Abstract
Atherosclerosis is the major cause of coronary artery disease (CAD) which includes unstable angina, myocardial infarction, and heart failure. The onset of atherogenesis, a process of atherosclerotic lesion formation in the intima of arteries, is driven by lipid accumulation, a vicious cycle of reactive oxygen species (ROS)-induced oxidative stress and inflammatory reactions leading to endothelial cell (EC) dysfunction, vascular smooth muscle cell (VSMC) activation, and foam cell formation which further fuel plaque formation and destabilization. In recent years, there is a surge in the number of publications reporting the involvement of circular RNAs (circRNAs) in the pathogenesis of cardiovascular diseases, cancers, and metabolic syndromes. These studies have advanced our understanding on the biological functions of circRNAs. One of the most common mechanism of action of circRNAs reported is the sponging of microRNAs (miRNAs) by binding to the miRNAs response element (MRE), thereby indirectly increases the transcription of their target messenger RNAs (mRNAs). Individual networks of circRNA–miRNA–mRNA associated with atherogenesis have been extensively reported, however, there is a need to connect these findings for a complete overview. This review aims to provide an update on atherogenesis-related circRNAs and analyze the circRNA–miRNA–mRNA interactions in atherogenesis. The atherogenic mechanisms and clinical relevance of each atherogenesis-related circRNA were systematically discussed for better understanding of the knowledge gap in this area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atherosclerotic cardiovascular diseases remain a leading cause of death globally. The presence of atherosclerotic plaque is the hallmark of coronary artery disease (CAD) [1, 2]. The development and progression of atherosclerosis involves cell adhesion, differentiation, migration, proliferation, and cell interaction with the extracellular matrix which are controlled by a complex network of cytokines and growth regulatory peptides [3,4,5]. The process of atherosclerotic lesion formation, atherogenesis, involves several sequential events and is initiated when low-density lipoproteins (LDLs) accumulate in the tunica intima and activate the endothelium. Apolipoprotein B100 containing-LDL transports high levels of circulating cholesterol through vascular endothelium, which leads to retention of LDL within the blood vessel wall [6, 7]. Reactive oxidative species (ROS), myeloperoxidase, lipoperoxidase, and nicotinamide adenine dinucleotide phosphate (NADH/NADPH) induce lipid peroxidation on the trapped LDL and form oxidized LDL (ox-LDL) [8]. Ox-LDL induces endothelial activation by facilitating the production and secretion of inflammatory mediators (e.g., interleukin-8), adhesion molecules (e.g., vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1) and selectins), chemoattractants (e.g., platelets-activating factors), and monocyte chemoattractant protein-1 (MCP-1) that drive inflammation, recruitment, and adhesion of monocytes at the lesion site. Recruited monocytes transmigrate through intercellular junctions to the subendothelial space and differentiate into macrophages following the stimulation by macrophage-colony stimulating factor. Macrophages up-regulate scavenger receptors and mediate the internalization of modified or oxidized lipoproteins such as ox-LDLs, native lipoproteins, and anionic phospholipids and leads to the foam cell lesion formation [9, 10]. These triggers worsen inflammation in the vascular wall and attract more monocytes/macrophages to the subendothelial space in a vicious cycle. Inflammatory mediators from the endothelium and macrophages also convert the contractile phenotype of vascular smooth muscle cells (VSMC) into the active synthetic phenotype with increased proliferative and migration potentials. The accumulation of lipid-laden foam cells, as well as the parallel proliferation and migration of VSMC from tunica media to tunica intima, eventually lead to atherosclerotic plaque formation. Ox-LDL also induces matrix metalloproteinase-1 (MMP-1) and MMP-9 in vascular wall matrix causing degradation of extracellular collagen leading to weak and unstable atherosclerotic plaque.
Circular RNA (circRNA) is an emerging yet interesting class of non-coding RNA (ncRNA) in which a covalently closed circular loop is generated through the backsplicing of linear RNA. Although circRNA was first identified in the RNA viruses [11], it was regarded as an artifact of the splicing process for decades until a group of endogenous circRNAs was detected in the 1990s [12]. With the improvement in high throughput sequencing, biochemical analysis, and bioinformatic algorithms, circRNAs were later found to be ubiquitously expressed throughout the eukaryotic kingdom and recently, in viruses as well. Not only are circRNAs evolutionarily conserved across species, the expression of circRNAs is reported to be regulated in a tissue/organ or developmental stage-dependent manner with the majority being derived from protein-coding genes [13,14,15]. The abundance of circRNA is usually low, which is a hundred to a thousand times lower than the co-expressed linear RNA [16] and are predominantly localized in the cytoplasm [17,18,19]. Due to its circular structure that lacks 3′ polyadenylation (poly-A) tail and 5′ cap, circRNAs are immune to various cellular exonucleases. Although circRNAs are less abundant, they are more stable, some with a half-life of more than 48 h compared to its linear counterparts that have an average half-life of 10 h [17].
The roles played by circRNAs in human diseases such as cancers, diabetes, cardiovascular, and neurodegenerative diseases have been widely reported (reviewed by [20]). CircRNAs are expressed in vascular cells and are involved in the pathogenesis of cardiovascular diseases. In recent years, there is an increasing trend of published studies on the roles of various circRNAs in atherosclerosis. However, literatures detailing the interactions involving circRNAs, miRNAs, and mRNAs on the whole in driving atherosclerosis are limited. This review provides a summary of circRNAs involved in atherogenesis and categorized them according to their pro-atherogenic or atheroprotective effects for a clearer understanding of the biological functions of the various circRNAs identified thus far. We also attempted to investigate if there are common circRNA–miRNA–mRNA networks from these reports to provide a deeper understanding on the clinical significance of these micromanagers in cardiovascular diseases. In particular, we attempted to analyze the interactions of circRNAs, miRNAs, and mRNAs that have been identified thus far via Cytoscape. A total of 12 circRNAs was identified and connected at a certain degree through their target miRNAs or mRNAs. This number is surprisingly low considering that numerous circRNAs and miRNAs have been reportedly involved in atherogenesis and cardiovascular diseases. Our analyses, hence, have highlighted the need for future studies to delineate a cohesive network of circRNA–miRNA–mRNA interactions to gain a more holistic view on the functional mechanisms of these micromanagers in the initiation and progression of atherosclerosis for better therapeutic design and development.
The endothelium
The endothelium is a monolayer of endothelial cells (EC) which forms the inner cellular lining of the blood vessels and lymphatic system in the body. The vascular endothelium acts as a barrier between circulating blood cells and the underlying tissues. It is selectively permeable and important in maintaining vascular homeostasis balance by regulating the exchange of nutrients, fluids, gases, and waste products between the surrounding cells or tissues and flowing blood. The endothelium controls the degree of vascular relaxation and constriction, detects and reacts to blood-borne signals and changes in hemodynamic forces. It regulates vascular tone by secreting vasodilators such as nitric oxide (NO) and prostacyclin (PGI2) in response to hormones and physical stimulus such as blood flow-induced shear stress [21, 22]. EC also produces vasoconstrictors such as angiotensin II (Ang II), endothelin, superoxide, and thromboxane A2 (TxA2) [23].
In addition, the endothelium is important in maintaining the balance of anti-thrombotic and anti-coagulant activities via the Protein C anti-coagulant pathway and prevents thrombosis in order to ensure normal blood flow [24]. Upon endothelial inflammation or injury, the anti-coagulant pathway is suppressed where both plasminogen activator (tPA) and plasminogen activator inhibitor-1 (PAI-1) are secreted into the bloodstream [25]. Clot formation is allowed in an injured site but still maintains blood fluidity in the surrounding area [23]. Production of NO also prevents adhesion and activation of platelet and leukocyte to the vessel wall (Fig. 1) through both cyclic guanosine monophosphate (cGMP)-dependent and -independent (endothelial-platelet interaction) [26, 27] and ICAM-1-dependent (endothelial-leukocyte interaction) [28] mechanisms.
The comparison of a normal and dysfunctional endothelium. Endothelial dysfunction is characterized by reduced nitric oxide (NO) bioavailability, increased reactive oxygen species (ROS), endothelin-1 (ET-1), and cell adhesion molecule (CAM) production and the imbalance of vasodilators and vasoconstrictors which favor a pro-inflammatory, proliferative and pro-coagulatory states that contribute to atherogenesis. Ang II Angiotensin II; EDHF endothelium-derived hyperpolarizing factor; eNOS endothelial nitric oxide synthase; ICAM-1 intercellular adhesion molecule-1; LDL low-density lipoprotein; NO nitric oxide; NF-κB nuclear factor-kappa B; ONOO2 peroxynitrite; PGI2 prostacyclin; TGF-β transforming growth factor β; TF-1 transcription factor 1; tPA tissue plasminogen activator; TxA2 thromboxane A2; VCAM-1 vascular cell adhesion molecule; vWF von Willebrand factor. The figure was created with Motifolio Toolkit (Motifolio Inc, Ellicott City, MD)
Endothelial dysfunction
Endothelial cell dysfunction is a key event in the initiation of atherosclerotic process and is characterized by the modification of the endothelial phenotype from a quiescent state (non-atherogenic/non-thrombogenic) to an activated state (atherogenic/thrombotic) in response to pathophysiological changes. The endothelium is incapable of maintaining vascular homeostasis balance in an activated state [29]. Dysfunctional endothelial is characterized by the reduction in production or availability of NO and/or an imbalance in the relative contribution of endothelium-derived relaxing factors (EDRF) (e.g., NO and PGI2) and endothelium-derived contracting factors (EDCF) (e.g., TxA2, endothelin-1 (ET-1), and Ang II) [30] (Fig. 1). Endothelium in the quiescent state is mainly maintained by NO and laminar shear stress [31]. NO is generated from l-arginine via endothelial nitric oxide synthase (eNOS) in the presence of cofactors such as tetrahydrobiopterin (BH4) and nicotinamide adenine dinucleotide phosphate (NADPH). Reduction of NO bioavailability may be caused by reduction of eNOS expression, impaired eNOS activation, or NO inactivation by oxidative stress.
Nitric oxide is an important vasodilator for balancing the vasodilation and vasoconstriction in the endothelium and diminished NO bioavailability is one of the major causes leading to vasoconstriction resulting in the lack of VSMC relaxation. NO diffuses to the VSMC and stimulates guanylate cyclase and leads to cGMP-mediated cell relaxation and subsequently vasodilation [32, 33]. Activation of cGMP-dependent protein kinase (cGK) by NO in VSMC inhibits VSMC proliferation, migration, and fibrotic gene expression [34]. Reduced NO bioavailability will lead to the proliferation and migration of VSMC and thus accelerates vascular hypertrophy and finally culminates in endothelial dysfunction [35,36,37]. In addition, reduction in NO bioavailability also inhibits platelet aggregation and contributes to thrombosis [38]. Upon vascular injury, inflammation, or mechanical perturbation, decreased NO level promotes the secretion of selectin family proteins (P-selectin, E-selectin, and L-selectin) which recruit and induce both platelet and leukocyte adhesion to endothelial surface before transmigrating to damaged tissue site from the blood vessels [39]. These endothelial modifications promote inflammation within the vessel wall, setting the stage for the initiation, and progression of an atherosclerotic lesion.
Endothelial activation by oxidative stress and inflammation
Apart from endothelium-dependent vasodilation impairment, EC dysfunction is also characterized by “endothelial activation” which comprises a milieu of pro-inflammatory, proliferative, and pro-coagulatory activations that favor all phases in atherogenesis [40, 41]. EC activation is characterized by the increment of the expressions of cell-surface adhesion molecules, such as ICAM-1, VCAM-1, and endothelial leukocyte adhesion molecule (E-selectin) which convert quiescent EC to migratory and proliferative phenotypes, and differentiates into an angiogenic phenotype [7]. Pro-inflammatory cytokines such as tumor necrosis factor α (TNF-α) and interleukin-6 (IL-6) activate EC and promote the recruitment and attachment of circulating leukocytes to the vessel wall [42].
The vascular endothelium is a major target of oxidative stress. Typical atherosclerotic risk factors such as aging, diabetes mellitus, hypertension, hyperglycemia, hypercholesterolemia, and smoking have been linked to the increased production of ROS in cells [43, 44]. Oxidative stress refers to the imbalance between ROS production and the efficacy of antioxidant system in the body. ROS such as hydrogen peroxide (H2O2), superoxide anion (O2−), nitric oxide (NO·), and peroxynitrite (ONOO−) and extrinsic factors such as environmental pollution, UV light, radiation or high-glucose and high-fat food intake, induce oxidative stress and tissue damage through glycoxidation and advanced glycation end-products (AGE) formation [45]. This can trigger the production of oxidants which reduce the level of antioxidants and lead to an imbalance in the redox potential of cells. ROS activated-NADPH oxidase reduces O2 to O2− and leads to the production of ONOO−. Highly reactive ONOO− inactivates eNOS, reduces NO bioavailability, and leads to endothelial dysfunction [45]. In addition, ox-LDL increases synthesis of caveolin-1, which binds to calmodulin to inhibit eNOS activity and thus reduces the production of NO [46]. The disruption of redox balance in EC under oxidative stress results in the activation of the nuclear factor erythroid 2-related factor 2 (Nrf2)/antioxidant responsive element (ARE) pathway which targets multiple ARE-regulated antioxidants, regulates the expression of antioxidative enzymes, such as heme oxygenase-1 (HO-1) [47]. Nrf2 plays an important role in atherosclerosis resistance by targeting multiple ARE-regulated antioxidants.
Plasma and macrophage content of ox-LDL in coronary plaques are well associated with the severity of acute coronary syndrome. Formation of ox-LDL is mediated by ROS in the subendothelial space and serves as a major trigger of atherogenesis. Binding of ox-LDL to the lectin-like oxidized LDL receptor-1 (LOX-1) activates nuclear factor-kappa B (NF-κB) in EC [48]. Activation of NF-κB induces the production of pro-inflammatory cytokines (e.g., TNF-α and IL-6), chemotactic factors, and adhesion molecules which then leads to inflammation and infiltration of immune cells at lesion sites, whereas NF-κB in monocytes facilitates inflammatory gene expression and converts macrophages into foam cells [49]. Phagocytosis of ox-LDL by macrophages via scavenger receptor amplifies and retains ox-LDL. Accumulative formation of ox-LDL in the atherosclerotic lesions acts as potent stimulus for ROS production within the vascular wall and further propagates oxidative stress at atherosclerotic plaques producing inflammation in a vicious cycle [50].
VSMC activation
Progression of atherosclerotic plaque formation continues with the accumulation of lipid and lipid-engorges cells. Proliferation, migration, and synthesis of collagen by VSMC facilitate the evolution of fatty streak toward a complex lesion. The activities of VSMC are stimulated by the continuous release of cytokines such as MCP-1 by activated EC and T cells [51]. Expansion of the atherosclerotic lesion within coronary arteries results in lumen obstruction and hence reduces blood flow which drive angina in clinical condition. Other than macrophages, T cells also localize within the lesions and play a role in the growth of atherosclerotic plaque. Activated T cells secrete interferon (IFN)-γ, a pro-inflammatory cytokines which limits the synthesis of new collagen required for fibrous cap preservation [52] and secretion of transforming growth factor β (TGF-β) restricts the inflammation and VSMC proliferation [53]. Accumulation of ox-LDL causes apoptosis of macrophages and VSMC, forming the necrotic core of the advancing atheroma [54], whereas inhibition of lipoprotein-associated phospholipase A2 reduces macrophage death [55, 56]. The death of macrophage foam cells leads to lipid spillage, further promoting inflammation [57], while VSMC death further reduces collagen synthesis and promotes fibrous cap thinning [58]. The thinning of the fibrous cap is enhanced by the overexpression of MMPs, interstitial collagenases, and gelatinases, which degrade supportive collagen [59]. MMP overexpression and activation within the plaque are mediated by IL-1β, TNF-α, ox-LDL, and CD40L. Once the fibrous cap is weakened, the plaque is vulnerable to rupture, precipitating acute thrombotic complications.
Circular RNAs
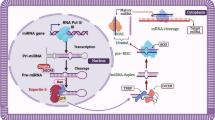
CircRNAs are generated when an upstream splice acceptor is covalently joined to a downstream splice donor and can be classified into 3 major subtypes based on their compositions, which are exonic circRNA (ecircRNA), intronic circRNA (ciRNA), and exon–intron circRNA (EIciRNA) [60]. Nevertheless, circRNA can also be derived from the intron of transfer RNA (tricRNA), intergenic region, and anti-sense RNAs. To date, three models have been proposed for circRNA biogenesis mechanisms which are RNA-binding protein (RBP)-mediated circularization, intron pairing-driven circularization, and lariat-driven circularization (Fig. 2). During RBP-mediated circularization, RBPs bind to specific sequence motifs on the upstream and downstream introns flanking the exons of a linear pre-mRNA in a trans manner, followed by dimerization of the RBPs to facilitate backsplicing. For example, Quaking (QKI) can bring the circle-forming exons into close proximity through binding to available intronic QKI binding motifs [13] while immune factors NF90/NF110 [61] and RNA editing enzyme, ADAR1 [62], both can regulate the expression of circRNAs through stabilizing and destabilizing the intronic RNA pairing step of the circularization process, respectively. On the other hand, the presence of inverted complementary sequences in flanking intronic regions (e.g., Alu repeats) may promote alternative circularization through intron pairing-driven circularization model, resulting in the generation of various circRNAs [17]. For example, the presence of ~ 400 bp long inverted repeats flanking the mouse Sry gene leads to the formation of the circSry in HEK 293 EBNA cells [63]. In humans, Ivanoc et al. revealed that 88% of human circRNAs contain complementary Alu elements (~ 300 nt long) in their flanking introns which could facilitate exon circularization [64]. In some cases, relatively short (30–40 nt) inverted repeats are sufficient for intron pairing-driven circularization [65]. The third circRNA biogenesis model, lariat-driven circularization involves an exon-skipping event during pre-mRNA transcription. The process begins with canonical splicing, resulting in the production of linear RNA with skipped exons within a long intron lariat. Subsequently, internal splicing facilitates the removal of flanking intronic sequence, allowing the generation of ecircRNA [66]. Whereas retention of the intronic sequences results in the production of ElciRNAs, having a consensus motif containing a 7 nt GU-rich element near the 5' splice site and an 11 nt C-rich element close to the branchpoint site would facilitate the generation of ciRNAs [67].
