Abstract
Neurological complications are common after liver transplantation (LT) and they are associated with a significant morbidity. Long-term effects of LT on cognitive and psychological outcomes are not clear. The objective of this study was to summarize the present knowledge on the neurological and cognitive complications of LT, resulting from a systematic review of the literature in the last 10 years. Several studies have investigated the incidence and the pathophysiology of neurological complications; in contrast, the knowledge of cognitive and psychological status after LT is poor. Currently, the effect of LT on mental performance is debated. Some studies have shown an improvement of cognitive function after OLTX and, at the same time, a persistence of different cognitive deficits. In addition, the quality of life (QoL) and the psychological status after LT seem to improve but LT recipients have significant deficiencies in most QoL domains. Consequently, future studies are necessary in order to investigate cognitive alterations and QoL in LT recipients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liver transplantation (LT) is the only curative treatment in patients with end-stage liver disease. The first LT was performed in 1963 (Starzl et al. 1963). Initially, the most common reason for death post-LT was an acute or chronic rejection of the graft. Then, the introduction of immunosuppressive treatment increased the survival rate from 20% to nearly 80% (Starzl et al. 1985), but postoperative complications remain a significant source of morbidity and mortality (Mazariegos et al. 1999). Of all the complications post-LT, the neurological complications (NC) are particularly relevant, since they affect up to a third of transplanted patients (Wang et al. 2000). Patients with neuropsychiatric complications following LT show a high mortality rate, especially when the complications occur in the first period after transplantation (Bronster et al. 1994; Guarino 1999). In addition, patients with NC have longer hospitalization (Pujol et al. 1994; Kim et al. 2007; Dhar et al. 2008), higher need of re-transplantation, more infections (Lewis and Howdle 2003) and lower self-sufficiency and social reintegration (Kaplan et al. 1996) than patients without NC. In contrast, in other studies the survival rate of cadaveric-donor LT patients with and without NC were comparable (Wijdicks et al. 1994; Saner et al. 2007). In a recent study, Saner et al. (2009) confirmed this observation also in adult living-donor LT: they observed that the occurrence of NC in adult living-donor LT did not influence the clinical outcome, since mortality rate and length of hospital stay were similar between the groups.
Neurological complications have been attributed to several pathogenetic factors, such as poorly functioning graft, intracranial hemorrhage, cerebral infarction, infection, or immunosuppressant toxicity.
Moreover, a considerable number of recent studies have observed persistence of various cognitive deficits after LT. The emerging evidence of possible persistence of some neuropsychiatric deficits within months after LT raises the question of whether these deficits are completely reversible. Only a few studies have analyzed the relationship of neurocognitive alterations to important preoperative variables, including a history of hepatic encephalopathy (HE), to identify factors that place recipients at higher risk for postoperative neuropsychiatric dysfunction.
Metabolic and electrolyte disturbances, cardiovascular events, infections, acute graft rejection and immunosuppressant therapy are at the basis of LT-related brain dysfunction (Mueller et al. 1994a; Wijdicks et al. 1995).
Methods
Studies pertaining to the neuropsychiatric complications of LT in human adults were searched within one on-line data base (PubMed) in the period 2005–2009, using the key words “liver transplantation and neurological complication”, “liver transplantation and neuropsychiatric effects”, and “liver transplantation and quality of life”. Only articles in English were considered. We found 450 articles strictly pertinent to the topic, of which 50 were both properly focused and available in our library, so these papers and papers cited in their reference lists were finally considered for this review.
Neurological complications after LT
LT recipients usually develop NC within the first month after the procedure (Stracciari and Guarino 2001) and these are associated with significant mortality and morbidity (Wang et al. 2000). The rate of NC following LT is reported between 10–47% (Pujol et al. 1994; Stracciari and Guarino 2001; Lewis and Howdle 2003; Saner et al. 2006). Moreover, 60–70% of LT recipients display neuropathological abnormalities at autopsy (Blanco et al. 1995). Living-donor LT is associated with lower incidence of NC than cadaveric LT (20.4 % vs 26.7 %, respectively) (Saner et al. 2006). The reason for this phenomenon remains uncertain: it could be correlated with a better quality of the transplanted graft and also an improved detoxification power in comparison with longer cold ischemia time (Saner et al. 2006) In addition, NC occurring in patients after living-donor LT seem to be predominantly transient in nature, and without major impact on clinical outcome (Saner et al. 2009).
Menegaux et al. (1994) showed an age-related rate of NC. The occurrence of NC in pediatric transplantation is significantly lower than in adults.
A previous study reported a higher rate of NC after LT for primary biliary cirrhosis and alcoholic cirrhosis (Philips et al. 1998; Lewis and Howdle 2003; Saner et al. 2009). Ghaus et al. (2001) reported a very high incidence of NC following LT, regardless of liver diagnosis. Other studies could not find a correlation between diagnosis and NC (Dhar et al. 2008; Kim et al. 2007; Saner et al. 2007)
NC following LT are more common than with other solid organ transplants (for example kidney or heart). The reason was attributed to the complexity of surgical procedure, the unfavorable clinical conditions of the patients awaiting transplantation (malnutrition, coagulopathy, low platelet count) and HE before the transplant (Bronster et al. 1994; Bronster et al. 2000)
Neurological complications following LT can be classified in different ways. On the basis of clinical findings and severity (Philips et al. 1998), they can be classified into minor or major complications (Fig. 1). Minor complications usually disappear spontaneously, their treatment is merely symptomatic, and therefore their prognosis is good. In contrast, major complications usually have ominous consequences. On the basis of the time of occurrence, NC after LT can be subdivided into: NC during LT (or surgery-related complications) and after LT (or post-surgical complications) (Stracciari and Guarino 2001; Ardizzone et al. 2006). The latter can be distinguished as: early (within 1 month after surgery) and late complications.
Prevalence of major neurological complication after liver transplantation. (pooled data from Amodio et al. 2007)
During liver transplantation (or surgery-related complications)
Central pontine myelinolysis (CPM), cerebrovascular autoregulation impairment, and paradoxical cerebral embolism are the main NC during LT.
