:25–28
Ausó E, Lavado-Autric R, Cuevas E, Del Rey FE, Morreale De Escobar G, Berbel P (2004) A moderate and transient deficiency of maternal thyroid function at the beginning of fetal neocorticogenesis alters neuronal migration. Endocrinology 145:4037–4047
Bartus RT, Dean RL 3rd, Beer B, Lippa AS (1982) The cholinergic hypothesis of geriatric memory dysfunction. Science 217:408–414
Battastini AM, da Rocha JB, Barcellos CK, Dias RD, Sarkis JJ (1991) Characterization of an ATP diphosphohydrolase (EC 3.6.1.5) in synaptosomes from cerebral cortex of adult rats. Neurochem Res 16:1303–1310
Battie CA, Verity MA (1979) Membrane enzyme development in nerve ending mitochondria during neonatal hypothyroidism. Dev Neurosci 2:139–148
Baudier J, Deloulme JC, Van Dorsselaer A, Black D, Matthes HW (1991) Purification and characterization of a brain-specific protein kinase C substrate, neurogranin (p17). Identification of a consensus amino acid sequence between neurogranin and neuromodulin (GAP43) that corresponds to the protein kinase C phosphorylation site and the calmodulin-binding domain. J Biol Chem 266:229–237
Bayer SA (1980) Development of the hippocampal region in the rat. I. Neurogenesis examined with 3H-thymidine autoradiography. J Comp Neurol 190:87–114
Berbel P, Marco P, Cerezo JR, DeFelipe J (1996) Distribution of parvalbumin immunoreactivity in the neocortex of hypothyroid adult rats. Neurosci Lett 204:65–68
Bernal J (2005) Thyroid hormones and brain development. Vitam Horm 71:95–122
Bernal J (2007) Thyroid hormone receptors in brain development and function. Nat Clin Pract Endocrinol Metab 3:249–259
Braganhol E, Bruno AN, Bavaresco L, Barreto-Chaves ML, Sarkis JJ, Battastini AM (2006) Neonatal hypothyroidism affects the adenine nucleotides metabolism in astrocyte cultures from rat brain. Neurochem Res 31:449–454
Broude E, McAtee M, Kelley MS, Bregman BS (1997) c-Jun expression in adult rat dorsal root ganglion neurons: differential response after central or peripheral axotomy. Exp Neurol 148:367–377
Bruno AN, Ricachenevsky FK, Pochmann D, Bonan CD, Battastini AM, Barreto-Chaves ML, Sarkis JJ (2005) Hypothyroidism changes adenine nucleotide hydrolysis in synaptosomes from hippocampus and cerebral cortex of rats in different phases of development. Int J Dev Neurosci 23:37–44
Burgard EC, Sarvey JM (1990) Muscarinic receptor activation facilitates the induction of long-term potentiation (LTP) in the rat dentate gyrus. Neurosci Lett 116:34–39
Calloni GW, Penno CA, Cordova FM, Trentin AG, Neto VM, Leal RB (2005) Congenital hypothyroidism alters the phosphorylation of ERK1/2 and p38MAPK in the hippocampus of neonatal rats. Brain Res Dev Brain Res 154:141–145
Calzà L, Aloe L, Giardino L (1997) Thyroid hormone-induced plasticity in the adult rat brain. Brain Res Bull 44:549–557
Carageorgiou H, Pantos C, Zarros A, Stolakis V, Mourouzis I, Cokkinos D, Tsakiris S (2007) Changes in acetylcholinesterase, Na+, K + -ATPase, and Mg2 + -ATPase activities in the frontal cortex and the hippocampus of hyper- and hypothyroid adult rats. Metabolism 56:1104–1110
Chang L, Karin M (2001) Mammalian MAP kinase signalling cascades. Nature 410:37–40
Chiquet-Ehrismann R, Hagios C, Schenk S (1995) The complexity in regulating the expression of tenascins. Bioessays 17:873–878
Clos J, Legrand C (1990) An interaction between thyroid hormone and nerve growth factor promotes the development of hippocampus, olfactory bulbs and cerebellum: a comparative biochemical study of normal and hypothyroid rats. Growth Factors 3:205–220
