Abstract
Metastatic breast cancer remains a serious health concern and numerous investigations recommended medicinal plants as a complementary therapy. Crocin is one of the known anticancer bio-component. Recently, the inhibitory effect of metformin has been studied on the various aspects of cancer. However, no study reported their combination effects on metastatic breast cancer. In the present study, we have assessed their anti-metastatic effects on in vitro and in vivo breast cancer models. Using MTT assay, scratch, and adhesion tests, we have evaluated the cytotoxic, anti-invasive and anti-adhesion effects of crocin and metformin on 4T1 cell line, respectively. Their protective effects and MMP9 as well as VEGF protein expression levels (Western blotting) investigated in the 4T1 murine breast cancer model. Our results showed that both crocin and metformin reduced cell viability, delayed scratch healing and inhibited the cell adhesion, in vitro. While crocin alone restored the mice’s weight reduction, crocin, metformin, and their combination significantly reduced the tumor volume size and enhanced animal survival rate in murine breast cancer model, responses that were associated with VEGF and MMP9 down-regulation. These findings suggest that a combination of crocin and metformin could serve as a novel therapeutic approach to enhance the effectiveness of metastatic breast cancer therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the first common diagnosed malignancy in women worldwide and its metastatic subtype resulted in a growing number of cancer-related death [1]. Metastasis, defined as the onset of tumor cells in other organs, is the main accused of cancer mortality [2, 3]. In the metastatic stage of breast cancer, tumor cells usually migrate to the lungs, liver, brain, and bones. Triple-negative breast cancer (TNBC) is the most malignant form of breast cancer with a maximum capacity of metastasis which could not be repressed by common therapeutic approaches [4].
Metastasis is the most important phenomenon in cancer progression and applying therapeutic strategies which target metastatic pathways would be effective in cancer therapy [3]. Metastatic cascade has consisted of a series of sequential steps involving cell adhesion, invasion, and migration. Disturbance of the metastasis pathway holds preclinical and clinical promise for cancer patients with metastatic breast cancer [5].
Wnt/β-catenin signaling pathway has an impressive role in controlling epithelial–mesenchymal transition (EMT) and metastasis [6, 7], which could be in some extent due to upregulation of Vascular endothelial growth factor (VEGF) in several cancers. VEGF is a growth factor sub-family that has an important role in angiogenesis and mostly up-regulated in cancers [8]. Matrix metalloproteinase (MMPs) are also the other metastatic proteins that are directly related to EMT. MMP9 is a member of matrix-metalloproteinase which is involved in the degradation of extracellular matrix and mostly up-regulated in the metastasis process [9].
The current chemotherapeutic agents for breast cancer are not completely effective which might be due to their single-target nature, high toxicity resulted in unwanted side effects and resistance of tumor cells. The later one causes tumor relapse and metastasis [10]. Therefore, applying the novel therapeutic approaches that target multiple pathways especially metastasis-related ones could be beneficial. Currently, there is a growing interest to use combination of chemical agents with medicinal herbs to overcome this problem. On the other hand, multi-targeted therapy with combinational strategies leads to improve treatment efficiency.
Metformin is the first-line medication for type-2 diabetes and its inhibitory effect on metastatic cancer has been studied in recent years [11]. Several epidemiologic studies reported that the cancer risk in diabetic patients receiving metformin is less than those taking other anti-diabetic drugs. Moreover, it was shown that there is a meaningful relationship between metformin and cancer risk reduction [11,12,13]. On the other hand, numerous studies investigate the anti-cancer effects of saffron (Crocus sativus) and its compounds [14, 15]. Crocin which its pro-apoptosis, anti-proliferative, -metastasis and -angiogenesis effects have been proved, can be utilized as a food preservative [16,17,18,19].
Although numerous studies evaluated the effects of crocin and/or metformin in metastatic breast cancer [19,20,21], no study has been reported their combination effects in this context. Therefore, we conducted the present study to evaluate the anti-metastatic effects of the metformin, crocin and their combination on in vitro (4T1) and in vivo murine model of metastatic (BALB/c mouse) of TNBC.
Materials and methods
Crocin preparation
Repeated cycles of extraction with n-hexane and Alumina-90-active column chromatography were used to prepare crocin from Iranian saffron powder as reported previously [22]. The purity of extracted crocin (~99.8%) was determined by UV–Vis spectroscopy.
Cell culture
Mouse mammary carcinoma cell line (4T1) was purchased from the national cell bank of Pasteur Institute (Tehran, Iran). They were cultured in Roswell Park Memorial Institute medium (RPMI 1640; Gibco BRL, USA) with 10% heat-inactivated fetal bovine serum (FBS; Gibco BRL, USA), 100 units/ml penicillin and 100 mg/ml streptomycin (Gibco BRL, USA) and incubated in a humidified atmosphere containing 5% CO2 at 37 °C [18, 23].
Cell viability assay
The cytotoxic effects of different concentrations of crocin, metformin and their combinations on 4T1 cells were evaluated using MTT assay (Sigma–Aldrich; M2128-1G). Briefly, a total of 7 × 103 cells/well were seeded in a 96-well plate and 24 h after incubation treated with crocin (0–4.5 mM) and metformin (0–20 mM) at various time intervals (0–72 h). MTT assay was performed to analyze cell proliferation as previously reported [17, 24, 25]. Finally, the absorbance was measured at 570 nm using an Eliza reader (Epoch, BioTek, USA) and the IC50 values (defined as a concentration of drug that reduced the absorbance of treated cells by 50% compared to unchallenged cells) were calculated as a percentage of cell viability.