Surgery-related complications mostly affect the central nervous system (CNS), mainly via cerebrovascular damage: hypoxic-ischemic encephalopathy, due to blood loss; hypotension; air embolism; and embolic stroke and cerebral hemorrhage, often related to hypertension and coagulopathy. Changes in cerebral blood flow and metabolism associated with the hemodynamic imbalance frequently encountered during LT may be dangerous for brain perfusion. In fact, cerebral autoregulation might be impaired before graft reperfusion not only in patients with acute liver failure, but also in patients with end-stage liver failure (Larsen et al. 1995). Patients may experience increased intracranial pressure, with a severely compromised cerebral perfusion pressure. Hypotension may develop, leading to hypoxic-ischemic event. Cerebral ischemia can be also due to perioperative detachment of arterial emboli from carotid or intracranial arteries, from endocarditis in post LT or from mycotic arteritis (angioinvasive aspergillosis) (Adams et al. 1987). Paradoxical emboli of thrombotic material originating from the deep leg or pelvic veins via a cardiac or extracardiac right-to-left shunt can cause an ischemic stroke (Ward et al. 1995). Occasionally, patients may also experience arrhythmias and right ventricular insufficiency. Spinal anterior artery syndrome may also occur (Goss et al. 1997). This is due to the accidental damage of the great radicular artery of Adamkiewicz during liver explantation. Such a lesion prevents blood supply to the anterior and lateral columns of the lower spinal cord, damaging them. As a total, cardiovascular complications after LT reach about 4% (Adams et al. 1987; Lewis and Howdle 2003)
Peripheral nervous system (PNS) damage may also occur, mainly because of compression or particular postures and, rarely, compressive masses (like hematoma). The most common features are mononeuropathies, caused by stretching, compression or direct injury. Perioperative mononeuropathies had an incidence of 2–13% (Wijdicks et al. 1996b).
Central pontine myelinolysis is one of the most severe NC of LT; it’s frequency after LT was reported to reach 2–3% at least untill five years ago (Lewis and Howdle 2003; Yu et al. 2004). It is a clinical syndrome of tetraplegia and pseudobulbar palsy, often accompanied by a depressed state of consciousness associated with an electrolyte disturbance, particularly hyponatremia, and its rapid correction (Wijdicks et al. 1996a). Although occasionally reversible, the clinical course often progresses to death over days to weeks. Symmetric myelinolytic foci found in parts of the brain include: basal ganglia, thalami, cerebral peduncles, as well as corticomedullary junctions of the cerebrum, cerebellum and spinal cord. All of these changes have been documented by pathological studies (Maraganore et al. 1992). In severe cases, patients may develop a “locked-in syndrome”: this means patients are awake but unable to move or communicate. There is no definite therapy for CPM; sporadic suggestions include the use of steroids and intravenous immunoglobulin with some benefits. Prevention is based on a slow correction of perioperative hyponatremia (Yu et al. 2004) not exceeding 8 mM/L per day (Martin 2004).
After liver transplantation (or post-surgical complications)
Early post-surgical complications
These include the toxin effects of immunosuppressive drugs and those related to the residual effect of the drugs commonly used in the operative stage and in the intensive care unit (ICU). The most commonly used immunosuppressants in LT are the calcineurin inhibitors (CIs): cyclosporine (CS) and tacrolimus (FK506). Mycophenolate mofetil and sirolimus have recently been introduced. Corticosteroids, OKT3 and anti- timocyte globulin complete the immunosuppressive regimen. Their use is vital for the survival of the graft, but it entails an important risk of neurotoxicity, which includes direct toxic effects of the drug and the consequences of the chronic condition of immunosuppression.
Neurotoxicity is mainly associated with CS and FK506, amounting to 10–30% of neurological complications of LT for CS (Guarino 1999) and up to 32% for FK506 (Wijdicks et al. 1994). Sirolimus and mycophenolate mofetil lack the neurotoxicity of CIs. The mechanism of neurotoxicity of CS and FK506 is unclear. Neurotoxicity may depend on the same mechanisms causing the immunosuppressive action of CIs (Dumont 2000; Kochi et al. 1999). They express their immunosuppressive action by binding to immunophilins, intracellular proteins ubiquitous in the CNS, which have a protective action on neuronal function (Dawson 1996). The drug-immunophilins complex blocks the activity of calcineurin. The mechanisms by which CIs produce neurotoxic effects are not completely elucidated, but vascular and microvascular or endothelial dysfunction seem to play a crucial role (Amodio et al. 2007).
Immunosuppressant-related neurotoxicity often occurs immediately after the transplant, due to the high doses of drugs needed to induce immunosuppression, or later, possibly due to a cumulative effect of the drugs. At any rate, the neurological toxicity of immunosuppressants is hardly correlated with their plasma levels. Sometimes, it can be only indirectly inferred from the resolution of the clinical symptoms on treatment discontinuation (Bryan et al. 1998; Wang et al. 2000).
The neurological toxic effects of CS and FK506 can produce either major or minor complications. Manifestations are various, mainly affecting the CNS but the PNS can also be involved. Major NC induced by CIs include headaches, altered mental status, seizures, cortical blindness, auditory and visual hallucinations, spasticity, paresis and ataxia.
Corticosteroids can cause neuropsychiatric complications by means of a direct toxic effect or by the induced state of immunosuppression. The most common neurological problems are myopathy and behavioral disorders (Patchell 1994). Adjustment of therapy usually removes these effects.
