Abstract
Retinopathy of prematurity (ROP) is a retinal vasoproliferative disorder that represents an important cause of childhood visual impairment and blindness. Although oxidative stress has long been implicated in ROP etiology, other prenatal and perinatal factors are also involved. This review focuses on current research involving inflammation and genetic factors in the pathogenesis of ROP. Increasing evidence suggests that perinatal inflammation or infection contributes to ROP pathogenesis. Cytokines and chemokines with a fundamental role in inflammatory responses and that significantly contributing to angiogenesis are analyzed. Microglia cells, the retinal-resident macrophages, are crucial for retinal homeostasis, however, under sustained pathological stimuli release exaggerated amounts of inflammatory mediators and can promote pathological neovascularization. Current modulation of angiogenic cytokines, such as treatment with antibodies to vascular endothelial growth factor (anti-VEGF), has shown efficacy in the treatment of ocular neovascularization; however, some patients are refractory to anti-VEGF agents, suggesting that other angiogenic or anti-angiogenic cytokines need to be identified. Much evidence suggests that genetic factors contribute to the phenotypic variability of ROP. Several studies have implicated the involvement of candidate genes from different signaling pathways in the development of ROP. However, a genetic component with a major impact on ROP has not yet been discovered. Most studies have limitations and did not replicate results. Future research involving bioinformatics, genomics, and proteomics may contribute to finding more genes associated with ROP and may allow discovering better solutions in the management and treatment of ROP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Retinopathy of prematurity (ROP) is a retinal vasoproliferative disorder that affects preterm infants (PTI) and represents an important cause of blindness and childhood visual impairment [1] in developed and developing countries [2, 3].
The development of the retinal vascularization begins around 16 weeks of gestation, proceeds centrifugally from the optical disc to the peripheral retina, and is completed approximately at term [4]. For this reason, the retina of PTI is incompletely vascularized, with a peripheral avascular area that depends on the immaturity of the newborn infant [4, 5].
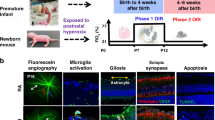
Clinical findings in PTI and studies in animal models of oxygen-induced retinopathy (OIR), of which the mouse OIR model (5 days of exposure to 75% oxygen after birth, followed by room air) is one of the most used [6], have shown that ROP has two phases (Fig. 1) [7]. The exposition of the immature retinal blood vessels (BV) to a relatively hyperoxic environment due to premature birth interrupts the vascular development [8]. This leads to microvascular retinal degeneration with an arrest in the vascularization of the peripheral retina (phase 1) [9]. The resulting retinal ischemia triggers the release of growth factors responsible for pathological angiogenesis (phase 2) [7, 10]. The new BV lead to the formation of a fibrovascular scar that may cause retinal detachment and vision loss [11]. It remains unclear why some PTI have a severe and rapidly progressive form of ROP, designated aggressive ROP (A-ROP) [12].
Development of retinopathy of prematurity. During fetal growth, retinal vascularization normally develops from the optic nerve in a centrifugal direction toward the periphery of the retina. Low oxygen tension in utero is the main stimulus for retinal vascular development, which is interrupted due to the increased bioavailability of oxygen caused by premature birth. ROP pathogenesis develops in two phases. In the first phase retinal vessel growth stops and regresses because the hyperoxic environment necessary to maintain adequate levels of circulating oxygen for the survival of the preterm infant inhibits retinal vascularization (Phase 1). As the newborn grows and the metabolic requirements of retina increase, retina becomes hypoxic, inducing a compensatory, albeit devastating and aberrant, neovascularization driven by oxygen-regulated angiogenic factors (Phase 2). EPO erythropoietin; IGF-1 insulin-like growth factor 1; ROP retinopathy of prematurity; VEGF vascular endothelial growth factor
Retinopathy of prematurity is classified in five stages according to its severity [12]. In stage 1 a fine demarcation line is visible between the vascular and the avascular area of the retina [12]. This flat line can progress to a ridge that defines stage 2 [12]. These two first stages are considered initial or mild ROP and can regress spontaneously. In stage 3 there is pathological vessel proliferation over the ridge and into the vitreous, a feature of severe ROP [13]. The new abnormal vessels can bleed into the vitreous chamber, causing fibrosis and traction and thereby lead to a partial detachment of the retina that defines phase 4 [13]. This can evolute to stage 5, in which retina is totally detached [13].
In most studies over time the incidence of ROP is approximately 60% and of severe ROP (stages 3 to 5) is approximately 15% in PTI with birth weight (BW) of less than 1500 g [14, 15]. However, in a multicenter study conducted in the USA and Canada that included 7,483 with BW of less than 1501 g, the incidence of ROP was 43.1% and that of severe ROP was 12.4% [16] (Table 1). Severe ROP occurs mostly among PTI with birth weight less than 1251 g [16]. Globally, of the 14.9 million PTI in 2010, approximately 184,700 developed any stage of ROP, over 30,000 of whom became visually impaired as a consequence of ROP [17]. Sixty-five percent of the visually impaired due to ROP were born in middle-income regions [17].
Retinopathy of prematurity is considered a multifactorial disease that involves prenatal and postnatal factors [18]. Oxidative stress (OS) which can result from inflammation [19, 20] has long been implicated in the etiology of ROP [20, 21]. It is recognized that inflammatory processes can interfere with normal retinal vascularization and, more recently, are also considered important factors in the pathogenesis of ROP [20].
Genetic polymorphisms involve one of two or more sequence variants of a specific DNA sequence and occur with a population frequency of at least 1% [23]. Genetic polymorphisms can influence the activity of encoded enzymes and the susceptibility to develop complications induced by reactive oxygen species (ROS) provided by genes involved in the regulation of OS [1, 22]. Studies have shown associations of genetic polymorphisms in genes involved in the pro-oxidant and pro-inflammatory response to premature birth and diseases related to OS in PTI [23].
Although ROP is strongly associated with extreme prematurity [24], environmental factors have also been implicated in the development of ROP, mainly high oxygen supplementation after birth and fluctuations in oxygenation [25, 26], but also nutrition [27], factors related to the causes of preterm birth [28], use of maternal medications [29], maternal smoking [30], altitude [31], length of day during early gestation [32], and assisted conception [33, 34]. These perinatal factors may alter gene expression through DNA acetylation and methylation, supporting the supposition that epigenetic modifications by external factors may affect gene expression and render PTI susceptible to severe ROP or PTI genetically prone to ROP not to develop retinopathy [35].”
In addition to the contribution of environmental factors, a marked genetic predisposition to ROP is suggested from research based on the candidate gene approach, twin studies, experimental, and clinical studies. The observation that ROP in a subset of PTI progresses to a severe stage, while in others with similar clinical characteristics regresses spontaneously is a strong indication of the genetic contribution to the etiology of ROP [36, 37].
Identifying susceptibility factors for ROP and a better comprehension of its pathogenesis is determinant for its proper prevention and treatment. It may also help to clarify the pathophysiology of other pediatric and adult neovascular retinal diseases. This review focuses on current research that involves inflammation and genetic factors in the pathogenesis of ROP.
The role of inflammation as a stress response: mediators of immune and inflammatory response in ROP
Prenatal and postnatal systemic inflammation might predispose to ROP, and this sensitization effect may constitute a pre-stage of the disease [38]. Inflammatory stimuli such as chorioamnionitis [39] and neonatal bacteremia [40] have been suggested in several studies to be risk factors for ROP, possibly due to systemic inflammation [38]. Systemic inflammation in animal models has also been shown to disrupt the development of retinal BV and leads to aberrant retinal vascularization [41].
Cytokines
Cytokines are intercellular signaling polypeptides released by activated immune cells that are produced during inflammatory processes and in which they participate [42]. There is an overlap in molecular signaling between oxidative and inflammatory compounds, in which complex networks of signaling pathways link oxidative agents and pro-inflammatory cytokines [43] (Fig. 2). The vascular damage of the ischemic phase of proliferative retinopathies is followed by an inflammatory response with the production of pro-inflammatory cytokines, which cause an increase in vascular permeability, immune and other cells recruitment, activation and differentiation, apoptosis, and angiogenesis [44].
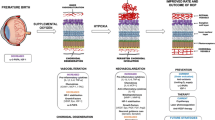
Role of oxidative stress and inflammation in the pathogenesis of ROP. After preterm birth, premature infants are exposed to an excess of supplemental oxygen, leading to retinal vascular obliteration due to suppression of pro-angiogenic factors regulated by oxygen, oxidant stress, and excessive production of pro-inflammatory factors by damaged tissues. The vascular dropout results in hypoxia and HIF stabilization with subsequent production of growth factors. The microenvironment of retinal ischemia is characterized by microglial activation and release of many pro-inflammatory cytokines and chemokines, which cause pathological vasoproliferation. The major pro-inflammatory cytokines responsible for early responses are IL-1β, IL-6, and TNF-α. Other pro-inflammatory mediators include IL-17, IL-18, IL-23, IL-33, TGF-β, bFGF, and a variety of other cytokines and chemokines. These cytokines upregulate the synthesis of secondary inflammatory mediators and pro-inflammatory cytokines. IL-6 and TGF-β act as either pro-inflammatory or anti-inflammatory cytokines, under various circumstances. Proangiogenic cytokines, such as IL-1, TNF-α, and VEGF, directly or indirectly stimulate endothelial cells proliferation, migration, and tube formation. IL-1Ra, IL-4, IL-10, IL-11, and IL-13 are major anti-inflammatory cytokines. Except for IL-1Ra, anti-inflammatory cytokines also have at least some pro-inflammatory properties. In ischemic areas, the enhanced production of ROS can further increase the level of pro-inflammatory cytokines. ECM degradation by MMPs activated by inflammatory cytokines, growth factors, and ROS, as well as proteolytic enzymes released from MFs allows EC migration and growth factors recruitment to form new capillaries. AA arachidonic acid; ANGs angiopoietins; bFGF basic fibroblast growth factor; ECM extracellular matrix; EPO erythropoietin; ICAM-1 intercellular adhesion molecule-1; IGF-1 insulin-like growth factor 1; IL Interleukin; IL-1Ra Interleukin 1 receptor antagonist; I-TAC Interferon-inducible T-cell alpha chemoattractant; MCP-1 monocyte chemotactic protein 1; MMPs matrix metalloproteinases; PC prostacyclin; PGs prostaglandins; PLA2 phospholipase A2; PLGF placental growth factor; PPARγ proliferator-activated receptor gamma; ROS reactive oxygen species; TGF-β transforming growth factor beta; TA thromboxane; TNF-α Tumor necrosis factor alpha; VEGF vascular endothelial growth factor; VEGFR vascular endothelial growth factor receptor
Angiogenesis is strongly orchestrated by a variety of angiogenic cytokines, such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), transforming growth factor beta (TGF-β), and interleukin (IL)-1β [45, 46], and anti-angiogenic cytokines [46]. These cytokines contribute to the proliferation and migration of endothelial cells (EC), which is considered the hallmark of angiogenesis [46, 47].