Constant EL, de Volder AG, Ivanoiu A, Bol A, Labar D, Seghers A, Cosnard G, Melin J, Daumerie C (2001) Cerebral blood flow and glucose metabolism in hypothyroidism: a positron emission tomography study. J Clin Endocrinol Metab 86:3864–3870
Correia N, Mullally S, Cooke G, Tun TK, Phelan N, Feeney J, Fitzgibbon M, Boran G, O'Mara S, Gibney J (2009) Evidence for a specific defect in hippocampal memory in overt and subclinical hypothyroidism. J Clin Endocrinol Metab 94:3789–3797
Delange F (2000) The role of iodine in brain development. Proc Nutr Soc 59:75–79
Demchenko IT (1983) Blood supply in the conscious brain. Nauka, Leningrad
Desouza LA, Ladiwala U, Daniel SM, Agashe S, Vaidya RA, Vaidya VA (2005) Thyroid hormone regulates hippocampal neurogenesis in the adult rat brain. Mol Cell Neurosci 29:414–426
Dong J, Yin H, Liu W, Wang P, Jiang Y, Chen J (2005) Congenital iodine deficiency and hypothyroidism impair LTP and decrease c-fos and c-jun expression in rat hippocampus. Neurotoxicology 26:417–426
Farahvar A, Darwish NH, Sladek S, Meisami E (2007) Marked recovery of functional metabolic activity and laminar volumes in the rat hippocampus and dentate gyrus following postnatal hypothyroid growth retardation: A quantitative cytochrome oxidase study. Exp Neurol 204:556–568
Friauf E, Wenz M, Oberhofer M, Nothwang HG, Balakrishnan V, Knipper M, Löhrke S (2008) Hypothyroidism impairs chloride homeostasis and onset of inhibitory neurotransmission in developing auditory brainstem and hippocampal neurons. Eur J Neurosci 28:2371–2380
Fukunaga K, Miyamoto E (1998) Role of MAP kinase in neurons. Mol Neurobiol 16:79–95
Furley AJ, Morton SB, Manalo D, Karagogeos D, Dodd J, Jessell TM (1990) The axonal glycoprotein TAG-1 is an immunoglobulin superfamily member with neurite outgrowth-promoting activity. Cell 61:157–170
Gabrichidze GO, Lazrishvili NI, Metreveli DS, Bekaya GL, Mitagvariya NP (2007) Local blood flow in the dorsal hippocampus and cerebellar cortex in the offspring of iodine-deficient rats. Neurosci Behav Physiol 37:495–498
Gage FH (2002) Neurogenesis in the adult brain. J Neurosci 22:612–613
García-Fernández LF, Rausell E, Urade Y, Hayaishi O, Bernal J, Muñoz A (1997) Hypothyroidism alters the expression of prostaglandin D2 synthase/beta trace in specific areas of the developing rat brain. Eur J Neurosci 9:1566–1573
Gerges NZ, Alkadhi KA (2004) Hypothyroidism impairs late LTP in CA1 region but not in dentate gyrus of the intact rat hippocampus: MAPK involvement. Hippocampus 14:40–45
Gerges NZ, Stringer JL, Alkadhi KA (2001) Combination of hypothyroidism and stress abolishes early LTP in the CA1 but not dentate gyrus of hippocampus of adult rats. Brain Res 922:250–260
Gilbert ME (2004) Alterations in synaptic transmission and plasticity in area CA1 of adult hippocampus following developmental hypothyroidism. Brain Res Dev Brain Res 148:11–18
Gilbert ME, Paczkowski C (2003) Propylthiouracil (PTU)-induced hypothyroidism in the developing rat impairs synaptic transmission and plasticity in the dentate gyrus of the adult hippocampus. Brain Res Dev Brain Res 145:19–29
Gilbert ME, Sui L, Walker MJ, Anderson W, Thomas S, Smoller SN, Schon JP, Phani S, Goodman JH (2007) Thyroid hormone insufficiency during brain development reduces parvalbumin immunoreactivity and inhibitory function in the hippocampus. Endocrinology 148:92–102
Gilroy J, Meyer JS (1975) Medical Neurology, 2nd edn. MacMillan, New York, pp 239–241