OKT3 can have rare neurological side effects, such as: acute aseptic meningitis with headache, nuchal rigidity and fever, and an encephalopathy-like syndrome with impairment of consciousness, myoclonic activity and seizures. Cerebral edema might be at the basis of this syndrome (Coleman and Norman 1990; Adair et al. 1991)
The relationship between immunosuppressive therapy and the development of NC has been well established for CIs (Mueller et al. 1994b). Previous studies reported a higher incidence of NC in FK506-based immunosuppressive regimens as compared with CS (Mueller et al. 1994b; McDiarmid et al. 1995). McDiarmid et al. (1995) reported a higher incidence of minor NCs, like insomnia or tremor in FK506-based immunosuppression compared with CS; and major neurological events, such as coma, seizures and encephalopathy occurred only in the FK506 group in this study (Mueller et al. 1994b; McDiarmid et al. 1995). While minor NC of tremor and headaches seem to reflect direct effects of the drug, the major complications may be multifactorial in origin, not ascribable to FK506 alone. However, subsequent studies did not find higher rates of NC in patients treated with FK506 as compared with CS (Lewis and Howdle 2003; Dhar et al. 2008; Saner et al. 2009). Only mycophenolate mofetil does not have neurotoxic side effects, the major adverse effects of this drug being gastrointestinal and hematological (Braun et al. 2002). In contrast, the other immunosuppressant drugs, i.e. corticosteroids and OKT3 do have sensible neurotoxic potential.
Conditions increasing the neurotoxic effects of immunosuppressant agents are: pre- existing CNS damage and pre-existing BBB alterations causing toxic intracerebral drug levels (Craven 1991); chronic HE; electrolyte disorders (hyper- and hyponatremia, hypomagnesemia); dysmetabolic alterations (hyperglycemia); and hypocholesterolemia because it increases brain uptake of immunosuppressant drugs and drug interactions (Craven 1991; Lewis and Howdle 2003).
To treat neurotoxicity, a reduction of doses and conversion from CS to FK506 and vice versa have been suggested (Pratschke et al. 1997). The recent use of novel combinations of drugs (CIs plus mycophenolate mofetil or sirolimus) allows lower dosages of CS and FK506 (McAlister et al. 2001). In most cases, these approaches lead to a resolution of symptoms and reversal of neuroimaging abnormalities. Prevention requires minimum efficacious doses, oral administration as soon as possible, strict monitoring of plasma levels (including metabolites), electrolyte imbalance (i.e. hypomagnesemia) and hypertension check and correction, and attention to pharmacological interactions (Guarino et al. 2006)
The most common NC reported in many studies (Pujol et al. 1994; Guarino et al. 1996; Bronster et al. 2000; Lewis and Howdle 2003; Saner et al. 2007; Dhar et al. 2008; Saner et al. 2009) is encephalopathy. In contrast to these studies, Kim et al. (2007) found that peripheral motor disturbance was the most common complication and the encephalopathy rate was only 12.2%. The aetiology of encephalopathy may be difficult to identify as it is usually multifactorial, related to metabolic derangements, organ failure and drug toxicity. Otherwise, the picture may include sleep disorders, apathy, disorientation, and confusion, acute psychotic episodes with agitation, crying, repetition of illogical sentences, rambling speech, confusion, dysperceptive disorders, and autonomic dysfunction, evolving to stupor and coma. Acute psychotic reactions were reported in 43% of patients treated with high doses of CS after transplantation (Wijdicks et al. 1995).
Previous studies reported a higher rate of encephalopathy after transplant in patients with alcoholic cirrhosis (Buis et al. 2002) but other studies have not confirmed this observation (Dhar et al. 2008). In fact, in the study by Buis et al. (2002), the patients with acute confusional states had higher preoperative ammonia levels. It is likely that alcohol-misuser patients with prior episodes of HE and high levels of ammonia had more pre-existing brain injury, placing them at higher risk for complications (Dhar et al. 2008). In a recent study, Dhar et al. (2008) demonstrated that the stronger predictor of postoperative neurological morbidity was the presence of active HE at the time of transplantation. It was hypothesized that alterations of the blood-brain barrier due to pre-existing HE might have made these patients more vulnerable to neurotoxicity than other patients (Bechstein 2000). This association has also been suggested in a previous study by Pujol et al. (1994) who observed that an abnormal neurological examination prior to LT was the strongest predictor of postoperative NC.
Seizures are the second most common neurological complication reported after LT (Lewis and Howdle 2003; Yu et al. 2004; Ardizzone et al. 2006; Saner et al. 2006, 2007, 2009). Seizures can be partial or generalized, most frequently of the tonic-clonic type. They can occur with or without structural brain lesions and with or without EEG alterations within the inter-critical period. Immediately after LT, their prevalence may reach 10–40% (Ardizzone et al. 2006); later, their prevalence decreases to about 10% (Saner et al. 2009). The incidence of seizures after LT has been declining in recent years, possibly thanks to closer monitoring of drug levels and cautious dose initiation and adjustment.
Posterior leukoencephalopathy syndrome (PLE) is a severe but reversible syndrome, presenting with nausea, emesis, headache, fever, and loss of vision. Visual hallucinations, cortical blindness, occipital headache, seizures and consciousness disorders can also occur (Hinchey et al. 1996; Nakamura et al. 1998). The true incidence of this encephalopathy is difficult to establish, but it appears to occur in about 5% of patients after LT (Adams et al. 1987; Moreno et al. 1993). Cerebral MRI shows focal regions of symmetric hemispheric edema; the parietal and occipital lobes are the most commonly affected (Bartynski et al. 1997). MR diffusion-weighted imaging (DWI) demonstrates that the areas of abnormabily represent vasogenic edema. After LT, PLE typically occurs early, (within 2 mounths after transplantation), blood pressure tends to be normal, and the extent of brain edema is significant (Bartynski et al. 2008). CS an induce endothelial injury/dysfunction leading to enhanced vasoconstrictive effects, increased symapathetic activation, and coagulation effetcs. Immune challenge from the transplant, effects of chemotherapy, ad the risks of infection in the immunosuppressed state may contribute to toxicity (Bartynski 2008).
The cerebellar syndrome seems associated with cyclosporine treatment (Eidelman 1999). The disorder is heralded by headache, nausea, dizziness, emesis, encephalopathy, nystagmus and ataxia.
In LT recipients, a state of akinetic mutism may occur. In this syndrome, patients appear alert, but they make no attempt to communicate and eye contact is also absent. The pathophysiology of this condition is largely unknown, but it may be due to damage or dysfunction of the dopaminergic mesencephalic-frontal pathways. Reversibility with interruption of CIs has been reported, suggesting a relationship with drug neurotoxicity (Stracciari and Guarino 2001; Eidelman 1999; Saner et al. 2006).
