Abstract
The objective of the present study was to investigate the in vitro effects of the branched-chain α-keto acids accumulating in maple syrup urine disease, namely L-2-ketoisocaproic acid, L-2-keto-3-methylvaleric acid and L-2-ketoisovaleric acid on Na+, K+-ATPase activity in synaptic plasma membranes from cerebral cortex of 35-day-old rats. All keto acids significantly inhibited Na+, K+-ATPase activity at concentrations similar (1 mM) or even lower (0.5 mM) than those found in blood and cerebrospinal fluid of maple syrup urine disease patients. We also tested the effects of alanine on this enzyme activity. Alanine per se did not alter Na+, K+-ATPase activity, but totally prevented the branched-chain α-keto acids-induced Na+, K+-ATPase inhibition, indicating that alanine and the keto acids may possibly bind to the same site on the enzyme. We also observed that the branched-chain amino acids leucine, isoleucine and valine also inhibited Na+ K+-ATPase activity to a similar degree as that of the branched-chain α-keto acids and that alanine was able to fully prevent these effects. Considering that Na+, K+-ATPase is a critical enzyme for normal brain development and functioning, it is presumed that these findings may be involved in the pathophysiology of the neurological dysfunction of maple syrup urine disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Na+, K+-ATPase (EC 3.6.1.37) is a crucial enzyme responsible for the generation of the membrane potential through the active transport of sodium and potassium ions in the Central Nervous System (CNS) necessary to maintain neuronal excitability and cell volume (Erecinska and Silver, 1994). A failure of Na+, K+-ATPase activity induces depolarization and cell swelling. These changes are potentially toxic to the cell as indicated by the observation that ouabain, a potent and specific Na+, K+-ATPase inhibitor, causes neuronal death in vivo (Lees, 1991). Inhibition of this activity is found in various neuropathological conditions, including cerebral ischemia (Wyse et al., 2000), epilepsy (Grisar, 1984), neurodegenerative disorders (Yu, 2003), in secondary spinal cord edema (Yang and Piao, 2003) and in some inborn errors of metabolism (Wyse et al., 1998; Kolker et al., 2002; Streck et al., 2002; Sgaravatti et al., 2003; Bavaresco et al., 2004).
Maple syrup urine disease (MSUD; branched-chain keto aciduria) is an inborn error of metabolism with encephalopathic presentation caused by deficiency of the activity of the mitochondrial enzyme complex branched-chain L-2-ketoacid dehydrogenase (BCKD). Due to BCKD deficiency, high concentrations of the branched-chain amino acids (BCAA) L-leucine (Leu), L-isoleucine (Ile) and L-valine (Val) and their corresponding branched-chain α-keto acids (BCKA) α-ketoisocaproic acid (KIC), α-keto-β-methylvaleric acid (KMV) and α-ketoisovaleric acid (KTV) accumulate in patients on an unrestricted diet or during episodes of catabolic stress (Chuang and Shih, 2001). Clinically, and according to its responsiveness to thiamine administration, MSUD can be classified into five groups (Chuang and Shih, 2001). Patients with the classical form (approximately 80% of MSUD patients) show their first signs during the neonatal period, presenting severe neurological deterioration and convulsions, and generally die within months if treatment is not instituted. A marked increase of serum and urine concentrations of BCAA and BCKA is the biochemical hallmark of the classical disorder. The other variants are characterized by a milder phenotype, with lesser accumulation of the characteristic metabolites, but the affected patients usually present a variable degree of psychomotor delay or mental retardation (Chuang and Shih, 2001). Neurological sequelae are present in most patients, but the mechanisms underlying the neurotoxicity of this disorder are yet unclear. However, Leu and mainly its keto acid KIC are considered to be the main neurotoxic metabolites in MSUD, since increased plasma concentrations of these compounds are associated with the appearance of neurological symptoms (Snyderman et al., 1964; Chuang and Shih, 2001). Neuroradiological studies in untreated encephalopathic newborns with MSUD show, in addition to a generalized cerebral edema, myelin deficiency and spongy degeneration of the white matter (Chuang and Shih, 2001; Schonberger et al., 2004). Although the mechanisms of brain damage in MSUD are still unclear, it has been demonstrated that the accumulating metabolites may affect energy metabolism in rat brain (Halestrap et al., 1974; Land et al., 1976; Yudkoff et al., 1994; Zielke et al., 2002; Pilla et al., 2003, 2003a) and cause significant alterations of the concentrations of the neurotransmitters glutamate, aspartate and γ-aminobutyric acid (GABA) in the brain (Yudkoff et al., 1994; Prensky and Moser, 1967; Dodd et al., 1992; Tavares et al., 2001). On the other hand, brain injury in this disorder may also be related to reduction of brain uptake of essential amino acids (Araujo et al., 2001), apoptosis of neural cells (Jouvet et al., 2000) and oxidative stress (Fontella et al., 2002; Bridi et al., 2003, 2005, 2005a).