Biogenesis and functions of circRNAs. CircRNAs can be derived through RNA-binding proteins (RBP)-mediated circularization, intron pairing and lariat-driven circularization. Their roles include sponging of miRNAs and RBP, as regulators of parental gene through cis or trans-actions, as modulators of protein–protein interactions, and as templates for protein translation
Emerging evidence reveals that circRNAs have different biological functions (Fig. 2), but the mechanism behind most of these functions remains unclear. MicroRNA (miRNA) sponge is the most reported role of circRNAs in many studies. MiRNAs can regulate protein production through transcriptional repression of messenger RNAs (mRNAs). With the miRNA response elements (MRE) on the circRNA sequence, circRNA are able to sponge and sequester away the miRNAs, indirectly regulating the miRNA-target genes that form part of the complex competing endogenous RNA (ceRNA) network [68]. A prominent example is the cytoplasmic CDR1 antisense (CDR1as), also known as ciRS-7 which contains miR-7 and miR-671 binding sites [69,70,71]. MiR-7 is a well-known regulatory molecule that functions as a tumor suppressor by repressing the expression of multiple oncogenes, hence, binding of miR-7 on ciRS-7 promotes tumorigenesis. The perfect complementarity binding of miR-671 on ciRS-7 on the other hand, will cause the slicing of ciRS-7. CircRNAs could also be a risk factor in disease development as well, for example circTMEM45A is a reported oncogenic circRNA that promotes hepatocellular carcinoma through the circTMEM45A/miR-665/IGF2 axis [72]. It is possible for circRNA to act as an RBP sponge or RBP assembly platform that may have a role in disease development [73]. Mapping of RBPs onto human circRNAs by Dudekula et al. indicated that many circRNAs could bind different RBPs for example, EIF4A3, HuR, and FMRP either at the body or backsplice junctions [74]. Binding of HuR to circPABPN1 (previously known as hsa_circ_0031288) suppressed the translation of PABPN1 by preventing the binding of HuR to linear PABPN1 mRNA [75]. In addition, Fang and team reported a nuclear-retained circFAT1(e2) acts as a tumor suppressor that specifically interacts with an RBP, namely YBX1 to prevent tumorigenesis in gastric carcinoma [76].
Other than being sponges, circRNA could regulate the expression of parental gene through cis- or trans-actions. For instance, nuclear-retained circEIF3J and circPAIP2 regulate transcription of its parental genes by binding to U1 snRNP through RNA-RNA interaction. Such complexes will further interact with the Pol II transcription complex at the promoters of their parental genes to enhance gene expression [77]. In addition, Chen et al. demonstrated that a circRNA, circFECR1 derived from FLI1 gene promotes breast cancer metastasis through epigenetic regulation mechanism [78]. CircFECR1 binds to its parental gene in cis manner and recruits TET1 demethylase to induce DNA demethylation of FLI1 promoter. Concurrently, circFECR1 also interacts with DNMT1 promoter in a trans manner that results in DNMT1 silencing. CircRNAs can also act as an mRNA trap, in which formation of circRNA competes with the linear mRNA splicing process. A good example of the mRNA trap is circMbl, which contains MBL binding sites on its flanking introns, thus it is strongly and specifically bound by MBL proteins [79]. When the MBL protein is in excess, it binds to the Mbl pre-mRNA and favors the biosynthesis of cirMbl through backsplicing, in turn reduces the production of the parental mRNA.
On the other hand, circRNAs are able to bind, store, sort, and sequester proteins to particular subcellular locations, and act as dynamic scaffolding molecules that modulate protein–protein interactions. CircFOXO3, is a circRNA that is negatively correlated to cancer by regulating protein in cells. CircFOXO3 interacts with cyclin-dependent kinase 2 (CDK2) and CDK inhibitor p21, forming a ternary complex. Such complex enhances the inhibitory effect of p21 on CDK2 preventing the formation of cyclin E/CDK2 complex which is essential for G1-S transition [80]. Apart from acting as a scaffold, circFOXO3 is also able to alter subcellular localization of proteins by sequestration. CircFOXO3 sequesters several proteins that are involved in cell survival (e.g., ID1 and E2F1) and stress response (e.g., FAK and HIF-1α) pathways to co-localize in the cytoplasm [81]. Sequestration by circFOXO3 prevents nuclear translocation of ID-1, E2F, and HIF-1α, as well as mitochondrial translocation of FAK, which in turn leads to cellular senescence.
Based on its circular structure, circRNA was initially presumed to be untranslated. By performing ribosome footprinting, a subgroup of circRNAs was found to be translated into protein due to its association with translating ribosomes. CircRNA translation initiation is cap-independent and mediated by IRESs and/or m6A motifs [82, 83]. Two of the best examples are the muscle-enriched circZNF609 and liver cancer-associated circβ-catenin. Both of them contain an open reading frame spanning from the start codon that is similar with their linear mRNA and terminating at the stop codon that was created upon circularization [84, 85]. Translated circZNF609 was reported to be involved in controlling the proliferation of myoblast [84], and positively associated with rhabdomyosarcoma [86]. On the other hand, a small peptide translated from circβ-catenin acts as a decoy for GSK3β-induced β-catenin phosphorylation and degradation to protect full-length β-catenin, resulting in Wnt-β-catenin pathway activation [85]
Due to the involvement of circRNAs in modulating signaling pathways that play important roles in human diseases, circRNAs are being extensively studied today to identify clinically relevant circRNAs as potential pharmacological targets and disease markers. The roles of circRNAs have been summarized by Verduci et al. [20] and here we present an update of circRNAs involved in endothelial dysfunction and vascular injury leading to atherosclerosis and other vascular-related diseases and complications (Tables 1, 2, 3, Fig. 3).
Pro-atherogenic and atheroprotective circRNAs involved in atherogenesis initiation and progression. ICAM-1 intercellular adhesion molecule-1; I/R ischemic/reperfusion; LDL low-density lipoprotein; LPS lipopolysaccharide; ox-LDL oxidized low-density lipoprotein; VCAM-1 vascular cell adhesion molecule. The figure was created with Motifolio Toolkit (Motifolio Inc, Ellicott City, MD)
CircRNAs that promote endothelial injury
Common cellular models of oxidative stress-induced vascular endothelium injury include treatment of EC lines such as human umbilical vein endothelial cells (HUVECs) and human aortic endothelial cells (HAECs) with ox-LDL or high glucose (HG). Ox-LDL contributes to the pathogenesis of atherosclerosis and atherosclerotic plaque rupture by promoting lipid accumulation, pro-inflammatory responses and apoptosis of EC and VSMC as documented in vivo [87]. In vitro, ox-LDL induces HUVECs proliferation at low concentrations (5 to 10 μg/mL) and apoptosis at higher concentrations (10 to 300 μg/mL) and is mediated by O−2 formation, with NADPH oxidase as the major source for O−2 [88]. In many of the studies reviewed, EC injury is induced using high concentrations of ox-LDL, and then EC health were measured by determining proliferation, migration, tube formation, and apoptosis in vitro. EC dysfunction protective or worsening effect of circRNAs is based on whether the overexpression or knockdown of a particular circRNA worsens or improves EC injury by these measures. On the other hand, hyperglycemic condition produces advanced glycation end products that initiate oxidative reactions and induce vascular hyperpermeability. Using these in vitro models, a number of circRNAs were found upregulated in oxidative stress-induced EC injury (Fig. 3, Table 1).
Circ_USP36 (circ_0003204)
Circ_USP36 (chr17:76,798,405–76,800,060) is derived from exon 16 and 17 regions within ubiquitin specific peptidase 36 (USP36) locus. The expression of circ_USP36 is reported to be elevated in HUVECs exposed to ox-LDL, whereby, it aggravates ox-LDL-induced EC injury [89, 90]. Its downstream target, miR-942-5p regulates histone deacetylase 9 (HDAC9) which promotes aortic calcification in mice and human aortic smooth muscle contractility [91]. Increased HDAC9 is associated with MMP1 and MMP2 expression in pro-inflammatory macrophages of human carotid plaque [92]. It was also shown that HDAC9 mediates ox-LDL-induced apoptosis and inflammatory reactions [90, 93].
MiR-942-5p also targets HMGB1, which stimulates pro-inflammatory cytokine synthesis of IL-1β, IL-6, and TNF-α in human monocytes and macrophages [94]. In addition to miR-942-5p, circ_USP36 also sponges miR-330-5p which regulates TLR4 [95]. TLR4 activates NF-κB-mediated inflammatory response [96] and has been associated with the initiation and progression of atherosclerotic disease [97].
Circ_USP36 targets miR-98-5p [98] which has an endothelial protective role. MiR-98-5p has been shown to protect brain endothelium and improves neurological outcomes in mouse ischemia/reperfusion stroke model [99] and inhibits HUVECs apoptosis by targeting caspase-3, MAPK6, LOX-1 [100,101,102]. Moreover, miR-98-5p also directly targets VCAM-1, an adhesion molecule that is highly expressed in atherosclerosis and HMGB1, a DNA binding protein that produces pro-inflammatory response [103]. In addition, circ_USP36 also mediates ox-LDL-induced cell cycle arrest, apoptosis, and inflammatory response in HUVECs by targeting miR-20a-5p which directly binds to Rho-associated coiled-coil kinase 2 (ROCK2). The inhibitory effects were reversed upon circ_USP36 knockdown, supporting its role in mediating EC dysfunction and inflammatory response [104].
Circ_0004104
Circ_0004104 was found significantly up-regulated in CAD patients compared to healthy controls [108]. Circ_0004104 overexpression resulted in increased pro-atherosclerotic genes expression such as indoleamine 2,3-dioxygenase 1 (IDO1), MMP8, soluble CD40 and decreased anti-atherosclerotic gene expression, apolipoprotein AI (ApoA I) in THP-1-derived macrophages [108]. In ox-LDL-induced EC injury model, circ_0004104 level was also up-regulated together with increased oxidative stress and released of pro-inflammatory mediators TNF-α and IL-1β. These effects were mediated by sponging miR-328-3p and in turn increased TRIM14 [109]. TRIM14 has been shown to promote endothelial activation by activating NF-κB signaling [110]. Indeed, another study has shown that miR-328-3p by targeting FOXO4 can protect HUVECs against ox-LDL induced injury. The suppression of miR-328-3p led to the overexpression of FOXO4 and decreased cell viability, migration and invasion and induced apoptosis and inflammation [111]. It was also reported that miR-328-3p is also a target of circHIPK3 where it drives the pathogenesis of pulmonary arterial hypertension via the miR-328-3p-STAT3 axis [112].
Circ_0124644
Circ_0124644 was found significantly up-regulated in the peripheral blood of CAD patients compared to healthy subjects and appears to have relatively high specificity and sensitivity in diagnosing CAD [113]. Subsequently, its pro-atherogenic role was demonstrated by mediating EC injury through miR-149-5p/PAPP-A axis [114]. The role of miR-149-5p in CAD is also well established clinically, whereby, its plasma level is down-regulated in CAD patients [115, 116]. MiR-149-5p is also shown to reduce the production of pro-inflammatory mediators, including NF-κB, TNF-α, and IL-6 in macrophages [117]. The role of miR-149-5p in vascular injury was first identified in HG-induced endothelial dysfunction by targeting TNF-α, resulting in decreased levels of ET-1, vWF, and ICAM-1 and increased level of NO and eNOS expression and reduced apoptosis [118].
Circ_RELL1 (circ_0002194) and circ_NOL12
In ox-LDL-induced EC inflammation, circ_RELL1 was significantly up-regulated compared to the untreated cells, whereas, knockdown of circ_RELL1 reduced the expression of adhesion molecules ICAM1 and VCAM1. Circ_RELL1 also sponges miR-6873-3p, resulting in the up-regulation of its target gene, MyD88 (myeloid differentiation primary response 88) which activates NF-κB and promotes inflammatory response [119]. MiR-6873-3p is also a target of circ_NOL12, which is derived from nucleolar protein 12 (NOL12) gene. Circ_NOL12 expression was increased following ox-LDL induced HUVECs injury. Overexpression of circ_NOL12 inhibited miR-6873-3p expression in HUVECs and decreased HUVECs viability, oxidative stress and inflammatory response [120]. Under the regulation of circ_NOL12, the effects of miR-6873-3p, which are mediated by targeting fibroblast growth factor receptor substrate 2 (FRS2) regulate cell proliferation via MAPK signaling [121]. In addition, the miR-6873-3p/FRS2 axis is also modulated by lncANRIL in the progression of atherosclerosis [122]. Collectively, these studies revealed that several circRNAs can target the same miRNA and produce various outcomes depending on the target mRNA.
Circ_BPTF (circ_0045462)
EC dysfunction is also a diabetes-related vascular complication. Hence, a HG-induced vascular inflammatory cellular model is used to study endothelial dysfunction. Circ_BPTF is up-regulated in HG-induced oxidative stress in HUVECs, but its knockdown improves cell viability and suppresses cell apoptosis, the release of pro-inflammatory cytokines and oxidative stress. Circ_BPTF mediates these effects by sponging miR-384, which in turns up-regulates LIN28B. MiR-384 has been reported to play a protective role on EC [123]. In the middle cerebral artery occlusion mouse model, overexpression of miR-384 increases the proliferation and angiogenesis of endothelial progenitor cells (EPC) [124]. MiR-384 has also been shown to be protective against retinal neovascularization caused by ischemia in microvascular impairment of diabetic retinopathy mice model [125]. The effects of miR-384 is mediated through targeting Lin-28 homolog B (LIN28B), Delta-like ligand 4 (DLL4), and hexokinase 2 (HK2). LIN28B up-regulation was previously shown to induce angiogenesis in human retinal EC and promotes diabetic retinopathy progression [126]. DLL4 is an endothelium-specific Notch ligand that is involved in vascular development and angiogenesis [127] and a high expression of DLL4 is associated with activation of the Notch signaling pathway and suppression of EC growth [128]. HK2 is a key enzyme of glucose metabolism [129] and decreased HK2 expression in coronary EC of type 2 diabetes mice leads to an increase in mitochondrial calcium level and excess ROS production, whereas, overexpression of HK2 restores endothelial function [130].
Circ_0068087
HG condition also up-regulates circ_0068087 in HUVECs and knockdown of circ_0068087 suppresses the secretion of pro-inflammatory mediators, TNF-α, IL-6, IL-1β, and MCP-1 [131]. Circ_0068087 promotes HG-induced TLR4/NF-κB/NLRP3 inflammasome-mediated inflammation and EC dysfunction by suppressing miR-197 which directly targets TLR4. MiR-197 has been reported to be a prognostic marker for CAD patients [132]. Circ_0068087 also promotes ox-LDL-induced oxidative injury in HUVECs by sponging miR-186-5p [133]. MiR-186-5p exerts protective effects from ox-LDL-induced endothelial dysfunction by suppressing its target Roundabout guidance receptor 1 (ROBO1), a receptor of slit guidance ligand 1 (SLIT1) and SLIT2. SLIT2-ROBO signaling plays a role in vascular injury where it can exert either pro-angiogenic or anti-angiogenic effects in EC, depending on the cell types [134].
CircVEGFC (circ_0071465)
In a HG-induced endothelial injury model, Wei et al. [135] showed that circVEGFC was up-regulated, concomitant with decreased proliferative ability induced by HG exposure. Whereas the knockdown of circVEGFC attenuated apoptosis and increased the survival of HUVECs. CircVEGFC inhibits EC proliferation by sponging miR-338-3p which targets the transcription factor, hypoxia-inducible factor 1 alpha (HIF-1α). Up-regulation of HIF-1α then leads to increased transcription of vascular endothelial growth factor A (VEGFA), an angiogenesis stimulating factor. However, it is unclear how VEGFA contributed to apoptosis in this study [135] since VEGFA overexpression when regulated by miR-320 in HG-treated HUVECs has been shown to enhance protein kinase C (PKC) and receptor for advanced glycation endproducts (RAGE) protein levels which subsequently promotes proliferation and angiogenesis but suppresses HUVECs apoptosis instead [136].
Contrary to the study by Wei et al. (2020), another study has observed an increase of miR-338-3p following ox-LDL-induced endothelial injury concomitant with decreased cell viability and increased apoptosis. These inhibitory effects were reversed following the inhibition of miR-338-3p [137]. It was shown that bone morphogenetic protein (BMP) and activin membrane‐bound inhibitor (BAMBI) is a direct target of miR-338-3p [137]. BAMBI regulates cell proliferation and differentiation by stably associating with TGFβ family receptors and represses TGFβ/Smad signaling [138, 139]. As discussed earlier, TGFβ signaling plays a protective role in vascular injury, whereby it is rapidly activated following vascular injury to stimulate proliferation and migration of EC and VSMC for vascular repair [105,106,107]. Hence, down-regulation of miR-338p may also increases BAMBI which in turn represses TGFβ signaling activation, thereby decreasing EC survival. Collectively, these studies revealed that circRNA-miRNA regulations in different EC injury models may produce different outcomes.
CZNF609
With regards to clinical relevance, cZNF609 levels in peripheral blood leukocytes and plasma of CAD and hypertensive patients were found decreased compared to healthy volunteers, concomitant with the up-regulation of circulating levels of miR-615-5p [140, 141]. In HG-treated HUVECs, cZNF609 expression was increased. Silencing of cZNF609 increased the cell viability and reduced oxidative stress or hypoxic stress-induced apoptosis in HUVECs. By contrast, overexpression of cZNF609 worsen oxidative stress or hypoxic stress-induced HUVEC apoptosis. In vivo, cZNF609 knockdown in both diabetic retinopathy and oxygen-induced retinopathy mouse model also decreased retinal vascular leakage and pathological angiogenesis, respectively. CZNF609 causes in vitro EC and in vivo vascular dysfunction by sponging miR-615-5p, which in turn increases its target, myocyte enhancer factor 2A (MEF2A). MEF2A is a transcription factor that is highly expressed in the endothelium of coronary arteries and has been implicated with the pathogenesis of CAD and myocardial infarction (MI) [142]. Overexpression of miR-615-5p could decrease retina vascular leakage and capillary degeneration in diabetic retinopathy and also pathological angiogenesis in oxygen-induced retinopathy mouse model. Moreover, MEF2A overexpression could also rescue cZNF609 silencing-mediated effects on HUVEC migration, tube formation, and apoptosis [141]. These comprehensive clinical, in vivo and in vitro findings strongly support the pro-atherosclerotic and abnormal angiogenic roles of cZNF609 in the pathogenesis of diabetes, hypertension, and CAD.
CircAFF1
EC dysfunction can also be induced by hypoxia, which is an important contributor of ischemic injury [143]. Using a hypoxic cellular model, Wang et al. [144] showed that circAFF1 was up-regulated in HUVECs and human brain endothelial cells (HBEC-5i) treated with CoCl2, an inducer of hypoxia and also in sera of patients with subarachnoid hemorrhage. The viability of EC in the hypoxic condition overall was further affected by the overexpression of circAFF1 which resulted in apoptosis, impaired wound healing, and impaired tube formation [144]. These effects were mediated by suppression of its target, miR-516b, whereby, miR-516b overexpression could attenuate EC suppression induced by circAFF1. MiR-516b targets Salvador family protein 1 (SAV1), which leads to the down-regulation of SAV1 and reduces the phosphorylation of Yes-associated protein 1 (YAP1). SAV1 and YAP1 are components of Hippo signaling involved in the regulation of cell proliferation. Phosphorylation of YAP1 by SAV1 will prevent its entry into the nucleus and transcription of relevant genes important for cell viability [145, 146].