One study reported significant correlations between levels of different cytokines in the first 3 weeks after birth and ROP development [48]. In an OIR model, the investigation of 94 selected genes known to be related to inflammation showed that many of them were upregulated in association with the clinical appearance of OIR [49]. The same authors analyzed the vitreous levels of 27 cytokines in PTI with stage 4 ROP and found higher levels of interleukin (IL) -6, IL-7, IL-10, IL-15, Eotaxin, bFGF, Granulocyte colony-stimulating factor (G-CSF), interferon-gamma-inducible protein (IP) -10, and mainly, VEGF [50].
Molecules of the IL-1 family acting as the first line of defense against invasive pathogenic microorganisms and physical damage play an important role in inflammatory and immune responses. However, many cytokines in the IL-1 family, such as IL-1α, IL-1β, IL-18, IL-33, and IL-37, contribute significantly to angiogenesis [46, 51]. IL-1β, an important mediator of inflammation [46, 47], in ischemic conditions of the retina is markedly increased in recruited neutrophils, EC, and retinal glial cells [52, 53] and has been implicated in the development of vasoproliferative retinopathies [53]. It has been suggested that in the hypoxic neonatal retina, activated microglial cells produce increased amounts of IL-1β and tumor necrosis factor alpha (TNF-α) that can induce retinal ganglion cell death [54]. TNF-α is also known to contribute to the breakdown of the blood–retinal barrier [54, 55].
In an OIR model, it was shown that retinal microglia is induced to produce IL-1β, leading to microvascular injury by the release of semaphorin 3A (Sema3A) from adjacent neurons [53]. Inhibition of the IL-1 β receptor prevented microglial activation and Sema3A expression in the retina, resulting in a significant decrease in vaso-obliteration and in the subsequent pathological pre-retinal neovascularization [53]. In another OIR model, inhibition of the IL-1 β receptor preserved the choroid and prevented external neuroretinal abnormalities, suggesting IL-1 β as a potential therapeutic target in ROP [56].
A mouse model of premature birth, in which chorioamnionitis was induced with an injection of IL-1β in utero, revealed that IL-1β causes sustained eye inflammation accompanied by delayed development of the retinal BV and thinning of the choroid, with all deleterious effects being prevented by antenatal administration of IL-1 receptor antagonist (IL-1Ra) [57]. However, in a study with preterm infants (PTI), the levels of IL-1β in the vitreous were identical and below detectable levels in patients with ROP and in control patients [50].
Cytokines have pro- and anti-inflammatory properties and regulate the human immune response acting in conjunction with specific cytokine inhibitors and soluble cytokine receptors [58]. IL-1Ra, IL-4, IL-10, IL-11, and IL-13 are considered anti-inflammatory cytokines [58].
The IL-1Ra was found at significantly elevated levels in the vitreous and tears of PTI with ROP, along with increased levels of VEGF, complement component proteins, and metalloproteinase 9 [59], possibly as a compensatory mechanism to prevent angiogenic effects of IL-18 and IL-1β [60].
In vitro, the inflammatory response induced in microglial cells was markedly reduced by IL-10 which inhibited the expression of TNF-α, MIP-1α, and regulated on activation, normal T cell expressed and secreted (RANTES) [61]. However, in an OIR mouse model, hypoxia guided the behavior of the macrophage to a pro-angiogenic phenotype via IL-10-activated pathways, implicating IL-10 in promoting pathological angiogenesis [62].
IL-38 is a novel cytokine from the IL-1 family that shares high-sequence homology with IL-1Ra [46, 63] and lower homology with IL-1β and other IL-1 family proteins [46]. A recent study in the OIR mice found that administration of IL-38 may help prevent pathogenic neovascularization and inflammation, suggesting that IL-38 is an anti-angiogenic cytokine and may have therapeutic potential for angiogenesis-related diseases [46].
IL-18 is a pleiotropic pro-inflammatory cytokine with an immunoregulatory activity [48]. Studies have suggested that the association of IL-18 with ROP may be as an immunoregulator and modulator of angiogenesis [48], promoting the regression of pathological neovascularization instead of inhibiting its development [64]. IL-6 is known to be a strong inducer of the acute-phase protein response; however, it has both pro-inflammatory and anti-inflammatory properties [58]. Twenty-four hours after birth, elevated levels of IL-6 and TNF-α were observed in PTI who subsequently needed treatment for ROP [65].
Tetrahydrobiopterin (BH4) is a crucial cofactor in several metabolic processes, with a fundamental role in maintaining inflammatory and neurovascular homeostasis [66]. A deficiency in BH4 can produce the uncoupling of endothelial nitric oxide synthase (eNOS), causing a reduction in nitric oxide bioavailability and increased ROS production [66, 67]. BH4 is involved in retinal vascular damage induced by oxygen due to its reduction caused by hyperoxia, which can result in decreased eNOS activity and increased superoxide [66, 68].
Chemokines
Chemokines are a family of low-molecular weight peptides that induce the activation and migration of specific cells, especially immune cells, such as leukocytes and microglia, and are involved in the inflammatory responses [69, 70]. The participation of chemokines in angiogenesis, growth control, and hematopoiesis has also been demonstrated [69].
IL-8, the first chemokine to be characterized, plays important roles in both eye inflammation and pathological neovascularization [71]. In a study involving PTI with early-onset clinical sepsis, elevated plasma levels of IL-8 in the first days of life were associated with later development of ROP requiring treatment [72]. In another study, high concentrations of IL-8 during the first three weeks after premature birth were associated with an increased risk for pre-threshold ROP [73]. According to these results, in an OIR rat model, an increased level of an IL-8 homolog was observed during the peak of pathological neovascularization [69].
Monocyte chemotactic protein 1 (MCP-1), one of the most produced and transitory chemokines during inflammation [74], is expressed by activated microglia of the neuroretina and simultaneously an attraction factor for various cells of the immune system, including macrophages/microglia [75]. MCP-1 was found to be significantly increased in umbilical cord blood from PTI who developed ROP compared to PTI who did not develop ROP [76].
Low concentrations of the chemokine RANTES in the blood [48, 77] and vitreous [50] have been found in PTI who have developed severe ROP, suggesting that RANTES may play a protective role. In agreement, high concentrations of RANTES have been associated with a lower risk of ROP [73].
The current modulation of well-known angiogenic cytokines such as anti-VEGF therapy demonstrated efficacy in ocular neovascularization [46, 78]. However, some patients are refractory to anti-VEGF agents, suggesting that other angiogenic or anti-angiogenic cytokines that contribute in a coordinated manner to angiogenesis need to be identified [46, 51].
Microglia
Microglia cells, the retinal-resident macrophages that provide neuroprotection against transient pathophysiological insults and play an important role in neuronal homeostasis, under sustained pathological stimuli become overactivated and release exaggerated amounts of inflammatory mediators that may promote tissue damage [79]. IL -1β, IL-6, TNF-α, interferon-gamma (IFN- γ), and TGF-β are produced by a variety of cell types, being macrophages and monocytes the most important sources at inflammatory sites [42].
The macrophage population residing in many tissues is mainly derived from the yolk sac and fetal liver; however, after tissue injury, inflammatory monocytes recruited from the bone marrow complement it [80, 81]. Recent studies have indicated that macrophages play different roles in the process of intraocular neovascularization (Fig. 3) [82]. Macrophages can be divided into at least two main phenotypes with different functions: pro-inflammatory M1 macrophages and anti-inflammatory M2 macrophages with a major role in resolving inflammation [82, 83]. After tissue hypoxia, it has been proved that cytokines are involved in the recruitment of monocytes and polarization of macrophages, as well as in angiogenesis [82, 84]. The inflammatory microenvironment leads the macrophages to M1 polarization in an initial phase [85]. The change in the microenvironment in the late inflammatory phase drives macrophages toward the M2 polarization [85].
Mechanisms that influence the main macrophage polarization phenotypes and response to its activation. After injury, resident and recruited macrophages experience remarkable phenotypic and functional variations in response to mediators released into the tissue microenvironment. The dominant phenotype regulates inflammation and tissue repair and may play an active role in the development or inhibition of retinal neovascularization in the OIR model. Cytokines are involved in pathogenesis, monocyte recruitment, and macrophage polarization and therefore are also key factors in the regulation of angiogenesis. IGF-1 insulin-like growth factor 1; IL Interleukin; IFN-γ interferon-gamma; iNOS inducible nitric oxide synthase; LPS lipopolysaccharide; MCP-1 monocyte chemotactic protein 1; MMPs matrix metalloproteinases; OIR oxygen-induced retinopathy; PDGF Platelet-derived growth factor; PLGF placental growth factor; TGF-β transforming growth factor beta; TNF-α Tumor necrosis factor alpha; VEGF vascular endothelial growth factor
The M1 phenotype can be polarized by lipopolysaccharide and IFN-γ, while other cytokines such as IL-4, IL-10, and IL-13 can induce M2 polarization [82, 86]. In the hypoxic microenvironment, it has been suggested that MCP-1 may play a role in the recruitment of monocytes to the vitreous and retina [82].
It is also increasingly clear that epigenetic modifiers can regulate the fate of macrophages [87]. Differentiation toward M1 or M2 polarization and inflammation in situ are regulated by defined microRNAs subsets [85, 88].
M1 macrophages are seen as phagocytic and pro-inflammatory, secreting large amounts of pro-inflammatory cytokines, such as IL-1β, IL-23, and proteases, reactive nitrogen and oxygen intermediates, and little amount of anti-inflammatory IL-10 [85].
M2 macrophages, instead of M1, have been reported to increase angiogenesis in vivo and highly express bFGF, insulin-like growth factor 1 (IGF-1), placental growth factor (PLGF), and MCP-1 [82, 83]. In the OIR model, the M2 phenotype was concentrated around neovascular tufts, promoting the development of retinal neovascularization [89].
Specific molecular targets associated with macrophages can be considered as a potential treatment in the future for retinal neovascularization; however, further studies are needed [82].
Genetic contribution
There is growing evidence that ROP is influenced by genetic predisposition, epigenetic regulation, and environmental factors [35, 90]. The fact that PTI of the same gestational age (GA) and exposed to identical environmental risk factors can develop ROP characterized by different degrees of severity strongly supports a genetic contribution to the etiopathogenesis of ROP [36, 37]. A study concluded that in PTI with extreme phenotypes, the known clinical risk factors were not significantly associated with the development of ROP, suggesting that other clinical, maternal, or genetic factors may predispose or protect from ROP [2].
The evidence of a genetic influence in ROP also comes from two studies with monozygotic and dizygotic twins that obtained an estimated heritability for ROP of 70% and 73%, respectively [91, 92] (Table 1).
In animal models of OIR, studies of different strains of rats observed differences in the avascular area of the retina and the expression of VEGF between the strains, these phenotypic differences also support the influence of a genetic factor [93, 94].