Glinoer D (2007) The importance of iodine nutrition during pregnancy. Public Health Nutr 10:1542–1546
Gu Y, Ihara Y (2000) Evidence that collapsin response mediator protein-2 is involved in the dynamics of microtubules. J Biol Chem 275:17917–17920
Guadaño Ferraz A, Escobar del Rey F, Morreale de Escobar G, Innocenti GM, Berbel P (1994) The development of the anterior commissure in normal and hypothyroid rats. Brain Res Dev Brain Res 81:293–308
Hemmings SJ, Shuaib A (1998) Hypothyroidism-evoked shifts in hippocampal adrenergic receptors: implications to ischemia-induced hippocampal damage. Mol Cell Biochem 185:161–169
Herdegen T, Skene P, Bähr M (1997) The c-Jun transcription factor—bipotential mediator of neuronal death, survival and regeneration. Trends Neurosci 20:227–231
Huang XW, Zhao ZY, Ji C (2005a) Effects of hypothyroidism on apoptosis and the expression of Bcl-2 and Bax gene in the neonatal rat hippocampus neurons. Zhonghua Er Ke Za Zhi 43:48–52
Huang XW, Yang RL, Zhao ZY, Ji C, Yang RW (2005b) Mechanism for apoptosis of hippocampus neuron induced by hypothyroidism in perinatal rats. Zhejiang Da Xue Xue Bao Yi Xue Ban 34:298–303
Huang XW, Yin HM, Ji C, Qin YF, Yang RW, Zhao ZY (2008a) Effects of perinatal hypothyroidism on rat behavior and its relation with apoptosis of hippocampus neurons. J Endocrinol Invest 31:8–15
Huang YH, Tsai MM, Lin KH (2008b) Thyroid hormone dependent regulation of target genes and their physiological significance. Chang Gung Med J 31:325–334
Ibarrola N, Rodríguez-Peña A (1997) Hypothyroidism coordinately and transiently affects myelin protein gene expression in most rat brain regions during postnatal development. Brain Res 752:285–293
Iglesias T, Caubín J, Stunnenberg HG, Zaballos A, Bernal J, Muñoz A (1996) Thyroid hormone-dependent transcriptional repression of neural cell adhesion molecule during brain maturation. EMBO J 15:4307–4316
Iniguez MA, De Lecea L, Guadano-Ferraz A, Morte B, Gerendasy D, Sutcliffe JG, Bernal J (1996) Cell-specific effects of thyroid hormone on RC3/neurogranin expression in rat brain. Endocrinology 137:1032–1041
Juárez de Ku LM, Sharma-Stokkermans M, Meserve LA (1994) Thyroxine normalizes polychlorinated biphenyl (PCB) dose-related depression of choline acetyltransferase (ChAT) activity in hippocampus and basal forebrain of 15-day-old rats. Toxicology 94:19–30
Karin M, Liu Z, Zandi E (1997) AP-1 function and regulation. Curr Opin Cell Biol 9:240–246
Katyare SS, Bangur CS, Howland JL (1994) Is respiratory activity in the brain mitochondria responsive to thyroid hormone action? A critical re-evaluation. Biochem J 302:857–860
Kobayashi H, Koppel AM, Luo Y, Raper JA (1997) A role for collapsin-1 in olfactory and cranial sensory axon guidance. J Neurosci 17:8339–8352
Kobayashi K, Tsuji R, Yoshioka T, Kushida M, Yabushita S, Sasaki M, Mino T, Seki T (2005) Effects of hypothyroidism induced by perinatal exposure to PTU on rat behaviour and synaptic gene expression. Toxicology 212:135–147
Kosaka T, Katsumaru H, Hama K, Wu JY, Heizmann CW (1987) GABAergic neurons containing the Ca2+-binding protein parvalbumin in the rat hippocampus and dentate gyrus. Brain Res 419:119–130
Kouniniotou-Krontiri P, Tsakiris S (1989) Time dependence of Li+ action on acetylcholinesterase activity in correlation with spontaneous quantal release of acetylcholine in rat diaphragm. Jpn J Physiol 39:429–440
Lass P, Slawek J, Derejko M, Rubello D (2008) Neurological and psychiatric disorders in thyroid dysfunctions. The role of nuclear medicine: SPECT and PET imaging. Minerva Endocrinol 33:75–84