In the present work we focused on the effects of the BCKA accumulating in MSUD, namely KIC, KMV and KIV on the activity of Na+, K+-ATPase in synaptic plasma membranes from rat cerebral cortex. We also investigated the role of Ala on the effects elicited by these α-keto acids since this amino acid has been previously demonstrated to prevent in vitro and in vivo the inhibitory effect of phenylalanine towards the activity of Na+, K+-ATPase in synaptic plasma membranes from rat cerebral cortex by competition at the same binding site (Wyse et al., 1995, 1998, 1999).
Material and methods
Animals and reagents
Forty 21-day-old Wistar rats bred in the Departmento de Bioquímica, Universidade Federal do Rio Grande do Sul, were used in the experiments. At this age, Wistar rat development of the brain is equivalent to the brain development of 10-month-old child. The animals were kept with dams until they were sacrificed. The dams had free access to water and to a standard commercial chow (Supra, Porto Alegre, RS, Brazil) containing 20.5% protein (predominantly soybean supplemented with methionine), 54% carbohydrate, 4.5% fiber, 4% lipids, 7% ash and 10% moisture. Temperature was maintained at 24 ± 1°C, with a 12–12 h light-dark cycle. The “Principles of Laboratory Animal Care” (NIH publication 85-23, revised 1985) were followed in all the experiments, and the Ethics Committee for Animal Research of the Federal University of Rio Grande do Sul approved the experimental protocol. All chemicals were purchased from Sigma Chemical Co., St. Louis, MO, USA.
Tissue preparation and isolation of synaptic plasma membrane from cerebral cortex
The animals were sacrificed by decapitation without anesthesia. The brain was rapidly removed and dissected on an ice-cold glass plate. Pons, medulla, and cerebellum were discarded, and the cerebral cortex was separated and homogenized in 10 vol of 0.32 mM sucrose solution containing 5.0 mM HEPES and 1.0 mM EDTA.at 900 rpm (10 strokes) using an ice-chilled ground glass Potter-Elvejhem homogenizer.
Membranes were prepared from the cortical homogenates according to the method of Jones and Matus (1974) using a discontinuous sucrose density gradient consisting of successive layers of 0.3, 0.8 and 1.0 mM. After centrifugation at 69,000 g for 2 h, the fraction at the 0.8–1.0 mM sucrose interface was taken as the membrane enzyme preparation.
The BCKA and Ala were dissolved in Tris-HCl 400 mM buffer, pH 7.4 on the same day of the enzymatic assays and added to the assays at varying concentrations (BCKA: 0.1–1 mM; alanine: 1 mM). Controls did not contain the BCKA or alanine.
Na+, K+-ATPase activity assay
Na+, K+-ATPase activity were assayed according to Tsakiris and Deliconstantinos (1984). Briefly, the reaction mixture for the Na+, K+-ATPase assay contained 5 mM MgCl2, 80 mM NaCl, 20 mM KC1 and 40 mM Tris-HCl buffer, pH 7.4, in a final volume of 200 μL. Total ATPase activities was measured at 37°C. The reaction was initiated by the addition of ATP (disodium salt, vanadium free) to a final concentration of 3 mM and was stopped after 5 min incubation by the addition of 200 μL of 10% trichloroacetic acid. ATPases ouabain-insensitive were assayed under the same conditions with the addition of 1 mM ouabain. Na+, K+-ATPase activity was calculated by the difference between the two assays. Released inorganic phosphate (Pi) was colorimetrically measured at 630 nm by the method of Chan et al. (1986). Na+, K+-ATPase activity of these preparations ranged from 800 to 1500 nmol Pi·mg protein per min. The enzyme activity was determined after a 20 min pre-incubation with various concentrations of each tested substance, whereas control groups did not contain any of the metabolites in the incubation medium. Some experiments were pre-incubated in the presence of Ala and the BCAA. None of the substances added to the assay medium interfered with the color development or spectrophotometric readings.