Epidemiological data and the role of β-adrenergic receptor
The influence of a genetic component in the disease was initially based on racial and regional risk profiles resulting from epidemiological studies [95, 96]. The CRYO-ROP study found that although ROP occurred with similar incidence rates in the Caucasian and black populations, severe ROP was less common in black PTI [95, 97]. This result was found in other later studies [2, 98], although one study found an opposite result, a higher incidence of ROP requiring treatment in black PTI than in Caucasian PTI [99]. Studies have found a higher risk of ROP in Asians and Alaskan natives than in Caucasians [100, 101]. One mechanism to explain some of the racial differences observed in ROP is polymorphisms of the β-blocker receptor. A polymorphism of the G protein-coupled 5 kinase receptor desensitizes β-adrenergic receptors causing resistance to noradrenergic stimuli. Retinal EC have β-adrenergic receptors and this theory of polymorphism is reinforced by reports showing an association of cutaneous hemangiomas with ROP, indicating possible common pathogenesis [102]. Cutaneous hemangiomas show a profound reduction with systemic β-blocker treatment [103, 104] and a β-blocker in eye drops, propranolol 0.2%, reduced the progression of ROP in a recent multicenter clinical trial, being promising in the treatment of ROP [105].
Some studies have reported that the incidence of ROP [106] or progression to severe stages [98, 107,108,109] is more frequent in males than in females. However, in other studies, no difference was observed in the incidence of ROP by gender [110, 111].
Wnt pathway
There are several studies on genetic polymorphisms in genes of the canonical Wnt pathway (dependent on beta-catenin) in ROP (Table 2). Variants of genes in the Wnt pathway cause familial exudative vitreoretinopathy (FEVR) or Norrie disease, which are diseases of the retinal development with characteristics similar to ROP although occurring in full-term infants [36, 90, 112]. Both of these diseases are hereditary disorders and have in common dysgenesis of the retinal vessels with a variable breakdown of the blood–retinal barrier, often leading to exudative and tractional retinal detachment [112, 113]. Molecular genetic studies have identified four genes that cause FEVR (NDP, FZD4, LRP5, and TSPAN12), which when mutated cause X-linked, AD, and AR FEVR (also some sporadic cases) [36]. Norrie disease results from mutations in the NDP gene [114].
Several studies correlate variants in genes of the Wnt pathway associated with FEVR or Norrie disease with an increased risk of severe ROP, suggesting involvement of genes associated with the Wnt pathway at least in a low portion of patients with severe ROP [36, 112, 115]. An important limitation of these results is the difficulty in distinguishing between PTI with severe ROP and PTI with FEVR [36]. Due to overlapping phenotypes, ROP is generally differentiated from FEVR by premature birth and lack of family history [112]. However, an ambiguous birth history can confuse the diagnosis [112] and some authors proposed the designation of ROPER (ROP vs. FEVR) to more accurately classify these patients [116].
Vascular endothelial growth factor
VEGF-A plays a key role in physiological and pathological angiogenesis [117, 118]. Studies had implicated VEGF and VEGF receptor (VEGFR)-2 in ROP development [119] and the VEGFA-VEGFR system is the main target for anti-angiogenic treatment [120].
In two studies of the VEGFA -634 polymorphism, the G allele had a higher frequency between PTI with ROP [121, 122]. However, another study reported a higher frequency of C allele in severe ROP [123] and other studies have not confirmed an association between this polymorphism and ROP [124, 125].
VEGFA gene − 460C > T and + 13553C > T polymorphisms have also been associated with ROP [123, 126]. Although − 460C > T has been included in other studies, it was not associated with ROP [125, 127].
The frequency of polymorphisms in the VEGFR-1 and -2 genes did not affect the development of ROP in two studies [125, 126].
Nitric oxide and endothelial nitric oxide synthase
The isoform of nitric oxide-producing enzymes fairly specific for EC, eNOS, has been found to play a notable role in angiogenesis and vasculogenesis [128, 129]. Functional polymorphisms of the eNOS gene affect the expression of eNOS [129] and have been reported to be associated with cardiovascular diseases [130] and diabetic retinopathy in type 1 diabetes [129, 131].
ROP was associated with single-nucleotide polymorphisms (SNPs) of the eNOS gene (T-786C [132, 133] and G894T [132]), but these results have not been replicated in other studies [129, 134].
Neurotrophins and serotonine
Neurotrophins (NTs) are members of a family of polypeptide growth factors that control several aspects related to the survival, differentiation, and function of neurons in the central and peripheral nervous systems and with important functions in non-neuronal cells [135, 136]. NTs family includes nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4/5 (NT-4/5) [137].
NTs bind to tropomyosin kinase (Trk) receptors, NGF with high affinity for TrkA receptor, BDNF for TrkB receptor, and NT-3 for TrkC, resulting in activation of signaling pathways, namely phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT), mitogen-activated protein kinase (MAPK)/extracellular-signal-regulated kinase (ERK), and phospholipase C gamma (PLCγ)/protein kinase C (PKC) [138]. BDNF and NGF have been described as playing an important role in the process of angiogenesis [137]. In different cell models, both NGF and BDNF promote the proliferation, migration, differentiation, and survival of EC [139, 140]. Moreover, both have been shown to induce VEGF expression, thus also having an indirect angiogenic role [141, 142].
Studies have associated lower-serum BDNF concentrations in the first week of life in PTI with the development of severe ROP [48]. A large-scale study of candidate genes in a cohort of PTI found SNPs (rs7934165 and rs2049046) in intronic regions of the BDNF gene associated with severe ROP [143]. This result supports the line of thought that neurovascular connections play a role in the development of ROP [144].
Different studies have shown a close relationship between BDNF and glial cell line-derived neurotrophic factor (GDNF) with the serotonergic system in brain development and neuroplasticity [145, 146]. The synthesis and release of the neurotransmitter serotonin in the retina, and the existence of several types of serotonin receptors expressed in the retina, support its retinal neuromodulatory role [147]. Several data show that serotonin is implicated in retinal physiology and pathophysiology and photoreceptor survival; however, retinal signaling pathways activated by serotonin receptors have been little investigated to date [147].
Hypoxia-inducible factor
Hypoxia-responsive genes, such as VEGF and erythropoietin (EPO), are regulated mainly through hypoxia-inducible factor (HIF) [148], a heterodimeric transcription factor consisting of two subunits, HIF-1-alpha (or HIF-2-alpha and HIF3-alpha, their analogs) and HIF1-beta (also known as ARNT) [149]. During hypoxia, the HIF-1α level increases and it binds to HIF-1β in the nucleus to trigger the transcription of genes involved in angiogenesis and adaptation of cells to hypoxia [149].
Endothelial PAS Domain Protein 1 (EPAS1), also called HIF-2α, has high homology to HIF-1α and, like HIF-1α, EPAS1 is stabilized during hypoxia and forms a heterodimer with the ARNT translocator and transactivates the VEGF promoter [150]. In addition, this heterodimer complex has also been shown to transactivate Flt-1, which encodes VEGFR-1 [150].
In a study with a hyperoxia/normoxia treatment, using a murine model of ROP, HIF-2α-knockdown mice showed no evidence of retinal neovascularization when compared to wild-type mice [151]. The expression of EPO mRNA was also significantly decreased when compared to control mice [151]. A candidate gene study in PTI found an association between EPAS1 and the development of severe ROP [152]. It is possible that polymorphisms in the EPAS1 gene in PTI predispose to increased expression of angiogenic factors, such as VEGF and EPO [152].
Erythropoietin
EPO is an oxygen-regulated growth factor [44] and also an important angiogenic factor, its production being regulated by HIF [149]. EPO plays an important role in both the first and second phases of ROP [153]. A candidate gene study investigated the influence of an EPO polymorphism in the development of ROP, but no statistical significance was observed [152].
Insulin-like growth factor 1
IGF-1 is a growth factor supplied by the placenta and amniotic fluid that is crucial for fetal development, including healthy retinal angiogenesis [153]. It has been reported that it is also essential for postnatal vascular eye development and that a prolonged period of low levels of IGF-1 can predict the development of ROP and other diseases related to prematurity [154]. In patients with a genetic defect in the production of IGF-1, a reduction in retinal vascularization was observed, which has not been restored after the administration of IGF-1 [155].
Because the level of IGF-1 is determined by the IGF-1 receptor [156], it is possible that the most prevalent IGF-1 receptor polymorphism (c.3174G > A), which exhibits low levels of free plasma IGF-1, has a role in ROP [36]. However, the association of this polymorphism with the risk of advanced ROP has not been proven by studies in different populations [157, 158].
Angiopoietins
Angiopoietins (ANGs) are growth factors that regulate physiological and pathological neovascularization, specifically in association with VEGF [159, 160]. Although ANG-1 and ANG-2 bind to the Tie2 tyrosine kinase receptor, ANG-2 is a functional ANG-1 antagonist [161, 162]. ANG-1 contributes to the maintenance of vascular integrity [163], while ANG-2 stimulates neovascularization [164] and is upregulated by VEGF and hypoxia [165, 166].
The influence of the ANG-2 (− 35G > C) gene polymorphism on ROP was investigated in two studies; however, no significance was reported [167, 168].
Mediators of immune and inflammatory response
Studies involving PTI have demonstrated the presence of multiple and complex associations of polymorphisms that occur in genes involved in the pro-inflammatory and pro-oxidant response with premature birth and the occurrence of SO diseases complicating prematurity [1, 23]
Regarding the association with ROP, one study suggested an increased risk of ROP progression with the presence of SNPs from the IL-10, IL-1β, and TNF-α genes, without changing the risk with an SNP of the Toll-like receptor-4 (TLR-4) gene, although none of these trends reached formal statistical significance [169].
Heme oxygenase 1
Heme oxygenase 1 (HMOX-1) is an enzyme that breaks down heme into iron ions, carbon monoxide, and biliverdin [134]. HMOX-1 products perform important physiological functions in the vascular system, related to the protection of the endothelium through a cytoprotective, promitogenic, and anti-inflammatory action [170].
The effectiveness of this enzyme is affected by repeated polymorphisms in the HMOX-1 gene promoter [170]. Despite this, no significant association was found between HMOX-1 and ROP in a candidate gene study [134].
Renin–angiotensin system
It has been shown that the renin–angiotensin system may influence the early stages of retinal vascularization [171] and retinal neovascularization was prevented by blocking the renin–angiotensin system in a rat model of ROP [172].
Angiotensin-converting enzyme gene polymorphism has been associated with ROP in the population of Kuwait [173], but not in another population [174].
A study of Angiotensin gene polymorphism found no association with ROP [134], while studies on the angiotensin receptor 1 gene found an association with the development of ROP [152] or found no association [134].
Matrix metalloproteinases
Matrix metalloproteinases (MMPs) are endopeptidases that hydrolyze the extracellular matrix (ECM) [175] and play an important role in inflammatory responses and angiogenesis among other various biological processes [176]. “A” disintegrin and metalloproteinase (ADAM) is also a family of enzymes involved in the degradation of components of the ECM [177].
Based on studies in animal models of OIR, some subtypes of the ADAM family have been suggested to be implicated in the ROP pathogenesis [178, 179]. Genetic studies in humans are needed to assess the influence of ECM, metalloproteinases, and the ADAM family on the pathogenesis of ROP.
Other studies
Apart from the studies described involving factors related to the pathogenesis of ROP, a large study mentioned above identified candidate genes with an unknown relationship with ROP, such as the complement factor H gene [152].
From the above, several evidences suggest a genetic contribution to ROP; however, it is not yet clear which genes or genetic polymorphisms are significantly associated with the development and progression of ROP. Many of the ROP candidate gene studies have limitations, essentially small sample size, non-replicable, or conflicting results from several studies, the latter of which may be at least in part, due to differences in neonatal care and clinical characterization in different countries or populations. In some studies, it may also be difficult to separate the contribution of genetic factors associated with ROP from those associated with prematurity itself.
