Abstract
As alterations in purinergic signaling have been observed in bladder diseases, we aimed to assess the potential prognostic role of purinergic receptors in bladder cancer in a translational approach based on clinical databases and in vitro data. The prognostic role of purinergic receptors in the survival of patients with bladder cancer and the expression profile of the altered P2 receptors in normal and in tumor samples were determined using The Cancer Genome Atlas databank. In T24 and RT4 human bladder cancer cell lines, the P2 purinergic receptors were characterized by RT-PCR and RT-qPCR analysis including radiotherapy exposure as treatment. The cell number and the cumulative population doubling were also assessed. The expression profile of P2X6 receptor in the cancer pathological stage and in the nodal metastasis status was in agreement with Kaplan–Meier analysis, indicating that high expression of this receptor was related to an increased survival rate in patients with bladder cancer. Of all the P2 receptors expressed on T24 cell line, P2X6 presented high expression after radiotherapy, while it was not altered in RT4 cells. In addition, irradiation promoted a decrease of T24 cell number, but did not change the cell number of RT4 after the same time and radiation dose. Along 7 days after irradiation exposure, both cells regrew. However, while P2X6 receptor was downregulated in T24 cells, it was upregulated in RT4 cells. Our findings indicated that high P2X6 receptor expression induced by radiation in T24 cell line may predict a good survival prognostic factor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bladder cancer is one of the most prevalent malignancies affecting the urinary tract and the sixth most common cancer in men [1]. Although radical cystectomy remains the standard treatment for muscle invasive bladder cancer, it strongly affects the patient’s quality of life [2]. An alternative treatment is radiation, which is a non-invasive technique that preserves the urinary bladder [3, 4]. However, many tumors present resistance to radiotherapy [4, 5]. In this way, finding molecular markers that allow the classification of patients with bladder cancer as susceptible or resistant to this treatment would be of value for improving the efficacy of radiotherapy.
In the urinary bladder, the adenosine 5′-triphosphate (ATP) signaling molecule has a crucial role both in mediating the sensation of fullness of the bladder and in many bladder disorders [6, 7]. ATP is a purine nucleotide that, in addition to its intracellular role, also acts as an important extracellular signaling molecule [8] as it can be released by cells in response to different stimuli, including bladder stretch, inflammation, or cell death [8,9,10]. ATP interacts and activates the purinergic receptors that are classified as ionotropic P2X receptors and metabotropic P2Y receptors [11]. P2X receptors are subdivided into P2X 1–7 whereas P2Y are subdivided into P2Y 1,2,4,6,11–14 [12]. Alterations in ATP release and in purinergic receptors expression have been observed in human bladder diseases as well as in animal models of bladder pathologies [13,14,15,16,17]. In addition, ATP signaling is controlled by ectonucleotidases, a family of enzymes that act in a coordinate manner to degrade ATP to adenosine [8]. We have previously showed that the altered expression of ecto-5′nucleotidase/CD73 is involved in bladder cancer tumorigenesis [18, 19] and that extracellular adenosine generated by this enzyme contributes to the radiosensitization of human bladder cancer cells [20]. ATP also reduced glioma cell proliferation after radiotherapy, by sensitizing P2X7 receptor [21]. However, the effect of radiotherapy on P2 receptors (P2R) expression in human bladder cancer cells and a possible relationship between the survival of bladder cancer patients and the expression of P2R have not yet been evaluated. Therefore, in the current study, the potential prognostic value of P2 purinergic receptors in bladder cancer and radiation toxicity were assessed.
Materials and methods
Patient survival
Data were generated by The Cancer Genome Atlas (TCGA) databank. The Kaplan–Meier survival plot for Gene Expression Data was generated with the following entries as a gene symbol in all the plots: ‘P2RX1,’ ‘P2RX2,’ ‘P2RX3,’ ‘P2RX4,’ ‘P2RX5,’ ‘P2RX6,’ ‘P2RX7,’ ‘P2RY1,’ ‘P2RY2,’ ‘P2RY4,’ ‘P2RY6,’ ‘P2RY11,’ ‘P2RY12,’ ‘P2RY13,’ and ‘P2RY14.’ ‘EGFR’ and ‘VIMENTIN,’ whose prognostic role was already reported [22,23,24], were included as two 'positive controls.' ‘BLADDER UROTHELIAL CARCINOMA’ was used as the sample group. The TCGA Browser was accessed to compile the Kaplan–Meier plots (http://tcgabrowser.ethz.ch:3838/PROD/—accessed in October 2020). Data were confirmed in the KMplot (KMplot.com—accessed in October 2020) [25]. The group of 194 patients was separated by the median overall survival (mOS) into two quartiles to reflect extreme low and high expression of the receptors.