Protein determination
Protein concentration was determined by the method of Lowry et al. (1951) using bovine serum albumin as standard.
Statistical analysis
Unless otherwise stated, results are presented as mean ± standard deviation of four to eleven animals in each group and expressed as nmol Pi· mg protein−1· min−1. All assays were performed in triplicate and the median was used for the calculations. Data were analyzed using the one-way analysis of variance (ANOVA) followed by the post-hoc Tukey test when F was significant. The statistical analyses were performed using the SPSS (Statistical Package for the Social Sciences) software. Differences between the groups were rated significant at P < 0.05.
Results
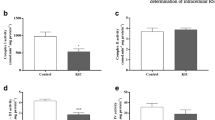
We observed that KIC significantly inhibited Na+, K+-ATPase activity (up to 31% inhibition) [F (4,25) = 3.4; P < 0.05] only at 1 mM concentration (Fig. 1), whereas KMV [F (4,25) = 3.22; P < 0.05] (up to 31% inhibition) and KIV [F (4,25) = 2.57; P < 0.05] (up to 20% inhibition) inhibited this enzyme activity at 0.5 mM and higher doses (Figs. 2 and 3).
It was previously shown that Ala totally prevented the inhibitory property of phenylalanine and its derivative keto acids phenylpyruvate, phenyllactate and phenylacetate on Na+, K+-ATPase activity from rat cerebral cortex in vitro and in vivo by competition at the same binding site (Wyse et al., 1995, 1998, 1999). Therefore, we next tested whether 1.0 mM Ala could prevent the inhibitory effect of the BCKA (1.0 mM) on synaptic membrane Na+, K+-ATPase activity. In these experiments we also added 1.0 mM of Leu, Ile and Val, which are the corresponding transaminated products of the BCKA. We observed that Ala by itself had not effect on Na+, K+-ATPase activity but totally prevented the enzyme activity reduction caused by KIC and KIV and partially by KMV (Figs. 4–6). Furthermore, it can be seen in the figures that the BCAA similarly inhibited Na+, K+-ATPase activity and that Ala was able to fully prevent this inhibition.
In vitro effect of alanine (Ala) on the inhibition provoked by α-ketoisocaproic acid (KIC) and leucine (Leu) on Na+, K+-ATPase activity in synaptic plasma membrane from cerebral cortex of young rats. All substances were added at 1 mM final concentrations. Data are means ± SD of six independent experiments (animals) performed in triplicate. *P < 0.05 compared to control (Tukey test)
In vitro effect of alanine (Ala) on the inhibition provoked by α-keto-β-methylvaleric acid (KMV) and isoleucine (Ileu) on Na+, K+-ATPase activity in synaptic plasma membrane from cerebral cortex of young rats. All substances were added at 1 mM final concentrations. Data are means ± SD of eight independent experiments (animals) performed in triplicate. *P < 0.05 compared to control (Tukey test)
In vitro effect of alanine (Ala) on the inhibition provoked by α-ketoisovaleric acid (KIV) and valine (Val) on Na+, K+-ATPase activity in synaptic plasma membrane from cerebral cortex of young rats. All substances were added at 1 mM final concentrations. Data are means ± SD of eight independent experiments (animals) performed in triplicate. *P < 0.05 compared to control (Tukey test)
Discussion
BCAA and BCKA accumulate in plasma and tissues of MSUD affected patients. Leu and KIC are thought to be the most neurotoxic agents in this disease. However, some studies have demonstrated that IQ do not correlate with average blood concentrations of Leu (Rousson and Guibaud, 1984), neither to Leu concentrations on presentation nor to the time taken for Leu plasma levels to fall following treatment (Naughten et al., 1982). Therefore, it may be presumed that KIC is the main neurotoxin in MSUD because of its high tissue concentrations. Furthermore, although neurological dysfunction is the hallmark of this disease, the mechanisms underlying the pathophysiology of this disorder seem to be multiple and poorly known. However, brain energy deficit may represent an important factor contributing to