Cerebrovascular complications occur in about 4% of cases in clinical series (Adams et al. 1987; Pujol et al. 1994), mostly with cerebral hemorrhage and usually within 2 months after surgery (Guarino et al. 1996). Several risk factors are recognized: those directly associated with hepatic failure such as coagulation disturbances, and those secondary to immunosuppressive therapy such as hypercholesterolemia, diabetes, and hypertension (Wang et al. 2000). Perioperative events, such as cerebral hypoperfusion and massive transfusion, may also favor cerebrovascular injury (Wang et al. 2000). Adjustment of cerebrovascular risk factors before, during and after LT is the main preventive measure. Prevention includes correction of coagulopathies before surgery, avoiding perioperative cerebral hypoperfusion and control of cerebrovascular risk factors after LT (especially hypertension) (Guarino et al. 2006).
Major complications affecting the PNS are less frequent and usually occur weeks to months after starting immunosuppressive treatment. Both the nerve and the muscle may be involved (Wijdicks et al. 1996b). Axonal and demyelinating neuropathy have been reported. The more severe forms have been observed during FK506 therapy (Bronster et al. 1995).
Among minor CNS disorders, the most frequent is tremor, almost invariably present on the first day of treatment and related to immunosuppressive drugs. It is fine, postural, and responsive to beta blockers. In most instances tremor is self-limiting, not needing a pharmacological treatment (Wijdicks et al. 1995). Other minor CNS disorders included: headache, sleep disorders, mood alterations, peripheral neuropathy and restless leg syndrome.
Late post-surgical complications
These mainly include CNS infections, “de novo” neoplasm and psychiatric disorders. Infectious processes involving the CNS occur in approximately 5% of patients who have undergone LT (Adams et al. 1987; Pujol et al. 1994) with a high mortality (Bronster et al. 2000). In other recent studies (Saner et al. 2007), no patient experienced CNS infections. The most commonly involved pathogens are Lysteria monocytogenes, Aspergillus fumigatus and Cryptococcus neoformans. Autopsy studies have shown that Aspergillus is the most frequently encountered pathogen within the CNS. Viral infections are rare, related to Herpesvirus-6 and Cytomegalovirus (Singh et al. 2000). Clinical patterns include meningitis, encephalitis, abscesses, or a combination.
Patients who are chronically immunosuppressed are at risk of developing primary CNS lymphoma. Typically a B-cell tumor, CNS lymphoma has developed in approximately 2% of all immunosuppressed transplant recipients (Penn 1999). Nevertheless, a careful approach to recognition and diagnosis of CNS lesions after LT and prompt treatment will help to improve the management of LT recipients. Recently, Guarino et al. (2006) published the EFNS guidelines on the management of NC in LT.
Neurocognitive complications after LT
Most studies indicate that, as expected, the condition of the patients improved markedly after LT. However, residual cognitive deficits are common and may reflect the extent of pre-transplant morbidity. Memory impairment, psychomotor slowing, anxiety, and depression have been commonly observed in patients after LT. Few studies have systematically compared the cognitive performance of patients with cirrhosis before and after LT. In addition, scant information was given on long-term effects regarding the range of improvements and their duration and stability (Mattarozzi et al. 2004).
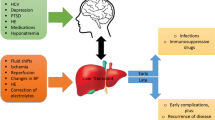
The brain, functionally and structurally altered in patients with liver disease (Weissenborn et al. 2004), may be preferentially vulnerable to perioperative insults and to the toxicity of immunosuppressive treatments. Cognitive dysfunction after LT can be related to prior HE, but many other causes of neurological dysfunction can be implicated: anoxic and ischemic intra-operative damage, osmotic myelinolysis, immunosuppressive toxicity, recurrence of liver disease (Stracciari and Guarino 2001), comorbidities (Guarino et al. 2006), and other metabolic and nutritional alterations. A schema of these interacting factors is reported in Table 1.
Liver cirrhosis patients without overt HE may have mild cognitive alterations, defined as minimal hepatic encephalopathy (MHE). MHE predicts overt HE (OHE) (Amodio et al. 2001), and it is often clinically missed, notwithstanding it can impair occupational and social functioning and safety (Groeneweg et al. 1998). It is important to identify this neuropsychiatric syndrome in patients awaiting LT, for proper interpretation of disorders occurring after transplantation (Amodio et al. 2007). As a rule, LT improves the cognitive deficits due to HE (Mattarozzi et al. 2004) but both new pathogenic conditions can damage cerebral dysfunction, and incomplete reversal of pre-existing dysfunction can occur. Patients with limited reserves of organic osmolytes, depleted in attempting to compensate for active preoperative HE, are unable to counteract the increased swelling precipitated by these additional perioperative stressors and so are at greater risk of cerebral dysfunction after LT (Dhar et al. 2008).
Limited studies in patients with minimal HE after LT suggest improvement of cognitive function; however, complete normalization is doubtful (Mechtcheriakov et al. 2004). Accurate screening for MHE before transplantation can enable its adequate treatment prior to surgery.
Tarter et al. (1990) studied 62 patients before and after LT on a battery of neuropsychological tests measuring HE. The profile of cognitive dysfunction was in line with the typical profile of MHE: deficits of selective attention and fine motor skills, with spared general intellectual ability (Amodio et al. 1998). Cognitive function was restored to a large extent after transplantation, but not completely. This observation was also confirmed by Mattarozzi et al. (2004) who evaluated neuropsychological performance 6 and 18 months after LT. They observed that visuospatial and selective attention, visuospatial short-term and long-term memory, and language tasks improved after 6 months post-LT. Selective attention continued to improve slightly, but significantly, until an 18-month assessment, while no other cognitive functions varied over time. These results were in accordance with neuroimaging studies of patients with cirrhosis before and after LT. On magnetic resonance imaging (MRI), basal ganglia abnormalities seen in patients with cirrhosis decrease after LT, but are still evident after 6 months following the procedure (Naegele et al. 2000)
Riether et al. (1992) found that cognitive performance approaches normal values by 1 year after LT, with the greatest improvement occurring between 3 and 6 months after surgery. Moore et al. (2000) reported improvement as early as 3 months after LT, which remained stable during subsequent testing.