Expression profile of the P2X1, P2X6, and P2Y11 receptors
To explore the expression profile of the gene of interest based on pathologic factors, the data generated by the TCGA databank were used. Normal (n = 19) and primary tumor (n = 408) samples were evaluated. The Box-Whisker plot was generated with the following entries: ‘P2RX1,’ ‘P2RX6,’ and ‘P2RY11.’ ‘BLADDER UROTHELIAL CARCINOMA’ was used as the sample group. The UALCAN Portal was accessed to compile the plots (http://ualcan.path.uab.edu/analysis.html—accessed in March 2021) [26].
Cell culture and radiation treatment
The human bladder cancer cell lines T24 (RRID:CVCL_0554) and RT4 (RRID:CVCL_0036) were obtained from ATCC (Rockville, MD, USA) and maintained in the culture mediums RPMI and DMEM, respectively. Both mediums contained 0.5 U/mL penicillin/streptomycin and 10% FBS. Cells were cultured in a 5% CO2, 95% air atmosphere at 37 °C. The cell lines were gamma irradiated with one pulse at a dose of 4 Gray (Gy) using Cobalt Theratron Phoenix equipment (Theratronics Ltd, Ontario, Canada) at a source-to-target distance of 54.5 cm. After radiation, the cells were incubated for 48 h, or 4 and 7 days under the same conditions described above. For both cell lines, the radiation effective dose and the time of incubation followed the optimal conditions described for T24 cells by Dietrich et al. [20].
RT-PCR and quantitative real-time PCR (RT-qPCR)
T24 and RT4 cells were seeded in 6-well plates, grown for 24 h, and irradiated with a dose of 4 Gy. After 48 h of radiation, the cells were reseeded in another 6-well plates to evaluate P2X6 receptor expression over time. Total mRNA was isolated from the cell lines with Trizol reagent (Life Tecnologies) after 48 h of the irradiation process, and at days 4 and 7 after the 48 h irradiation. The cDNA was synthesized by reaction with the reverse transcriptase (Promega Corporation, Madison, WI, USA). RT-PCR and RT-qPCR were used to evaluate P2R gene expression. The primer sequences for the P2R are described in Table 1. For RT-PCR qualitative analysis, results were analyzed by electrophoresis gel. For RT-qPCR, the efficiency of all reactions was > 95%, and the ΔΔCt parameter was used to determine the relative expression data, using GAPDH gene expression as an endogenous control for normalization.
Cumulative population doubling
To assess cumulative population doubling (CPD), T24 and RT4 cell lines were seeded in 6-well plates and grown for 24 h. Both cells were irradiated with a dose of 4 Gy. Forty-eight hours after the irradiation, the cells were seeded in 24-well plates and after 4 and 7 days of growth in the culture medium, the number of cells was counted and the CPD was calculated as previously described [27]. The sum of the CPDs was plotted versus the duration of exposure of the cultures to radiation.
Cell counting
T24 and RT4 cell lines were seeded in 24-well plates, grown for 24 h, and then irradiated with a dose of 4 Gy. Forty-eight hours after the irradiation process, and at days 4 and 7 of the CPD, cells were counted in a hemocytometer using the trypan blue dye exclusion test. The 48-h result was expressed as the number of viable cells in relation to the control group.
Statistical analysis
The median overall survival of bladder cancer patients, separated according to the highest and lowest quartiles considering gene expression, was compared by a Kaplan–Meier survival plot. The hazard ratio with 95% confidence intervals and the log rank p value were calculated. The Box–Whisker plot were used to show the gene expression levels in normal and in primary tumor samples, expressed as median. We also compared the expression levels of genes of interest segregating according to individual cancer stages and by nodal metastasis status. Student’s t-test was used to evaluate the statistical differences between the comparisons made: (a) normal versus tumor tissue, (b) tumor stages, and (c) nodal metastasis status, as indicated in the results and legend of Fig. 2. A probability level of < 0.05 was considered statistically significant.
For the cell lines experiments, the results presented are representative of at least three independent experiments and are expressed as mean ± SD. Unpaired t test was used to evaluate the significant differences between control (Ctr) and irradiated (Irr) group. A probability level of < 0.05 was considered statistically significant. Calculations and graphical illustrations were made using GraphPad Prism version 8.01 for Windows (GraphPad Software, La Jolla, CA, USA).