Latini S, Pedata F (2001) Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J Neurochem 79:463–484
Legrand J (1982-1983) Thyroid hormones and maturation of the nervous system. J Physiol (Paris) 78:603–652
Madeira MD, Paula-Barbosa M, Cadete-Leite A, Tavares MA (1988) Unbiased estimate of hippocampal granule cell numbers in hypothyroid and in sex-age-matched control rats. J Hirnforsch 29:643–650
Madeira MD, Cadete-Leite A, Andrade JP, Paula-Barbosa MM (1991a) Effects of hypothyroidism upon the granular layer of the dentate gyrus in male and female adult rats: a morphometric study. J Comp Neurol 314:171–186
Madeira MD, Cadete-Leite A, Sousa N, Paula-Barbosa MM (1991b) The supraoptic nucleus in hypothyroid and undernourished rats: an experimental morphometric study. Neuroscience 41:827–839
Madeira MD, Sousa N, Lima-Andrade MT, Calheiros F, Cadete-Leite A, Paula-Barbosa MM (1992) Selective vulnerability of the hippocampal pyramidal neurons to hypothyroidism in male and female rats. J Comp Neurol 322:501–518
Martínez-Galán JR, Pedraza P, Santacana M, Escobar del Ray F, Morreale de Escobar G, Ruiz-Marcos A (1997) Early effects of iodine deficiency on radial glial cells of the hippocampus of the rat fetus. A model of neurological cretinism. J Clin Invest 99:2701–2709
Mash DC, Potter LT (1986) Autoradiographic localization of M1 and M2 muscarine receptors in the rat brain. Neuroscience 19:551–564
Mellström B, Pipaón C, Naranjo JR, Perez-Castillo A, Santos A (1994) Differential effect of thyroid hormone on NGFI-A gene expression in developing rat brain. Endocrinology 135:583–588
Montero-Pedrazuela A, Venero C, Lavado-Autric R, Fernández-Lamo I, García-Verdugo JM, Bernal J, Guadaño-Ferraz A (2006) Modulation of adult hippocampal neurogenesis by thyroid hormones: implications in depressive-like behavior. Mol Psychiatry 11:361–371
Moreno M, de Lange P, Lombardi A, Silvestri E, Lanni A, Goglia F (2008) Metabolic effects of thyroid hormone derivatives. Thyroid 18:239–253
Moskovkin GN, Marshak TL (1982) Action of hypothyroidism on the metabolic maturation of the pyramidal neurons of the rat hippocampus. Biull Eksp Biol Med 94:112–114
Peeters RP (2008) Thyroid hormones and aging. Hormones (Athens) 7:28–35
Pérez-Delgado MM, Ferres-Torres R, Castañeyra-Perdomo A, González-Hernández T (1987) Effects of hypothyroidism on the karyometric development of pyramidal neurons of the hippocampus (CA1), area 6 and area 17 in the male mouse. J Anat 150:23–29
Rabié A, Patel AJ, Clavel MC, Legrand J (1979) Effect of thyroid deficiency on the growth of the hippocampus in the rat. A combined biochemical and morphological study. Dev Neurosci 2:183–194
Rami A, Rabié A, Patel AJ (1986a) Thyroid hormone and development of the rat hippocampus: cell acquisition in the dentate gyrus. Neuroscience 19:1207–1216
Rami A, Patel AJ, Rabié A (1986b) Thyroid hormone and development of the rat hippocampus: morphological alterations in granule and pyramidal cells. Neuroscience 19:1217–1226
Rami A, Rabie A, Clos J (1989) The time course of hippocampal cholinergic innervation in the developing hypothyroid rat. A combined histochemical and biochemical study of acetylcholinesterase activity. Int J Dev Neurosci 7:301–308
Royland JE, Parker JS, Gilbert ME (2008) A genomic analysis of subclinical hypothyroidism in hippocampus and neocortex of the developing rat brain. J Neuroendocrinol 20:1319–1338