Circ_0010729
The knockdown of circ_0010729 has been previously shown to have a protective role from cardiomyocyte injury by several studies [147, 148]. The role of circ_0010729 on EC dysfunction has also been reported. It was shown that hypoxia-induced HUVECs injury up-regulates circ_0010729 expression, concomitant with proliferation and migration inhibition and the up-regulation of hypoxia-inducible factor, HIF-1α [149]. HIFs are transcription factors that respond to low oxygen tension in tissue and induce genes that promote angiogenesis, energy metabolism and cell survival, which then promote EC migration, growth, and differentiation [150]. However, the EC inhibitory effects caused the hypoxic condition were reversed by the knockdown of circ_0010729. It was further shown that circ_0010729 targets miR-186, resulting in the increased expression of HIF-1α [149]. These findings, hence suggest that circ_0010729 drives EC dysfunction caused by hypoxia and its knockdown plays a protective role.
Circ_ADAM9
Endothelial progenitor cells (EPC) which are immature EC are vital for wound healing. These cells differentiate into mature EC, proliferate, and form new blood vessels at the injured site [151]. These cells also secrete angiogenic factors to stimulate angiogenesis. EPC are also vulnerable to oxidative stress caused by hyperglycemia, resulting in EPC apoptosis and suppression of angiogenesis [152]. In diabetic EPC or EPC subjected to HG condition, these cells undergo apoptosis and angiogenic suppression. Overexpression of circ_ADAM9 has been shown to promote EPC apoptosis under HG treatment, whereas its knockdown significantly suppressed apoptosis [153]. Furthermore, down-regulation of circ_ADAM9 also promotes angiogenesis in the hind limbs of diabetic mice. The apoptosis promotion effect was mediated through sponging miR-20a-5p, which has apoptosis and autophagy suppressive actions by directly targeting PTEN and ATG7. Suppression of the PI3K/AKT/mTOR pathway promotes autophagy, whereas activation of PI3K/AKT/mTOR inhibits autophagy by regulating ATG7. PTEN negatively regulates PI3K/AKT/mTOR pathway [154]. Hence, its increased expression following the binding of miR-20a-5p by circ_ADAM9, leads to increased autophagy and apoptosis [153]. As discussed earlier, miR-20a-5p is also a target of circ_0003204 (circ_USP36), which mediates ox-LDL-induced HUVECs injury via ROCK2 [104].
CZBTB44 (circ_0002484)
CZBTB44 is reported to be up-regulated in a laser-induced choroidal neovascularization (CNV) mouse model and in choroid-retinal endothelial RF/6A cells subjected to CoCl2-induced hypoxia stress [155]. Abnormal angiogenesis and increased vessel sprouting occurred concurrently with the overexpression of cZBTB44 in hypoxia, but knockdown of cZBTB44 decreased choroidal sprouting assay ex vivo, reduced CNV lesion are in laser-induced CNV in mouse and also inhibited the viability, proliferation, migration, and tube formation of RF/6A cells under basal and hypoxic conditions. The effects of cZBTB44 were mediated through the sponging of miR-578 which targets VEGFA and VCAM1. Inhibition of miR-578 leads to increased VEGFA and VCAM1 expression which promotes abnormal angiogenesis, whereas overexpression of miR-578 mimicked cZBTB44 knockdown-mediated anti-angiogenic effects in vivo and in vitro [155]. These findings suggest that cZBTB44-miR-578-VEGFA/VCAM1 crosstalk promotes pathological neovascularization by regulating EC function.
CircHECW2
Endothelial to mesenchymal transition (EndMT) is also known to drive vascular inflammation and the progression of atherosclerotic plaque [156, 157]. EndMT is a process by which EC dedifferentiate into mesenchymal phenotype and lose their expression markers and functions. Acquisition of the mesenchymal phenotype enables these cells to migrate into underlying tissues and compromise vascular integrity. A genome-wide bioinformatic analysis has identified that circHECW2, which is derived from exon 12 and exon 13 of the HECW2 gene can sponge miR-30d and drives the EndMT process [158]. Lipopolysaccharide (LPS) stimulation induced EndMT, as characterized by increased expression of mesenchymal markers COL1A2/Collagen I and ACTA2/α-SMA (alpha-actin-2/alpha-smooth muscle actin) and decreased expressions of endothelial tight junction proteins (TJP-1, occludin, and claudin 5). Yang et al. showed that miR-30d was down-regulated and ATG5 up-regulated in LPS-stimulated human brain microvascular endothelial cells (HBMECs) and in mice administered with LPS [158]. Subsequent experiments revealed that miR-30d regulates ATG5 in the EndMT process. These effects were confirmed with the knockdown of circHECW2 which resulted in the inhibition of EndMT in vitro and in vivo. The decrease of ATG5 caused by circHECW2 knockdown was reversed by silencing of miR-30d.
Endothelial dysfunction protective circRNAs
CircHIPK3
CircHIPK3 is abundantly expressed in various tissues and it promotes EC growth by regulating its target miRNAs: miR-124, miR-193, miR-379, and miR-654 [159]. Although its role may be conflicting, several studies have reported that it plays protective roles in endothelial dysfunction (Table 2). Overexpression of circHIPK3 (i) inhibits HG-induced cell death and apoptosis in HG-treated HUVECs by sponging miR-124 which up-regulates pro-survival targets such as SphK1 and STAT3 [160]; (ii) promotes autophagy of ox-LDL-stimulated HUVECs and inhibits lipid accumulation by targeting miR-190b which in turns target ATG7, a driver of autophagy [161]; (iii) promotes human coronary aortic artery EC proliferation and angiogenesis after myocardial infarction by suppressing its target miR-133a and promotes connective tissue growth factor (CTGF) expression, which is associated with angiogenesis [162]. Moreover, exosomal circHIPK3 released from hypoxic cardiomyocytes has been shown to protect cardiac microvascular EC (CMVECs) following internalization by inhibiting miR-29a, thereby increasing IGF-1 expression that modulates oxidative damage and protects CMVECs from oxidative stress [163].
Nevertheless, the pro-angiogenic activity of circHIPK3 can be deleterious in pulmonary arterial hypertension and diabetic retinopathy. The stimulatory effects of circHIPK3 on human pulmonary artery endothelial cells (hPAECs) proliferation, migration, and angiogenesis, however, can promote the pathogenesis of pulmonary arterial hypertension. These effects are mediated via miR-328-3p-STAT3 axis [112]. The pro-angiogenic effect of circHIPK3 is also detrimental for diabetic retinopathy, whereby it is up-regulated in diabetic retinas and retinal EC stimulated with HG. Silencing circHIPK3 decreased retinal acellular capillaries, vascular leakage, and inflammation and alleviated retinal vascular dysfunction. The abnormal EC growth was found mediated by the suppression of miR-30a-3p which led to increased VEGF-c, FZDA and WNT2 expression [164].
Although circHIPK3 in general has a protective role in EC dysfunction, a study has shown that circHIPK3 can enhance the proliferative ability of VSMC, leading to the conversion to synthetic active VSMC phenotype that is pro-atherosclerotic. It was shown that knockdown of circHIPK3 up-regulates miR-637 expression which then decreases its target, CDK6 levels [165]. Whereas overexpression of circHIPK3 increases CDK6 levels and subsequently promotes the proliferation of VSMC that accelerates the progression of atherosclerosis. Hence, circHIPK3 modulates different outcomes in cell types and vascular diseases [165].
CircANRIL
Antisense noncoding RNA in the INK4 locus (ANRIL) is transcribed on chromosome 9p21.3 locus, which has been identified by GWAS and robustly replicated as a genetic risk factor for CAD [166, 167]. According to NCBI, three ANRIL transcripts were annotated, respectively, as NR_003529 (the 3,857-bp full-length ANRIL transcript), DQ485454 (2,659-bp transcript), and EU741058 (short 688-bp transcript). ANRIL regulates glucose and fatty acid metabolism [168]. Up-regulation of the circulatory form, circANRIL is associated with lower risk of CAD development [169, 170], whereas its linear counterpart, linANRIL (NR_003529 and EU741058) is associated with increased risk for CAD [171]. In the modulation of atherosclerosis, circANRIL was shown to increase apoptosis and decrease viability of VSMC and macrophages by inhibiting pescadillo homolog 1 (PES1), a 60S pre-ribosomal assembly factor vital for ribosome biogenesis. Impairment of pre-ribosomal RNA processing and ribosome biogenesis lead to nucleolar stress and activation of p53 which in turn, promotes apoptosis and inhibits proliferation of VSMC and macrophages [170]. Similarly, overexpression of circANRIL in EC isolated from atherosclerotic rats also resulted in reduced oxidative stress and inflammatory mediators released [172], further supporting the protective role of circANRIL in atherosclerosis. On the contrary, Song et al. showed that overexpression of circANRIL increases serum lipids level, exacerbates endothelial inflammation by increasing IL-1, IL-6, MMP-9, and CRP levels and induces endothelial apoptosis in coronary arteries that can cause vessel regression in rat atherosclerotic model [173]. Whereas opposite effects were observed in rats injected with circANRIL inhibitor. Therefore, circANRIL’s role in atherosclerosis may also be dependent on its expression level. It is also worth noting that miRNAs regulated by circANRIL in atherosclerosis have yet to be reported.
CircDNMT3B
CircDNMT3B and BAMBI expressions were found down-regulated in retinal EC of diabetic retinopathy patients and diabetic rats [174]. BAMBI is a target of miR-20b-5p and circDNMT3B has been shown to sponge miR-20b-5p. In human retinal microvascular endothelial cells (HRMECs) treated with HG as a mimic of diabetic condition, it was shown that up-regulation of miR-20b-5p promotes abnormal proliferation, migration, and tube formation of HRMECs, all of which contributes to diabetic retinopathy. MiR-20b-5p induced excessive EC proliferation, migration, and tube formation under HG conditions was mediated through the suppression of BAMBI. In diabetic nephropathy, BAMBI ensures endothelial stability by reducing capillary growth and migration [175,176,177]. These results show that circDNMT3B can control abnormal EC growth and migration that can contribute to vascular dysfunction.
CircDLGAP4
Endothelial dysfunction also contributes to ischemic heart disease. Conversion of quiescent EC to the migratory and angiogenic phenotype could result in abnormal angiogenesis as seen in late stages of ischemic/reperfusion (I/R) injury [178]. Bai et al. reported that circDLGAP4 levels were significantly decreased in the plasma of acute ischemic stroke patients and in a mouse stroke model [179]. In a subsequent study, Chen et al. showed that circDLGAP4 was also down-regulated in HUVECs following I/R-induced injury, whereas its overexpression attenuated the I/R-induced increase in EC migration [180]. Increased endothelial migration contributes to the progression of atherosclerotic lesion formation [9, 181]. EndMT was also attenuated as evidenced by reduced expression of mesenchymal markers following microinjection of circDLGAP4 into mice with cerebral ischemia-induced injury [179]. In both studies, the expression of miR-143 which is a target of circDLGAP4 were up-regulated in I/R-induced injury in HUVECs and mice with cerebral ischemia-induced injury. It was further shown that miR-143 mediates endothelial dysfunction and EndMT by inhibiting HECT domain E3 ubiquitin protein ligase 1 (HECTD1). The role of HECTD1 in EndMT and metastasis in tumorigenesis has been previously reported [182]. MiR-143 was also previously implicated in EndMT [183] and also up-regulated in clinical atherosclerotic plaque samples [180]. In HUVECs, overexpression of miR-143 suppresses glycolysis by directly targeting hexokinase 2 (HK2), a key enzyme in glucose metabolism, and leads to endothelial dysfunction [184]. Collectively, these findings suggest the protective role of circDLGAP4 in endothelial dysfunction.
Circ_0058092
EPC are important for wound healing as they differentiate into mature EC and proliferate to promote new blood vessels formation of the injury site [151]. CircRNA has also been shown to affect EPC. For example, circ_0058092 was down-regulated in type 2 diabetic patients. A further study has revealed that the expression of circ_0058092 is decreased in EPC subjected to hyperglycemic condition, whereas circ_0058092 overexpression preserved EPC survival, proliferation, migration, and angiogenic differentiation as well as decreased oxidative stress and inflammatory response [185]. These protective effects were mediated through increased FOXO3 expression as overexpression of circ_0058092 sponges miR-217 which targets FOXO3 [185]. Although this study has shown the EPC protective effects of increased FOXO3 expression in HG-induced EPC injury [180], other studies have also shown that overexpression of FOXO3 could suppress human EPC proliferation by modulating cell cycle regulators [186]. Knockdown of FOXO3 gene could also protect EC in FOXO-knocked out mice from vascular dysfunction and atherosclerosis [187] and increases HUVECs sprouting and migration [188]. These findings suggest the pleiotropic role of FOXO3 in endothelial health depending on the study system or the injury inducer used.
Circ_0003575
Circ_0003575 was reportedly up-regulated in ox-LDL-induced endothelial damage and its silencing promoted the proliferation and angiogenesis ability of HUVECs [189]. Subsequently, Shang et al. demonstrated using aortic EC isolated from an atherosclerosis in vivo model, ApoE deficient mouse, that overexpression of circ_0003575 suppresses FOXO3 and FOXO4 protein expressions [190]. However, ectopic expression of miR-148a-3p impaired the circ_0003575-mediated up-regulation of FOXO4 and FOXO3 [190]. As discussed above, knockdown of FOXO is protective of EC dysfunction [187, 188]. Shang et al. also showed that overexpression of miR-148a-3p, a regulator of lipid metabolism [191] enhances EC proliferation and inhibits apoptosis [190]. However, in this study, there was no direct evidence of circ_0003575 sponging miR-148a-3p, suggesting that circ_0003575 and miR-148a-3p interaction may involve other regulators.
CircRSF1 (circ_0000345)
In sera of patients with atherosclerosis, the expression of circRSF1 was reportedly down-regulated together with the up-regulation of miR-758 [192]. This observation was further demonstrated in ox-LDL-treated HAECs, whereby overexpression of circRSF1 and silencing miR-758 improved HAECs viability, tube formation, and migration while apoptosis was attenuated. It was demonstrated that circRSF1 positively regulates cyclin D2 (CCND2) expression by sponging miR-758 [192]. CCND2 complexes with CDK4 or CDK6 and initiates cell cycle progression from G1 to S phase by phosphorylating retinoblastoma protein [193]. It has been reported that CCDN2 is also a target of miR-98. In a HG-induced EC injury, miR-98 expression was found down-regulated while CCDN2 was also up-regulated [194]. This further supports the role of CCDN2 in regulating EC growth under stress conditions. In ox-LDL treated HUVECs, the expression of circRSF1 was similarly down-regulated, whereas its target, miR-135b-5p was up-regulated [195]. MiR-135b-5p induced EC apoptosis and inflammation by regulating its target gene, histone deacetylase 1 (HDAC1), which has been reported to increase VCAM-1 expression, promoting the early events of atherogenesis [196]. Overexpression of circRSF1 rescued the inhibitory effects of EC [195]. Collectively, these findings support the protective role of circRSF1 in endothelial dysfunction by regulating miR-758 and miR-135b-5p.
Circ_CLASP2 (circ_0064772)
CircRNA CLIP–associating protein 2 (circ_CLASP2) expression was initially found decreased in HUVECs exposed to HG condition in a circRNA profiling study using RNA sequencing [197]. Subsequent mechanistic study revealed that circ_CLASP2 overexpression promotes proliferation and inhibits apoptosis in HUVECs under HG conditions by interacting with miR-140-5p which directly targets FBXW7 [198]. FBXW7 is an important regulator of endothelial barrier and angiogenesis [199, 200]. MiR-140-5p is up-regulated in HUVECs under HG conditions and knockdown of miR-140-5p promoted proliferation, inhibited apoptosis, and increased VEGF expression. An earlier study has shown that miR-140-5p expression is increased in mice with atherosclerosis, whereby its up-regulation increases oxidative stress and ROS levels by suppressing the protein expression of Nrf2, sirtuin 2 (Sirt2), Kelch-like enoyl-CoA hydratase-associated protein 1 (Keap1), and heme oxygenase 1 (HO-1) in HUVECs. By contrast, down-regulation of miR-140-5p decreased oxidative stress and ROS levels [201].
Circ_0054633
Circ_0054633, first found up-regulated in the peripheral blood of type 2 diabetes mellitus patients [202] has been subsequently validated to play a protective role in HG-induced endothelial dysfunction [203]. Under HG-induced HUVECs injury, circ_0054633 was up-regulated and the down-regulation of circ_0054633 further suppressed EC proliferation, increased apoptosis, migration, and tube formation [203]. The down-regulation of circ_0054633 has led to increased expression of its target, miR-218 which then decreased ROBO1 and VEGF expression levels, which are vital angiogenic drivers for vascular injury. Furthermore, decreased circ_0054633 also resulted in the reduction of HO-1, which is another target of miR-218 [203]. HO-1 is an anti-oxidant and cytoprotective enzyme vital for protection from oxidative stress and inflammation [204]. Hence, these findings are supportive of the protective role of circ_0054633 in EC dysfunction.
CircRNAs that regulate the functions of VSMC
Physiologically, VSMC assumes a quiescent contractile state which performs contractile function to regulate blood flow and blood vessel diameter by vasoconstriction and vasodilation [205]. The maintenance of VSMC in the contractile phenotype is dependent on the interaction between EC and VSMC. However, in response to inflammatory responses from EC and macrophages, VSMC is converted to the active synthetic phenotype with increased proliferation and migration capacity but decreased contractility [206]. Active synthetic VSMC plays a key role in pathological vascular remodeling, which is a pathophysiological process that contributes to atherosclerosis and hypertension. Recent literature has discussed the role of proliferation of VSMC, which can be argued as reparative or detrimental depending on the stages of atherosclerosis [58, 207]. Increased VSMC proliferation is observed following vascular injury and during early atherogenesis [208], suggesting a reparative role. Whereas human VSMC derived from both aged vessels and advanced atherosclerotic plaques showed reduced proliferation [209]. This suggests that in the later stage of atherosclerosis, enhancement of proliferation could be beneficial for plaque stability since plaque rupture commonly occurs in areas with reduced VSMC, increased VSMC apoptosis, and increased macrophages. VSMC apoptosis, hence, could drive atherogenesis and progression of established lesions. The roles of circRNAs in regulating VSMC in the various events leading to atherosclerosis has also begun to unfold and are discussed below (Fig. 3, Table 3).