Results
Prognostic role of purinergic receptors in the survival of patients with bladder cancer
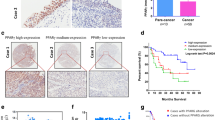
From all P2R analyzed (Fig. 1a and Supplementary Fig. S1), mRNA levels of P2X1, P2X6, and P2Y11 receptors were associated with different median overall survival (mOS) in the cohort of 194 bladder cancer patients from TCGA (Fig. 1a). Kaplan–Meier analysis indicated that patients with high P2X6 and P2Y11 receptors expression had higher median survival of 65 months when compared to 23 months for patients with low expression of these two receptors (p = 0.03 and p = 0.006, respectively, Fig. 1a), whereas patients with high P2X1 receptor expression had a lower median survival of 27 months (p = 0.01, Fig. 1a). The mOS was not available (NA) in patients expressing low levels of P2X1 receptor, since the probability of survival has not reduced to less than 50% (Fig. 1a). Kaplan–Meier analysis for two 'positive controls,' EGFR and VIMENTIN (VIM), were also included. Accordingly, patients who expressed high levels of these markers had reduced mOS (p = 0.001 and p = 0.03, respectively, Fig. 1b). No significant differences for the remaining P2R (P2X 2–5,7 and P2Y 1,2,4,6,12–14) (Supplementary Fig. S1) were observed.
Comparative median overall survival (mOS) for bladder cancer patients expressing low (red) versus high (gray) levels of (a) P2X1, P2X6, and P2Y11 receptors. (b) Data for mOS using the EGFR and VIMENTIN (VIM) bladder cancer prognostic markers. Bladder cancer patients (n = 194) from TCGA databank were separated according to the two highest and lowest quartiles. The two patient cohorts were compared by a Kaplan–Meier survival plot, and the hazard ratio with 95% confidence intervals and log rank p value were calculated. The p values are provided in the left corner of each graph
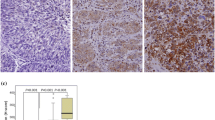
For the receptors P2X1, P2X6, and P2Y11 associated with different mOS, their expression profile was evaluated both in normal tissue and in cancer samples (Fig. 2a), as well as in individual cancer stages (left) and in nodal metastasis status (right) (Fig. 2b, c, d) using data generated by the TCGA databank. Expression levels of P2X1 receptor were lower in primary tumors samples (p < 0.002, Fig. 2a), while P2Y11 receptor expression was higher in the same samples (p < 0.0001, Fig. 2a) when compared to normal tissue. Levels of the P2X6 receptor did not differ between the two groups (Fig. 2a).
Expression profile of P2X1, P2X6, and P2Y11 receptors generated by the TCGA databank were evaluated both in (a) normal tissue and in primary tumor samples and also in (b, c, d) individual cancer stages (left) and in nodal metastasis status (right). Cancer stages were classified as stage 1–4. Nodal metastasis was classified as N0 (no regional lymph node), N1 (metastases in 1–3 axillary lymph nodes) N2 (metastases in 4–9 axillary lymph nodes) or N3 (metastases in 10 or more axillary lymph nodes). Box–Whisker plot showed gene expression levels. The horizontal lines indicate the median, whose values are described above each box. Statistical difference is shown by comparing each tumor stage (1–4) or nodal status (N0–N3) in relation to normal tissue and is represented by asterisks. Differences between tumor stage (1–4) or nodal metastasis (N0–N3) are shown using square brackets and hashtag. */#p < 0.05, **/##p < 0.01, ***/###p = or < 0.001 as determined by Student’s t-test, considering unequal variance
According to the tumor stage, stage 1 to 4 tumors showed lower expression levels of P2X1 receptor than normal tissue (stage 1 and 2 p = 0.001; stage 3 and 4 p = 0.002, Fig. 2b). However, stage 3 and 4 tumors showed higher levels than stage 1 tumors (p = 0.001 and p < 0.0001, respectively, Fig. 2b), and these results were in agreement with Kaplan–Meier data, where higher receptor expression was associated with poor survival. Lower expression levels of P2X1 receptor were observed in those tumors with increased nodal metastases (N0 and N1 p = 0.002; N2 p = 0.003; N3 p = 0.001, Fig. 2b).
Lower expression levels of P2X6 receptor in stage 4 tumors in relation to normal tissue (p = 0.02) and also in relation to stage 2 tumors (p = 0.0001) were observed (Fig. 2c). Furthermore, we found lower expression levels of P2X6 receptor in those tumors with progressive increment in the number of node metastasis in relation to non-metastatic tumor (N1 p = 0.0001, N2 p = 0.03 and N3 p = 0.01, Fig. 2c). These results are in agreement with Kaplan–Meier survival curve, which showed that lower levels of P2X6 receptor were associated with poorer survival.