brain injury in MSUD. It has been demonstrated that KIC and KIV strongly reduce respiration in rat brain slices (Howell and Lee, 1963; de Castro Vasques et al., 2004) and that KIC inhibits pyruvate dehydrogenase and α-ketoglutarate dehydrogenase activities, as well as the transport of pyruvate into mitochondria in rat and human brain (Halestrap et al., 1974; Coitinho et al., 2001). In this context, we have recently reported that the keto acids accumulating in MSUD significantly compromised the respiratory chain function (Sgaravatti et al., 2003). In the present study, we evaluated the influence of these metabolites on Na+, K+-ATPase activity from synaptic membrane of cerebral cortex of young rats. We observed that all BCKA significantly inhibited (20–30% inhibition) this enzyme activity at concentrations of 0.5 mM and/or higher and that Ala supplementation to the medium completely prevented the inhibitory effects of KIC and KIV and partially of KMV. Since it has been reported a competition between Ala and the keto acids of phenylalanine phenylpyruvate, phenyllactate and phenylacetate at the same binding site of the enzyme (Wyse et al., 1995, 1998, 1999), it is possible that Ala prevented the inhibition caused by the BCKA through competition with these keto acids for a critical site in the enzyme. Interestingly, we also found that Leu, Ile and Val also inhibited Na+, K+-ATPase activity to a similar degree as that seen for the BCKA and that Ala also prevented these effects.
Our present results may be of pathophysiological significance since the concentrations of KIC and KIV provoking significant inhibitions of Na+, K+-ATPase activity (0.5–1.0 mM) were within the pathological values found in blood and CSF of MSUD patients (Snyderman et al., 1984; Chuang and Shih, 2001). KIC accumulates in MSUD, reaching concentrations of about 3 to 5 mM in plasma, while plasma levels of KMV and KIV, have been reported to range from 0.2 to 1.5 mM (KMV) and from 0.2 to 0.35 mM (KIV), respectively (Steele, 1984). Furthermore, although the concentrations of these metabolites within the neuronal cells are unknown, it has been postulated that during crises of metabolic decompensation they may reach brain levels equal to or higher than plasma levels (Hoffmann et al., 1993). In this scenario, transport of branched-chain amino acids and keto acids into brain through the blood-brain barrier seems to be effective and quick (Oldendorf, 1973; Steele, 1986). On the other hand, inhibitions of Na+, K+-ATPase activity of the order of 20% may lead to an imbalance of intra and extracellular concentrations of Na+ and K+ because of the high amount of this enzyme present in the brain (Henver et al., 1992). This imbalance may alter the membrane potential, cell volume and fluxes of organic molecules whose transport is associated with Na+, such as glucose, amino acids and neurotransmitters, therefore altering neurotransmission (Geering, 1990).
As regards to the mechanisms underlying the inhibitory effect of the metabolites accumulating in MSUD on Na+, K+-ATPase activity, increasing evidence is emerging demonstrating that Na+, K+-ATPase is highly vulnerable to free radical attack (Lees, 1993; Kurella et al., 1997; Yousef et al., 2002). Therefore, considering that it has been well documented that KIC, KMV and KIV significantly increase lipid peroxidation and decrease the antioxidant defenses in the brain (Fontella et al., 2000; Bridi et al., 2005, 2005a), it might be presumed that Na+, K+-ATPase inhibition was caused, at least in part, by lipid peroxidation of the synaptic membrane in which the enzyme is embedded. However, this is unlikely since purified plasma synaptic membranes were used in the enzymatic assays and these membranes do not have the whole cell machinery necessary to free radical production. We cannot therefore exclude that the hydrophobicity of the compounds tested may also be responsible for some of our results, as it has been shown for phenylalanine (Wyse et al., 1998, 1999).