In a prospective study by Mechtcheriakov et al. (2004) patients were investigated before and, on average, 21 months after LT using a neuropsychological test battery that measured visuoconstructive and visuomotor ability, verbal fluency, and memory function. The visuoconstructive performance score (VMCP) was abnormal in the patients listed for LT, but other neuropsychological functions such as short- and long- term memory, as well as verbal intelligence, were no different from controls. After LT, the patients did not demonstrate a significant increase of VMCP and, additionally, showed a significantly lower VMCP score compared with the control group. These data indicate that the cirrhosis-associated visuomotor deficits subside or disappear only in some of the patients after LT, whereas a significant number of patients show no improvement of the visuomotor and visuoconstructive function. It was concluded that monitoring of cognitive and visuomotor functioning is important for the post-transplant rehabilitation of patients with liver cirrhosis.
The role of pre-transplant bouts of OHE on the post-transplant cognitive profile has been recently explored by Sotil et al. (2009) who suggested that HE before LT has important implications for persistent neurological deficits after LT. They studied the impact of episodes of OHE prior to transplantation on neuropsychological performance after 18 months following transplantation. The authors showed that the transplanted patients with a history of HE prior to LT had worse neuropsychological performance measured by the PHES battery (in particular as far as the NCT-A and the Digit Symbol were concerned) and lower critical flicker frequency (CFF) compared with subjects without HE, while their QoL score measured by the SF-36 was similar. This study suggests that a history of HE prior to transplantation is associated with persisting neurological deficits post-LT. As a consequence, the authors suggest that MELD might be incomplete as a system for organ allocation, as it does not include HE.
Auditory and visual P300 cognitive evoked potentials were studied in 12 subjects prior to LT and again 3–6 months subsequent to transplantation (Reeves et al. 2007). Visual P300 amplitude increases and its latency decreases after transplantation.
On MRI, typical hyperintensity T1-weighted pictures of the basal ganglia, possibly due to manganese accumulation, have been frequently observed in patients with HE (Krieger et al. 1995). However, these changes are neither sensitive nor specific to HE (Thuluvath et al. 1995). They disappear within a few months of transplantation and after the recovery of the biochemical changes by magnetic resonance spectroscopy (MRS) (Naegele et al. 2000).
Rovira et al. (2007) used MRI to measure the volume of supratentorial focal brain white matter lesions (WMLs) and neuropsychological examination to assess cognitive function before OLTX as well as 6 and 14 months after LT in 27 patients with cirrhosis without signs of OHE who had focal brain WMLs on MRI T2-weighted images. These abnormalities, which are radiologically indistinguishable from the features of small-vessel disease or normal aging, were found to be partially reversible and parallel to the improvement of cognitive dysfunction (Minguez et al. 2007).
Psychological status and quality of life
Long-term effects of LT on psychological outcome are unclear. Many studies have reported significant improvements in QoL and satisfactory psychological outcome after LT (Bryan et al. 1998; O’Carroll et al. 2003). In addition, QoL and psychological distress were significantly better in most of the post-LT groups than in cirrhotic patients (De Bona et al. 2000). The topic was recently reviewed in detail by Tome et al. (2008) who observed that general QoL improves dramatically after LT, compared with health status prior to transplantation; however, when compared with the general population, the vast majority of LT patients have significant deficits in most QoL domains. This observation is in contrast with many previous studies (Pereira et al. 2000; Karam et al. 2003), which found QoL similar to that of the general population. A relationship between the etiology of liver disease and QoL has been also reported: hepatitis-C virus (HCV) infections impinge on QoL mainly in the patients with recurrence of liver disease (Bona et al. 1998). The same trend was observed in the patients who relapse into heavy drinking after transplantation (Bravata et al. 2001). Recent data indicate that women have worse scores than men (Blanch et al. 2004) and sexual function after LT tends to show no improvement; in some cases it even deteriorates (Sorrell and Brown, 2006). An intriguing issue is provided by the interrelation across QoL, psychological status and brain function. One year post- transplantation, O’Carroll et al. (2003) observed a significant improvement in most psychological domains. Interestingly, elevated levels of anxiety and neuroticism on pre- transplantation assessment were associated with worse psychological outcome 1 year after transplantation, while the severity of liver disease was not related to psychological outcome. These finding suggest that personality and affective status may be important determinants of long-term psychological health after LT. At any rate, within six months after LT, patients with HCV who experience HCV recurrence showed significantly greater depression, anxiety, phobic anxiety, and paranoid ideation than anti-HCV- negative patients (O’Carroll et al. 2003).
Addiction is a significant complication: resumption of drinking, often difficult to assess, can occur in 10–30% of cases and results in increased morbidity and mortality (Tome et al. 2008).
These studies imply that successful LT, while improving QoL, psychological and cognitive status, does not fully reverse the patient’s condition (Rose and Jalan 2004). Many factors may influence the wellbeing of the patient after transplantation, including pre-transplant mental condition (concerning cognition, emotion, and personality), transplant procedure, side effects of drugs, recurrence of liver disease, anxiety about disease recurrence, inability to resume work with the ensuing economic restraints, and changes in social relationships.
Conclusions
Neurological, cognitive and psychiatric disorders are common after LT. HE occurring before transplantation is a probable risk factor for neuropsychiatric symptoms after transplantation, even if they are multifactorial. The diagnosis and interpretation of neuropsychiatric disorders occurring after transplantation need accurate investigation, which is facilitated by detailed evaluation of cognitive, psychological, emotional and neurological conditions prior to transplantation.