P2Y11 receptor showed higher expression levels in stage 2, 3, and 4 tumors (p < 0.0001, Fig. 2d) compared to normal tissue. Despite not being significative, levels of P2Y11 receptor expression in stage 4 tumors were lower than stage 2 and 3 tumors, which may explain the Kaplan–Meier survival plot. In relation to those tumors with metastases in 0 to 2 axillary lymph nodes, lower levels of P2Y11 receptor expression in normal tissue were observed (N0 and N2 p < 0.0001; N1 p = 0.03, Fig. 2d).
Expression of P2 receptors in bladder cancer cell lines after radiotherapy
Based on results presented in Figs. 1 and 2, and considering that radiotherapy is an alternative treatment for bladder cancer, we sought to deeper investigate, in vitro, the involvement of purinergic receptors in the susceptibility or resistance to this treatment. For this purpose, T24 bladder cancer cell line was used. Firstly, RT-PCR analysis showed that control T24 cells expressed mRNA for the P2X4, 5, 6, 7 (Fig. 3a) and P2Y1, 2, 4, 6, 12 receptors (Fig. 3c). RT-qPCR analysis demonstrated that 4 Gy dose radiation induced a significantly increased in the expression of P2X5 and P2X6 receptors (p = 0.0003 and p = 0.005, respectively, Fig. 3b) and in the gene expression of P2Y2, 6, 12 receptors when compared to T24 control cells (p = 0.0001; p = 0.003; p = 0.04, respectively, Fig. 3d). In contrast, P2Y1 receptor demonstrated a lower gene expression when compared to their respective control cells (p < 0.0001, Fig. 3d).
Expression of the P2 purinergic receptors in control (Ctr) and 4 Gy-dose irradiated (Irr) T24 human bladder cancer cell line. The expression of (a, b) P2X and (c, d) P2Y receptors were evaluated by (a, c) RT-PCR and (b, d) RT-qPCR 48 h after irradiation, as described in materials and methods. Expression was normalized to GAPDH expression. The experiments were performed at least three times in triplicate. *p < 0.05, **p < 0.01, ***p < 0.001 in relation to the T24 control cell line, as determined by Unpaired t test
Although the mOS in bladder cancer patients expressing high or low levels of P2X1 and P2Y11 receptors was significantly different (Fig. 1a) and was detected in primary tumors (Fig. 2a), both receptors were not expressed in the T24 human bladder cancer cell line (Fig. 3a, c). Therefore, we focused our attention on the P2X6 receptor (Fig. 3a, b), whose levels of mRNA increased by approximately 2.5-fold 48 h after irradiation (Fig. 3b). Moreover, in order to investigate our findings in another bladder cancer cell line, the expression of the P2R was also analyzed before and after the radiotherapy in the RT4 cells, which has a less aggressive phenotype (Supplementary Fig. S2). P2X6 receptor gene expression was almost undetectable by RT-PCR in control RT4 cell line (Supplementary Fig. S2a), and 48 h after radiotherapy, no change was observed in relation to its control (Supplementary Fig. S2a, b).
Cell counting and cumulative population doubling
Confirming previous published data [20], the dose of 4 Gy of radiation led to a significant reduction in the cell number of the T24 cell line after 48 h (p = 0.01, Fig. 4a). However, at the same time and radiation dose, RT4 cell line showed to be less sensitive (Fig. 4b). Next, we sought to investigate the long-term effects of irradiation on cell proliferation and P2X6 receptor mRNA expression in the two cell lines (schematic experimental protocol in Fig. 4c). Analysis of cell proliferation over time showed that the cumulative population doubling (CPD) of T24 cell line was reduced until 4 days after radiation (p < 0.0001; Fig. 4d); at 7 days, the cells return to proliferate at a similar ratio as the control cells (p < 0.0001; Fig. 4d), which coincide with a decrease of the P2X6 receptor expression levels, similar to the control levels (Fig. 4f). RT4 cell line, which was less affected by radiation (Fig. 4b) compared to the T24 cells (Fig. 4a), presented a different profile (Fig. 4e). While T24 cell line reached their lowest CPD 4 days after radiation (Fig. 4d), RT4 cell line had already doubled once after radiation at this time (p = 0.003; Fig. 4e).