Epigenetic studies on ROP have not been found in the literature, which could be an important contribution to the emergence of new methods for the treatment of this pathology. Future studies involving next-generation sequencing and genome-wide association, integrated with metabolomics and proteomics, may provide a better understanding of the genetic risk factors and pathophysiology of ROP and contribute to finding new solutions in the management and treatment of ROP.
Expected clinical applicability of studies on genetics and inflammatory pathways in ROP
The search for biomarkers to detect PTI with increased risk of developing ROP allowed the identification of potential indicators involved in inflammatory and angiogenic pathways. Circulating and genetic biomarkers can be incorporated into models to predict the risk of developing ROP. A risk analysis system that includes biomarkers and clinical risk factors can help neonatologists and ophthalmologists identify high-risk PTI. This may allow the development of more adequate follow-up strategies depending on the risk level of the PTI and reduce the number of ROP screening tests for those at lower risk.
A better understanding of the genetic contribution to the pathogenesis of ROP may also help to find new targets that lead to the development of therapeutic approaches that are more effective and less harmful than current ones. In addition, knowledge of the influence of genetic polymorphisms on phenotypic biomarkers (biochemical and cellular) can contribute to defining the ROP phase and, thus, choosing the most appropriate therapeutic approach over time.
Furthermore, a deeper knowledge of the molecular and genetic mechanisms involved in ROP may help to better understand and treat other oxidative stress diseases associated with prematurity with which ROP shares etiopathogenic factors.
Many molecules and related signaling pathways suspected to be involved in the pathogenesis of ROP are common to other pediatric and adult ischemic retinopathies. Thus, a deeper comprehension of molecular mechanisms in ROP may provide important insights to other retinal neovascular pathologies.
Conclusion
Several pieces of evidence suggest that the pathogenesis of ROP begins in utero. Perinatal inflammation and genetic factors may contribute to the development and progression of ROP.
Studies have implicated the involvement of factors linked to the inflammatory process, such as leukocytes, monocytes, macrophages, cytokines, chemokines, and growth factors, in angiogenesis and pathological vascular development of ROP. Cytokines are also involved in monocyte recruitment and macrophage polarization. Macrophages may be recruited by long-term pathological neovascularization, but they can also promote pathological neovascularization.
Several studies have found genetic polymorphisms in candidate genes associated with ROP or severe ROP. Many pathways and their signaling molecules have been studied due to their connection with the pathogenesis of ROP. Associations were found between genes involved in the WNT signaling pathway, the VEGFA gene and the eNOS gene, and the development of ROP. A large multicenter study found polymorphisms in the BDNF gene associated with severe ROP.
Although multiple genes have been implicated in several investigations, a genetic component with a major impact on ROP has not yet been discovered. Several of these studies have not replicated findings mainly because of limitations in aspects such as sample size, non-replicable or conflicting results, and differences in neonatal care or inclusion criteria. The knowledge of such a genetic component would possibly allow the identification of possible targets to improve the screening and treatment of ROP.
New technologies involving bioinformatics, genomics, and proteomics may contribute to find genes or pathways associated with ROP and help in the future to find better solutions in the management and treatment of ROP.
Data availability
Not applicable.
Abbreviations
- AA:
-
Arachidonic acid
- ADAM:
-
“A” disintegrin and metalloproteinase
- ANGs:
-
Angiopoietins
- BDNF:
-
Brain-derived neurotrophic factor
- bFGF:
-
Basic fibroblast growth factor
- BH4:
-
Tetrahydrobiopterin
- BV:
-
Blood vessels
- BW:
-
Birth weight
- EC:
-
Endothelial cells
- eNOS:
-
Endothelial nitric oxide synthase
- ECM:
-
Extracellular matrix
- EPAS1:
-
Endothelial PAS Domain Protein 1
- EPO:
-
Erythropoietin
- FEVR:
-
Familial exudative vitreoretinopathy
- GA:
-
Gestational age
- HIF:
-
Hypoxia-inducible factor
- HMOX-1:
-
Heme oxygenase 1
- ICAM-1:
-
Intercellular adhesion molecule-1
- IFN-γ:
-
Interferon gamma
- IGF-1:
-
Insulin-like growth factor 1
- IL:
-
Interleukin
- IL-1Ra:
-
Interleukin 1 receptor antagonist
- iNOS:
-
Inducible nitric oxide synthase
- I-TAC:
-
Interferon-inducible T-cell alpha chemoattractant
- LPS:
-
Lipopolysaccharide
- MCP-1:
-
Monocyte chemotactic protein 1
- MMPs:
-
Matrix metalloproteinases
- NGF:
-
Nerve growth factor
- NTs:
-
Neurotrophins
- OIR:
-
Oxygen-induced retinopathy
- OS:
-
Oxidative stress
- PC:
-
Prostacyclin
- PDGF:
-
Platelet-derived growth factor
- PGs:
-
Prostaglandins
- PLA2:
-
Phospholipase A2
- PLGF:
-
Placental growth factor
- PPARγ:
-
Proliferator-activated receptor gamma
- PTI:
-
Preterm infants
- RANTES:
-
Regulated on activation, normal T cell expressed and secreted
- ROP:
-
Retinopathy of prematurity
- ROS:
-
Reactive oxygen species
- Sema3A:
-
Semaphoring 3A
- SNPs:
-
Single-nucleotide polymorphisms
- TA:
-
Thromboxane
- TGF-β:
-
Transforming growth factor beta
- TNF-α:
-
Tumor necrosis factor alpha
- Trk:
-
Tropomyosin kinase
- VEGF:
-
Vascular endothelial growth factor
- VEGFR:
-
Vascular endothelial growth factor receptor
- Wk:
-
Weeks
References
Giusti B, Vestrini A, Poggi C, Magi A, Pasquini E, Abbate R, Dani C (2012) Genetic polymorphisms of antioxidant enzymes as risk factors for oxidative stress-associated complications in preterm infants. Free Radic Res 46:1130–1139. https://doi.org/10.3109/10715762.2012.692787
Port AD, Chan RVP, Ostmo S, Choi D, Chiang MF (2014) Risk factors for retinopathy of prematurity: insights from outlier infants. Graefe’s Arch Clin Exp Ophthalmol 252(10):1669–1677. https://doi.org/10.1007/s00417-014-2716-1
Aydin H, Gunay M, Celik G, Gunay BO, Taka U, Karaman A (2016) Evaluation of Factor V Leiden, Prothrombin G20210A, MTHFR C677T and MTHFR A1298C gene polymorphisms in retinopathy of prematurity in a Turkish cohort. Ophthalmic Genet 37(4):415–418. https://doi.org/10.3109/13816810.2015.1126611
Jang JH, Kim YC (2020) Retinal vascular development in an immature retina at 33–34 weeks postmenstrual age predicts retinopathy of prematurity. Sci Rep 10(1):18111. https://doi.org/10.1038/s41598-020-75151-0
Smith LEH (2004) Pathogenesis of retinopathy of prematurity. Growth Horm IGF Res. https://doi.org/10.1016/j.ghir.2004.03.030
Liu CH, Wang Z, Sun Y, Chen J (2017) Animal models of ocular angiogenesis: from development to pathologies. FASEB J 31(11):4665–4681. https://doi.org/10.1096/fj.201700336R
Suwanpradid J, Rojas M, Behzadian MA, Caldwell RW, Caldwell RB (2014) Arginase 2 deficiency prevents oxidative stress and limits hyperoxia-induced retinal vascular degeneration. PLoS ONE 9(11):e110604. https://doi.org/10.1371/journal.pone.0110604
Perrone S, Santacroce A, Longini M, Proietti F, Bazzini F, Buonocore G (2018) The free radical diseases of prematurity: from cellular mechanisms to bedside. Oxid Med Cell Longev 2018:7483062. https://doi.org/10.1155/2018/7483062
Hartnett ME (2015) Pathophysiology and mechanisms of severe retinopathy of prematurity. Ophthalmology 122(1):200–210. https://doi.org/10.1016/j.ophtha.2014.07.050
Smith LEH (2008) Through the eyes of a child: understanding retinopathy through ROP—The Friedenwald Lecture. Invest Ophthalmol Vis Sci 49(12):5177–5182. https://doi.org/10.1167/iovs.08-2584
Sapieha P, Hamel D, Shao Z, Rivera JC, Zaniolo K, Joyal JS, Chemtob S (2010) Proliferative retinopathies: angiogenesis that blinds. Int J Biochem Cell Biol 42(1):5–12. https://doi.org/10.1016/j.biocel.2009.10.006
Chiang MF, Quinn GE, Fielder AR et al (2021) International classification of retinopathy of prematurity, third edition. Ophthalmology 128(10):e51–e68. https://doi.org/10.1016/j.ophtha.2021.05.031
Hartnett ME (2010) Studies on the pathogenesis of avascular retina and neovascularization into the vitreous in peripheral severe retinopathy of prematurity (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc 108:96–119
Zin A, Gole GA (2013) Retinopathy of prematurity-incidence today. Clin Perinatol 40(2):185–200. https://doi.org/10.1016/j.clp.2013.02.001
Chang JW (2019) Risk factor analysis for the development and progression of retinopathy of prematurity. PLoS ONE 14(7):e0219934. https://doi.org/10.1371/journal.pone.0219934
Quinn GE, Ying GS, Bell EF, Donohue PK, Morrison D, Tomlinson LA, Binenbaum G (2018) Incidence and early course of retinopathy of prematurity: secondary analysis of the Postnatal Growth and Retinopathy of Prematurity (G-ROP) Study. JAMA Ophthalmol 136(12):1383–1389. https://doi.org/10.1001/jamaophthalmol.2018.4290
Blencowe H, Lawn JE, Vazquez T, Fielder A, Gilbert C (2013) Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res 74(Suppl 1):35–49. https://doi.org/10.1038/pr.2013.205
Goldstein GP, Leonard SA, Kan P, Koo EB, Lee HC, Carmichael SL (2019) Prenatal and postnatal inflammation-related risk factors for retinopathy of prematurity. J Perinatol 39(7):964–973. https://doi.org/10.1038/s41372-019-0357-2