References
Adair JC, Woodley SL, O’Connell JB, Call GK, Baringer JR (1991) Aseptic meningitis following cardiac transplantation: clinical characteristics and relationship to immunosuppressive regimen. Neurology 41:249–252
Adams DH, Ponsford S, Gunson B, Boon A, Honigsberger L, Williams A, Buckels J, Elias E, McMaster P (1987) Neurological complications following liver transplantation. Lancet 1:949–951
Amodio P, Marchetti P, Del Piccolo F, Campo G, Rizzo C, Iemmolo RM, Gerunda G, Caregaro L, Merkel C, Gatta A (1998) Visual attention in cirrhotic patients: a study on covert visual attention orienting. Hepatology 27:1517–1523
Amodio P, Del Piccolo F, Petteno E, Mapelli D, Angeli P, Iemmolo R, Muraca M, Musto C, Gerunda G, Rizzo C, Merkel C, Gatta A (2001) Prevalence and prognostic value of quantified electroencephalogram (EEG) alterations in cirrhotic patients. J Hepatol 35:37–45
Amodio P, Biancardi A, Montagnese S, Angeli P, Iannizzi P, Cillo U, D’Amico D, Gatta A (2007) Neurological complications after orthotopic liver transplantation. Dig Liver Dis 39:740–747
Ardizzone G, Arrigo A, Schellino MM, Stratta C, Valzan S, Skurzak S, Andruetto P, Panio A, Ballaris MA, Lavezzo B, Salizzoni M, Cerutti E (2006) Neurological complications of liver cirrhosis and orthotopic liver transplant. Transplant Proc 38:789–792
Bartynski WS (2008) Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol 29:1036–1042
Bartynski WS, Grabb BC, Zeigler Z, Lin L, Andrews DF (1997) Watershed imaging features and clinical vascular injury in cyclosporin A neurotoxicity. J Comput Assist Tomogr 21:872–880
Bartynski WS, Tan HP, Boardman JF, Shapiro R, Marsh JW (2008) Posterior reversible encephalopathy syndrome after solid organ transplantation. AJNR Am J Neuroradiol 29:924–930
Bechstein WO (2000) Neurotoxicity of calcineurin inhibitors: impact and clinical management. Transpl Int 13:313–326
Blanch J, Sureda B, Flavia M, Marcos V, de Pablo J, De Lazzari E, Rimola A, Vargas V, Navarro V, Margarit C, Visa J (2004) Psychosocial adjustment to orthotopic liver transplantation in 266 recipients. Liver Transpl 10:228–234
Blanco R, De Girolami U, Jenkins RL, Khettry U (1995) Neuropathology of liver transplantation. Clin Neuropathol 14:109–117
Bona MD, Rupolo G, Ponton P, Iemmolo RM, Boccagni P, Destro C, Ermani M, Naccarato R, Burra P (1998) The effect of recurrence of HCV infection of life after liver transplantation. Transpl Int 11(Suppl 1):S475–S479
Braun KP, Glander P, Hambach P, Bohler T, Waiser J, Mai I, Neumayer HH, Budde K (2002) Pharmacokinetics and pharmacodynamics of mycophenolate mofetil under oral and intravenous therapy. Transplant Proc 34:1745–1747
Bravata DM, Olkin I, Barnato AE, Keeffe EB, Owens DK (2001) Employment and alcohol use after liver transplantation for alcoholic and nonalcoholic liver disease: a systematic review. Liver Transpl 7:191–203
Bronster DJ, Emre S, Mor E, Sheiner P, Miller CM, Schwartz ME (1994) Neurologic complications of orthotopic liver transplantation. Mt Sinai J Med 61:63–69
Bronster DJ, Yonover P, Stein J, Scelsa SN, Miller CM, Sheiner PA (1995) Demyelinating sensorimotor polyneuropathy after administration of FK506. Transplantation 59:1066–1068
Bronster DJ, Emre S, Boccagni P, Sheiner PA, Schwartz ME, Miller CM (2000) Central nervous system complications in liver transplant recipients-incidence, timing, and long-term follow-up. Clin Transplant 14:1–7
Bryan S, Ratcliffe J, Neuberger JM, Burroughs AK, Gunson BK, Buxton MJ (1998) Health-related quality of life following liver transplantation. Qual Life Res 7:115–120
Buis CI, Wiesner RH, Krom RA, Kremers WK, Wijdicks EF (2002) Acute confusional state following liver transplantation for alcoholic liver disease. Neurology 59:601–605
Coleman AE, Norman DJ (1990) OKT3 encephalopathy. Ann Neurol 28:837–838
Craven JL (1991) Cyclosporine-associated organic mental disorders in liver transplant recipients. Psychosomatics 32:94–102
Dawson TM (1996) Immunosuppressants, immunophilins, and the nervous system. Ann Neurol 40:559–560
De Bona M, Ponton P, Ermani M, Iemmolo RM, Feltrin A, Boccagni P, Gerunda G, Naccarato R, Rupolo G, Burra P (2000) The impact of liver disease and medical complications on quality of life and psychological distress before and after liver transplantation. J Hepatol 33:609–615
Dhar R, Young GB, Marotta P (2008) Perioperative neurological complications after liver transplantation are best predicted by pre-transplant hepatic encephalopathy. Neurocrit Care 8:253–258
Dumont FJ (2000) FK506, an immunosuppressant targeting calcineurin function. Curr Med Chem 7:731–748
Eidelman BH (1999) Neurological Complications of immunosuppressive agents. Gastroenterol Int Congress Proc 12:128–134
Ghaus N, Bohlega S, Rezeig M (2001) Neurological complications in liver transplantation. J Neurol 248:1042–1048
Goss JA, Seu P, Shackleton CR, Busuttil RW (1997) Lower extremity paralysis after use of the supraceliac aorta for hepatic arterial reconstruction of the transplanted liver. Transplantation 63:163–164
Groeneweg M, Quero JC, De B, Hartmann IJ, Essink-bot ML, Hop WC, Schalm SW (1998) Subclinical hepatic encephalopathy impairs daily functioning. Hepatology 28:45–49
Guarino M (1999) Immunosuppressive therapy. Gastroenterol Int Congress Proc 12:140–145