A cell counting assay after the exposure of (a) T24 and (b) RT4 cell lines to a dose of 4 Gy of radiation for 48 h. The experiments were performed three times in triplicate. *p < 0.05 in relation to the control cells as determined by Unpaired t test. (c) Schematic representation of the experimental protocol. 1–means the day where T24 and RT4 cell lines were seeded in plates and grown for 24 h; 2–means the day where both cells were irradiated with a dose of 4 Gy; 4–means 48 h after irradiation when the cells were seeded in new plates; D4 and D7–means 4 and 7 days of growth after 48 h of irradiation (4), when the cells were analyzed for cumulative population doubling (CPD) and RT-qPCR. CPD of (d) T24 and (e) RT4 cell line was determined 4 and 7 days after 48 h of irradiation (considered day 0 in the graph) in control (Ctr) and irradiated (Irr) cells. The experiments were performed six times in triplicate. **p < 0.01, ***p < 0.001 in relation to the respective control cells as determined by UNPAIRED t test. P2X6 mRNA expression in control (Ctr) and irradiated (Irr) (f) T24 and (g) RT4 bladder cancer cell line after 48 h (considered day 0 in the graph), 4 and 7 days. Irradiated cells were analyzed in relation to their respective controls (0, 4, and 7 days). Control cells from the respective days exhibited similar Ct values. P2X6 receptor expression was determined by RT-qPCR analysis, as described in materials and methods. Expression was normalized to GAPDH expression. The experiments were performed three times in triplicate
To determine the relationship between the observed increase in P2X6 receptor expression and radiation, RT-qPCR was used to determine the levels of the gene expression of this receptor in T24 and RT4 cells along 7 days after the treatment with radiation. After a dose of 4 Gy, T24 cells presented higher P2X6 protein levels than the control cells, with the highest expression levels of the receptor achieved 48 h after radiation (considered day zero) and a progressive long-term decrease in the level of the mRNA (Fig. 4f). An opposite profile was observed for the RT4 cells (Fig. 4g).
Discussion
The present study shows that patients with bladder cancer who express high levels of P2X6 or P2Y11 receptors presented a greater survival rate than those who express lower levels of these receptors. In contrast, low P2X1 receptor expression was associated with increased median overall survival (mOS). Considering the cancer pathological stage and the nodal metastasis status in normal tissue and in primary bladder tumors, the expression profile of these receptors was in agreement with the Kaplan–Meier survival curves. Among these three receptors, P2X6 was the only expressed receptor and whose levels appeared altered after irradiation in the T24 human bladder cancer cell line. Specifically, we found low levels of basal expression of the P2X6 mRNA in RT4 and T24 bladder cancer cell lines, while radiation induced an increase in these levels after 48 h only in the T24 cells. Although there is a complexity in the translationality of preclinical to clinical research, cell lines are useful models to accelerate the understanding of diseases and treatment actions and design of future trials in human cancer. Therefore, we argue in favor of the adequacy of our model to explore changes in the purinergic receptor P2X6 associated with radiation.
Little is known about the involvement of P2X6 in cancer and the expression of this receptor in bladder cancer has not been reported before. Although P2X6 receptor is poorly expressed in most tumors (http://ualcan.path.uab.edu/cgi-bin/Pan-cancer.pl?genenam=P2RX6—accessed in October 2020), a recent clinical study associated overexpression of the P2X6 with progression and poor prognosis in renal cell carcinoma [28]. In T24 bladder cancer cell line, a transient induction of ecto-5′nucleotidase/CD73 enzyme by radiotherapy was reported by our group, pointing out the generated adenosine as the probable responsible for the observed cell death [20]. In order to better understand the involvement of purinergic signaling with the response to radiotherapy, more studies have been carried out, and here we demonstrate that radiotherapy induces increased expression of the P2X6 receptor in the same cell line.
P2X6 receptor does not form stable and functional homomeric structure [29, 30]; however, the P2X6 is thought to act as a modulatory subunit that alters the pharmacology of the P2X2 and P2X4 by interacting and assembling heteromers with these receptors [31]. As such, we suggest that the P2X6 can assemble with the P2X4 subunit (present in the T24 cell line) to form stable and functional heteromers that are delivered to the cell membrane. We postulate that the upregulation of the P2X6 mRNA by irradiation may represent a compensatory mechanism to provide an intracellular pool of subunits ready for incorporation into heteromers receptors, as previously proposed [31]. It can be supposed that the ATP released as a result of radiotherapy [32, 33] may contribute to the cell death of T24 cells by binding and activating the P2X4/6 heteromer. Part of this extracellular ATP can also be hydrolyzed by the NPP1 and ecto-5′nucleotidase/CD73 ectoenzymes, generating the cytotoxic metabolite adenosine in the extracellular medium, as previously showed [20]. Unfortunately, a specific antagonist of the P2X6 or P2X4/6 receptor is not currently available, which hinders further studies at present.