CircNRG-1
CircNRG-1 has been shown to inhibit apoptosis of mouse aortic smooth muscle cells (MASMCs) by regulating miR-193b-5p/NRG-1 [210]. Neuregulin-1 (NRG-1), a member of the epidermal growth factor (EGF) family, is expressed in vascular EC and VSMC. It has been shown that NRG-1 expression can be decreased by Ang II, an effector peptide of the renin–angiotensin–aldosterone-system (RAAS) involved in the regulation of blood pressure and fluid balance. Ang II is also known for its role in regulating VSMC proliferation or apoptosis in the early stages of vascular remodeling [211]. Sun et al. showed that Ang II inhibits apoptosis of MASMCs by decreasing both NRG-1 and circNRG-1 levels [210]. Whereas, overexpression of NRG-1 reversed the inhibitory action of Ang II on MASMC apoptosis. Luciferase reporter assays revealed that circNRG-1 up-regulates NRG-1 expression by acting as miR-193b-5p sponge. MiR-193b-5p has been shown to target the 3′-UTR of NRG-1 and suppresses NRG-1 expression in MASMCs. VSMC apoptosis is implicated in vascular remodeling and these findings suggest that circNRG-1/miR-193b-5p/NRG-1 axis is modulated by Ang II in apoptosis resistance of VSMC [210].
Circ_0010283
In an ox-LDL treated VSMC, circ_0010283 and HMGB1 were found significantly up-regulated, whereas the expression of miR-370-3p was down-regulated [212]. Ox-LDL promotes VSMC proliferation and migration and knockdown of circ_0010283 suppresses VSMC viability and migration, as evidenced by the suppression of cell cycle regulator cyclin D1 and proliferating cell nuclear antigen, and migration-associated proteins MMP2 and MMP9 [212]. Hence, circ_0010283 promotes the viability and migration of ox-LDL-induced VSMC. Circ_0010283 was shown to sponge miR-370-3p which targets HMGB1. The inhibition of miR-370-3p reversed the circ_0010283 silencing-mediated inhibitory effects on VMSC viability and migration. Additionally, the miR-370-3p-mediated suppressive effects on cell viability and migration were rescued by overexpression of HMGB1. Hence, circ_0010283 mediated cell viability and migration via a miR-370-3p/HMGB1 axis in ox-LDL-induced VSMC. HMGB1 has been reported to accelerate foam cell formation and cholesterol accumulation in VSMC [213] and is involved in pro-inflammatory response [103].
Circ_CHFR (circ_0029589)
The checkpoint with forkhead-associated and ring-finger domains (circ_CHFR), was identified by microarray profiling of VSMC exposed to ox-LDL treatment [214]. A subsequent study also showed that circ_CHFR is up-regulated in serum of atherosclerosis patients and in ox-LDL-stimulated VSMC [215]. In both studies, knockdown of circ_CHFR suppressed the ox-LDL-induced promotion of cell growth, migration, and inflammation in VSMC. Yang et al. showed that circ_CHFR increases the expression of FOXO1 by sponging miR-370 [214]. In addition, miR-370 has also been reported as a target of circ_0010283 [212]. These studies revealed that both circ_CHFR and circ_USP36 indirectly increase the expression of FOXO1 by suppressing miR-370 and promoting apoptosis of VSMC and EC. In addition to miR-370, circ_CHFR also targets miR-214-3p and increases the expression of Wnt3 [215]. On the contrary, knockdown of miR-214-3p and overexpression of Wnt3 attenuated circ_CHFR induced cell migration and inflammation triggered by ox-LDL exposure in VSMC [215]. Previously, it has been shown that Wnt3a produces pro-migratory effects on EC [216]. Collectively, these studies show the existence of an indirect network of common circRNAs-miRNAs-mRNA in regulating the active synthetic VSMC phenotype that contributes to atherogenesis.
Circ_SIRT1 (circ_0093887)
Circ_SIRT1 which is derived from the SIRT1 host gene, is expressed in VSMC and is enriched in response to vascular injury induced by TNF-α or platelet-derived growth factor-BB (PDGF-BB) [217]. Its expression is decreased in the plasma of CAD patients as well as in human renal arterial neointima, concomitant with the increased expression of pro-inflammatory cytokine MCP-1 and adhesion molecules VCAM-1 and ICAM-1. In contrast, overexpression of circ_SIRT1 decreased TNF-α-induced expression of VCAM-1, ICAM-1, and MCP-1 levels in VSMC, by inhibiting the nuclear translocation of NF-κB p65, which is vital for the transcription of pro-inflammatory cytokines and adhesion molecules [217]. These findings show that circ_SIRT1 inhibits inflammatory phenotypic switching of VSMC. Kong et al. further showed that circ_SIRT1 directly binds to miR-132/212 which targets SIRT1 resulting in enhanced SIRT1 activity [217]. This then increases deacetylation and inactivation of the nuclear NF-κB p65 and prevents inflammatory phenotype switching of VSMC. Other studies have also shown the protective effects of enhanced SIRT1 activity by inhibiting VSMC proliferation and migration that underlie neointima formation [218], vascular endothelial dysfunction, and cardiovascular diseases [219].
Circ_0020397
Neointimal thickening occurs in response to vascular injury in atherosclerosis [220]. It is characterized by VSMC proliferation and migration from the media to the neointima. Circ_0020397 has been shown to modulate VSMC proliferation and migration via the miR-138/VEGFR2 axis [221]. VEGFR2 is the principal receptor of VEGFA which enhances proliferation, migration, and survival of ECs [222]. Studies have shown that VEGFR2 also plays an important role in regulating VSMC proliferation [223, 224]. The expression of circ_0020397 was found significantly decreased in the arterial smooth muscle cells isolated from intracranial aneurysm patients, in association with increased expression of miR-138 and decreased the protein expression of VEGFR2 [221]. It was reported that overexpression of circ_0020397 in human umbilical artery smooth muscle cells (HUASMCs) promotes cell proliferation, suppresses miR-138 expression but down-regulates VEGFR2 expression. MiR-138 was also shown to negatively regulate VEGFR2 by binding with the 3’UTR of VEGFR2 mRNA. In contrast, overexpression of miR-138 induces HUASMC apoptosis [221].
Circ_0044073
Circ_0044073 was found up-regulated in blood cells of atherosclerosis patients, in association with the down-regulation of miR-107 expression and up-regulation of the levels of JAK1 and STAT3 [225]. In vitro validation revealed that under the stimulation of LPS, the migration and invasion potentials of human vascular smooth muscle cells (HUVSMC) and HUVECs increased together with a significant increase of circ_0044073 expression. Circ_0044073 increases the proliferative and invasive potentials of these cells by sponging miR‐107, which targets JAK1 [225]. The JAK/STAT signaling pathway is known to promote vascular cell inflammation, proliferation, migration, and adhesion [226]. In agreement to this, overexpression of circ_0044073 activated the JAK/STAT signaling pathway along with up-regulation of Bcl-2, c-myc, IL-1β, IL-6, and TNF-α level [225]. Contrary to the effects seen with the overexpression of circ_0044073, transfection with mimic miR-107 inhibited the proliferation of HUVSMC and HUVECs. MiR-107 is linked to insulin sensitivity and inflammation [227] and our previous study has also shown that overexpression of miR-107 promotes EC senescence and inhibits tube formation by increasing MTORC1 activity [228]. Collectively, these findings are supportive of the role of circ_0044073 in promoting the pro-atherosclerotic VSMC and EC phenotype.
CircPTPRA (circ_102984)
CircPTPRA was reported as a tumor suppressor that represses EndMT and metastasis in non-small cell lung carcinomas (NSCLC) [229]. However, up-regulation of circPTPRA and down-regulation of miR-636 level were reported in plasma of patients with atherosclerosis compared to healthy individuals. Inhibition of miR-636 via the direct sponging by circPTPRA increased SP1 and further promoted cell proliferation and repressed apoptosis in VSMC [230]. Inhibition of circPTPRA or SP1 in VSMC promoted G0/G1 cell cycle arrest with down-regulation of Cyclin D1 and cyclin E protein levels.
CircRNAs that modulate foam cell formation and progression
Circ_0092317/circ_0003546 and circ_0028198/circ_0092317
Foam cell formation is a hallmark of atherosclerosis progression. Foam cell lesions are cholesterol ester-enriched macrophages while some of them are macrophage-like cells originating from VSMC. The formation of foam cells is determined by the combination effect of lipid uptake, cholesterol esterification, and cholesterol efflux. Bioinformatic analysis revealed the impact of circRNAs in the cholesterol transport process in THP-1 macrophages. Circ_0092317/circ_0003546 and circ_0028198/circ_0092317 possibly sponged miR-326 and miR‐543, respectively, in reducing the cholesterol efflux in macrophages by targeting aspartate beta-hydroxylase (ASPH) and phosphodiesterase 3B (PDE3B) [231, 232]. ASPH regulates cellular response to calcium ions, while PDE3B plays important roles in the hydrolysis of cyclic adenosine monophosphate (cAMP) and prohibits the activation of cAMP-dependent protein kinases which phosphorylates ABCA1.
CircDENND1B
High density lipoprotein (HDL) is important in removing cholesterol from cells and tissues involved in the early stages of atherosclerosis back to the liver for excretion. Down-regulation of circDENND1B was reported in ox-LDL-induced monocytes. CircDENND1B produces anti-atherosclerotic effect by regulating adenosine triphosphate (ATP)-binding cassette transporter A1 (ABCA1) through sponging miR-17-5p and hence promotes cholesterol efflux in foam [233]. ABCA1 is an important receptor involved in the initiation of cholesterol efflux to HDL in macrophage foam cells. Pro-inflammatory cytokines such as IL-1β, IFN-γ, and TNF-α activate macrophages by regulating ABCA1 expression and drive foam cell formation. Increased circulating miR-17-5p was reported in plasma of CAD patients [234]. MiR-17-5p binds directly to ABCA1 mRNA and down-regulation of miR-17-5p significantly up-regulated ABCA1 in RAW264.7 mouse macrophage cells accompanied with decreased inflammatory cytokines production and inhibition of lipid accumulation [235].
CircTM7SF3
CircRNA transmembrane 7 superfamily member 3 (circTM7SF3) was significantly up-regulated in the plasma of atherosclerosis patients and ox-LDL-induced THP-1-derived macrophages compared to their corresponding counterparts [236]. Silencing of circTM7SF3 partly attenuated the production of inflammatory cytokines such as TNF-α and IL-6 and oxidative stress in ox-LDL-induced human monocyte-derived macrophages (hMDMs) and THP-1 derived macrophages. The macrophage cell injury was induced through circTM7SF3/miR-206/ASPH signal axis. The role of miR-206 in atherosclerosis has been revealed in a previous study, whereby, decreased level of miR-206 in atherosclerosis tissue samples and overexpression of miR-206 markedly reduced VSMC relative survival rate [237].
CircRNA–miRNA–mRNA interactions
To date, most circRNA studies on atherogenesis have focused on a single circRNA, as shown in Tables 1, 2, 3. We attempted to identify common circRNA–miRNA–mRNA axis involved in atherogenesis using Cytoscape 3.8.2. Cytoscape is an open-source bioinformatic platform that allows visualization of interaction between biomolecules [238]. After removing unconnected clusters, we identified 12 circRNAs (circHIPK3, circ_0004104, circ-ADAM9, circ_USP36, circ_0068087, circ_0010283, circ_CHFR, circ_0054633, circVEGFC, circ_0010729, circ_RELL1, and circ_NOL12) that have demonstrated some degree of connectivity, either through miRNAs or their target mRNAs (Fig. 4). We noted three clusters of circRNAs that target a common miRNA and exert either pro-atherogenic or atheroprotective effects. The first cluster consists of circ_0010283 and circ_CHFR which can sponge miR-370, thereby up-regulating targets of miR-370 such as HMGB1 and FOXO1, resulting in endothelial inflammation and injury as well as VSMC activation, all of which favor atherogenesis. The second cluster includes circ_RELL1 and circ_NOL12 which target miR-6878-3p and produces pro-atherogenic effects such as induction of oxidative stress, EC inflammation, and apoptosis. In contrast, circHIPK3 and circ_0004104 both act on miR-328-3p which targets STAT3 and TRIM14, respectively, but produce opposing effects. Modulation of STAT3 is protective of EC health, whereas modulation of TRIM14 promotes EC apoptosis and inflammation. Nevertheless, other studies have identified that circHIPK3 engages several other miRNAs to exert an overall protective effect from EC dysfunction.
It is well established that a single miRNA can have multiple target mRNAs. In this regard, both miR-338-3p and miR-186, which can be sponged by circVEGFC and circ_0010729, respectively, thereby, increasing HIF1 and promote EC apoptosis, inhibit growth and migration. Likewise, both circHIPK3 and circ-ADAM9 also modulate ATG7 by targeting miR-190b and miR-20a-5p, respectively, and promote autophagy in EC and EPC. MiR-197 and miR-330-5p similarly target TLR4 and activate NF-κB pathway in promoting EC activation, whereby, miR-197 and miR-330-5p can be sponged by circ_USP36 and circ_0068087, respectively. On the other hand, targeting of ROBO1 by miR-186-5p and miR-218 produce opposing effects on EC health. MiR-186-5p and miR-218 are regulated by circ_0068087 and circ_0054633, respectively. In this scenario, opposing effects were observed likely because of the difference in the two EC injury models used in their studies, i.e., ox-LDL and HG-induced EC injury. Collectively, these findings revealed that while there is accumulating evidence of the role of numerous circRNAs in atherogenesis, identification of common circRNA–miRNA–mRNA networks in atherosclerosis remains limited. Identification of cohesive networks is warranted to gain deeper understanding to the complexity of circRNA-miRNA roles in atherogenesis as combination use of these small RNAs have great potential as diagnostic and preventive strategies.
Clinical applicability of circRNAs
The greater aim would be to develop clinical diagnostic and prognostic circRNA biomarkers as well as therapeutics for human diseases including CAD. Although the regulatory functions of circRNAs on disease development and progression are yet to be fully elucidated, the reported stability along with the cell-type, tissue, and developmental stage specificity of circRNAs [15] present an immense potential for circRNAs to be developed into useful diagnostic and prognostic biomarkers. Notably, circRNAs were reported to be stably detected in blood cells of whole blood or circulating cell-free components such as exosomes in plasma, serum, and saliva, making it ideal for liquid biopsy that is easily accessible yet non-invasive in clinical setting [239]. Many circRNAs have been identified as biomarkers for human diseases especially cancers, cardiovascular diseases, neurodegenerative diseases, and diabetes [20, 240].
In addition, effort to understand the mechanism of actions of disease-associated circRNAs will provide new treatment avenues such as circRNA-based therapy. Possible concepts for therapeutic modulation of disease-associated circRNAs include overexpressing native protective circRNAs or depleting endogenous disease-promoting circRNAs. The overview of pro-atherogenic or atheroprotective circRNAs in EC, VSMC, and macrophages which was extensively discussed in the current review highlights the potential of circRNAs as therapeutic target in inhibiting the initiation or delaying the progression of atherogenesis. Furthermore, the use of evolutionarily optimized artificial circRNAs as inhibitor of miRNA activity could be made and introduced into the patients to absorb one or multiple specific over-expressed disease-linked miRNAs, respectively, making them as disease suppressors. The engineered circRNAs were reported to possess higher stability and efficiency in action compared to their typical linear miRNA sponges as showed in human diseases other than CAD [241, 242]. Such concept can be extended to miRNA and RNA-binding protein sponges necessary for the development and progression of CAD. However, effective targeting of circRNAs and reduction of off-target effects and activation of innate immune system [243, 244] warrant the development of effective and safe tools for delivering and silencing circRNAs in vivo. Generation of a large amount and delivery of synthetic circRNAs is challenging. Nanoparticles and exosome-based delivery methods seem feasible but the safety aspect must be addressed. Similarly, the safety aspect of the use of CRISPR and siRNA technology for silencing circRNA must be evaluated. Lastly, questions remain on whether targeting circRNAs could produce the expected and long-lasting clinical outcome in human and this awaits further studies.
Concluding remarks
The pathophysiological roles of miRNAs of EC, VSMC, and macrophage in atherosclerosis have been well established in vitro, in vivo, and in clinical studies (reviewed in [245,246,247]). Notably are also groups of miRNAs identified as potential diagnostic markers for CAD and acute myocardial infarction based on their detections in PBMCs or plasma or serum samples in patients (reviewed by [248]). Some of the well-established anti-atherosclerotic miRNAs include miR-126, miR-24, and miR146a, whereas miR-92a, miR-30, miR-33, and miR-342-5p are pro-atherosclerotic. We noted that miR-370 (regulated by circ_USP36 in THP cells; regulated by circ_0010283 and circ_CHFR in VSMC), miR-20a-5p (regulated by circ_USP36 and circ-ADAM9) and miR-197 (regulated by circ_0068087) have been reported as miRs involved in CAD [247]. However, many other well-defined miRNAs involved in atherosclerosis are not among those regulated by the circRNAs identified from our literature review.
The vascular EC are enriched with miR-126, which is responsive to oxidative and hyperlipidemic stress and maintains the proliferative capacity, survival of EC, reduces lesions formation and hence an overall protective effect on atherosclerosis [249]. Yet, circRNA(s) that may regulate miR-126 has yet to be identified in HUVECs or other common EC subjected to oxidative and hyperlipidemic stress. Similarly, circRNA that may regulate miR-92a whose expression is increased in EC exposed to ox-LDL to reduce NF-κB activity and promote the expression of eNOS has yet to be identified by studies listed in Table 1. One possible reason is that miR-92a is a mechanosensitive atheromiR whose expression is dependent on shear stress or flow but was not recapitulated in in vitro EC injury models used in many circRNA studies. In VSMCs, circRNAs that modulate the actions of miR-26a, miR-490-3p, and miR-143/145 which promote VSMC proliferation, migration, and acquisition of contractile phenotype also remain to be identified [249,250,251]. It can be argued that the EC injury models used are dissimilar but it is also observed that clinical samples used for the detection of circRNAs expression were also less well-defined than those used in studies involving miRNAs. In vitro models may not accurately reflect in vivo angiogenesis as there is lack of cell–cell contact/signaling, shear stress, or even clearance of apoptotic cells seen in vivo.
Notably, the circRNA studies reporting a miRNA sponging model during atherogenesis only have a single to, at most, a few miRNA binding sites. This implies that the endogenous circRNA must be expressed at a high level and/or localized to the same location as its targeted miRNA to sufficiently exert a significant sponging effect to perturb the expression of the miRNA targets [252]. This is due to the fact that most circRNAs are present in relatively low abundance compared to their cognate linear counterpart and miRNA target derepression is only detected at a high threshold of alternative miRNA binding sites available [253, 254]. However, most atherogenesis circRNA studies to date, did not address the stoichiometric quantifications, e.g., copy number measurement, between the endogenous circRNA, and its targeted miRNA. Caution needs to be applied on interpreting results of forced overexpression of circRNAs, a method that is commonly used for functional validation of the particular circRNA as the increased fold change in multitudes of hundreds or thousands folds may not be physiological relevant. In order to prove that the circRNA act as a miRNA sponge, it is important to demonstrate the over-expression of circRNA with mutated miRNA binding site could abolish the sponging effect, whereas the effect of circRNA knockdown can be rescued with the overexpression of the respective circRNA [252].