As regards to the possible consequences of the inhibition of Na+, K+-ATPase activity to neural cellular metabolism and function, it should be stressed that there is increasing evidence suggesting that alterations in Na+, K+-ATPase activity may be a link between many common neurotoxic mechanisms in neurons (Bavaresco et al., 2004; Wyse et al., 1998, 1998). In this context, blockage of the Na+, K+-ATPase activity secondary to energy depletion and free radical attack may be a common event in the apoptotic cascade (Wang et al., 2003). Furthermore, neuronal death associated with failure of the Na+, K+ pump may lead to apoptosis and necrosis mediated by depletion of K+ and accumulation of Na+ and Ca2+, respectively (Xiao et al., 2002). On the other hand, a reduction of Na+, K+-ATPase activity in the cerebral cortex of a neonate was considered to be directly involved in its status convulsivus and spongiform encephalopathy (Renkawek et al., 1992). Inhibition of Na+, K+-ATPase has been also associated with excitotoxicity and epilepsy (Grisar, 1984; Ben-Ari, 1985; Choi and Rothman, 1990; Hilliges et al., 1993; Cousin et al., 1995). We cannot therefore rule out a possible link between our present findings of an inhibition of Na+, K+-ATPase activity caused by the BCKA and the convulsions presented by MSUD patients during crises when the levels of the accumulating metabolites dramatically increase. We cannot also exclude a synergistic action of KIC, KMV and KIV and also of the BCAA since they are simultaneously accumulated in the disorder and can easily cross the blood-brain barrier. In fact, transport of BCAA and their keto acids into brain through the blood-brain barrier seems to be effective and quick (Steele, 1986).
Treatment of MSUD patients has been directed to reduce plasma BCAA levels, but despite dietary amino acid and protein restriction, which results in normal plasma BCAA concentrations, “well treated” MSUD patients have a variable degree of psychomotor delay (Hilliges et al., 1993). Thus, we propose that the plasma, urine and CSF concentrations of the BCKA, which primarily accumulate in this disorder, should be also measured and correlated with the clinical symptoms and brain abnormalities of these patients, in order to verify which metabolites (BCAA or BCKA) give the best correlation and should be measured at regular intervals for the follow up of the affected patients.
Finally, it should be emphasized that various pathomechanisms are probably responsible for the neurologic damage in MSUD. Inhibition of Na+, K+-ATPase activity by the metabolites that accumulate in this disorder is possibly one of them, so that preventing this inhibition by Ala may not prevent all abnormalities. Additional biochemical and behavioral studies on the effects of oral administration of Ala to MSUD patients are therefore necessary before pharmacokinetics and clinical trials are applied to human MSUD.
In conclusion, the present study demonstrated for the first time that the BCKA strongly inhibit a crucial enzyme of energy metabolism which is necessary for maintaining the basal membrane potential necessary for a normal neurotransmission and that uses 40–60% of the ATP generated in the brain. It is difficult to extrapolate our in vitro data to the human in vivo condition. However, if this is the case, it is feasible that inhibition of Na+, K+-ATPase activity might contribute, at least in part, to the encephalopathy of MSUD, especially during crises when the concentrations of these toxic metabolites dramatically increase.