Although radiotherapy is used to treat patients with bladder cancer [3], many tumors are radioresistant [5], constituting a major obstacle for the use of radiation therapy as a primary strategy for the treatment of this disease [34, 35]. As such, the identification of biomarkers that allow the selection of patients with a high potential for responding to radiotherapy is of great interest. Data obtained in this study indicate that the advantages of radiotherapy may be greater in those patients with tumors that express the P2X6 receptor. Therefore, we hypothesize that the stimulation of the P2X6 receptor (with radiotherapy or another stimulus) may be a therapeutic alternative for treating bladder cancer as a Kaplan–Meier analysis demonstrated that a higher expression of this receptor was associated with a better prognosis and higher mOS in bladder cancer patients. Finally, the radiation dose of 4 Gy, in addition to stimulating the P2X6 receptor expression, also significantly radiosensitized the T24 cell line, as already reported [20]. A different profile was observed for the RT4 cell line, which was less affected by radiation compared to the T24 cells. In addition to be radioresistant, the P2X6 receptor expression was not modified after 48 h of radiation in the RT4 cell line. Thus, screening patients using the P2X6 receptor as a promising treatment marker may represent an important approach for identifying patients that may respond to this conduct. It is difficult to draw conclusive statements based on this study, but our finding further supports the analysis of a panel of markers that may be extremely relevant for directing patients to specific treatments, and here, we demonstrated that the P2X6 receptor may become a possible molecular marker for classifying patients with bladder cancer into those potentially responsive, or not, to radiotherapy. Although the presence of the P2X6 receptor may sensitize tumor cells to radiotherapy, this may not be sufficient to eradicate cancer and, therefore, association with other therapies may be necessary.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Jacobs BL, Lee CT, Montie JE (2010) Bladder cancer in 2010: how far have we come? CA Cancer J Clin 60(4):244–272. https://doi.org/10.3322/caac.20077
Kotwal S, Choudhury A, Johnston C, Paul AB, Whelan P, Kiltie AE (2008) Similar treatment outcomes for radical cystectomy and radical radiotherapy in invasive bladder cancer treated at a United Kingdom specialist treatment center. Int J Radiat Oncol Biol Phys 70(2):456–463. https://doi.org/10.1016/j.ijrobp.2007.06.030
James ND, Hussain SA, Hall E, Jenkins P, Tremlett J, Rawlings C, Crundwell M, Sizer B, Sreenivasan T, Hendron C, Lewis R, Waters R, Huddart RA (2012) Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med 366(16):1477–1488. https://doi.org/10.1056/NEJMoa1106106
Park JC, Citrin DE, Agarwal PK, Apolo AB (2014) Multimodal management of muscle-invasive bladder cancer. Curr Probl Cancer 38(3):80–108. https://doi.org/10.1016/j.currproblcancer.2014.06.001
Burnstock G (2014) Purinergic signalling in the urinary tract in health and disease. Purinergic Signal 10(1):103–155. https://doi.org/10.1007/s11302-013-9395-y
Winder M, Tobin G, Zupančič D, Romih R (2014) Signalling molecules in the urothelium. Biomed Res Int 2014:297295. https://doi.org/10.1155/2014/297295
Yegutkin GG (2008) Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta 1783(5):673–694. https://doi.org/10.1016/j.bbamcr.2008.01.024
Wang ECY, Lee JM, Ruiz WG, Balestreire EM, von Bodungen M, Barrick S, Cockayne DA, Birder LA, Apodaca G (2005) ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. J Clin Invest 115(9):2412–2422. https://doi.org/10.1172/JCI24086
Birder L, Andersson KE (2013) Urothelial Signaling. Physiol Rev 93(2):653–680. https://doi.org/10.1152/physrev.00030.2012
Burnstock G, Kennedy C (1985) Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol 16(5):433–440. https://doi.org/10.1016/0306-3623(85)90001-1