Lastly, many of the studies have thus far focused on a simplified model, which delineate the effects of individual circRNAs, their downstream targets and outcomes on EC health and atherogenesis. Undeniably, these studies have advanced our understanding on the circRNA–miRNA–mRNA axis as drivers of atherosclerosis. Nevertheless, the fact that a single circRNA can regulate several miRNAs and similarly, a single mRNA can be regulated by the simultaneous coordinated actions of a number of different miRNAs cannot be overlooked. There is a need for future studies to establish a complete network of circRNAs and miRNAs to gain a deeper understanding on the concerted simultaneous actions of these micromanagers in the development and progression of atherosclerosis to enable the design of diagnostic and preventive strategies for early intervention. It is evident now that the role of circRNAs as regulators of atherogenesis and cardiovascular diseases are gaining importance but there remains the need to identify true “atherocircs” that are clinically relevant as diagnostics, prognostics, or therapeutic targets.
Data availability
Data sharing is not applicable—the article is a review of published research findings.
References
Ambrose JA, Singh M (2015) Pathophysiology of coronary artery disease leading to acute coronary syndromes. F1000Prime Rep 7:8
Kumar A, Cannon CP (2009) Acute coronary syndromes: diagnosis and management, part I. Mayo Clin Proc 84:917–938. https://doi.org/10.1016/S0025-6196(11)60674-5
Raines EW, Ross R (1995) Biology of atherosclerotic plaque formation: possible role of growth factors in lesion development and the potential impact of soy. J Nutr 125:624S-630S
Tedgui A, Mallat Z (2006) Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev 86:515–581. https://doi.org/10.1152/physrev.00024.2005
Tousoulis D, Oikonomou E, Economou EK, Crea F, Kaski JC (2016) Inflammatory cytokines in atherosclerosis: current therapeutic approaches. Eur Heart J 37:1723–1732. https://doi.org/10.1093/eurheartj/ehv759
Tabas I, Williams KJ, Borén J (2007) Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation 116:1832–1844. https://doi.org/10.1161/circulationaha.106.676890
Weber C, Noels H (2011) Atherosclerosis: current pathogenesis and therapeutic options. Nat Med 17:1410–1422. https://doi.org/10.1038/nm.2538
Gianazza E, Brioschi M, Martinez Fernandez A, Casalnuovo F, Altomare A, Aldini G, Banfi C (2021) Lipid peroxidation in atherosclerotic cardiovascular diseases. Antioxid Redox Signal 34:49–98. https://doi.org/10.1089/ars.2019.7955
Lusis AJ (2000) Atherosclerosis Nature 407:233–241. https://doi.org/10.1038/35025203
Weissberg PL (2000) Atherogenesis: current understanding of the causes of atheroma. Heart 83:247–252. https://doi.org/10.1136/heart.83.2.247
Sanger HL, Klotz G, Riesner D, Gross HJ, Kleinschmidt AK (1976) Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci USA 73:3852–3856. https://doi.org/10.1073/pnas.73.11.3852
Jeck WR, Sharpless NE (2014) Detecting and characterizing circular RNAs. Nat Biotechnol 32:453–461. https://doi.org/10.1038/nbt.2890
Conn SJ, Pillman KA, Toubia J, Conn VM, Salmanidis M, Phillips CA, Roslan S, Schreiber AW, Gregory PA, Goodall GJ (2015) The RNA binding protein quaking regulates formation of circRNAs. Cell 160:1125–1134. https://doi.org/10.1016/j.cell.2015.02.014
Mahmoudi E, Cairns MJ (2019) Circular RNAs are temporospatially regulated throughout development and ageing in the rat. Sci Rep 9:2564. https://doi.org/10.1038/s41598-019-38860-9
Mayer A, Mosler G, Just W, Pilgrim C, Reisert I (2000) Developmental profile of Sry transcripts in mouse brain. Neurogenetics 3:25–30
Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO (2012) Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS ONE 7:e30733. https://doi.org/10.1371/journal.pone.0030733
Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE (2013) Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 19:141–157. https://doi.org/10.1261/rna.035667.112
Suenkel C, Cavalli D, Massalini S, Calegari F, Rajewsky N (2020) A highly conserved circular RNA is required to keep neural cells in a progenitor state in the mammalian brain. Cell Rep 30:2170-2179.e5. https://doi.org/10.1016/j.celrep.2020.01.083
Wu W, Ji P, Zhao F (2020) CircAtlas: an integrated resource of one million highly accurate circular RNAs from 1070 vertebrate transcriptomes. Genome Biol 21:101. https://doi.org/10.1186/s13059-020-02018-y
Verduci L, Tarcitano E, Strano S, Yarden Y, Blandino G (2021) CircRNAs: role in human diseases and potential use as biomarkers. Cell Death Dis 12:468. https://doi.org/10.1038/s41419-021-03743-3
Mitchell JA, Ali F, Bailey L, Moreno L, Harrington LS (2008) Role of nitric oxide and prostacyclin as vasoactive hormones released by the endothelium. Exp Physiol 93:141–147. https://doi.org/10.1113/expphysiol.2007.038588
Pittner J, Wolgast M, Casellas D, Persson AE (2005) Increased shear stress-released NO and decreased endothelial calcium in rat isolated perfused juxtamedullary nephrons. Kidney Int 67:227–236. https://doi.org/10.1111/j.1523-1755.2005.00073.x
Rajendran P, Rengarajan T, Thangavel J, Nishigaki Y, Sakthisekaran D, Sethi G, Nishigaki I (2013) The vascular endothelium and human diseases. Int J Biol Sci 9:1057–1069. https://doi.org/10.7150/ijbs.7502
Bouwens EA, Stavenuiter F, Mosnier LO (2013) Mechanisms of anticoagulant and cytoprotective actions of the protein C pathway. J Thromb Haemost 11(Suppl 1):242–253. https://doi.org/10.1111/jth.12247
Sillen M, Declerck PJ (2020) Targeting PAI-1 in cardiovascular disease: structural insights into PAI-1 functionality and inhibition. Front Cardiovasc Med 7:622473. https://doi.org/10.3389/fcvm.2020.622473
Marcondes S, Cardoso MH, Morganti RP, Thomazzi SM, Lilla S, Murad F, De Nucci G, Antunes E (2006) Cyclic GMP-independent mechanisms contribute to the inhibition of platelet adhesion by nitric oxide donor: a role for alpha-actinin nitration. Proc Natl Acad Sci USA 103:3434–3439. https://doi.org/10.1073/pnas.0509397103
Morrell CN, Matsushita K, Chiles K, Scharpf RB, Yamakuchi M, Mason RJ, Bergmeier W, Mankowski JL, Baldwin WM 3rd, Faraday N, Lowenstein CJ (2005) Regulation of platelet granule exocytosis by S-nitrosylation. Proc Natl Acad Sci USA 102:3782–3787. https://doi.org/10.1073/pnas.0408310102
Gao F, Lucke-Wold BP, Li X, Logsdon AF, Xu LC, Xu S, LaPenna KB, Wang H, Talukder MAH, Siedlecki CA, Huber JD, Rosen CL, He P (2017) Reduction of endothelial nitric oxide increases the adhesiveness of constitutive endothelial membrane ICAM-1 through Src-mediated phosphorylation. Front Physiol 8:1124. https://doi.org/10.3389/fphys.2017.01124
Chiu JJ, Chien S (2011) Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev 91:327–387. https://doi.org/10.1152/physrev.00047.2009
Cai H, Harrison DG (2000) Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87:840–844. https://doi.org/10.1161/01.res.87.10.840
Gimbrone MA Jr, García-Cardeña G (2013) Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. Cardiovasc Pathol 22:9–15. https://doi.org/10.1016/j.carpath.2012.06.006
Corson MA, James NL, Latta SE, Nerem RM, Berk BC, Harrison DG (1996) Phosphorylation of endothelial nitric oxide synthase in response to fluid shear stress. Circ Res 79:984–991. https://doi.org/10.1161/01.res.79.5.984
Förstermann U, Münzel T (2006) Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113:1708–1714. https://doi.org/10.1161/circulationaha.105.602532
Hofmann F, Feil R, Kleppisch T, Schlossmann J (2006) Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol Rev 86:1–23. https://doi.org/10.1152/physrev.00015.2005
Bonetti PO, Lerman LO, Lerman A (2003) Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol 23:168–175. https://doi.org/10.1161/01.atv.0000051384.43104.fc
Masha A, Dinatale S, Allasia S, Martina V (2011) Role of the decreased nitric oxide bioavailability in the vascular complications of diabetes mellitus. Curr Pharm Biotechnol 12:1354–1363. https://doi.org/10.2174/138920111798281054
Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R (1994) ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb 14:133–140. https://doi.org/10.1161/01.atv.14.1.133
Chen JY, Ye ZX, Wang XF, Chang J, Yang MW, Zhong HH, Hong FF, Yang SL (2018) Nitric oxide bioavailability dysfunction involves in atherosclerosis. Biomed Pharmacother 97:423–428. https://doi.org/10.1016/j.biopha.2017.10.122
Hossain M, Qadri SM, Liu L (2012) Inhibition of nitric oxide synthesis enhances leukocyte rolling and adhesion in human microvasculature. J Inflamm (Lond) 9:28. https://doi.org/10.1186/1476-9255-9-28
Anderson TJ (1999) Assessment and treatment of endothelial dysfunction in humans. J Am Coll Cardiol 34:631–638. https://doi.org/10.1016/s0735-1097(99)00259-4
Chhabra N (2009) Endothelial dysfunction—a predictor of atherosclerosis. Internet J Med 4:33–41
Čejková S, Králová-Lesná I, Poledne R (2016) Monocyte adhesion to the endothelium is an initial stage of atherosclerosis development. Cor Vasa 58:e419–e425. https://doi.org/10.1016/j.crvasa.2015.08.002
Davalli P, Mitic T, Caporali A, Lauriola A, D’Arca D (2016) ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxid Med Cell Longev 2016:3565127. https://doi.org/10.1155/2016/3565127
Niemann B, Rohrbach S, Miller MR, Newby DE, Fuster V, Kovacic JC (2017) Oxidative stress and cardiovascular risk: obesity, diabetes, smoking, and pollution: part 3 of a 3-part series. J Am Coll Cardiol 70:230–251. https://doi.org/10.1016/j.jacc.2017.05.043
Moldogazieva NT, Mokhosoev IM, Mel’nikova TI, Porozov YB, Terentiev AA (2019) Oxidative stress and advanced lipoxidation and glycation end products (ALEs and AGEs) in aging and age-related diseases. Oxid Med Cell Longev 2019:3085756. https://doi.org/10.1155/2019/3085756
Luchetti F, Crinelli R, Nasoni MG, Benedetti S, Palma F, Fraternale A, Iuliano L (2020) LDL receptors, caveolae and cholesterol in endothelial dysfunction: oxLDLs accomplices or victims? Br J Pharmacol. https://doi.org/10.1111/bph.15272
Chen B, Lu Y, Chen Y, Cheng J (2015) The role of Nrf2 in oxidative stress-induced endothelial injuries. J Endocrinol 225:R83-99. https://doi.org/10.1530/joe-14-0662
Cominacini L, Pasini AF, Garbin U, Davoli A, Tosetti ML, Campagnola M, Rigoni A, Pastorino AM, Lo Cascio V, Sawamura T (2000) Oxidized low density lipoprotein (ox-LDL) binding to ox-LDL receptor-1 in endothelial cells induces the activation of NF-kappaB through an increased production of intracellular reactive oxygen species. J Biol Chem 275:12633–12638. https://doi.org/10.1074/jbc.275.17.12633
Yu XH, Zheng XL, Tang CK (2015) Nuclear factor-κB activation as a pathological mechanism of lipid metabolism and atherosclerosis. Adv Clin Chem 70:1–30. https://doi.org/10.1016/bs.acc.2015.03.004
Alfarisi HAH, Mohamed ZBH, Ibrahim MB (2020) Basic pathogenic mechanisms of atherosclerosis. Egypt J Basic Appl Sci 7:116–125. https://doi.org/10.1080/2314808X.2020.1769913
Viedt C, Vogel J, Athanasiou T, Shen W, Orth SR, Kübler W, Kreuzer J (2002) Monocyte chemoattractant protein-1 induces proliferation and interleukin-6 production in human smooth muscle cells by differential activation of nuclear factor-kappaB and activator protein-1. Arterioscler Thromb Vasc Biol 22:914–920. https://doi.org/10.1161/01.atv.0000019009.73586.7f
Voloshyna I, Littlefield MJ, Reiss AB (2014) Atherosclerosis and interferon-γ: new insights and therapeutic targets. Trends Cardiovasc Med 24:45–51. https://doi.org/10.1016/j.tcm.2013.06.003
Gao P, Wu W, Ye J, Lu YW, Adam AP, Singer HA, Long X (2018) Transforming growth factor β1 suppresses proinflammatory gene program independent of its regulation on vascular smooth muscle differentiation and autophagy. Cell Signal 50:160–170. https://doi.org/10.1016/j.cellsig.2018.07.002
Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, Tokgözoğlu L, Lewis EF (2019) Atherosclerosis. Nat Rev Dis Primers 5:56. https://doi.org/10.1038/s41572-019-0106-z
Carpenter KL, Dennis IF, Challis IR, Osborn DP, Macphee CH, Leake DS, Arends MJ, Mitchinson MJ (2001) Inhibition of lipoprotein-associated phospholipase A2 diminishes the death-inducing effects of oxidised LDL on human monocyte-macrophages. FEBS Lett 505:357–363. https://doi.org/10.1016/s0014-5793(01)02840-x
Wang WY, Zhang J, Wu WY, Li J, Ma YL, Chen WH, Yan H, Wang K, Xu WW, Shen JH, Wang YP (2011) Inhibition of lipoprotein-associated phospholipase A2 ameliorates inflammation and decreases atherosclerotic plaque formation in ApoE-deficient mice. PLoS ONE 6:e23425. https://doi.org/10.1371/journal.pone.0023425
Tabas I, Bornfeldt KE (2016) Macrophage phenotype and function in different stages of atherosclerosis. Circ Res 118:653–667. https://doi.org/10.1161/circresaha.115.306256
Bennett MR, Sinha S, Owens GK (2016) Vascular smooth muscle cells in atherosclerosis. Circ Res 118:692–702. https://doi.org/10.1161/CIRCRESAHA.115.306361
Ruddy JM, Ikonomidis JS, Jones JA (2016) Multidimensional contribution of matrix metalloproteinases to atherosclerotic plaque vulnerability: multiple mechanisms of inhibition to promote stability. J Vasc Res 53:1–16. https://doi.org/10.1159/000446703
Zhang Y, Zhao Y, Liu Y, Wang M, Yu W, Zhang L (2020) Exploring the regulatory roles of circular RNAs in Alzheimer’s disease. Transl Neurodegeneration 9:35–35. https://doi.org/10.1186/s40035-020-00216-z
Li X, Liu CX, Xue W, Zhang Y, Jiang S, Yin QF, Wei J, Yao RW, Yang L, Chen LL (2017) Coordinated circRNA biogenesis and function with NF90/NF110 in viral infection. Mol Cell 67:214-227.e7. https://doi.org/10.1016/j.molcel.2017.05.023
Rybak-Wolf A, Stottmeister C, Glažar P, Jens M, Pino N, Giusti S, Hanan M, Behm M, Bartok O, Ashwal-Fluss R, Herzog M, Schreyer L, Papavasileiou P, Ivanov A, Öhman M, Refojo D, Kadener S, Rajewsky N (2015) Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol Cell 58:870–885. https://doi.org/10.1016/j.molcel.2015.03.027
Dubin RA, Kazmi MA, Ostrer H (1995) Inverted repeats are necessary for circularization of the mouse testis Sry transcript. Gene 167:245–248. https://doi.org/10.1016/0378-1119(95)00639-7
Ivanov A, Memczak S, Wyler E, Torti F, Porath HT, Orejuela MR, Piechotta M, Levanon EY, Landthaler M, Dieterich C, Rajewsky N (2015) Analysis of intron sequences reveals hallmarks of circular RNA biogenesis in animals. Cell Rep 10:170–177. https://doi.org/10.1016/j.celrep.2014.12.019
Liang D, Wilusz JE (2014) Short intronic repeat sequences facilitate circular RNA production. Genes Dev 28:2233–2247. https://doi.org/10.1101/gad.251926.114
Barrett SP, Wang PL, Salzman J (2015) Circular RNA biogenesis can proceed through an exon-containing lariat precursor. Elife 4:e07540. https://doi.org/10.7554/eLife.07540
Zhang Y, Chen T, Xiang JF, Yin QF, Xing YH, Zhu S, Yang L, Chen LL (2013) Circular intronic long noncoding RNAs. Mol Cell 51:792–806. https://doi.org/10.1016/j.molcel.2013.08.017
Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP (2011) A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell 146:353–358. https://doi.org/10.1016/j.cell.2011.07.014
Hansen TB, Kjems J, Damgaard CK (2013) Circular RNA and miR-7 in cancer. Cancer Res 73:5609–5612. https://doi.org/10.1158/0008-5472.can-13-1568
Huang H, Wei L, Qin T, Yang N, Li Z, Xu Z (2019) Circular RNA ciRS-7 triggers the migration and invasion of esophageal squamous cell carcinoma via miR-7/KLF4 and NF-κB signals. Cancer Biol Ther 20:73–80. https://doi.org/10.1080/15384047.2018.1507254
Zhao J, Tao Y, Zhou Y, Qin N, Chen C, Tian D, Xu L (2015) MicroRNA-7: a promising new target in cancer therapy. Cancer Cell Int 15:103. https://doi.org/10.1186/s12935-015-0259-0