Saugstad OD (2005) Oxidative stress in the newborn—a 30-year perspective. Biol Neonate 88(3):228–236. https://doi.org/10.1159/000087586
Dammann O (2010) Inflammation and retinopathy of prematurity. Acta Paediatr Int J Paediatr 99(7):975–977. https://doi.org/10.1111/j.1651-2227.2010.01836.x
Sullivan JL (1986) Retinopathy of prematurity and iron: a modification of the oxygen hypothesis. Pediatrics 78(6):1171–1172
Miao L, St. Clair DK (2009) Regulation of superoxide dismutase genes: Implications in disease. Free Radic Biol Med 47(4):344–356. https://doi.org/10.1016/j.freeradbiomed.2009.05.018
Poggi C, Giusti B, Vestri A, Pasquini E, Abbate R, Dani C (2012) Genetic polymorphisms of antioxidant enzymes in preterm infants. J Matern Neonatal Med 25(Suppl 4):131–134. https://doi.org/10.3109/14767058.2012.714976
Good WV, Hardy RJ, Dobson V et al (2005) The incidence and course of retinopathy of prematurity: findings from the early treatment for retinopathy of prematurity study. Pediatrics 116(1):15–23. https://doi.org/10.1542/peds.2004-1413
Patz A (1980) Studies on retinal neovascularization. Friedenwald lecture. Investig Ophthalmol Vis Sci 19(10):1133–1138
York JR, Landers S, Kirby RS, Arbogast PG, Penn JS (2004) Arterial oxygen fluctuation and retinopathy of prematurity in very-low-birth-weight infants. J Perinatol 24(2):82–87. https://doi.org/10.1038/sj.jp.7211040
Lenhartova N, Matasova K, Lasabova Z, Javorka K (2017) Impact of early aggressive nutrition on retinal development in premature infants. Physiol Res 66(Suppl 2):S215–S226. https://doi.org/10.33549/physiolres.933677
Gagliardi L, Rusconi F, Da Frè M, Mello G, Carnielli V, Di Lallo D, Macagno F, Miniaci S, Corchia C, Cuttini M (2013) Pregnancy disorders leading to very preterm birth influence neonatal outcomes: results of the population-based ACTION cohort study. Pediatr Res 73(6):794–801. https://doi.org/10.1038/pr.2013.52
Gallo J, Jacobson L, Broberger U (1993) Perinatal factors associated with retinopathy of prematurity. Acta Pædiatrica 82(10):829–834. https://doi.org/10.1111/j.1651-2227.1993.tb12573.x
Spiegler J, Jensen R, Segerer H, Ehlers S, Kühn T, Jenke A, Gebauer C, Möller J, Orlikowsky T, Heitmann F, Boeckenholt K, Herting E, Göpel W (2013) Influence of smoking and alcohol during pregnancy on outcome of VLBW infants. Z Geburtshilfe Neonatol 217(6):215–219. https://doi.org/10.1055/s-0033-1361145
Reem RE, Nguyen T, Yu Y, Ying G-S, Tomlinson LA, Binenbaum G (2021) Effects of altitude on retinopathy of prematurity. J Am Assoc Pediatr Ophthalmol Strabismus 61(7):2190. https://doi.org/10.1016/j.jaapos.2021.08.254
Yang MB, Rao S, Copenhagen DR, Lang RA (2013) Length of day during early gestation as a predictor of risk for severe retinopathy of prematurity. Ophthalmology 120(12):2706–2713. https://doi.org/10.1016/j.ophtha.2013.07.051
Chan RV, Yonekawa Y, Morrison MA, Sun G, Wong RK, Perlman JM, Chiang MF, Lee TC, Hartnett ME, Deangelis MM (2010) Association between assisted reproductive technology and advanced retinopathy of prematurity. Clin Ophthalmol 4:1385–1390. https://doi.org/10.2147/OPTH.S15587
Barker L, Bunce C, Husain S, Adams GGW (2017) Is artificial reproductive technology a risk factor for retinopathy of prematurity independent of the generation of multiple births? Eur J Ophthalmol 27(2):174–178. https://doi.org/10.5301/ejo.5000832
Hartnett ME (2017) Advances in understanding and management of retinopathy of prematurity. Surv Ophthalmol 62(3):257–276. https://doi.org/10.1016/j.survophthal.2016.12.004
Shastry BS (2010) Genetic susceptibility to advanced retinopathy of prematurity (ROP). J Biomed Sci 17(1):69. https://doi.org/10.1186/1423-0127-17-69
Pietrzyk JJ, Kwinta P, Bik-Multanowski M, Madetko-Talowska A, Jagła M, Tomasik T, Mitkowska Z, Wollen EJ, Nygård S, Saugstad OD (2013) New insight into the pathogenesis of retinopathy of prematurity: assessment of whole-genome expression. Pediatr Res 73(4 Pt 1):476–483. https://doi.org/10.1038/pr.2012.195
Lee J, Dammann O (2012) Perinatal infection, inflammation, and retinopathy of prematurity. Semin Fetal Neonatal Med 17(1):26–29. https://doi.org/10.1016/j.siny.2011.08.007
Ahn YJ, Hong KE, Yum HR, Lee JH, Kim KS, Youn YA, Park SH (2017) Characteristic clinical features associated with aggressive posterior retinopathy of prematurity. Eye 31(6):924–930. https://doi.org/10.1038/eye.2017.18
Wang X, Tang K, Chen L, Cheng S, Xu H (2019) Association between sepsis and retinopathy of prematurity: a systematic review and meta-analysis. BMJ Open 9(5):e025440. https://doi.org/10.1136/bmjopen-2018-025440
Hong HK, Lee HJ, Ko JH, Park JH, Park JY, Choi CW, Yoon CH, Ahn SJ, Park KH, Woo SJ, Oh JY (2014) Neonatal systemic inflammation in rats alters retinal vessel development and simulates pathologic features of retinopathy of prematurity. J Neuroinflam 11:87. https://doi.org/10.1186/1742-2094-11-87
Gabay C, Kushner I (1999) Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 340(6):448–454. https://doi.org/10.1056/NEJM199902113400607
Wang H, Zhang SX, Hartnett ME (2013) Signaling pathways triggered by oxidative stress that mediate features of severe retinopathy of prematurity. Arch Ophthalmol 131(1):80–85. https://doi.org/10.1001/jamaophthalmol.2013.986
Mataftsi A, Dimitrakos SA, Adams GGW (2011) Mediators involved in retinopathy of prematurity and emerging therapeutic targets. Early Hum Dev 87(10):683–690. https://doi.org/10.1016/j.earlhumdev.2011.05.009
Folkman J, D’Amore PA (1996) Blood vessel formation: what is its molecular basis? Cell 87(7):1153–1155. https://doi.org/10.1016/s0092-8674(00)81810-3
Zhang J, Zhao R, Chen J, Jin J, Yu Y, Tian Y, Li W, Wang W, Zhou H, Su SB (2017) The effect of interleukin 38 on angiogenesis in a model of oxygen-induced retinopathy. Sci Rep 7(1):256. https://doi.org/10.1038/s41598-017-03079-z
Chung AS, Ferrara N (2011) Developmental and pathological angiogenesis. Annu Rev Cell Dev Biol 27:563–584. https://doi.org/10.1146/annurev-cellbio-092910-154002
Sood BG, Madan A, Saha S, Schendel D, Thorsen P, Skogstrand K, Hougaard D, Shankaran S, Carlo W (2010) Perinatal systemic inflammatory response syndrome and retinopathy of prematurity. Pediatr Res 67(4):394–400. https://doi.org/10.1203/PDR.0b013e3181d01a36
Sato T, Kusaka S, Hashida N, Saishin Y, Fujikado T, Tano Y (2009) Comprehensive gene-expression profile in murine oxygen-induced retinopathy. Br J Ophthalmol 93(1):96–103. https://doi.org/10.1136/bjo.2008.142646
Sato T, Kusaka S, Shimojo H, Fujikado T (2009) Simultaneous analyses of vitreous levels of 27 cytokines in eyes with retinopathy of prematurity. Ophthalmology 116(11):2165–2169. https://doi.org/10.1016/j.ophtha.2009.04.026
Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN (2003) IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci USA 100(5):2645–2650. https://doi.org/10.1073/pnas.0437939100
Hangai M, Yoshimura N, Yoshida M, Yabuuchi K, Honda Y (1995) Interleukin-1 gene expression in transient retinal ischemia in the rat. Investig Ophthalmol Vis Sci 36(3):571–578
Rivera JC, Sitaras N, Noueihed B, Hamel D, Madaan A, Zhou T, Honoré JC, Quiniou C, Joyal JS, Hardy P, Sennlaub F, Lubell W, Chemtob S (2013) Microglia and interleukin-1β in ischemic retinopathy elicit microvascular degeneration through neuronal semaphorin-3A. Arterioscler Thromb Vasc Biol 33(8):1881–1891. https://doi.org/10.1161/ATVBAHA.113.301331
Sivakumar V, Foulds WS, Luu CD, Ling EA, Kaur C (2011) Retinal ganglion cell death is induced by microglia derived pro-inflammatory cytokines in the hypoxic neonatal retina. J Pathol 224:245–260. https://doi.org/10.1002/path.2858
Luna JD, Chan CC, Derevjanik NL, Mahlow J, Chiu C, Peng B, Tobe T, Campochiaro PA, Vinores SA (1997) Blood-retinal barrier (BRB) breakdown in experimental autoimmune uveoretinitis: Comparison with vascular endothelial growth factor, tumor necrosis factor and interleukin-1β-mediated breakdown. J Neurosci Res 49(3):268–280. https://doi.org/10.1002/(SICI)1097-4547(19970801)49:3%3c268::AID-JNR2%3e3.0.CO;2-A
Zhou TE, Rivera JC, Bhosle VK et al (2016) Choroidal Involution Is Associated with a Progressive Degeneration of the Outer Retinal Function in a Model of Retinopathy of Prematurity: Early Role for IL-1β. Am J Pathol 186(12):3100–3116. https://doi.org/10.1016/j.ajpath.2016.08.004
Beaudry-Richard A, Nadeau-Vallée M, Prairie É et al (2018) Antenatal IL-1-dependent inflammation persists postnatally and causes retinal and sub-retinal vasculopathy in progeny. Sci Rep 8:11875. https://doi.org/10.1038/s41598-018-30087-4
Opal SM, DePalo VA (2000) Anti-inflammatory cytokines. Chest 117(4):1162–1172. https://doi.org/10.1378/chest.117.4.1162
Rathi S, Jalali S, Patnaik S et al (2017) Abnormal complement activation and inflammation in the pathogenesis of retinopathy of prematurity. Front Immunol 8:1868. https://doi.org/10.3389/fimmu.2017.01868
Wooff Y, Man SM, Aggio-Bruce R, Natoli R, Fernando N (2019) IL-1 family members mediate cell death, inflammation and angiogenesis in retinal degenerative diseases. Front Immunol 10:1618. https://doi.org/10.3389/fimmu.2019.01618
Kremlev SG, Palmer C (2005) Interleukin-10 inhibits endotoxin-induced pro-inflammatory cytokines in microglial cell cultures. J Neuroimmunol 162(1–2):71–80. https://doi.org/10.1016/j.jneuroim.2005.01.010
Dace DS, Khan AA, Kelly J, Apte RS (2008) Interleukin-10 promotes pathological angiogenesis by regulating macrophage response to hypoxia during development. PLoS ONE 3(10):e3381. https://doi.org/10.1371/journal.pone.0003381