Sastry BS, Phillis JW (1977) Antagonism of biogenic amine-induced depression of cerebral cortical neurones by Na+, K + -ATPase in inhibitors. Can J Physiol Pharmacol 55:170–179
Sawin S, Brodish P, Carter CS, Stanton ME, Lau C (1998) Development of cholinergic neurons in rat brain regions: dose-dependent effects of propylthiouracil-induced hypothyroidism. Neurotoxicol Teratol 20:627–635
Schlessinger AR, Cowan WM, Gottlieb DI (1975) An autoradiographic study of the time of origin and the pattern of granule cell migration in the dentate gyrus of the rat. J Comp Neurol 159:149–175
Schlessinger AR, Cowan WM, Swanson LW (1978) The time of origin of neurons in Ammon’s horn and the associated retrohippocampal fields. Anat Embryol (Berl) 154:153–173
Schoonover CM, Seibel MM, Jolson DM, Stack MJ, Rahman RJ, Jones SA, Mariash CN, Anderson GW (2004) Thyroid hormone regulates oligodendrocyte accumulation in developing rat brain white matter tracts. Endocrinology 145:5013–5020
Stanfield BB, Cowan WM (1979) The development of the hippocampus and dentate gyrus in normal and reeler mice. J Comp Neurol 185:423–459
Sui L, Gilbert ME (2003) Pre- and postnatal propylthiouracil-induced hypothyroidism impairs synaptic transmission and plasticity in area CA1 of the neonatal rat hippocampus. Endocrinology 144:4195–4203
Sui L, Anderson WL, Gilbert ME (2005) Impairment in short-term but enhanced long-term synaptic potentiation and ERK activation in adult hippocampal area CA1 following developmental thyroid hormone insufficiency. Toxicol Sci 85:647–656
Sui L, Wang F, Li BM (2006) Adult-onset hypothyroidism impairs paired-pulse facilitation and long-term potentiation of the rat dorsal hippocampo-medial prefrontal cortex pathway in vivo. Brain Res 1096:53–60
Sutcliffe JG (1988) The genes for myelin revisited. Trends Genet 4:211–213
Thomas GM, Huganir RL (2004) MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci 5:173–183
Ujihara M, Urade Y, Eguchi N, Hayashi H, Ikai K, Hayaishi O (1988) Prostaglandin D2 formation and characterization of its synthetases in various tissues of adult rats. Arch Biochem Biophys 260:521–531
Wagner JA, Kostyk SK (1990) Regulation of neural cell survival and differentiation by peptide growth factors. Curr Opin Cell Biol 2:1050–1057
White AM, Matthews DB, Best PJ (2000) Ethanol, memory, and hippocampal function: a review of recent findings. Hippocampus 10:88–93
Williams GR (2008) Neurodevelopmental and neurophysiological actions of thyroid hormone. J Neuroendocrinol 20:784–794
Wong CC, Leung MS (2001) Effects of neonatal hypothyroidism on the expressions of growth cone proteins and axon guidance molecules related genes in the hippocampus. Mol Cell Endocrinol 184:143–150
Wotta DR, Wattenberg EV, Langason RB, el-Fakahany EE (1998) M1, M3 and M5 muscarinic receptors stimulate mitogen-activated protein kinase. Pharmacology 56:175–186
Acknowledgements
The authors wish to express their appreciation to the medical students Marianna Almpani, Ioanna Loupasi and Ioannis-Angelos Trantos for their assistance in manuscript preparation. The authors are grateful to Mr Ioannis Mantzikos (BSc, PGDip, MSc) for his assistance in manuscript improvement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koromilas, C., Liapi, C., Schulpis, K.H. et al. Structural and functional alterations in the hippocampus due to hypothyroidism. Metab Brain Dis 25, 339–354 (2010). https://doi.org/10.1007/s11011-010-9208-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-010-9208-8