Zhang T, Jing B, Bai Y, Zhang Y, Yu H (2020) Circular RNA circTMEM45A acts as the sponge of MicroRNA-665 to promote hepatocellular carcinoma progression. Mol Ther Nucleic Acids 22:285–297. https://doi.org/10.1016/j.omtn.2020.08.011
Hentze MW, Preiss T (2013) Circular RNAs: splicing’s enigma variations. EMBO J 32:923–925. https://doi.org/10.1038/emboj.2013.53
Dudekula DB, Panda AC, Grammatikakis I, De S, Abdelmohsen K, Gorospe M (2016) CircInteractome: a web tool for exploring circular RNAs and their interacting proteins and microRNAs. RNA Biol 13:34–42. https://doi.org/10.1080/15476286.2015.1128065
Abdelmohsen K, Panda AC, Munk R, Grammatikakis I, Dudekula DB, De S, Kim J, Noh JH, Kim KM, Martindale JL, Gorospe M (2017) Identification of HuR target circular RNAs uncovers suppression of PABPN1 translation by CircPABPN1. RNA Biol 14:361–369. https://doi.org/10.1080/15476286.2017.1279788
Fang J, Hong H, Xue X, Zhu X, Jiang L, Qin M, Liang H, Gao L (2019) A novel circular RNA, circFAT1(e2), inhibits gastric cancer progression by targeting miR-548g in the cytoplasm and interacting with YBX1 in the nucleus. Cancer Lett 442:222–232. https://doi.org/10.1016/j.canlet.2018.10.040
Li Z, Huang C, Bao C, Chen L, Lin M, Wang X, Zhong G, Yu B, Hu W, Dai L, Zhu P, Chang Z, Wu Q, Zhao Y, Jia Y, Xu P, Liu H, Shan G (2015) Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol 22:256. https://doi.org/10.1038/nsmb.2959
Chen N, Zhao G, Yan X, Lv Z, Yin H, Zhang S, Song W, Li X, Li L, Du Z, Jia L, Zhou L, Li W, Hoffman AR, Hu JF, Cui J (2018) A novel FLI1 exonic circular RNA promotes metastasis in breast cancer by coordinately regulating TET1 and DNMT1. Genome Biol 19:218. https://doi.org/10.1186/s13059-018-1594-y
Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N, Kadener S (2014) circRNA biogenesis competes with pre-mRNA splicing. Mol Cell 56:55–66. https://doi.org/10.1016/j.molcel.2014.08.019
Du WW, Yang W, Liu E, Yang Z, Dhaliwal P, Yang BB (2016) Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res 44:2846–2858. https://doi.org/10.1093/nar/gkw027
Du WW, Yang W, Chen Y, Wu ZK, Foster FS, Yang Z, Li X, Yang BB (2016) Foxo3 circular RNA promotes cardiac senescence by modulating multiple factors associated with stress and senescence responses. Eur Heart J 38:1402–1412. https://doi.org/10.1093/eurheartj/ehw001
Pamudurti NR, Bartok O, Jens M, Ashwal-Fluss R, Stottmeister C, Ruhe L, Hanan M, Wyler E, Perez-Hernandez D, Ramberger E, Shenzis S, Samson M, Dittmar G, Landthaler M, Chekulaeva M, Rajewsky N, Kadener S (2017) Translation of CircRNAs. Mol Cell 66:9-21.e7. https://doi.org/10.1016/j.molcel.2017.02.021
Yang Y, Fan X, Mao M, Song X, Wu P, Zhang Y, Jin Y, Yang Y, Chen LL, Wang Y, Wong CCL, Xiao X, Wang Z (2017) Extensive translation of circular RNAs driven by N6-methyladenosine. Cell Res 27:626–641. https://doi.org/10.1038/cr.2017.31
Legnini I, Di Timoteo G, Rossi F, Morlando M, Briganti F, Sthandier O, Fatica A, Santini T, Andronache A, Wade M, Laneve P, Rajewsky N, Bozzoni I (2017) Circ-ZNF609 Is a circular RNA that can be translated and functions in myogenesis. Mol Cell 66:22-37.e9. https://doi.org/10.1016/j.molcel.2017.02.017
Liang WC, Wong CW, Liang PP, Shi M, Cao Y, Rao ST, Tsui SKW, Waye MMY, Zhang Q, Fu WM (2019) Translation of the circular RNA circβ-catenin promotes liver cancer cell growth through activation of the Wnt pathway. Genome Biol 20:84. https://doi.org/10.1186/s13059-019-1685-4
Rossi F, Legnini I, Megiorni F, Colantoni A, Santini T, Morlando M, Di Timoteo G, Dattilo D, Dominici C, Bozzoni I (2019) Circ-ZNF609 regulates G1-S progression in rhabdomyosarcoma. Oncogene 38:3843–3854. https://doi.org/10.1038/s41388-019-0699-4
Norata GD, Tonti L, Roma P, Catapano AL (2002) Apoptosis and proliferation of endothelial cells in early atherosclerotic lesions: possible role of oxidised LDL. Nutr Metab Cardiovasc Dis 12:297–305
Galle J, Heinloth A, Wanner C, Heermeier K (2001) Dual effect of oxidized LDL on cell cycle in human endothelial cells through oxidative stress. Kidney Int Suppl 78:S120–S123. https://doi.org/10.1046/j.1523-1755.2001.59780120.x
Liu H, Ma X, Mao Z, Shen M, Zhu J, Chen F (2020) Circular RNA has_circ_0003204 inhibits oxLDL-induced vascular endothelial cell proliferation and angiogenesis. Cell Signal 70:109595. https://doi.org/10.1016/j.cellsig.2020.109595
Wan H, You T, Luo W (2021) circ_0003204 regulates cell growth, oxidative stress, and inflammation in ox-LDL-induced vascular endothelial cells via regulating miR-942-5p/HDAC9 Axis. Front Cardiovasc Med 8:646832. https://doi.org/10.3389/fcvm.2021.646832
Malhotra R, Mauer AC, Lino Cardenas CL, Guo X, Yao J, Zhang X, Wunderer F, Smith AV, Wong Q, Pechlivanis S, Hwang SJ, Wang J, Lu L, Nicholson CJ, Shelton G, Buswell MD, Barnes HJ, Sigurslid HH, Slocum C, Rourke CO, Rhee DK, Bagchi A, Nigwekar SU, Buys ES, Campbell CY, Harris T, Budoff M, Criqui MH, Rotter JI, Johnson AD, Song C, Franceschini N, Debette S, Hoffmann U, Kälsch H, Nöthen MM, Sigurdsson S, Freedman BI, Bowden DW, Jöckel KH, Moebus S, Erbel R, Feitosa MF, Gudnason V, Thanassoulis G, Zapol WM, Lindsay ME, Bloch DB, Post WS, O’Donnell CJ (2019) HDAC9 is implicated in atherosclerotic aortic calcification and affects vascular smooth muscle cell phenotype. Nat Genet 51:1580–1587. https://doi.org/10.1038/s41588-019-0514-8
Schiano C, Benincasa G, Franzese M, Della Mura N, Pane K, Salvatore M, Napoli C (2020) Epigenetic-sensitive pathways in personalized therapy of major cardiovascular diseases. Pharmacol Ther 210:107514. https://doi.org/10.1016/j.pharmthera.2020.107514
Han X, Han X, Wang Z, Shen J, Dong Q (2016) HDAC9 regulates ox-LDL-induced endothelial cell apoptosis by participating in inflammatory reactions. Front Biosci (Landmark Ed) 21:907–917. https://doi.org/10.2741/4428
Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, Tracey KJ (2000) High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med 192:565–570. https://doi.org/10.1084/jem.192.4.565
Su Q, Dong X, Tang C, Wei X, Hao Y, Wu J (2021) Knockdown of circ_0003204 alleviates oxidative low-density lipoprotein-induced human umbilical vein endothelial cells injury: circulating RNAs could explain atherosclerosis disease progression. Open Med (Wars) 16:558–569. https://doi.org/10.1515/med-2021-0209
Roy A, Srivastava M, Saqib U, Liu D, Faisal SM, Sugathan S, Bishnoi S, Baig MS (2016) Potential therapeutic targets for inflammation in toll-like receptor 4 (TLR4)-mediated signaling pathways. Int Immunopharmacol 40:79–89. https://doi.org/10.1016/j.intimp.2016.08.026
Pasterkamp G, Van Keulen JK, De Kleijn DP (2004) Role of Toll-like receptor 4 in the initiation and progression of atherosclerotic disease. Eur J Clin Invest 34:328–334. https://doi.org/10.1111/j.1365-2362.2004.01338.x
Peng K, Jiang P, Du Y, Zeng D, Zhao J, Li M, Xia C, Xie Z, Wu J (2021) Oxidized low-density lipoprotein accelerates the injury of endothelial cells via circ-USP36/miR-98-5p/VCAM1 axis. IUBMB Life 73:177–187. https://doi.org/10.1002/iub.2419
Bernstein DL, Zuluaga-Ramirez V, Gajghate S, Reichenbach NL, Polyak B, Persidsky Y, Rom S (2020) miR-98 reduces endothelial dysfunction by protecting blood-brain barrier (BBB) and improves neurological outcomes in mouse ischemia/reperfusion stroke model. J Cereb Blood Flow Metab 40:1953–1965. https://doi.org/10.1177/0271678x19882264
Chen Z, Wang M, He Q, Li Z, Zhao Y, Wang W, Ma J, Li Y, Chang G (2017) MicroRNA-98 rescues proliferation and alleviates ox-LDL-induced apoptosis in HUVECs by targeting LOX-1. Exp Ther Med 13:1702–1710. https://doi.org/10.3892/etm.2017.4171
Hu C, Huang S, Wu F, Ding H (2018) miR-98 inhibits cell proliferation and induces cell apoptosis by targeting MAPK6 in HUVECs. Exp Ther Med 15:2755–2760. https://doi.org/10.3892/etm.2018.5735
Li HW, Meng Y, Xie Q, Yi WJ, Lai XL, Bian Q, Wang J, Wang JF, Yu G (2015) miR-98 protects endothelial cells against hypoxia/reoxygenation induced-apoptosis by targeting caspase-3. Biochem Biophys Res Commun 467:595–601. https://doi.org/10.1016/j.bbrc.2015.09.058
Zheng Z, Zhang G, Liang X, Li T (2021) LncRNA OIP5-AS1 facilitates ox-LDL-induced endothelial cell injury through the miR-98-5p/HMGB1 axis. Mol Cell Biochem 476:443–455. https://doi.org/10.1007/s11010-020-03921-5
Miao J, Wang B, Shao R, Wang Y (2021) CircUSP36 knockdown alleviates oxidized lowdensity lipoproteininduced cell injury and inflammatory responses in human umbilical vein endothelial cells via the miR20a5p/ROCK2 axis. Int J Mol Med. https://doi.org/10.3892/ijmm.2021.4873
Goumans MJ, Liu Z, ten Dijke P (2009) TGF-beta signaling in vascular biology and dysfunction. Cell Res 19:116–127. https://doi.org/10.1038/cr.2008.326
Grainger DJ (2004) Transforming growth factor beta and atherosclerosis: so far, so good for the protective cytokine hypothesis. Arterioscler Thromb Vasc Biol 24:399–404. https://doi.org/10.1161/01.Atv.0000114567.76772.33
Khan R, Agrotis A, Bobik A (2007) Understanding the role of transforming growth factor-beta1 in intimal thickening after vascular injury. Cardiovasc Res 74:223–234. https://doi.org/10.1016/j.cardiores.2007.02.012
Wang L, Shen C, Wang Y, Zou T, Zhu H, Lu X, Li L, Yang B, Chen J, Chen S, Lu X, Gu D (2019) Identification of circular RNA Hsa_circ_0001879 and Hsa_circ_0004104 as novel biomarkers for coronary artery disease. Atherosclerosis 286:88–96. https://doi.org/10.1016/j.atherosclerosis.2019.05.006
Zhang C, Wang L, Shen Y (2021) Circ_0004104 knockdown alleviates oxidized low-density lipoprotein-induced dysfunction in vascular endothelial cells through targeting miR-328-3p/TRIM14 axis in atherosclerosis. BMC Cardiovasc Disord 21:207. https://doi.org/10.1186/s12872-021-02012-7
Huang X, Li Y, Li X, Fan D, Xin HB, Fu M (2020) TRIM14 promotes endothelial activation via activating NF-κB signaling pathway. J Mol Cell Biol 12:176–189. https://doi.org/10.1093/jmcb/mjz040
Qin X, Guo J (2020) MicroRNA-328-3p protects vascular endothelial cells against oxidized low-density lipoprotein induced injury via targeting forkhead box protein O4 (FOXO4) in atherosclerosis. Med Sci Monit 26:e921877. https://doi.org/10.12659/msm.921877
Hong L, Ma X, Liu J, Luo Y, Lin J, Shen Y, Zhang L (2021) Circular RNA-HIPK3 regulates human pulmonary artery endothelial cells function and vessel growth by regulating microRNA-328-3p/STAT3 axis. Pulm Circ 11:20458940211000230. https://doi.org/10.1177/20458940211000234
Zhao Z, Li X, Gao C, Jian D, Hao P, Rao L, Li M (2017) Peripheral blood circular RNA hsa_circ_0124644 can be used as a diagnostic biomarker of coronary artery disease. Sci Rep 7:39918. https://doi.org/10.1038/srep39918
Wang G, Li Y, Liu Z, Ma X, Li M, Lu Q, Li Y, Lu Z, Niu L, Fan Z, Lei Z (2020) Circular RNA circ_0124644 exacerbates the ox-LDL-induced endothelial injury in human vascular endothelial cells through regulating PAPP-A by acting as a sponge of miR-149-5p. Mol Cell Biochem 471:51–61. https://doi.org/10.1007/s11010-020-03764-0
Ali Sheikh MS, Xia K, Li F, Deng X, Salma U, Deng H, Wei Wei L, Yang TL, Peng J (2015) Circulating miR-765 and miR-149: potential noninvasive diagnostic biomarkers for geriatric coronary artery disease patients. Biomed Res Int 2015:740301. https://doi.org/10.1155/2015/740301
Sayed AS, Xia K, Li F, Deng X, Salma U, Li T, Deng H, Yang D, Haoyang Z, Yang T, Peng J (2015) The diagnostic value of circulating microRNAs for middle-aged (40–60-year-old) coronary artery disease patients. Clinics (Sao Paulo) 70:257–263. https://doi.org/10.6061/clinics/2015(04)07
Xu G, Zhang Z, Xing Y, Wei J, Ge Z, Liu X, Zhang Y, Huang X (2014) MicroRNA-149 negatively regulates TLR-triggered inflammatory response in macrophages by targeting MyD88. J Cell Biochem 115:919–927. https://doi.org/10.1002/jcb.24734
Yuan J, Chen M, Xu Q, Liang J, Chen R, Xiao Y, Fang M, Chen L (2017) Effect of the diabetic environment on the expression of MiRNAs in endothelial cells: Mir-149-5p restoration ameliorates the high glucose-induced expression of TNF-α and ER stress markers. Cell Physiol Biochem 43:120–135. https://doi.org/10.1159/000480330
Huang HS, Huang XY, Yu HZ, Xue Y, Zhu PL (2020) Circular RNA circ-RELL1 regulates inflammatory response by miR-6873-3p/MyD88/NF-κB axis in endothelial cells. Biochem Biophys Res Commun 525:512–519. https://doi.org/10.1016/j.bbrc.2020.02.109
Li S, Hao M, Wu T, Wang Z, Wang X, Zhang J, Zhang L (2021) Kaempferol alleviates human endothelial cell injury through circNOL12/miR-6873-3p/FRS2 axis. Biomed Pharmacother 137:111419. https://doi.org/10.1016/j.biopha.2021.111419
Gotoh N (2008) Regulation of growth factor signaling by FRS2 family docking/scaffold adaptor proteins. Cancer Sci 99:1319–1325. https://doi.org/10.1111/j.1349-7006.2008.00840.x
Huang T, Zhao HY, Zhang XB, Gao XL, Peng WP, Zhou Y, Zhao WH, Yang HF (2020) LncRNA ANRIL regulates cell proliferation and migration via sponging miR-339-5p and regulating FRS2 expression in atherosclerosis. Eur Rev Med Pharmacol Sci 24:1956–1969. https://doi.org/10.26355/eurrev_202002_20373
Zhang W, Sui Y (2020) CircBPTF knockdown ameliorates high glucose-induced inflammatory injuries and oxidative stress by targeting the miR-384/LIN28B axis in human umbilical vein endothelial cells. Mol Cell Biochem 471:101–111. https://doi.org/10.1007/s11010-020-03770-2
Fan J, Xu W, Nan S, Chang M, Zhang Y (2020) MicroRNA-384-5p promotes endothelial progenitor cell proliferation and angiogenesis in cerebral ischemic stroke through the delta-likeligand 4-mediated notch signaling pathway. Cerebrovasc Dis 49:39–54. https://doi.org/10.1159/000503950
Xia F, Sun JJ, Jiang YQ, Li CF (2018) MicroRNA-384-3p inhibits retinal neovascularization through targeting hexokinase 2 in mice with diabetic retinopathy. J Cell Physiol 234:721–730. https://doi.org/10.1002/jcp.26871
Fu X, Ou B (2020) miR-152/LIN28B axis modulates high-glucose-induced angiogenesis in human retinal endothelial cells via VEGF signaling. J Cell Biochem 121:954–962. https://doi.org/10.1002/jcb.28978
Sharghi-Namini S, Tan E, Ong LL, Ge R, Asada HH (2014) Dll4-containing exosomes induce capillary sprout retraction in a 3D microenvironment. Sci Rep 4:4031. https://doi.org/10.1038/srep04031
Benedito R, Roca C, Sörensen I, Adams S, Gossler A, Fruttiger M, Adams RH (2009) The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell 137:1124–1135. https://doi.org/10.1016/j.cell.2009.03.025
Xia HG, Najafov A, Geng J, Galan-Acosta L, Han X, Guo Y, Shan B, Zhang Y, Norberg E, Zhang T, Pan L, Liu J, Coloff JL, Ofengeim D, Zhu H, Wu K, Cai Y, Yates JR, Zhu Z, Yuan J, Vakifahmetoglu-Norberg H (2015) Degradation of HK2 by chaperone-mediated autophagy promotes metabolic catastrophe and cell death. J Cell Biol 210:705–716. https://doi.org/10.1083/jcb.201503044
Pan M, Han Y, Basu A, Dai A, Si R, Willson C, Balistrieri A, Scott BT, Makino A (2018) Overexpression of hexokinase 2 reduces mitochondrial calcium overload in coronary endothelial cells of type 2 diabetic mice. Am J Physiol Cell Physiol 314:C732-c740. https://doi.org/10.1152/ajpcell.00350.2017
Cheng J, Liu Q, Hu N, Zheng F, Zhang X, Ni Y, Liu J (2019) Downregulation of hsa_circ_0068087 ameliorates TLR4/NF-κB/NLRP3 inflammasome-mediated inflammation and endothelial cell dysfunction in high glucose conditioned by sponging miR-197. Gene 709:1–7. https://doi.org/10.1016/j.gene.2019.05.012
Schulte C, Molz S, Appelbaum S, Karakas M, Ojeda F, Lau DM, Hartmann T, Lackner KJ, Westermann D, Schnabel RB, Blankenberg S, Zeller T (2015) miRNA-197 and miRNA-223 predict cardiovascular death in a cohort of patients with symptomatic coronary artery disease. PLoS ONE 10:e0145930. https://doi.org/10.1371/journal.pone.0145930