References
Araujo P, Wassermann GF, Tallini K, Furlanetto V, Vargas CR, Wannmacher CM, Dutra-Filho CS, Wyse AT, Wajner M (2001) Reduction of large neutral amino acid levels in plasma and brain of hyperleucinemic rats. Neurochem Int 38:529–537
Bavaresco CS, Zugno AI, Tagliari B, Wannmacher CMD, Wajner M, Wyse ATS (2004) Inhibition of Na+, K+-ATPase activity in rat striatum by the metabolites accumulated in Lesch-Nyhan disease. Int J Devel Neurosci 22:11–17
Ben-Ari Y (1985) Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience 14:375–403
Bridi R, Araldi J, Sgarbi MB, Testa CG, Durigon K, Wajner M, Dutra-Filho CS (2003) Induction of oxidative stress in rat brain by the metabolites accumulating in maple syrup urine disease. Int J Dev Neurosci 21:327–332
Bridi R, Braun CA, Zorzi GK, Wannmacher CM, Wajner M, Lissi EG, Dutra-Filho CS (2005) Alpha-keto acids accumulating in maple syrup urine disease stimulate lipid peroxidation and reduce antioxidant defenses in cerebral cortex from young rats. Metab Brain Dis 20:155–167
Bridi R, Latini A, Braum CA, Zorzi GK, Wajner M, Lissi E, Dutra-Filho CS (2005a) Evaluation of the mechanisms involved in leucine-induced oxidative damage in cerebral cortex of young rats. Free Radic Res 39:71–79
Chan KM, Delfert D, Junger KD (1986) A direct colorimetric assay for Ca2+-stimulated ATPase activity. Anal Biochem 157:375–380
Choi DW, Rothman SM (1990) The role of glutamate neurotoxicity in hypoxicischemic neuronal death. Ann Rev Neurosci 13:171–182
Chuang DT, Shih VE (2001) Maple syrup urine disease (branched-chain ketoaciduria). In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease, 8th edn. Ma Graw-Hill, New York, pp 1971–2005
Coitinho AS, de Mello CF, Lima TT, de Bastiani J, Fighera MR, Wajner M (2001) Pharmacological evidence that alpha-ketoisovaleric acid induces convulsions through GABAergic and glutamatergic mechanisms in rats. Brain Res 894:68–73
Cousin MA, Nicholls DG, Pocock JM (1995) Modulation of ion gradients and glutamate release in cultured cerebellar granule cells by ouabain. J Neurochem 64:2097–2104
de Castro Vasques V, Boer MA, Diligenti F, Brinco F, Mallman F, Mello CF, Wajner M (2004) Intrahippocampal administration of the α-keto acids accumulating in maple syrup urine disease provokes learning deficits in rats. Pharmacol Biochem Behav 77:183–190
Dodd PR, Williams SH, Gundlach AL, Harper PA, Healy PJ, Dennis JA, Johnston GA (1992) Glutamate and gama-aminobutyric acid neurotransmitter systems in the acute phase of maple syrup urine disease and citrullinemia encephalopathies in newborn calves. J Neurochem 59:582–590
Erecinska M, Silver IA (1994) Ions and energy in mammalian brain. Prog Neurobiol 43:37–71
Fontella FU, Gassen E, Pulronik V, Wannmacher CM, Klein AB, Wajner M, Dutra-Filho CS (2002) Stimulation of lipid peroxidation in vitro in rat brain by the metabolites accumulating in maple syrup urine disease. Metab Brain Dis 17:47–54
Fontella FU, Pulrolnik V, Gassen E, Wannmacher CM, Klein AB, Wajner M, Dutra-Filho CS (2000) Propionic and L-methylmalonic acids induce oxidative stress in brain of young rats. Neuroreport 11:541–544
Geering K (1990) Subunit assembly and functional maturation of Na+, K(+)-ATPase. J Membrane Biol 115:109–121
Grisar T (1984) Glial and neuronal Na+, K+-ATPase pump in epilepsy. Ann Neurol 16:128–134
Halestrap AP, Brand MD, Denton RM (1974) Inhibition of mitochondrial piruvate transport by phenylpyruvate and α-ketoisocaproate. Biochim Biophys Acta 367:102–108
Henver RF, Duff RS, Wong-Ryley MTT (1992) Coordination of ATP production and consumption in brain: parallel regulation of cytochrome oxidase and Na+, K+-ATPase. Neurosci Lett 138:188–192
Hilliges C, Awiszus D, Wendel U (1993) Intellectual performance of children with maple syrup urine disease. Eur J Pediatr 152:144–147