Burnstock G (2004) Introduction: P2 Receptors. Curr Top Med Chem 4(8):793–803. https://doi.org/10.2174/1568026043451014
Birder LA, Ruan HZ, Chopra B, Xiang Z, Barrick S, Buffington CA, Roppolo JR, Ford APDW, de Groat WC, Burnstock G (2004) Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. Am J Physiol Renal Physiol 287(5):F1084–F1091. https://doi.org/10.1152/ajprenal.00118.2004
Tempest HV, Dixon AK, Turner WH, Elneil S, Sellers LA, Ferguson DR (2004) P2X2 and P2X3 receptor expression in human bladder urothelium and changes in interstitial cystitis. BJU Int 93(9):1344–1348. https://doi.org/10.1111/j.1464-410X.2004.04858.x
Smith CP, Vemulakonda VM, Kiss S, Boone TB, Somogyi GT (2005) Enhanced ATP release from rat bladder urothelium during chronic bladder inflammation: effect of botulinum toxin A. Neurochem Int 47(4):291–297. https://doi.org/10.1016/j.neuint.2005.04.021
Sun Y, Chai TC (2006) Augmented extracellular ATP signaling in bladder urothelial cells from patients with interstitial cystitis. Am J Physiol Cell Physiol 290(1):C27–C34. https://doi.org/10.1152/ajpcell.00552.2004
Shabbir M, Ryten M, Thompson C, Mikhailidis D, Burnstock G (2008) Purinergic receptor-mediated effects of ATP in high-grade bladder cancer. BJU Int 101(1):106–112. https://doi.org/10.1111/j.1464-410X.2007.07286.x
Stella J, Bavaresco L, Braganhol E, Rockenbach L, Farias PF, Wink MR, Azambuja AA, Barrios CH, Morrone FB, Battastini AMO (2010) Differential ectonucleotidase expression in human bladder cancer cell lines. Urol Oncol 28(3):260–267. https://doi.org/10.1016/j.urolonc.2009.01.035
Rockenbach L, Braganhol E, Dietrich F, Figueiró F, Pugliese M, Edelweiss MIA, Morrone FB, Sévigny J, Battastini AMO (2014) NTPDase3 and ecto-5’-nucleotidase/CD73 are differentially expressed during mouse bladder cancer progression. Purinergic Signal 10(3):421–430. https://doi.org/10.1007/s11302-014-9405-8
Dietrich F, Figueiró F, Filippi-Chiela EC, Cappellari AR, Rockenbach L, Tremblay A, de Paula PB, Roesler R, Filho AB, Sévigny J, Morrone FB, Battastini AMO (2018) Ecto-5’-nucleotidase/CD73 contributes to the radiosensitivity of T24 human bladder cancer cell line. J Cancer Res Clin Oncol 144(3):469–482. https://doi.org/10.1007/s00432-017-2567-3
Gehring MP, Pereira TCB, Zanin RF, Borges MC, Filho AB, Battastini AMO, Bogo MR, Lenz G, Campos MM, Morrone FB (2012) P2X7 receptor activation leads to increased cell death in a radiosensitive human glioma cell line. Purinergic Signal 8(4):729–739. https://doi.org/10.1007/s11302-012-9319-2
Bryan RT, Regan HL, Pirrie SJ, Devall AJ, Cheng KK, Zeegers MP, James ND, Knowles MA, Ward DG (2015) Protein shedding in urothelial bladder cancer: prognostic implications of soluble urinary EGFR and EpCAM. Br J Cancer 112(6):1052–1058. https://doi.org/10.1038/bjc.2015.21
Rahmani AH, Babiker AY, AlWanian WM, Elsiddig SA, Faragalla HE, Aly SM (2015) Association of cytokeratin and vimentin protein in the genesis of transitional cell carcinoma of urinary bladder patients. Dis Markers 2015:204759. https://doi.org/10.1155/2015/204759
Lobo J, Monteiro-Reis S, Guimarães-Teixeira C, Lopes P, Carneiro I, Jerónimo C, Henrique R (2020) Practicability of clinical application of bladder cancer molecular classification and additional value of epithelial-to-mesenchymal transition: prognostic value of vimentin expression. J Transl Med 18(1):303. https://doi.org/10.1186/s12967-020-02475-w
Nagy Á, Munkácsy G, Győrffy B (2021) Pancancer survival analysis of cancer hallmark genes. Sci Rep 11(1):6047. https://doi.org/10.1038/s41598-021-84787-5
Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK, Varambally S (2017) UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia 19(8):649–658. https://doi.org/10.1016/j.neo.2017.05.002
Filippi-Chiela EC, Bueno e Silva MM, Thomé MP, Lenz G, (2015) Single-cell analysis challenges the connection between autophagy and senescence induced by DNA damage. Autophagy 11(7):1099–1113. https://doi.org/10.1080/15548627.2015.1009795