Li S, Huang T, Qin L, Yin L (2021) Circ_0068087 silencing ameliorates oxidized low-density lipoprotein-induced dysfunction in vascular endothelial cells depending on miR-186-5p-mediated regulation of roundabout guidance receptor 1. Front Cardiovasc Med 8:650374. https://doi.org/10.3389/fcvm.2021.650374
Yuen DA, Robinson LA (2013) Slit2-Robo signaling: a novel regulator of vascular injury. Curr Opin Nephrol Hypertens 22:445–451. https://doi.org/10.1097/MNH.0b013e32836235f4
Wei H, Cao C, Wei X, Meng M, Wu B, Meng L, Wei X, Gu S, Li H (2020) Circular RNA circVEGFC accelerates high glucose-induced vascular endothelial cells apoptosis through miR-338-3p/HIF-1α/VEGFA axis. Aging (Albany NY) 12:14365–14375. https://doi.org/10.18632/aging.103478
Gao J, Ailifeire M, Wang C, Luo L, Zhang J, Yuan L, Zhang L, Li X, Wang M (2020) miR-320/VEGFA axis affects high glucose-induced metabolic memory during human umbilical vein endothelial cell dysfunction in diabetes pathology. Microvasc Res 127:103913. https://doi.org/10.1016/j.mvr.2019.103913
Yin J, Hou X, Yang S (2019) microRNA-338-3p promotes ox-LDL-induced endothelial cell injury through targeting BAMBI and activating TGF-β/Smad pathway. J Cell Physiol 234:11577–11586. https://doi.org/10.1002/jcp.27814
Onichtchouk D, Chen YG, Dosch R, Gawantka V, Delius H, Massagué J, Niehrs C (1999) Silencing of TGF-beta signalling by the pseudoreceptor BAMBI. Nature 401:480–485. https://doi.org/10.1038/46794
Raykhel I, Moafi F, Myllymäki SM, Greciano PG, Matlin KS, Moyano JV, Manninen A, Myllyharju J (2018) BAMBI is a novel HIF1-dependent modulator of TGFβ-mediated disruption of cell polarity during hypoxia. J Cell Sci. https://doi.org/10.1242/jcs.210906
Liang B, Li M, Deng Q, Wang C, Rong J, He S, Xiang Y, Zheng F (2020) CircRNA ZNF609 in peripheral blood leukocytes acts as a protective factor and a potential biomarker for coronary artery disease. Ann Transl Med 8:741. https://doi.org/10.21037/atm-19-4728
Liu C, Yao MD, Li CP, Shan K, Yang H, Wang JJ, Liu B, Li XM, Yao J, Jiang Q, Yan B (2017) Silencing of circular RNA-ZNF609 ameliorates vascular endothelial dysfunction. Theranostics 7:2863–2877. https://doi.org/10.7150/thno.19353
Wang L, Fan C, Topol SE, Topol EJ, Wang Q (2003) Mutation of MEF2A in an inherited disorder with features of coronary artery disease. Science 302:1578–1581. https://doi.org/10.1126/science.1088477
Eltzschig HK, Bratton DL, Colgan SP (2014) Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat Rev Drug Discov 13:852–869. https://doi.org/10.1038/nrd4422
Wang HG, Yan H, Wang C, Li MM, Lv XZ, Wu HD, Fang ZH, Mo DL, Zhang ZY, Liang B, Lai KG, Bao JY, Yang XJ, Zhao HJ, Chen S, Fan YM, Tong XG (2020) circAFF1 aggravates vascular endothelial cell dysfunction mediated by miR-516b/SAV1/YAP1 Axis. Front Physiol 11:899. https://doi.org/10.3389/fphys.2020.00899
Plouffe SW, Hong AW, Guan KL (2015) Disease implications of the Hippo/YAP pathway. Trends Mol Med 21:212–222. https://doi.org/10.1016/j.molmed.2015.01.003
Rausch V, Hansen CG (2020) The Hippo pathway, YAP/TAZ, and the plasma membrane. Trends Cell Biol 30:32–48. https://doi.org/10.1016/j.tcb.2019.10.005
Lei D, Wang Y, Zhang L, Wang Z (2020) Circ_0010729 regulates hypoxia-induced cardiomyocyte injuries by activating TRAF5 via sponging miR-27a-3p. Life Sci 262:118511. https://doi.org/10.1016/j.lfs.2020.118511
Ma B, Zhao M, Guo Z (2021) Circular RNA circ_0010729 knockdown attenuates oxygen-glucose deprivation-induced human cardiac myocytes injury by miR-338-3p/CALM2 axis. J Cardiovasc Pharmacol 77:594–602. https://doi.org/10.1097/fjc.0000000000000988
Dang RY, Liu FL, Li Y (2017) Circular RNA hsa_circ_0010729 regulates vascular endothelial cell proliferation and apoptosis by targeting the miR-186/HIF-1α axis. Biochem Biophys Res Commun 490:104–110. https://doi.org/10.1016/j.bbrc.2017.05.164
Bartoszewski R, Moszyńska A, Serocki M, Cabaj A, Polten A, Ochocka R, Dell’Italia L, Bartoszewska S, Króliczewski J, Dąbrowski M, Collawn JF (2019) Primary endothelial cell-specific regulation of hypoxia-inducible factor (HIF)-1 and HIF-2 and their target gene expression profiles during hypoxia. FASEB J 33:7929–7941. https://doi.org/10.1096/fj.201802650RR
Kaushik K, Das A (2019) Endothelial progenitor cell therapy for chronic wound tissue regeneration. Cytotherapy 21:1137–1150. https://doi.org/10.1016/j.jcyt.2019.09.002
Muniyappa R, Sowers JR (2014) Glycogen synthase kinase-3β and cathepsin B in diabetic endothelial progenitor cell dysfunction: an old player finds a new partner. Diabetes 63:1194–1197. https://doi.org/10.2337/db14-0004
Tian D, Xiang Y, Tang Y, Ge Z, Li Q, Zhang Y (2020) Circ-ADAM9 targeting PTEN and ATG7 promotes autophagy and apoptosis of diabetic endothelial progenitor cells by sponging mir-20a-5p. Cell Death Dis 11:526. https://doi.org/10.1038/s41419-020-02745-x
Heras-Sandoval D, Perez-Rojas JM, Hernandez-Damian J, Pedraza-Chaverri J (2014) The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal 26:2694–2701. https://doi.org/10.1016/j.cellsig.2014.08.019
Zhou RM, Shi LJ, Shan K, Sun YN, Wang SS, Zhang SJ, Li XM, Jiang Q, Yan B, Zhao C (2020) Circular RNA-ZBTB44 regulates the development of choroidal neovascularization. Theranostics 10:3293–3307. https://doi.org/10.7150/thno.39488
Chen PY, Schwartz MA, Simons M (2020) Endothelial-to-mesenchymal transition, vascular inflammation, and atherosclerosis. Front Cardiovasc Med 7:53. https://doi.org/10.3389/fcvm.2020.00053
Souilhol C, Harmsen MC, Evans PC, Krenning G (2018) Endothelial–mesenchymal transition in atherosclerosis. Cardiovasc Res 114:565–577. https://doi.org/10.1093/cvr/cvx253
Yang L, Han B, Zhang Y, Bai Y, Chao J, Hu G, Yao H (2018) Engagement of circular RNA HECW2 in the nonautophagic role of ATG5 implicated in the endothelial-mesenchymal transition. Autophagy 14:404–418. https://doi.org/10.1080/15548627.2017.1414755
Zheng Q, Bao C, Guo W, Li S, Chen J, Chen B, Luo Y, Lyu D, Li Y, Shi G, Liang L, Gu J, He X, Huang S (2016) Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat Commun 7:11215. https://doi.org/10.1038/ncomms11215
Cao Y, Yuan G, Zhang Y, Lu R (2018) High glucose-induced circHIPK3 downregulation mediates endothelial cell injury. Biochem Biophys Res Commun 507:362–368. https://doi.org/10.1016/j.bbrc.2018.11.041
Wei MY, Lv RR, Teng Z (2020) Circular RNA circHIPK3 as a novel circRNA regulator of autophagy and endothelial cell dysfunction in atherosclerosis. Eur Rev Med Pharmacol Sci 24:12849–12858. https://doi.org/10.26355/eurrev_202012_24187
Si X, Zheng H, Wei G, Li M, Li W, Wang H, Guo H, Sun J, Li C, Zhong S, Liao W, Liao Y, Huang S, Bin J (2020) circRNA Hipk3 induces cardiac regeneration after myocardial infarction in mice by binding to Notch1 and miR-133a. Mol Ther Nucleic Acids 21:636–655. https://doi.org/10.1016/j.omtn.2020.06.024
Wang Y, Zhao R, Liu W, Wang Z, Rong J, Long X, Liu Z, Ge J, Shi B (2019) Exosomal circHIPK3 released from hypoxia-pretreated cardiomyocytes regulates oxidative damage in cardiac microvascular endothelial cells via the miR-29a/IGF-1 pathway. Oxid Med Cell Longev 2019:7954657. https://doi.org/10.1155/2019/7954657
Shan K, Liu C, Liu BH, Chen X, Dong R, Liu X, Zhang YY, Liu B, Zhang SJ, Wang JJ, Zhang SH, Wu JH, Zhao C, Yan B (2017) Circular noncoding RNA HIPK3 mediates retinal vascular dysfunction in diabetes mellitus. Circulation 136:1629–1642. https://doi.org/10.1161/circulationaha.117.029004
Kang L, Jia H, Huang B, Lu S, Chen Z, Shen J, Zou Y, Wang C, Sun Y (2021) Identification of differently expressed mRNAs in atherosclerosis reveals CDK6 is regulated by circHIPK3/miR-637 axis and promotes cell growth in human vascular smooth muscle cells. Front Genet 12:596169. https://doi.org/10.3389/fgene.2021.596169
Helgadottir A, Thorleifsson G, Manolescu A, Gretarsdottir S, Blondal T, Jonasdottir A, Jonasdottir A, Sigurdsson A, Baker A, Palsson A, Masson G, Gudbjartsson DF, Magnusson KP, Andersen K, Levey AI, Backman VM, Matthiasdottir S, Jonsdottir T, Palsson S, Einarsdottir H, Gunnarsdottir S, Gylfason A, Vaccarino V, Hooper WC, Reilly MP, Granger CB, Austin H, Rader DJ, Shah SH, Quyyumi AA, Gulcher JR, Thorgeirsson G, Thorsteinsdottir U, Kong A, Stefansson K (2007) A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science 316:1491–1493. https://doi.org/10.1126/science.1142842
McPherson R, Pertsemlidis A, Kavaslar N, Stewart A, Roberts R, Cox DR, Hinds DA, Pennacchio LA, Tybjaerg-Hansen A, Folsom AR, Boerwinkle E, Hobbs HH, Cohen JC (2007) A common allele on chromosome 9 associated with coronary heart disease. Science 316:1488–1491. https://doi.org/10.1126/science.1142447
Bochenek G, Häsler R, El Mokhtari NE, König IR, Loos BG, Jepsen S, Rosenstiel P, Schreiber S, Schaefer AS (2013) The large non-coding RNA ANRIL, which is associated with atherosclerosis, periodontitis and several forms of cancer, regulates ADIPOR1, VAMP3 and C11ORF10. Hum Mol Genet 22:4516–4527. https://doi.org/10.1093/hmg/ddt299
Burd CE, Jeck WR, Liu Y, Sanoff HK, Wang Z, Sharpless NE (2010) Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk. PLoS Genet 6:e1001233. https://doi.org/10.1371/journal.pgen.1001233
Holdt LM, Stahringer A, Sass K, Pichler G, Kulak NA, Wilfert W, Kohlmaier A, Herbst A, Northoff BH, Nicolaou A, Gäbel G, Beutner F, Scholz M, Thiery J, Musunuru K, Krohn K, Mann M, Teupser D (2016) Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat Commun 7:12429. https://doi.org/10.1038/ncomms12429
Holdt LM, Beutner F, Scholz M, Gielen S, Gäbel G, Bergert H, Schuler G, Thiery J, Teupser D (2010) ANRIL expression is associated with atherosclerosis risk at chromosome 9p21. Arterioscler Thromb Vasc Biol 30:620–627. https://doi.org/10.1161/atvbaha.109.196832
Shi P, Ji H, Zhang H, Yang J, Guo R, Wang J (2020) circANRIL reduces vascular endothelial injury, oxidative stress and inflammation in rats with coronary atherosclerosis. Exp Ther Med 20:2245–2251. https://doi.org/10.3892/etm.2020.8956
Song CL, Wang JP, Xue X, Liu N, Zhang XH, Zhao Z, Liu JG, Zhang CP, Piao ZH, Liu Y, Yang YB (2017) Effect of circular ANRIL on the inflammatory response of vascular endothelial cells in a rat model of coronary atherosclerosis. Cell Physiol Biochem 42:1202–1212. https://doi.org/10.1159/000478918
Zhu K, Hu X, Chen H, Li F, Yin N, Liu AL, Shan K, Qin YW, Huang X, Chang Q, Xu GZ, Wang Z (2019) Downregulation of circRNA DMNT3B contributes to diabetic retinal vascular dysfunction through targeting miR-20b-5p and BAMBI. EBioMedicine 49:341–353. https://doi.org/10.1016/j.ebiom.2019.10.004
Fan Y, Li X, Xiao W, Fu J, Harris RC, Lindenmeyer M, Cohen CD, Guillot N, Baron MH, Wang N, Lee K, He JC, Schlondorff D, Chuang PY (2015) BAMBI elimination enhances alternative TGF-β signaling and glomerular dysfunction in diabetic mice. Diabetes 64:2220–2233. https://doi.org/10.2337/db14-1397
Guillot N, Kollins D, Badimon JJ, Schlondorff D, Hutter R (2013) Accelerated reendothelialization, increased neovascularization and erythrocyte extravasation after arterial injury in BAMBI-/- mice. PLoS ONE 8:e58550. https://doi.org/10.1371/journal.pone.0058550
Salles C II, Monkman JH, Ahnström J, Lane DA, Crawley JT (2014) Vessel wall BAMBI contributes to hemostasis and thrombus stability. Blood 123:2873–2881. https://doi.org/10.1182/blood-2013-10-534024
Zhu T, Yao Q, Wang W, Yao H, Chao J (2016) iNOS induces vascular endothelial cell migration and apoptosis via autophagy in ischemia/reperfusion injury. Cell Physiol Biochem 38:1575–1588. https://doi.org/10.1159/000443098
Bai Y, Zhang Y, Han B, Yang L, Chen X, Huang R, Wu F, Chao J, Liu P, Hu G, Zhang JH, Yao H (2018) Circular RNA DLGAP4 ameliorates ischemic stroke outcomes by targeting mir-143 to regulate endothelial-mesenchymal transition associated with blood-brain barrier integrity. J Neurosci 38:32–50. https://doi.org/10.1523/jneurosci.1348-17.2017
Chen Y, Wang Y, Jiang Y, Zhang X, Sheng M (2019) High-glucose treatment regulates biological functions of human umbilical vein endothelial cells via Sirt1/FOXO3 pathway. Ann Transl Med 7:199. https://doi.org/10.21037/atm.2019.04.29
Ku YH, Cho BJ, Kim MJ, Lim S, Park YJ, Jang HC, Choi SH (2017) Rosiglitazone increases endothelial cell migration and vascular permeability through Akt phosphorylation. BMC Pharmacol Toxicol 18:62. https://doi.org/10.1186/s40360-017-0169-y
Li X, Zhou Q, Sunkara M, Kutys ML, Wu Z, Rychahou P, Morris AJ, Zhu H, Evers BM, Huang C (2013) Ubiquitylation of phosphatidylinositol 4-phosphate 5-kinase type I γ by HECTD1 regulates focal adhesion dynamics and cell migration. J Cell Sci 126:2617–2628. https://doi.org/10.1242/jcs.117044
Tiedt S, Prestel M, Malik R, Schieferdecker N, Duering M, Kautzky V, Stoycheva I, Böck J, Northoff BH, Klein M, Dorn F, Krohn K, Teupser D, Liesz A, Plesnila N, Holdt LM, Dichgans M (2017) RNA-Seq identifies circulating miR-125a-5p, miR-125b-5p, and miR-143-3p as potential biomarkers for acute ischemic stroke. Circ Res 121:970–980. https://doi.org/10.1161/circresaha.117.311572
Xu RH, Liu B, Wu JD, Yan YY, Wang JN (2016) miR-143 is involved in endothelial cell dysfunction through suppression of glycolysis and correlated with atherosclerotic plaques formation. Eur Rev Med Pharmacol Sci 20:4063–4071
Cheng J, Hu W, Zheng F, Wu Y, Li M (2020) hsa_circ_0058092 protects against hyperglycemia-induced endothelial progenitor cell damage via miR-217/FOXO3. Int J Mol Med 46:1146–1154. https://doi.org/10.3892/ijmm.2020.4664
Sang T, Cao Q, Wang Y, Liu F, Chen S (2014) Overexpression or silencing of FOXO3a affects proliferation of endothelial progenitor cells and expression of cell cycle regulatory proteins. PLoS ONE 9:e101703. https://doi.org/10.1371/journal.pone.0101703
Tsuchiya K, Tanaka J, Shuiqing Y, Welch CL, DePinho RA, Tabas I, Tall AR, Goldberg IJ, Accili D (2012) FoxOs integrate pleiotropic actions of insulin in vascular endothelium to protect mice from atherosclerosis. Cell Metab 15:372–381. https://doi.org/10.1016/j.cmet.2012.01.018
Potente M, Urbich C, Sasaki K, Hofmann WK, Heeschen C, Aicher A, Kollipara R, DePinho RA, Zeiher AM, Dimmeler S (2005) Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J Clin Invest 115:2382–2392. https://doi.org/10.1172/jci23126
Li CY, Ma L, Yu B (2017) Circular RNA hsa_circ_0003575 regulates oxLDL induced vascular endothelial cells proliferation and angiogenesis. Biomed Pharmacother 95:1514–1519. https://doi.org/10.1016/j.biopha.2017.09.064
Shang L, Quan A, Sun H, Xu Y, Sun G, Cao P (2019) MicroRNA-148a-3p promotes survival and migration of endothelial cells isolated from Apoe deficient mice through restricting circular RNA 0003575. Gene 711:143948. https://doi.org/10.1016/j.gene.2019.143948
Goedeke L, Rotllan N, Canfrán-Duque A, Aranda JF, Ramírez CM, Araldi E, Lin CS, Anderson NN, Wagschal A, de Cabo R, Horton JD, Lasunción MA, Näär AM, Suárez Y, Fernández-Hernando C (2015) MicroRNA-148a regulates LDL receptor and ABCA1 expression to control circulating lipoprotein levels. Nat Med 21:1280–1289. https://doi.org/10.1038/nm.3949
Wei Z, Ran H, Yang C (2020) CircRSF1 contributes to endothelial cell growth, migration and tube formation under ox-LDL stress through regulating miR-758/CCND2 axis. Life Sci 259:118241. https://doi.org/10.1016/j.lfs.2020.118241
Johnson DG, Walker CL (1999) Cyclins and cell cycle checkpoints. Annu Rev Pharmacol Toxicol 39:295–312. https://doi.org/10.1146/annurev.pharmtox.39.1.295