Hoffmann GF, Meier-Augenstein W, Stockler S, Surtees R, Rating D, Nyhan WL (1993) Physiology and pathophysiology of organic acids in cerebrospinal fluid. J Inherit Metab Dis 16:648–669
Howell RK, Lee M (1963) Influence of α-keto acids on the respiration of brain in vitro. Proc Soc Exp Biol Med 113:660–663
Jones DH, Matus AI (1974) Isolation of synaptic plasma membrane from brain by combined flotation-sedimentation density gradient centrifugation. Biochim Biophys Acta 356:276–287
Jouvet P, Rustin P, Taylor DL, Pocock JM, Felderhoff-Mueser U, Mazarakis ND, Sarraf C, Joashi U, Kozma M, Greenwood K, Edwards AD, Mehmet H (2000) Branched chain amino acids induce apoptosis in neural cells without mitochondrial membrane depolarization or cytochrome c release: implications for neurological impairment associated with maple syrup urine disease. Mol Biol Cell 1:1919–1932
Kolker S, Okun JG, Ahlemeyer B, Wyse AT, Horster F, Wajner M, Kohlmuller D, Mayatepek E, Kriglstein J, Hoffmann GF (2002) Chronic treatment with glutaric acid induces partial tolerance to excitotoxicity in neuronal cultures from chick embryo telencephalons. J Neurosci Res 68:424–431
Kurella E, Kukley M, Tyulina O, Dobrota D, Matejovicova M, Mezesova V, Boldyrev A (1997) Kinetic parameters of Na/K-ATPase modified by free radicals in vitro and in vivo. Ann NY Acad Sci 834:661–665
Land JM, Mowbray J, Clark JB (1976) Control of pyruvate and β-hydroxybutyrate utilization in rat brain mitochondria and its relevance to phenylketonuria and maple syrup urine disease. J Neurochem 26:823–830
Lees GJ (1993) Contributory mechanisms in the causation of neurodegenerative disorders. Neuroscience 54:287–322
Lees GJ (1991) Inhibition of sodium-potassium-ATPase: a potential ubiquitous mechanism contributing to central nevous system neuropathology. Brain Res Rev 16:283–300
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Naughten ER, Jenkings J, Francis DE, Leonard JV (1982) Outcome of maple syrup utine disease. Arch Dis Child 57:918–921
Oldendorf WH (1973) Carrier-mediated blood-brain barrier transport of short-chain monocarboxylic organic acids. Am J Physiol 224:1450–1453
Pilla C, Cardozo RF, Dutra-Filho CS, Wyse AT, Wajner M, Wannmacher CM (2003) Creatine kinase activity from rat brain is inhibited by branched-chain amino acids in vitro. Neurochem Res 28:675–679
Pilla C, Cardozo RF, Dutra-Filho CS, Wyse AT, Wajner M, Wannmacher CM (2003) Effect of leucine administration on creatine kinase activity in rat brain. Metab Brain Dis 18:17–25
Prensky AL, Moser HW (1967) Changes in the amino acid composition of proteolipids of white matter during maturation of the human nervous system. J Neurochem 14:117–121
Renkawek K, Renier WO, De Pont JJ, Vogels OJ, Gabreels FJ (1992) Neonatal status convulsivus, spongiform encephalopathy, and low activity of Na+/K(+)-ATPase in the brain. Epilepsy 33:58–64
Rousson R, Guibaud P (1984) Long term outcome of organic acidurias: survey of 105 French cases (1967–1983). J Inherit Metab Dis 7(Suppl 1):10–12
Satoh E, Nakasato Y (1992) On the mechanism of ouabain-induced released of acetylcholine from synaptosomes. J Neurochem 58:1038–1044
Schonberger S, Schweiger B, Schwahn B, Schwarz M, Wendel U (2004) Dysmyelination in the brain of adolescents and young adults with maple syrup urine disease. Mol Genet Metab 82:69–75
Sgaravatti AM, Rosa RB, Schuck PF, Ribeiro CA, Wannmacher CM, Wyse AT, Dutra-Filho CS, Wajner M (2003) Inhibition of brain energy metabolism by the alpha-keto acids accumulating in maple syrup urine disease. Biochim Biophys Acta 1639:232–238
Snyderman SE, Goldstein F, Sansaricq C, Norton PM (1984) The relationship between the branched chain amino acids and their α-ketoacids in maple syrup urine disease. Pediatr Res 18:851–853
Snyderman SE, Morton PM, Roitman E, Holt LE Jr (1964) Maple syrup urine disease with a particular reference to diet therapy. Pediatrics 34:454–472