Bensen JT, Dawson PA, Mychaleckyj JC, Bowden DW (2001) Identification of a novel human cytokine gene in the interleukin gene cluster on chromosome 2q12-14. J Interf Cytokine Res 21(11):899–904. https://doi.org/10.1089/107999001753289505
Qiao H, Sonoda K-H, Ikeda Y, Yoshimura T, Hijioka K, Jo Y-J, Sassa Y, Tsutsumi-Miyahara C, Hata Y, Akira S, Ishibashi T (2007) Interleukin-18 regulates pathological intraocular neovascularization. J Leukoc Biol 81(4):1012–1021. https://doi.org/10.1189/jlb.0506342
Hellgren G, Löfqvist C, Hansen-Pupp I, Gram M, Smith LE, Ley D, Hellström A (2018) Increased postnatal concentrations of pro-inflammatory cytokines are associated with reduced IGF-I levels and retinopathy of prematurity. Growth Horm IGF Res 39:19–24. https://doi.org/10.1016/j.ghir.2017.11.006
Rivera JC, Noueihed B, Madaan A, Lahaie I, Pan J, Belik J, Chemtob S (2017) Tetrahydrobiopterin (BH4) deficiency is associated with augmented inflammation and microvascular degeneration in the retina. J Neuroinflammation 14:181. https://doi.org/10.1186/s12974-017-0955-x
Brand MP, Heales SJR, Land JM, Clark JB (1995) Tetrahydrobiopterin deficiency and brain nitric oxide synthase in the hph1 mouse. J Inherit Metab Dis 18(1):33–39. https://doi.org/10.1007/BF00711370
Edgar KS, Matesanz N, Gardiner TA, Katusic ZS, McDonald DM (2015) Hyperoxia depletes (6R)-5,6,7,8-tetrahydrobiopterin levels in the neonatal retina: Implications for nitric oxide synthase function in retinopathy. Am J Pathol 185(6):1769–1782. https://doi.org/10.1016/j.ajpath.2015.02.021
Powers MR, Davies MH, Eubanks JP (2005) Increased expression of chemokine KC, an interleukin-8 homologue, in a model of oxygen-induced retinopathy. Curr Eye Res 30(4):299–307. https://doi.org/10.1080/02713680590923276
Hughes CE, Nibbs RJB (2018) A guide to chemokines and their receptors. FEBS J 285(16):2944–2971. https://doi.org/10.1111/febs.14466
Ghasemi H, Ghazanfari T, Yaraee R, Faghihzadeh S, Hassan ZM (2011) Roles of IL-8 in ocular inflammations: a review. Ocul Immunol Inflamm 19(6):401–412. https://doi.org/10.3109/09273948.2011.618902
Silveira RC, Fortes Filho JB, Procianoy RS (2011) Assessment of the contribution of cytokine plasma levels to detect retinopathy of prematurity in very low birth weight infants. Invest Ophthalmol Vis Sci 52(3):1297–1301. https://doi.org/10.1167/iovs.10
Holm M, Morken TS, Fichorova RN, VanderVeen DK, Allred EN, Dammann O, Leviton A (2017) Systemic inflammation-associated proteins and retinopathy of prematurity in infants born before the 28th week of gestation. Investig Ophthalmol Vis Sci 58(14):6419–6428. https://doi.org/10.1167/iovs.17-21931
Yao Y, Tsirka SE (2014) Monocyte chemoattractant protein-1 and the blood–brain barrier. Cell Mol Life Sci 71(4):683–697. https://doi.org/10.1007/s00018-013-1459-1
Yoshida S (2003) Role of MCP-1 and MIP-1alpha in retinal neovascularization during postischemic inflammation in a mouse model of retinal neovascularization. J Leukoc Biol 73(1):137–144. https://doi.org/10.1189/jlb.0302117
Yu H, Yuan L, Zou Y, Peng L, Wang Y, Li T, Tang S (2014) Serum concentrations of cytokines in infants with retinopathy of prematurity. APMIS 122(9):818–823. https://doi.org/10.1111/apm.12223
Hellgren G, Willett K, Engstrom E, Thorsen P, Hougaard DM, Jacobsson B, Hellstrom A, Lofqvist C (2010) Proliferative retinopathy is associated with impaired increase in BDNF and RANTES expression levels after preterm birth. Neonatology 98(4):409–418. https://doi.org/10.1159/000317779
Kamba T, McDonald DM (2007) Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer 96(12):1788–1795. https://doi.org/10.1038/sj.bjc.6603813
Rashid K, Akhtar-Schaefer I, Langmann T (2019) Microglia in retinal degeneration. Front Immunol 10:1975. https://doi.org/10.3389/fimmu.2019.01975
Davies LC, Jenkins SJ, Allen JE, Taylor PR (2013) Tissue-resident macrophages. Nat Immunol 14(10):986–995. https://doi.org/10.1038/ni.2705
Galli SJ, Borregaard N, Wynn TA (2011) Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol 12(11):1035–1044. https://doi.org/10.1038/ni.2109
Di ZY, Yoshida S, Peng YQ, Kobayashi Y, Zhang LS, Tang LS (2017) Diverse roles of macrophages in intraocular neovascular diseases: a review. Int J Ophthalmol 10(12):1902–1908. https://doi.org/10.18240/ijo.2017.12.18
Jetten N, Verbruggen S, Gijbels MJ, Post MJ, De Winther MPJ, Donners MMPC (2014) Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 17(1):109–118. https://doi.org/10.1007/s10456-013-9381-6
Sica A, Erreni M, Allavena P, Porta C (2015) Macrophage polarization in pathology. Cell Mol Life Sci 72(21):4111–4126. https://doi.org/10.1007/s00018-015-1995-y
Ribatti D (2017) The contribution of immune cells to angiogenesis in inflammation and tumor growth. Inflammation and angiogenesis. Springer, Cham, pp 27–84
Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M (2013) Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 229(2):176–185. https://doi.org/10.1002/path.4133
Chen S, Yang J, Wei Y, Wei X (2020) Epigenetic regulation of macrophages: from homeostasis maintenance to host defense. Cell Mol Immunol 17:36–49. https://doi.org/10.1038/s41423-019-0315-0
Ponomarev ED, Veremeyko T, Weiner HL (2013) MicroRNAs are universal regulators of differentiation, activation, and polarization of microglia and macrophages in normal and diseased CNS. Glia 61(1):91–103. https://doi.org/10.1002/glia.22363
Zhou Y, Yoshida S, Nakao S, Yoshimura T, Kobayashi Y, Nakama T, Kubo Y, Miyawaki K, Yamaguchi M, Ishikawa K, Oshima Y, Akashi K, Ishibashi T (2015) M2 macrophages enhance pathological neovascularization in the mouse model of oxygen-induced retinopathy. Investig Ophthalmol Vis Sci 56(8):4767–4777. https://doi.org/10.1167/iovs.14-16012
Hartnett ME, Cotten CM (2015) Genomics in the neonatal nursery: focus on ROP. Semin Perinatol 39(8):604–610. https://doi.org/10.1053/j.semperi.2015.09.007
Bizzarro MJ, Hussain N, Jonsson B, Feng R, Ment LR, Gruen JR, Zhang H, Bhandari V (2006) Genetic susceptibility to retinopathy of prematurity. Pediatrics 118(5):1858–1863. https://doi.org/10.1542/peds.2006-1088
Ortega-Molina JM, Anaya-Alaminos R, Uberos-Fernández J, Solans-Pérez De Larraya A, Chaves-Samaniego MJ, Salgado-Miranda A, Piñar-Molina R, Jerez-Calero A, García-Serrano JL (2015) Genetic and environmental influences on retinopathy of prematurity. Mediators Inflamm 2015:764159. https://doi.org/10.1155/2015/764159
Van Wijngaarden P, Coster DJ, Brereton HM, Gibbins IL, Williams KA (2005) Strain-dependent differences in oxygen-induced retinopathy in the inbred rat. Investig Ophthalmol Vis Sci 46(4):1445–1452. https://doi.org/10.1167/iovs.04-0708
Floyd BNI, Leske DA, Wren SME, Mookadam M, Fautsch MP, Holmes JM (2005) Differences between rat strains in models of retinopathy of prematurity. Mol Vis 11:524–530
Saunders RA, Donahue ML, Christmann LM, Pakalnis AV, Tung B, Hardy RJ, Phelps DL (1997) Racial variation in retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol 115(5):604–608. https://doi.org/10.1001/archopht.1997.01100150606005
Darlow BA, Hutchinson JL, Henderson-Smart DJ, Donoghue DA, Simpson JM, Evans NJ (2005) Prenatal risk factors for severe retinopathy of prematurity among very preterm infants of the Australian and New Zealand Neonatal Network. Pediatrics 115(4):990–996. https://doi.org/10.1542/peds.2004-1309
Schaffer DB, Palmer EA, Plotsky DF, Metz HS, Flynn JT, Tung B, Hardy RJ (1993) Prognostic factors in the natural course of retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology 100(2):230–237. https://doi.org/10.1016/S0161-6420(93)31665-9
Yang MB, Donovan EF, Wagge JR (2006) Race, Gender, and Clinical Risk Index for Babies (CRIB) Score as predictors of severe retinopathy of prematurity. J AAPOS 10(3):253–261. https://doi.org/10.1016/j.jaapos.2006.01.004
Aralikatti AKV, Mitra A, Denniston AKO, Haque MS, Ewer AK, Butler L (2010) Is ethnicity a risk factor for severe retinopathy of prematurity? Arch Dis Child Fetal Neonatal Ed 95(3):F174–F176. https://doi.org/10.1136/adc.2009.160366
Janevic T, Zeitlin J, Auger N, Egorova NN, Hebert P, Balbierz A, Howell EA (2018) Association of race/ethnicity with very preterm neonatal morbidities. JAMA Pediatr 172(11):1061–1069. https://doi.org/10.1001/jamapediatrics.2018.2029
Lang DM, Blackledge J, Arnold RW (2005) Is Pacific race a retinopathy of prematurity risk factor? Arch Pediatr Adolesc Med 159(8):771–773. https://doi.org/10.1001/archpedi.159.8.771
Hyland RM, Komlósi K, Alleman BW, Tolnai M, Wood LM, Bell EF, Ertl T (2013) Infantile hemangiomas and retinopathy of prematurity: clues to the regulation of vasculogenesis. Eur J Pediatr 172(6):803–809. https://doi.org/10.1007/s00431-013-1966-y
Léauté-Labrèze C, de la Roque ED, Hubiche T, Boralevi F, Thambo J-B, Taïeb A (2008) Propranolol for severe hemangiomas of infancy. N Engl J Med 358(24):2649–2651. https://doi.org/10.1056/nejmc0708819
Krowchuk DP, Frieden IJ, Mancini AJ et al (2019) Clinical practice guideline for the management of infantile hemangiomas. Pediatrics 143(1):e20183475. https://doi.org/10.1542/peds.2018-3475
Filippi L, Cavallaro G, Berti E et al (2019) Propranolol 0.2% eye micro-drops for retinopathy of prematurity: a prospective phase IIb study. Front Pediatr 7:180. https://doi.org/10.3389/fped.2019.00180
Van Sorge AJ, Termote JUM, Kerkhoff FT, Van Rijn LJ, Simonsz HJ, Peer PGM, Schalij-Delfos NE (2014) Nationwide inventory of risk factors for retinopathy of prematurity in the netherlands. J Pediatr 164(3):494-498.e1. https://doi.org/10.1016/j.jpeds.2013.11.015