Guarino M, Stracciari A, Pazzaglia P, Sterzi R, Santilli I, Donato F, D’Alessandro R (1996) Neurological complications of liver transplantation. J Neurol 243:137–142
Guarino M, Benito-Leon J, Decruyenaere J, Schmutzhard E, Weissenborn K, Stracciari A (2006) EFNS guidelines on management of neurological problems in liver transplantation. Eur J Neurol 13:2–9
Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, Pessin MS, Lamy C, Mas JL, Caplan LR (1996) A reversible posterior leukoencephalopathy syndrome. N Engl J Med 334:494–500
Kaplan PE, Clinchot DM, Arnett JA (1996) Cognitive deficits after hepatic transplantation: relevance to the rehabilitation potential. Brain Inj 10:599–607
Karam VH, Gasquet I, Delvart V, Hiesse C, Dorent R, Danet C, Samuel D, Charpentier B, Gandjbakhch I, Bismuth H, Castaing D (2003) Quality of life in adult survivors beyond 10 years after liver, kidney, and heart transplantation. Transplantation 76:1699–1704
Kim BS, Lee SG, Hwang S, Park KM, Kim KH, Ahn CS, Moon DB, Ha TY, Song GW, Kim DS, Moon KM, Jung DH (2007) Neurologic complications in adult living donor liver transplant recipients. Clin Transplant 21:544–547
Kochi S, Takanaga H, Matsuo H, Naito M, Tsuruo T, Sawada Y (1999) Effect of cyclosporin A or tacrolimus on the function of blood-brain barrier cells. Eur J Pharmacol 372:287–295
Krieger D, Krieger S, Jansen O, Gass P, Theilmann L, Lichtnecker H (1995) Manganese and chronic hepatic encephalopathy. Lancet 346:270–274
Larsen FS, Olsen KS, Ejlersen E, Hansen BA, Paulson OB, Knudsen GM (1995) Cerebral blood flow autoregulation and transcranial Doppler sonography in patients with cirrhosis. Hepatology 22:730–736
Lewis MB, Howdle PD (2003) Neurologic complications of liver transplantation in adults. Neurology 61:1174–1178
Maraganore DM, Folger WN, Swanson JW, Ahlskog JE (1992) Movement disorders as sequelae of central pontine myelinolysis: report of three cases. Mov Disord 7:142–148
Martin RJ (2004) Central pontine and extrapontine myelinolysis: the osmotic demyelination syndromes. J Neurol Neurosurg Psychiatry 75(Suppl 3):iii22–iii28
Mattarozzi K, Stracciari A, Vignatelli L, D’Alessandro R, Morelli MC, Guarino M (2004) Minimal hepatic encephalopathy: longitudinal effects of liver transplantation. Arch Neurol 61:242–247
Mazariegos GV, Molmenti EP, Kramer DJ (1999) Early complications after orthotopic liver transplantation. Surg Clin North Am 79:109–129
McAlister VC, Peltekian KM, Malatjalian DA, Colohan S, MacDonald S, Bitter-Suermann H, MacDonald AS (2001) Orthotopic liver transplantation using low-dose tacrolimus and sirolimus. Liver Transpl 7:701–708
McDiarmid SV, Busuttil RW, Ascher NL, Burdick J, D’Alessandro AM, Esquivel C, Kalayoglu M, Klein AS, Marsh JW, Miller CM (1995) FK506 (tacrolimus) compared with cyclosporine for primary immunosuppression after pediatric liver transplantation. Results from the U.S. Multicenter Trial. Transplantation 59:530–536
Mechtcheriakov S, Graziadei IW, Mattedi M, Bodner T, Kugener A, Hinterhuber HH, Marksteiner J, Vogel W (2004) Incomplete improvement of visuo-motor deficits in patients with minimal hepatic encephalopathy after liver transplantation. Liver Transpl 10:77–83
Menegaux F, Keeffe EB, Andrews BT, Egawa H, Monge H, Concepcion W, So SK, Esquivel CO (1994) Neurological complications of liver transplantation in adult versus pediatric patients. Transplantation 58:447–450
Minguez B, Rovira A, Alonso J, Cordoba J (2007) Decrease in the volume of white matter lesions with improvement of hepatic encephalopathy. AJNR Am J Neuroradiol 28:1499–1500
Moore KA, McL JR, Burrows GD (2000) Quality of life and cognitive function of liver transplant patients: a prospective study. Liver Transpl 6:633–642
Moreno E, Gomez SR, Gonzalez I, Loinaz C, Garcia I, Perez A, Palomo C, Alvarado A, Maffettone V, Perez-Cerda F (1993) Neurologic complications in liver transplantation. Acta Neurol Scand 87:25–31
Mueller AR, Platz KP, Bechstein WO, Schattenfroh N, Stoltenburg-Didinger G, Blumhardt G, Christe W, Neuhaus P (1994a) Neurotoxicity after orthotopic liver transplantation. A comparison between cyclosporine and FK506. Transplantation 58:155–170
Mueller AR, Platz KP, Christe W, Bechstein WO, Blumhardt G, Neuhaus P (1994b) Severe neurotoxicity afetr liver transplantation: associatiom between FK 506 therapy and hepatitis C virus disease. Transplant Proc 26:3131–3132
Naegele T, Grodd W, Viebahn R, Seeger U, Klose U, Seitz D, Kaiser S, Mader I, Mayer J, Lauchart W, Gregor M, Voigt K (2000) MR imaging and (1)H spectroscopy of brain metabolites in hepatic encephalopathy: time-course of renormalization after liver transplantation. Radiology 216:683–691
Nakamura M, Fuchinoue S, Sato S, Hoshino T, Sawada T, Sageshima J, Kitajima K, Tojinbara T, Fujita S, Nakajima I, Agishi T, Tanaka K (1998) Clinical and radiological features of two cases of tacrolimus-related posterior leukoencephalopathy in living related liver transplantation. Transplant Proc 30:1477–1478
O’Carroll RE, Couston M, Cossar J, Masterton G, Hayes PC (2003) Psychological outcome and quality of life following liver transplantation: a prospective, national, single-center study. Liver Transpl 9:712–720
Patchell RA (1994) Neurological complications of organ transplantation. Ann Neurol 36:688–703