Steele RD (1986) Blood-brain barrier transport of the alpha-keto acid analogs of amino acids. Fed Proc 45:2060–2064
Streck EL, Zugno AI, Tagliari B, Wannmacher CMD, Wajner M, Wyse ATS (2002) Inhibition of Na+-K+-ATPase activity by the metabolites accumulating in homocystinuria. Metab Brain Dis 17:83–91
Tavares RG, Santos CE, Tasca CI, Wajner M, Souza DO, Dutra-Filho CS (2001) Inhibition of glutamate uptake into synaptic vesicles from rat brain by 3-nitropropionic acid in vitro. Exp Neurol 172:250–254
Tsakiris S, Deliconstantinos G (1984) Influence of phosphatidylserine on (Na+ K+)-stimulated ATPase and acethylcholinesterase activities of dog brain synaptosomal plasma membranes. Biochem J 22:301–307
Wang XQ, Xiao AY, Sheline C, Hyrc K, Yang A, Goldberg MP, Choi DW, Yu SP (2003) Apoptotic insults impair Na+, K+-ATPase activity as a mechanism of neuronal death mediated by concurrent ATP deficiency and oxidant stress. J Cell Sci 116:2099–2110
Wyse AT, Brusque AM, Silva CG, Streck EL, Wajner M, Wannmacher CM (1998) Inhibition of Na+, K+-ATPase from rat brain cortex by propionic acid. Neuroreport 9:1719–1721
Wyse AT, Noriler ME, Borges LF, Floriano PJ, Silva CG, Wajner M, Wannmacher CM (1999) Alanine prevents the decrease of Na+, K+ -ATPase activity in experimental phenylketonuria. Metab Brain Dis 14:95–101
Wyse AT, Wajner M, Brusque A, Wannmacher CM (1995) Alanine reverses the inhibitory effect of phenylalanine and its metabolites on Na+, K(+)-ATPase in synaptic plasma membranes from cerebral cortex of rats. Biochem Soc Trans 23:227S
Wyse ATS, Streck EL, Worm P, Wajner A, Ritter F, Netto CA (2000) Preconditioning prevents the inhibition of Na+, K+-ATPase activity after brain ischemia. Neurochem Res 25:969–973
Wyse ATS, Wajner M, Wannmacher CMD (1998) Kinects of alanine reversal on the inhibition of Na+, K+-ATPase activity by phenylalanine and phenillactate in the synaptic plasma membrane from the cerebral cortex of rats. Med Sci Res 26:141–143
Xiao AY, Wei L, Xia S, Rothman S, Yu SP (2002) Ionic mechanism of ouabain-induced concurrent apoptosis and necrosis in individual cultured cortical neurons. J Neurosci 22:1350–1362
Yang YB, Piao YJ (2003) Effects of resveratrol on secondary damages after acute spinal cord injury in rats. Acta Pharmacol 24:703–710
Yousef MI, El-Hendy HA, El-Demerdash FM, Elagamy EI (2002) Dietary zinc deficiency induced-changes in the activity of enzymes and the levels of free radicals, lipids and protein electrophoretic behavior in growing rats. Toxicology 175:223–234
Yu SP (2003) Na+, K+-ATPase: the new face of an old player in pathogenesis and apoptotic/hybrid cell death Biochem. Pharmacol 66:1601–1609
Yudkoff M, Daikhim Y, Lin ZP, Nissim I, Sterm J, Pleasure D (1994) Interrelationships of leucine and glutamate metabolism in cultures astrocytes. J Neurochem 62:1191–1192
Zielke HR, Zielke CL, Baab PJ, Collins RM (2002) Large neutral amino acids auto exchange when infused by microdialysis into the rat brain: implications for maple syrup urine disease and phenylketonuria. Neurochem Int 40:347–354
Acknowledgments
This work was supported in part by grants from Conselho Nacional de Desenvolvimento Científico e tecnológico (CNPq-Brazil), Fundação de Amparo à Pesquisa do Rio Grande do Sul (FAPERGS, RS-Brazil) and Programa de Núcleos de Excelência-Financiadora de Estudos e Projetos (PRONEX II, FINEP-CNPq-Brazil).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wajner, A., Bürger, C., Dutra-Filho, C.S. et al. Synaptic Plasma Membrane Na+, K+-ATPase Activity is Significantly Reduced by the α-Keto Acids Accumulating in Maple Syrup Urine Disease in Rat Cerebral Cortex. Metab Brain Dis 22, 77–88 (2007). https://doi.org/10.1007/s11011-007-9046-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-007-9046-5