Ying GS, Quinn GE, Wade KC, Repka MX, Baumritter A, Daniel E (2015) Predictors for the development of referral-warranted retinopathy of prematurity in the telemedicine approaches to evaluating acute-phase retinopathy of prematurity (e-ROP) study. JAMA Ophthalmol 133(3):304–311. https://doi.org/10.1001/jamaophthalmol.2014.5185
Slidsborg C, Jensen A, Forman JL, Rasmussen S, Bangsgaard R, Fledelius HC, Greisen G, La Cour M (2016) Neonatal risk factors for treatment-demanding retinopathy of prematurity: a Danish National Study. Ophthalmology 123(4):796–803. https://doi.org/10.1016/j.ophtha.2015.12.019
Lundgren P, Kistner A, Andersson EM, Pupp IH, Holmström G, Ley D, Niklasson A, Smith LEH, Wu C, Hellström A, Löfqvist C (2014) Low birth weight is a risk factor for severe retinopathy of prematurity depending on gestational age. PLoS ONE 9(10):e109460. https://doi.org/10.1371/journal.pone.0109460
Palmer EA, Flynn JT, Hardy RJ et al (1991) Incidence and early course of retlnonathy of prematurity. Ophthalmology 98(11):1628–1640. https://doi.org/10.1016/S0161-6420(91)32074-8
Chiang MF, Arons RR, Flynn JT, Starren JB (2004) Incidence of retinopathy of prematurity from 1996 to 2000: Analysis of a comprehensive New York state patient database. Ophthalmology 111(7):1317–1325. https://doi.org/10.1016/j.ophtha.2003.10.030
Dailey WA, Gryc W, Garg PG, Drenser KA (2015) Frizzled-4 variations associated with retinopathy and intrauterine growth retardation: a potential marker for prematurity and retinopathy. Ophthalmology 122(9):1917–1923. https://doi.org/10.1016/j.ophtha.2015.05.036
Sızmaz S, Yonekawa Y, Trese MT (2015) Familial exudative vitreoretinopathy. Turk J Ophthalmol 45(4):164–168. https://doi.org/10.4274/tjo.67699
Chen ZY, Battinelli EM, Fielder A, Bundey S, Sims K, Breakefield XO, Craig IW (1993) A mutation in the norrie disease gene (NDP) associated with X linked familial exudative vitreoretinopathy. Nat Genet 5(2):180–183. https://doi.org/10.1038/ng1093-180
Li Y, Li J, Zhang X, Peng J, Li J, Zhao P, Armenti ST (2020) Identification of gene mutations in atypical retinopathy of prematurity cases. J Ophthalmol 2020:4212158. https://doi.org/10.1155/2020/4212158
John VJ, McClintic JI, Hess DJ, Berrocal AM (2016) Retinopathy of prematurity versus familial exudative vitreoretinopathy: report on clinical and angiographic findings. Ophthalmic Surg Lasers Imaging Retin 47(1):14–19. https://doi.org/10.3928/23258160-20151214-02
Kandasamy Y, Hartley L, Rudd D, Smith R (2017) The association between systemic vascular endothelial growth factor and retinopathy of prematurity in premature infants: a systematic review. Br J Ophthalmol 101(1):21–24. https://doi.org/10.1136/bjophthalmol-2016-308828
Nguyen QD, De Falco S, Behar-Cohen F, Lam WC, Li X, Reichhart N, Ricci F, Pluim J, Li WW (2018) Placental growth factor and its potential role in diabetic retinopathy and other ocular neovascular diseases. Acta Ophthalmol 96:e1–e9. https://doi.org/10.1111/aos.13325
Pieh C, Agostini H, Buschbeck C, Krüger M, Schulte-Mönting J, Zirrgiebel U, Drevs J, Lagrèze WA (2008) VEGF-A VEGFR-1, VEGFR-2 and Tie2 levels in plasma of premature infants: Relationship to retinopathy of prematurity. Br J Ophthalmol 92(5):689–693. https://doi.org/10.1136/bjo.2007.128371
Shibuya M (2011) Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes Cancer 2(12):1097–1105. https://doi.org/10.1177/1947601911423031
Cooke RW, Drury JA, Mountford R, Clark D (2004) Genetic polymorphisms and retinopathy of prematurity. Investig Ophthalmol Vis Sci 45(6):1712–1715. https://doi.org/10.1167/iovs.03-1303
Ali AA, Hussien NF, Samy RM, Al Husseiny K (2015) Polymorphisms of vascular endothelial growth factor and retinopathy of prematurity. J Pediatr Ophthalmol Strabismus 52(4):245–253. https://doi.org/10.3928/01913913-20150506-02
Vannay Á, Dunai G, Bányász I, Szabó M, Vámos R, Treszl A, Hajdú J, Tulassay T, Vásárhelyi B (2005) Association of genetic polymorphisms of vascular endothelial growth factor and risk for proliferative retinopathy of prematurity. Pediatr Res 57(3):396–398. https://doi.org/10.1203/01.PDR.0000153867.80238.E0
Lei XJ, Zhao YX, Qiao T (2018) Influence of polymorphisms in VEGF, ACE, TNF and GST genes on the susceptibility to retinopathy of prematurity among Chinese infants. Int J Ophthalmol 11(9):1451–1457. https://doi.org/10.18240/ijo.2018.09.04
Kaya M, Çokakli M, Berk AT, Yaman A, Yesilirmak D, Kumral A, Atabey N (2013) Associations of VEGF/VEGF-receptor and HGF/c-Met promoter polymorphisms with progression/regression of retinopathy of prematurity. Curr Eye Res 38(1):137–142. https://doi.org/10.3109/02713683.2012.731550
Kusuda T, Hikino S, Ohga S, Kinjo T, Ochiai M, Takahata Y, Tokunaga S, Ihara K, Hata Y, Hara T (2011) Genetic variation of vascular endothelial growth factor pathway does not correlate with the severity of retinopathy of prematurity. J Perinatol 31(4):246–250. https://doi.org/10.1038/jp.2010.111
Shastry BS, Qu X (2007) Lack of association of the VEGF gene promoter (-634 G→C and -460 C→T) polymorphism and the risk of advanced retinopathy of prematurity. Graefe’s Arch Clin Exp Ophthalmol 2(9):949–962. https://doi.org/10.1007/s00417-006-0480-6
Kimura H, Esumi H (2003) Reciprocal regulation between nitric oxide and vascular endothelial growth factor in angiogenesis. Acta Biochim Pol 50(1):49–59
Rusai K, Vannay A, Szebeni B, Borgulya G, Fekete A, Vásárhelyi B, Tulassay T, Szabó AJ (2008) Endothelial nitric oxide synthase gene T-786C and 27-bp repeat gene polymorphisms in retinopathy of prematurity. Mol Vis 14:286–290
Nishijima T, Nakayama M, Yoshimura M et al (2007) The endothelial nitric oxide synthase gene -786T/C polymorphism is a predictive factor for reattacks of coronary spasm. Pharmacogenet Genomics 17(8):581–587. https://doi.org/10.1097/01.fpc.0000239978.61841.1a
Taverna MJ, Sola A, Guyot-Argenton C, Pacher N, Bruzzo F, Chevalier A, Slama G, Reach G, Selam JL (2002) eNOS4 polymorphism of the endothelial nitric oxide synthase predicts risk for severe diabetic retinopathy. Diabet Med 19(3):240–245. https://doi.org/10.1046/j.1464-5491.2002.00681.x
Yanamandra K, Napper D, Pramanik A, Bocchini JA, Dhanireddy R (2010) Endothelial nitric oxide synthase genotypes in the etiology of retinopathy of prematurity in premature infants. Ophthalmic Genet 31(4):173–177. https://doi.org/10.3109/13816810.2010.497528
Shastry BS (2013) Endothelial nitric oxide synthase gene promoter polymorphism (T-786C) may be associated with advanced retinopathy of prematurity. Graefe’s Arch Clin Exp Ophthalmol 251(9):2251–2253. https://doi.org/10.1007/s00417-012-2231-1
Poggi C, Giusti B, Gozzini E, Sereni A, Romagnuolo I, Kura A, Pasquini E, Abbate R, Dani C, Rogers LK (2015) Genetic contributions to the development of complications in preterm newborns. PLoS ONE 10:e0131741. https://doi.org/10.1371/journal.pone.0131741
Skaper S (2011) Peptide mimetics of neurotrophins and their receptors. Curr Pharm Des 17(25):2704–2718. https://doi.org/10.2174/138161211797415995
Camerino C, Conte E, Cannone M, Caloiero R, Fonzino A, Tricarico D (2016) Nerve growth factor, brain-derived neurotrophic factor and osteocalcin gene relationship in energy regulation, bone homeostasis and reproductive organs analyzed by mrna quantitative evaluation and linear correlation analysis. Front Physiol 7:456. https://doi.org/10.3389/fphys.2016.00456
Sahay AS, Sundrani DP, Joshi SR (2017) Neurotrophins: role in placental growth and development. Vitam Horm 104:243–261. https://doi.org/10.1016/bs.vh.2016.11.002
Reichardt LF (2006) Neurotrophin-regulated signalling pathways. Philos Trans R Soc B Biol Sci 361(1473):1545–1564. https://doi.org/10.1098/rstb.2006.1894
Garrido MP, Vera C, Vega M, Quest AFG, Romero C (2018) Metformin prevents nerve growth factor-dependent proliferative and proangiogenic effects in epithelial ovarian cancer cells and endothelial cells. Ther Adv Med Oncol 10:1758835918770984. https://doi.org/10.1177/1758835918770984
Lam CT, Yang ZF, Lau CK, Tam KH, Fan ST, Poon RTP (2011) Brain-derived neurotrophic factor promotes tumorigenesis via induction of neovascularization: implication in hepatocellular carcinoma. Clin Cancer Res 17(10):3123–3133. https://doi.org/10.1158/1078-0432.CCR-10-2802
Julio-Pieper M, Lozada P, Tapia V, Vega M, Miranda C, Vantman D, Ojeda SR, Romero C (2009) Nerve growth factor induces vascular endothelial growth factor expression in granulosa cells via a trkA receptor/mitogen-activated protein kinase-extracellularly regulated kinase 2-dependent pathway. J Clin Endocrinol Metab 94(8):3065–3071. https://doi.org/10.1210/jc.2009-0542
Zhang Z, Zhang Y, Zhou Z, Shi H, Qiu X, Xiong J, Chen Y (2017) BDNF regulates the expression and secretion of VEGF from osteoblasts via the TrkB/ERK1/2 signaling pathway during fracture healing. Mol Med Rep 15(3):1362–1367. https://doi.org/10.3892/mmr.2017.6110
Hartnett ME, Morrison MA, Smith S et al (2014) Genetic variants associated with severe retinopathy of prematurity in extremely low birth weight infants. Investig Ophthalmol Vis Sci 55(10):6194–6203. https://doi.org/10.1167/iovs.14-14841
Hartnett ME, Capone A Jr (2016) Advances in diagnosis, clinical care, research, and treatment in retinopathy of prematurity. Eye Brain 8:27–29. https://doi.org/10.2147/EB.S105319
Rumajogee P, Madeira A, Vergé D, Hamon M, Miquel MC (2002) Up-regulation of the neuronal serotoninergic phenotype in vitro: BDNF and cAMP share Trk B-dependent mechanisms. J Neurochem 83(6):1525–1528. https://doi.org/10.1046/j.1471-4159.2002.01264.x