Penn I (1999) Posttransplant malignancies. Transplant Proc 31:1260–1262
Pereira SP, Howard LM, Muiesan P, Rela M, Heaton N, Williams R (2000) Quality of life after liver transplantation for alcoholic liver disease. Liver Transpl 6:762–768
Philips BJ, Armstrong IR, Pollock A, Lee A (1998) Cerebral blood flow and metabolism in patients with chronic liver disease undergoing orthotopic liver transplantation. Hepatology 27:369–376
Pratschke J, Neuhaus R, Tullius SG, Haller GW, Jonas S, Steinmueller T, Bechstein WO, Neuhaus P (1997) Treatment of cyclosporine-related adverse effects by conversion to tacrolimus after liver transplantation. Transplantation 64:938–940
Pujol A, Graus F, Rimola A, Beltran J, Garcia-Valdecasas JC, Navasa M, Grande L, Galofre J, Visa J, Rodes J (1994) Predictive factors of in-hospital CNS complications following liver transplantation. Neurology 44:1226–1230
Reeves RR, Struve FA, Rash CJ, Burke RS (2007) P300 cognitive evoked potentials before and after liver transplantation. Metab Brain Dis 22:139–144
Riether AM, Smith SL, Lewison BJ, Cotsonis GA, Epstein CM (1992) Quality-of-life changes and psychiatric and neurocognitive outcome after heart and liver transplantation. Transplantation 54:444–450
Rose C, Jalan R (2004) Is minimal hepatic encephalopathy completely reversible following liver transplantation? Liver Transpl 10:84–87
Rovira A, Minguez B, Aymerich FX, Jacas C, Huerga E, Cordoba J, Alonso J (2007) Decreased white matter lesion volume and improved cognitive function after liver transplantation. Hepatology 46:1485–1490
Saner F, Gu Y, Minouchehr S, Ilker K, Fruhauf NR, Paul A, Radtke A, Dammann M, Katsarava Z, Koeppen S, Malago M, Broelsch CE (2006) Neurological complications after cadaveric and living donor liver transplantation. J Neurol 253:612–617
Saner FH, Sotiropoulos GC, Gu Y, Paul A, Radtke A, Gensicke J, Kavuk I, Malago M, Broelsch CE (2007) Severe neurological events following liver transplantation. Arch Med Res 38:75–79
Saner FH, Gensicke J, Damink SW, Pavlakovic G, Treckmann J, Dammann M, Kaiser GM, Sotiropoulos GC, Radtke A, Koeppen S, Beckebaum S, Cicinnati V, Nadalin S, Malago M, Paul A, Broelsch CE (2009) Neurologic complications in adult living donor liver transplant patients: an underestimated factor? J Neurol published online 1 sept 2009
Singh N, Bonham A, Fukui M (2000) Immunosuppressive-associated leukoencephalopathy in organ transplant recipients. Transplantation 69:467–472
Sorrell JH, Brown JR (2006) Sexual functioning in patients with end-stage liver disease before and after transplantation. Liver Transpl 12:1473–1477
Sotil EU, Gottstein J, Ayala E, Randolph C, Blei AT (2009) Impact of preoperative overt hepatic encephalopathy on neurocognitive function after liver transplantation. Liver Transpl 15:184–192
Starzl TE, Marchioro TL, Vonkaulla KN, Hermann G, Brittain RS, Waddell WR (1963) Homotransplantation of the liver in humans. Surg Gynecol Obstet 117:659–676
Starzl TE, Iwatsuki S, Esquivel CO, Todo S, Kam I, Lynch S, Gordon RD, Shaw BW Jr (1985) Refinements in the surgical technique of liver transplantation. Semin Liver Dis 5:349–356
Stracciari A, Guarino M (2001) Neuropsychiatric complications of liver transplantation. Metab Brain Dis 16:3–11
Tarter RE, Switala JA, Arria A, Plail J, Van Thiel DH (1990) Subclinical hepatic encephalopathy. Comparison before and after orthotopic liver transplantation. Transplantation 50:632–637
Thuluvath PJ, Edwin D, Yue NC, deVilliers C, Hochman S, Klein A (1995) Increased signals seen in globus pallidus in T1-weighted magnetic resonance imaging in cirrhotics are not suggestive of chronic hepatic encephalopathy. Hepatology 21:440–442
Tome S, Wells JT, Said A, Lucey MR (2008) Quality of life after liver transplantation. A systematic review. J Hepatol 48:567–577
Wang WL, Yang ZF, Lo CM, Liu CL, Fan ST (2000) Intracerebral hemorrhage after liver transplantation. Liver Transpl 6:345–348
Ward R, Jones D, Haponik EF (1995) Paradoxical embolism. An underrecognized problem. Chest 108:549–558
Weissenborn K, Bokemeyer M, Ahl B, Fischer-Wasels D, Giewekemeyer K, van den HJ, Kostler H, Berding G (2004) Functional imaging of the brain in patients with liver cirrhosis. Metab Brain Dis 19:269–280
Wijdicks EF, Wiesner RH, Dahlke LJ, Krom RA (1994) FK506-induced neurotoxicity in liver transplantation. Ann Neurol 35:498–501
Wijdicks EF, Wiesner RH, Krom RA (1995) Neurotoxicity in liver transplant recipients with cyclosporine immunosuppression. Neurology 45:1962–1964
Wijdicks EF, Blue PR, Steers JL, Wiesner RH (1996a) Central pontine myelinolysis with stupor alone after orthotopic liver transplantation. Liver Transpl Surg 2:14–16
Wijdicks EF, Litchy WJ, Wiesner RH, Krom RA (1996b) Neuromuscular complications associated with liver transplantation. Muscle Nerve 19:696–700
Yu J, Zheng SS, Liang TB, Shen Y, Wang WL, Ke QH (2004) Possible causes of central pontine myelinolysis after liver transplantation. World J Gastroenterol 10:2540–2543
Conflict of interest:
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Campagna, F., Biancardi, A., Cillo, U. et al. Neurocognitive-neurological complications of liver transplantation: a review. Metab Brain Dis 25, 115–124 (2010). https://doi.org/10.1007/s11011-010-9183-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-010-9183-0