Gong D, Zhang J, Chen Y, Xu Y, Ma J, Hu G, Huang Y, Zheng J, Zhai W, Xue W (2019) The m6A-suppressed P2RX6 activation promotes renal cancer cells migration and invasion through ATP-induced Ca2+ influx modulating ERK1/2 phosphorylation and MMP9 signaling pathway. J Exp Clin Cancer Res 38(1):233. https://doi.org/10.1186/s13046-019-1223-y
Torres GE, Egan TM, Voigt MM (1999) Hetero-oligomeric assembly of P2X receptor subunits: specificities exist with regard to possible partners. J Biol Chem 274(10):6653–6659. https://doi.org/10.1074/jbc.274.10.6653
Barrera NP, Ormond SJ, Henderson RM, Murrell-Lagnado RD, Edwardson JM (2005) Atomic force microscopy imaging demonstrates that P2X2 receptors are trimers but that P2X6 receptor subunits do not oligomerize. J Biol Chem 280(11):10759–10765. https://doi.org/10.1074/jbc.M412265200
Ormond SJ, Barrera NP, Qureshi OS, Henderson RM, Edwardson JM, Murrell-Lagnado RD (2006) An uncharged region within the N terminus of the P2X6 receptor inhibits its assembly and exit from the endoplasmic reticulum. Mol Pharmacol 69(5):1692–1700. https://doi.org/10.1124/mol.105.020404
Ohshima Y, Tsukimoto M, Takenouchi T, Harada H, Suzuki A, Sato M, Kitani H (1800) Kojima S (2010) gamma-Irradiation induces P2X(7) receptor-dependent ATP release from B16 melanoma cells. Biochim Biophys Acta 1:40–46. https://doi.org/10.1016/j.bbagen.2009.10.008
Ohshima Y, Tsukimoto M, Harada H, Kojima S (2012) Involvement of connexin43 hemichannel in ATP release after γ-irradiation. J Radiat Res 53(4):551–557. https://doi.org/10.1093/jrr/rrs014
Chen RC, Shipley WU, Efstathiou JA, Zietman AL (2013) Trimodality bladder preservation therapy for muscle-invasive bladder cancer. J Natl Compr Canc Netw 11(8):952–960. https://doi.org/10.6004/jnccn.2013.0116
Shrivastava S, Mansure JJ, Almajed W, Cury F, Ferbeyre G, Popovic M, Seuntjens J, Kassouf W (2016) The role of HMGB1 in radioresistance of bladder cancer. Mol Cancer Ther 15(3):471–479. https://doi.org/10.1158/1535-7163.MCT-15-0581
Acknowledgements
We thank all staff from the Radiotherapy team at the São Lucas Hospital (Serviço de Radioterapia—SERP, Hospital São Lucas de Porto Alegre).
Funding
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico [CNPq/PQ project number 302879/2017-0], INCT.MCTI/CNPq/CAPES/FAPERGS [project number 465671/2014-4], FAPERGS PQG [project number 17/2551- 0000 970-3], Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), FAPERGS/PRONEX [project number 16/2551-0000473-0], and Fundo de Incentivo a Pesquisa do Hospital de Clínicas de Porto Alegre (FIPE/HCPA). FD is recipient of CAPES/PRINT fellowship [process 88887.583176/2020-00]. ARC and ECFC were recipient of PNPD/CAPES fellowships. PBdP and RA were recipient of CNPq fellowships. JBdS was recipient of BPA/PUCRS fellowship. SWA was recipient of BIC/UFRGS fellowship.
Author information
Authors and Affiliations
Contributions
FD, ARC, ECFC, and AMOB contributed to the study conception and design. Material preparation, data collection, and data analysis were performed by FD, ARC, ECFC, PBdP, JBdS, SWA, RA, and AMOB. The first draft of the manuscript was written by FD and all authors reviewed, read, and approved the final version of the manuscript. RR, FBM, and AMOB were responsible for the resources and funding acquisition. AMOB was the supervisor.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare that are relevant to the content of this article.
Ethical approval
The study includes data generated from a databank and laboratory studies on two stable established cell lines. No ethical or institutional approval was required.
Consent to participate
Not applicable.
Consent for publication
All authors read and approved the final manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dietrich, F., Cappellari, A.R., Filippi-Chiela, E.C. et al. High P2X6 receptor expression in human bladder cancer predicts good survival prognosis. Mol Cell Biochem 477, 2047–2057 (2022). https://doi.org/10.1007/s11010-022-04425-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04425-0