Popova NK, Ilchibaeva TV, Naumenko VS (2017) Neurotrophic factors (BDNF and GDNF) and the serotonergic system of the brain. Biochem 82(3):308–317. https://doi.org/10.1134/S0006297917030099
Masson J (2019) Serotonin in retina. Biochimie 161:51–55. https://doi.org/10.1016/j.biochi.2018.11.006
Chen PS, Chiu WT, Hsu PL, Lin SC, Peng IC, Wang CY, Tsai SJ (2020) Pathophysiological implications of hypoxia in human diseases. J Biomed Sci 27(1):63. https://doi.org/10.1186/s12929-020-00658-7
Fallah J, Rini BI (2019) HIF inhibitors: status of current clinical development. Curr Oncol Rep 21(1):6. https://doi.org/10.1007/s11912-019-0752-z
Takeda N, Maemura K, Imai Y, Harada T, Kawanami D, Nojiri T, Manabe I, Nagai R (2004) Endothelial PAS domain protein 1 gene promotes angiogenesis through the transactivation of both vascular endothelial growth factor and its receptor, Flt-1. Circ Res 95(2):146–153. https://doi.org/10.1161/01.RES.0000134920.10128.b4
Morita M, Ohneda O, Yamashita T et al (2003) HLF/HIF-2α is a key factor in retinopathy of prematurity in association with erythropoietin. EMBO J 22(5):1134–1146. https://doi.org/10.1093/emboj/cdg117
Mohamed S, Schaa K, Cooper ME, Ahrens E, Alvarado A, Colaizy T, Marazita ML, Murray JC, Dagle JM (2009) Genetic contributions to the development of retinopathy of prematurity. Pediatr Res 65(2):193–197. https://doi.org/10.1203/PDR.0b013e31818d1dbd
Hellström A, Smith LEH, Dammann O (2013) Retinopathy of prematurity. Lancet 382(9902):1445–1457. https://doi.org/10.1016/S0140-6736(13)60178-6
Hellström A, Engström E, Hård AL et al (2003) Postnatal serum insulin-like growth factor I deficiency is associated with retinopathy of prematurity and other complications of premature birth. Pediatrics 112(5):1016–1020. https://doi.org/10.1542/peds.112.5.1016
Hellström A, Carlsson B, Niklasson A et al (2002) IGF-I is critical for normal vascularization of the human retina. J Clin Endocrinol Metab 87(7):3413–3416. https://doi.org/10.1210/jc.87.7.3413
Bonafè M, Barbieri M, Marchegiani F, Olivieri F, Ragno E, Giampieri C, Mugianesi E, Centurelli M, Franceschi C, Paolisso G (2003) Polymorphic variants of insulin-like growth factor I (IGF-I) receptor and phosphoinositide 3-kinase genes affect IGF-I plasma levels and human longevity: cues for an evolutionarily conserved mechanism of life span control. J Clin Endocrinol Metab 88(7):3299–3304. https://doi.org/10.1210/jc.2002-021810
Shastry BS (2007) Assessment of the contribution of insulin-like growth factor I receptor 3174 G→A polymorphism to the progression of advanced retinopathy of prematurity. Eur J Ophthalmol 17(6):950–953. https://doi.org/10.1177/112067210701700613
Balogh Á, Derzbach L, Vannay Á, Vásárhelyi B (2006) Lack of association between insulin-like growth factor I receptor G+3174A polymorphism and retinopathy of prematurity. Graefe’s Arch Clin Exp Ophthalmol 244(8):1035–1038. https://doi.org/10.1007/s00417-005-0203-4
Sato T, Shima C, Kusaka S (2011) Vitreous levels of angiopoietin-1 and angiopoietin-2 in eyes with retinopathy of prematurity. Am J Ophthalmol 151(2):353-357.e1. https://doi.org/10.1016/j.ajo.2010.08.037
Stark A, Dammann C, Nielsen HC, Volpe MV (2018) A pathogenic relationship of bronchopulmonary dysplasia and retinopathy of prematurity? A review of angiogenic mediators in both diseases. Front Pediatr 6:125. https://doi.org/10.3389/fped.2018.00125
Maisonpierre PC, Suri C, Jones PF et al (1997) Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277(5322):55–60. https://doi.org/10.1126/science.277.5322.55
Takagi H, Koyama S, Seike H, Oh H, Otani A, Matsumura M, Honda Y (2003) Potential role of the angiopoietin/tie2 system in ischemia-induced retinal neovascularization. Investig Ophthalmol Vis Sci 44(1):393–402. https://doi.org/10.1167/iovs.02-0276
Geva E, Jaffe RB (2000) Role of angiopoietins in reproductive tract angiogenesis. Obstet Gynecol Surv 55(8):511–519. https://doi.org/10.1097/00006254-200008000-00024
Asahara T, Chen D, Takahashi T, Fujikawa K, Kearney M, Magner M, Yancopoulos GD, Isner JM (1998) Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res 83(3):233–240. https://doi.org/10.1161/01.res.83.3.233
Mandriota SJ, Pepper MS (1998) Regulation of angiopoietin-2 mRNA levels in bovine microvascular endothelial cells by cytokines and hypoxia. Circ Res 83(8):852–859. https://doi.org/10.1161/01.res.83.8.852
Oh H, Takagi H, Suzuma K, Otani A, Matsumura M, Honda Y (1999) Hypoxia and vascular endothelial growth factor selectively up-regulate angiopoietin-2 in bovine microvascular endothelial cells. J Biol Chem 274(22):15732–15739. https://doi.org/10.1074/jbc.274.22.15732
Shastry BS (2009) Lack of association of VEGF (-2578 C → A) and ANG 2 (-35 G → C) gene polymorphisms with the progression of retinopathy of prematurity. Graefe’s Arch Clin Exp Ophthalmol 247(6):859–860. https://doi.org/10.1007/s00417-008-0988-z
Bányász I, Bokodi G, Vannay Á, Szebeni B, Treszl A, Vásárhelyi B, Tulassay T, Szabó A (2006) Genetic polymorphisms of vascular endothelial growth factor and angiopoietin 2 in retinopathy of prematurity. Curr Eye Res 31(7–8):685–690. https://doi.org/10.1080/02713680600801123
Dammann O, Brinkhaus MJ, Bartels DB, Dördelmann M, Dressler F, Kerk J, Dörk T, Dammann CEL (2009) Immaturity, perinatal inflammation, and retinopathy of prematurity: a multi-hit hypothesis. Early Hum Dev 85(5):325–329. https://doi.org/10.1016/j.earlhumdev.2008.12.010
Taha H, Skrzypek K, Guevara I et al (2010) Role of heme oxygenase-1 in human endothelial cells: lesson from the promoter allelic variants. Arterioscler Thromb Vasc Biol 30(8):1634–1641. https://doi.org/10.1161/ATVBAHA.110.207316
Sarlos S, Wilkinson-Berka JL (2005) The renin-angiotensin system and the developing retinal vasculature. Investig Ophthalmol Vis Sci 46(3):1069–1077. https://doi.org/10.1167/iovs.04-0885
Moravski CJ, Kelly DJ, Cooper ME, Gilbert RE, Bertram JF, Shahinfar S, Skinner SL, Wilkinson-Berka JL (2000) Retinal neovascularization is prevented by blockade of the renin-angiotensin system. Hypertension 36(6):1099–1104. https://doi.org/10.1161/01.HYP.36.6.1099
Haider MZ, Devarajan LV, Al-Essa M, Kumar H (2002) Angiotensin-converting enzyme gene insertion/deletion polymorphism in Kuwaiti children with retinopathy of prematurity. Biol Neonate 82(2):84–88. https://doi.org/10.1159/000063092
Spiegler J, Gilhaus A, König IR et al (2009) Polymorphisms in the renin-angiotensin system and outcome of very-low-birthweight infants. Neonatology 97(1):10–14. https://doi.org/10.1159/000226602
Cockle JV, Gopichandran N, Walker JJ, Levene MI, Orsi NM (2007) Matrix metalloproteinases and their tissue inhibitors in preterm perinatal complications. Reprod Sci 14(7):629–645. https://doi.org/10.1177/1933719107304563
Visse R, Nagase H (2003) Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92(8):827–839. https://doi.org/10.1161/01.RES.0000070112.80711.3D
Zhong S, Khalil RA (2019) A disintegrin and metalloproteinase (ADAM) and ADAM with thrombospondin motifs (ADAMTS) family in vascular biology and disease. Biochem Pharmacol 164:188–204. https://doi.org/10.1016/j.bcp.2019.03.033
Weskamp G, Mendelson K, Swendeman S et al (2010) Pathological neovascularization is reduced by inactivation of ADAM17 in endothelial cells but not in pericytes. Circ Res 106(5):932–940. https://doi.org/10.1161/CIRCRESAHA.109.207415
Guaiquil VH, Hewing NJ, Chiang MF, Rosenblatt MI, Chan RVP, Blobel CP (2013) A murine model for retinopathy of prematurity identifies endothelial cell proliferation as a potential mechanism for plus disease. Investig Ophthalmol Vis Sci 54(8):5294–5302. https://doi.org/10.1167/iovs.12-11492
Kondo H, Kusaka S, Yoshinaga A, Uchio E, Tawara A, Tahira T (2013) Genetic variants of FZD4 and LRP5 genes in patients with advanced retinopathy of prematurity. Mol Vis 19:476–485
Hutcheson KA, Paluru PC, Bernstein SL, Koh J, Rappaport EF, Leach RA, Young TL (2005) Norrie disease gene sequence variants in an ethnically diverse population with retinopathy of prematurity. Mol Vis 11:501–508
Haider MZ, Devarajan LV, Al-Essa M, Kumar H (2002) A C597–>A polymorphism in the Norrie disease gene is associated with advanced retinopathy of prematurity in premature Kuwaiti infants. J Biomed Sci 9(4):365–370. https://doi.org/10.1159/000065008
Shastry BS, Pendergast SD, Hartzer MK, Liu X, Trese MT (1997) Identification of missense mutations in the Norrie disease gene associated with advanced retinopathy of prematurity. Arch Ophthalmol 115(5):651–655. https://doi.org/10.1001/archopht.1997.01100150653015
Acknowledgments
Special thanks to Ana Carolina Santos for her support in this work.
Funding
This work was supported by the Laboratório de Genética and the Instituto de Saúde Ambiental (ISAMB) of the Faculdade de Medicina of Universidade de Lisboa and the Instituto de Investigação Científica Bento da Rocha Cabral. The writing of the manuscript was also supported by funds from Fundação para a Ciência e a Tecnologia to ISAMB (ref. UIDB/04295/2020 and UIDP/04295/2020).
Author information
Authors and Affiliations
Contributions
The first draft of the manuscript was written by Mariza Fevereiro-Martins. Mariza Fevereiro-Martins and Manuel Bicho had the idea for the article. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fevereiro-Martins, M., Guimarães, H., Marques-Neves, C. et al. Retinopathy of prematurity: contribution of inflammatory and genetic factors. Mol Cell Biochem 477, 1739–1763 (2022). https://doi.org/10.1007/s11010-022-04394-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04394-4