Li XX, Liu YM, Li YJ, Xie N, Yan YF, Chi YL, Zhou L, Xie SY, Wang PY (2016) High glucose concentration induces endothelial cell proliferation by regulating cyclin-D2-related miR-98. J Cell Mol Med 20:1159–1169. https://doi.org/10.1111/jcmm.12765
Zhang X, Lu J, Zhang Q, Luo Q, Liu B (2021) CircRNA RSF1 regulated ox-LDL induced vascular endothelial cells proliferation, apoptosis and inflammation through modulating miR-135b-5p/HDAC1 axis in atherosclerosis. Biol Res 54:11. https://doi.org/10.1186/s40659-021-00335-5
Hu C, Peng K, Wu Q, Wang Y, Fan X, Zhang DM, Passerini AG, Sun C (2021) HDAC1 and 2 regulate endothelial VCAM-1 expression and atherogenesis by suppressing methylation of the GATA6 promoter. Theranostics 11:5605–5619. https://doi.org/10.7150/thno.55878
Jin G, Wang Q, Hu X, Li X, Pei X, Xu E, Li M (2019) Profiling and functional analysis of differentially expressed circular RNAs in high glucose-induced human umbilical vein endothelial cells. FEBS Open Bio 9:1640–1651. https://doi.org/10.1002/2211-5463.12709
Zhang Q, Long J, Li N, Ma X, Zheng L (2021) Circ_CLASP2 regulates high glucose-induced dysfunction of human endothelial cells through targeting miR-140-5p/FBXW7 axis. Front Pharmacol 12:594793. https://doi.org/10.3389/fphar.2021.594793
Izumi N, Helker C, Ehling M, Behrens A, Herzog W, Adams RH (2012) Fbxw7 controls angiogenesis by regulating endothelial Notch activity. PLoS ONE 7:e41116. https://doi.org/10.1371/journal.pone.0041116
Pronk MCA, Majolee J, Loregger A, van Bezu JSM, Zelcer N, Hordijk PL, Kovacevic I (2019) FBXW7 regulates endothelial barrier function by suppression of the cholesterol synthesis pathway and prenylation of RhoB. Mol Biol Cell 30:607–621. https://doi.org/10.1091/mbc.E18-04-0259
Liu QQ, Ren K, Liu SH, Li WM, Huang CJ, Yang XH (2019) MicroRNA-140-5p aggravates hypertension and oxidative stress of atherosclerosis via targeting Nrf2 and Sirt2. Int J Mol Med 43:839–849. https://doi.org/10.3892/ijmm.2018.3996
Zhao Z, Li X, Jian D, Hao P, Rao L, Li M (2017) Hsa_circ_0054633 in peripheral blood can be used as a diagnostic biomarker of pre-diabetes and type 2 diabetes mellitus. Acta Diabetol 54:237–245. https://doi.org/10.1007/s00592-016-0943-0
Pan L, Lian W, Zhang X, Han S, Cao C, Li X, Li M (2018) Human circular RNA-0054633 regulates high glucose-induced vascular endothelial cell dysfunction through the microRNA-218/roundabout 1 and microRNA-218/heme oxygenase-1 axes. Int J Mol Med 42:597–606. https://doi.org/10.3892/ijmm.2018.3625
Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J (2016) Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci 73:3221–3247. https://doi.org/10.1007/s00018-016-2223-0
Schachter M (1997) Vascular smooth muscle cell migration, atherosclerosis, and calcium channel blockers. Int J Cardiol 62(Suppl 2):S85-90. https://doi.org/10.1016/s0167-5273(97)00245-3
Owens GK (1995) Regulation of differentiation of vascular smooth muscle cells. Physiol Rev 75:487–517. https://doi.org/10.1152/physrev.1995.75.3.487
Jaminon A, Reesink K, Kroon A, Schurgers L (2019) The role of vascular smooth muscle cells in arterial remodeling: focus on calcification-related processes. Int J Mol Sci. https://doi.org/10.3390/ijms20225694
Lutgens E, de Muinck ED, Kitslaar PJ, Tordoir JH, Wellens HJ, Daemen MJ (1999) Biphasic pattern of cell turnover characterizes the progression from fatty streaks to ruptured human atherosclerotic plaques. Cardiovasc Res 41:473–479. https://doi.org/10.1016/s0008-6363(98)00311-3
Bennett MR, Evan GI, Schwartz SM (1995) Apoptosis of human vascular smooth muscle cells derived from normal vessels and coronary atherosclerotic plaques. J Clin Invest 95:2266–2274. https://doi.org/10.1172/JCI117917
Sun Y, Zhang S, Yue M, Li Y, Bi J, Liu H (2019) Angiotensin II inhibits apoptosis of mouse aortic smooth muscle cells through regulating the circNRG-1/miR-193b-5p/NRG-1 axis. Cell Death Dis 10:362. https://doi.org/10.1038/s41419-019-1590-5
Zhang M, Xu Y, Qiu Z, Jiang L (2019) Sulforaphane attenuates angiotensin II-induced vascular smooth muscle cell migration via suppression of NOX4/ROS/Nrf2 signaling. Int J Biol Sci 15:148–157. https://doi.org/10.7150/ijbs.28874
Ding P, Ding Y, Tian Y, Lei X (2020) Circular RNA circ_0010283 regulates the viability and migration of oxidized lowdensity lipoproteininduced vascular smooth muscle cells via an miR3703p/HMGB1 axis in atherosclerosis. Int J Mol Med 46:1399–1408. https://doi.org/10.3892/ijmm.2020.4703
Wang R, Wu W, Li W, Huang S, Li Z, Liu R, Shan Z, Zhang C, Li W, Wang S (2018) Activation of NLRP3 inflammasome promotes foam cell formation in vascular smooth muscle cells and atherogenesis via HMGB1. J Am Heart Assoc 7:e008596. https://doi.org/10.1161/JAHA.118.008596
Yang L, Yang F, Zhao H, Wang M, Zhang Y (2019) Circular RNA circCHFR facilitates the proliferation and migration of vascular smooth muscle via miR-370/FOXO1/Cyclin D1 pathway. Mol Ther Nucleic Acids 16:434–441. https://doi.org/10.1016/j.omtn.2019.02.028
Zhuang JB, Li T, Hu XM, Ning M, Gao WQ, Lang YH, Zheng WF, Wei J (2020) Circ_CHFR expedites cell growth, migration and inflammation in ox-LDL-treated human vascular smooth muscle cells via the miR-214-3p/Wnt3/beta-catenin pathway. Eur Rev Med Pharmacol Sci 24:3282–3292. https://doi.org/10.26355/eurrev_202003_20696
Samarzija I, Sini P, Schlange T, Macdonald G, Hynes NE (2009) Wnt3a regulates proliferation and migration of HUVEC via canonical and non-canonical Wnt signaling pathways. Biochem Biophys Res Commun 386:449–454. https://doi.org/10.1016/j.bbrc.2009.06.033
Kong P, Yu Y, Wang L, Dou YQ, Zhang XH, Cui Y, Wang HY, Yong YT, Liu YB, Hu HJ, Cui W, Sun SG, Li BH, Zhang F, Han M (2019) circ-Sirt1 controls NF-kappaB activation via sequence-specific interaction and enhancement of SIRT1 expression by binding to miR-132/212 in vascular smooth muscle cells. Nucleic Acids Res 47:3580–3593. https://doi.org/10.1093/nar/gkz141
Li L, Zhang HN, Chen HZ, Gao P, Zhu LH, Li HL, Lv X, Zhang QJ, Zhang R, Wang Z, She ZG, Zhang R, Wei YS, Du GH, Liu DP, Liang CC (2011) SIRT1 acts as a modulator of neointima formation following vascular injury in mice. Circ Res 108:1180–1189. https://doi.org/10.1161/CIRCRESAHA.110.237875
Kane AE, Sinclair DA (2018) Sirtuins and NAD(+) in the development and treatment of metabolic and cardiovascular diseases. Circ Res 123:868–885. https://doi.org/10.1161/CIRCRESAHA.118.312498
Majesky MW (1994) Neointima formation after acute vascular injury. Role of counteradhesive extracellular matrix proteins. Tex Heart Inst J 21:78–85
Wang Y, Wang Y, Li Y, Wang B, Miao Z, Liu X, Ma Y (2019) Decreased expression of circ_0020397 in intracranial aneurysms may be contributing to decreased vascular smooth muscle cell proliferation via increased expression of miR-138 and subsequent decreased KDR expression. Cell Adh Migr 13:220–228. https://doi.org/10.1080/19336918.2019.1619432
Kowanetz M, Ferrara N (2006) Vascular endothelial growth factor signaling pathways: therapeutic perspective. Clin Cancer Res 12:5018–5022. https://doi.org/10.1158/1078-0432.CCR-06-1520
Chanakira A, Dutta R, Charboneau R, Barke R, Santilli SM, Roy S (2012) Hypoxia differentially regulates arterial and venous smooth muscle cell proliferation via PDGFR-beta and VEGFR-2 expression. Am J Physiol Heart Circ Physiol 302:H1173–H1184. https://doi.org/10.1152/ajpheart.00411.2011
Yao JS, Zhai W, Fan Y, Lawton MT, Barbaro NM, Young WL, Yang GY (2007) Interleukin-6 upregulates expression of KDR and stimulates proliferation of human cerebrovascular smooth muscle cells. J Cereb Blood Flow Metab 27:510–520. https://doi.org/10.1038/sj.jcbfm.9600365
Shen L, Hu Y, Lou J, Yin S, Wang W, Wang Y, Xia Y, Wu W (2019) CircRNA0044073 is upregulated in atherosclerosis and increases the proliferation and invasion of cells by targeting miR107. Mol Med Rep 19:3923–3932. https://doi.org/10.3892/mmr.2019.10011
Wang R, Zhang Y, Xu L, Lin Y, Yang X, Bai L, Chen Y, Zhao S, Fan J, Cheng X, Liu E (2016) Protein inhibitor of activated STAT3 suppresses oxidized LDL-induced cell responses during atherosclerosis in apolipoprotein E-deficient mice. Sci Rep 6:36790. https://doi.org/10.1038/srep36790
Foley NH, O’Neill LA (2012) miR-107: a toll-like receptor-regulated miRNA dysregulated in obesity and type II diabetes. J Leukoc Biol 92:521–527. https://doi.org/10.1189/jlb.0312160
Khor ES, Wong PF (2018) Endothelial replicative senescence delayed by the inhibition of MTORC1 signaling involves MicroRNA-107. Int J Biochem Cell Biol 101:64–73. https://doi.org/10.1016/j.biocel.2018.05.016
Wei S, Zheng Y, Jiang Y, Li X, Geng J, Shen Y, Li Q, Wang X, Zhao C, Chen Y, Qian Z, Zhou J, Li W (2019) The circRNA circPTPRA suppresses epithelial-mesenchymal transitioning and metastasis of NSCLC cells by sponging miR-96-5p. EBioMedicine 44:182–193. https://doi.org/10.1016/j.ebiom.2019.05.032
Zhang LL (2020) CircRNA-PTPRA promoted the progression of atherosclerosis through sponging with miR-636 and upregulating the transcription factor SP1. Eur Rev Med Pharmacol Sci 24:12437–12449. https://doi.org/10.26355/eurrev_202012_24039
Soderling SH, Beavo JA (2000) Regulation of cAMP and cGMP signaling: new phosphodiesterases and new functions. Curr Opin Cell Biol 12:174–179. https://doi.org/10.1016/s0955-0674(99)00073-3
Wang L, Zheng Z, Feng X, Zang X, Ding W, Wu F, Zhao Q (2019) circRNA/lncRNA–miRNA–mRNA network in oxidized, low-density, lipoprotein-induced foam cells. DNA Cell Biol 38:1499–1511. https://doi.org/10.1089/dna.2019.4865
Xu F, Shen L, Chen H, Wang R, Zang T, Qian J, Ge J (2021) circDENND1B participates in the antiatherosclerotic effect of IL-1β monoclonal antibody in mouse by promoting cholesterol efflux via miR-17-5p/Abca1 axis. Front Cell Dev Biol 9:652032. https://doi.org/10.3389/fcell.2021.652032
Chen J, Xu L, Hu Q, Yang S, Zhang B, Jiang H (2015) MiR-17-5p as circulating biomarkers for the severity of coronary atherosclerosis in coronary artery disease. Int J Cardiol 197:123–124. https://doi.org/10.1016/j.ijcard.2015.06.037
Tan L, Liu L, Jiang Z, Hao X (2019) Inhibition of microRNA-17-5p reduces the inflammation and lipid accumulation, and up-regulates ATP-binding cassette transporterA1 in atherosclerosis. J Pharmacol Sci 139:280–288. https://doi.org/10.1016/j.jphs.2018.11.012
Wang X, Bai M (2021) CircTM7SF3 contributes to oxidized low-density lipoprotein-induced apoptosis, inflammation and oxidative stress through targeting miR-206/ASPH axis in atherosclerosis cell model in vitro. BMC Cardiovasc Disord 21:51. https://doi.org/10.1186/s12872-020-01800-x
Xing T, Du L, Zhuang X, Zhang L, Hao J, Wang J (2017) Upregulation of microRNA-206 induces apoptosis of vascular smooth muscle cells and decreases risk of atherosclerosis through modulating FOXP1. Exp Ther Med 14:4097–4103. https://doi.org/10.3892/etm.2017.5071
Saito R, Smoot ME, Ono K, Ruscheinski J, Wang PL, Lotia S, Pico AR, Bader GD, Ideker T (2012) A travel guide to Cytoscape plugins. Nat Methods 9:1069–1076. https://doi.org/10.1038/nmeth.2212
Wen G, Zhou T, Gu W (2021) The potential of using blood circular RNA as liquid biopsy biomarker for human diseases. Protein Cell 12:911–946. https://doi.org/10.1007/s13238-020-00799-3
Ren S, Lin P, Wang J, Yu H, Lv T, Sun L, Du G (2020) Circular RNAs: promising molecular biomarkers of human aging-related diseases via functioning as an miRNA sponge. Mol Ther Methods Clin Dev 18:215–229. https://doi.org/10.1016/j.omtm.2020.05.027
Jost I, Shalamova LA, Gerresheim GK, Niepmann M, Bindereif A, Rossbach O (2018) Functional sequestration of microRNA-122 from Hepatitis C Virus by circular RNA sponges. RNA Biol 15:1032–1039. https://doi.org/10.1080/15476286.2018.1435248
Liu X, Abraham JM, Cheng Y, Wang Z, Wang Z, Zhang G, Ashktorab H, Smoot DT, Cole RN, Boronina TN, DeVine LR, Talbot CC Jr, Liu Z, Meltzer SJ (2018) Synthetic circular RNA functions as a miR-21 sponge to suppress gastric carcinoma cell proliferation. Mol Ther Nucleic Acids 13:312–321. https://doi.org/10.1016/j.omtn.2018.09.010
Chen YG, Chen R, Ahmad S, Verma R, Kasturi SP, Amaya L, Broughton JP, Kim J, Cadena C, Pulendran B, Hur S, Chang HY (2019) N6-methyladenosine modification controls circular RNA immunity. Mol Cell 76:96-109.e9. https://doi.org/10.1016/j.molcel.2019.07.016
Wesselhoeft RA, Kowalski PS, Parker-Hale FC, Huang Y, Bisaria N, Anderson DG (2019) RNA circularization diminishes immunogenicity and can extend translation duration in vivo. Mol Cell 74:508-520.e4. https://doi.org/10.1016/j.molcel.2019.02.015
Gao Y, Peng J, Ren Z, He NY, Li Q, Zhao XS, Wang MM, Wen HY, Tang ZH, Jiang ZS, Wang GX, Liu LS (2016) Functional regulatory roles of microRNAs in atherosclerosis. Clin Chim Acta 460:164–171. https://doi.org/10.1016/j.cca.2016.06.044
Loyer X, Mallat Z, Boulanger CM, Tedgui A (2015) MicroRNAs as therapeutic targets in atherosclerosis. Expert Opin Ther Targets 19:489–496. https://doi.org/10.1517/14728222.2014.989835
Zhang Y, Zhang L, Wang Y, Ding H, Xue S, Qi H, Li P (2019) MicroRNAs or long noncoding RNAs in diagnosis and prognosis of coronary artery disease. Aging Dis 10:353–366. https://doi.org/10.14336/AD.2018.0617
Economou EK, Oikonomou E, Siasos G, Papageorgiou N, Tsalamandris S, Mourouzis K, Papaioanou S, Tousoulis D (2015) The role of microRNAs in coronary artery disease: from pathophysiology to diagnosis and treatment. Atherosclerosis 241:624–633. https://doi.org/10.1016/j.atherosclerosis.2015.06.037
Schober A, Nazari-Jahantigh M, Wei Y, Bidzhekov K, Gremse F, Grommes J, Megens RT, Heyll K, Noels H, Hristov M, Wang S, Kiessling F, Olson EN, Weber C (2014) MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med 20:368–376. https://doi.org/10.1038/nm.3487
Leeper NJ, Raiesdana A, Kojima Y, Chun HJ, Azuma J, Maegdefessel L, Kundu RK, Quertermous T, Tsao PS, Spin JM (2011) MicroRNA-26a is a novel regulator of vascular smooth muscle cell function. J Cell Physiol 226:1035–1043. https://doi.org/10.1002/jcp.22422
Sun Y, Chen D, Cao L, Zhang R, Zhou J, Chen H, Li Y, Li M, Cao J, Wang Z (2013) MiR-490-3p modulates the proliferation of vascular smooth muscle cells induced by ox-LDL through targeting PAPP-A. Cardiovasc Res 100:272–279. https://doi.org/10.1093/cvr/cvt172
Dodbele S, Mutlu N, Wilusz JE (2021) Best practices to ensure robust investigation of circular RNAs: pitfalls and tips. EMBO Rep 22:e52072. https://doi.org/10.15252/embr.202052072
Denzler R, Agarwal V, Stefano J, Bartel DP, Stoffel M (2014) Assessing the ceRNA hypothesis with quantitative measurements of miRNA and target abundance. Mol Cell 54:766–776. https://doi.org/10.1016/j.molcel.2014.03.045
Jens M, Rajewsky N (2015) Competition between target sites of regulators shapes post-transcriptional gene regulation. Nat Rev Genet 16:113–126. https://doi.org/10.1038/nrg3853
Chen L, Luo W, Zhang W, Chu H, Wang J, Dai X, Cheng Y, Zhu T, Chao J (2020) circDLPAG4/HECTD1 mediates ischaemia/reperfusion injury in endothelial cells via ER stress. RNA Biol 17:240–253. https://doi.org/10.1080/15476286.2019.1676114
Acknowledgements
Not applicable.
Funding
This work was funded by the Ministry of Higher Education, Malaysia, Fundamental Research Grant Scheme No. FRGS/2/2013/SKK01/UM/02/3.
Author information
Authors and Affiliations
Contributions
WPF and LYY conceived and designed the review. All authors were involved in literature search, analysis, and drafting the manuscript. WPF and LYY critically reviewed and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tong, KL., Tan, KE., Lim, YY. et al. CircRNA–miRNA interactions in atherogenesis. Mol Cell Biochem 477, 2703–2733 (2022). https://doi.org/10.1007/s11010-022-04455-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04455-8