Cell migration wound healing assay
To test the effect of crocin and metformin on cell migration, 4T1 cells (2 × 105 per well) were seeded in a 12-well plate and incubated overnight at 37 °C and wound healing assay was performed thereafter. A scratch was created in the monolayer by a yellow micropipette tip. Detached cells were removed with PBS, fresh medium was added, and cells were treated with different concentrations of crocin (2, 4.5 mM), metformin (8, 16 mM) and their combination at various time intervals (12–24 h). Finally, a phase-contrast microscope was used to monitor the relative width of scratch measured by ImageJ software and wound area at 0 h defined as 100% [18, 26].
Cell–matrix adhesion assay
The 4T1 cells treated with crocin (2–4.5 mM), metformin (8–16 mM) and their combination, trypsinized and suspended in RPMI 1640 and BSA (1:1), incubated at 37 °C for 90 min and seeded in precoated 96 well-plate (nearly 105 cells) with 100 ml of 2 mg/ml fibronectin (Sigma) and incubated again for 90 min at 37C. Then, cells were washed with PBS and the adherent cells were fixed in 70% ethanol and stained with Crystal Violet (0.1% [w/v] in 25% [v/v] methanol). Finally, the stained cells were dissolved in 0.2% Triton X-100, the optical density was measured at 550 nm by an Eliza reader and the relative percent of adhesion to the matrix was assessed [27, 28].
Animals
Forty female BALB/c mice, 6–8-week-old, were purchased from Iran Pasteur Institute. The mice were maintained in the standard conditions with 12-h dark and 12-h light cycle at 25 °C with water and pellets diet. The animal experiments were ethically approved under the regulations set by the Animal Care Committee of Birjand University of Medical Sciences guidelines (approval # ir.bums.REC.1396.268).
Tumor inoculation and therapy
To induce a tumor, 1 × 106 4T1 cells suspended in 0.2 ml phosphate-buffered saline and inoculated into the left dorsal flank regions of the mice [29]. After 14 days, the tumor-bearing mice were randomly divided into 4 groups (each = 8 mice) including a tumor control group, crocin treatment group, metformin treatment group, metformin/crocin treatment group and normal mice group as a negative control. The normal mice group (8 mice) was injected with PBS instead of 4T1 cells. The tumor control group and the normal group were injected with normal saline and treatment groups were injected with 200 mg/kg of crocin, 150 mg/kg metformin and their combination for 4 weeks (thrice per week). It would be noted that during the study period, the mice were screened for general health, the animal weights and tumor volumes (using a digital vernier caliper) were measured every 5 days. Also, the sacrifice date was recorded to evaluate the treatment effect on the survival rate of mice.
Western blotting analyses
Western blotting was accomplished as described before [23]. Membranes were incubated overnight at 4 °C in primary antibodies (anti-MMP9 (1:500), anti-VEGF-C (1:100); and anti-GAPDH (1:15,000); Abcam, Germany) and subsequently in horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibody (1:3000–1:5000), respectively at room temperature for 1 h. Peroxidase activity was visualized using an enhanced chemiluminescence kit. GAPDH was chosen as a loading control.
Statistics analysis
Results are illustrated as the means ± SEM of at least three independent experiments (n = 3). Data were analyzed using appropriate statistical tests such as one-way ANOVA and with Turkey’s post hoc test and statistical significances were inferred at p ≤ 0.05 (PRISM 6.07; Graph-Pad Software Inc.). p Values measured by comparison to non-treated mice as a control group.
Results
Crocin, metformin, and their combination decreased 4T1 cell viability
To evaluate the effects of crocin on cell viability, 4T1 cells were cultured in 96-well plate (7 × 103 cells/well), treated with crocin (0–4.5 mM) at different times (24–72 h) and cell viability was assessed using MTT assay. As shown in Fig. 1a–c, treatment of 4T1 cells with crocin caused a clear reduction of cell viability in a time- and concentration-dependent manner (p < 0.001). We have also extended the above experiment to examine the effects of metformin (0–20 mM; 24–72 h) on cell viability. Metformin also decreased the cell viability similarly in a time- and concentration-dependent manner (Fig. 1d–f, p < 0.001). Table 1 demonstrated the IC50 values for crocin and metformin which were decreased by increasing periods of cell treatment.
Crocin, metformin, and their combination modulate 4T1 cell viability. Crocin (a–c) and metformin (d–f) significantly reduced cell viability in breast cancer cells (p < 0.001, compared to non-treated cells). The 4T1 cells cultured with and without crocin (0–4.5 mM) and metformin (0–20 mM) at different times (24–72 h) and cell viability were determined using MTT assay. Metformin enhanced crocin-reduced cell viability (p < 0.001, compared to non-treated cells) in a time- and concentration-dependent manner (g–i). Cells cultured and treated with crocin (IC50 concentration) in the absence and presence of metformin (0–16 mM; 24–72 h). Crocin augmented metformin-decreased cell viability (p < 0.001, compared to non-treated cells) in a time- and concentration-dependent manner (j–l). Cells cultured and treated metformin (IC50 concentration) with and without crocin (0–4 mM; 24–72 h). *p < 0.05, **p < 0.01, ***p < 0.001; n = 3, all p values evaluated by comparison to non-treated cells (control group). Two signs – and + showed absence and presence of each component (IC50 concentration) in each MTT assay
To examine the possible synergistic effect of crocin and metformin, cells were cultured and treated with crocin (IC50: 4.5 mM, 24 h; 3.5 mM, 48 h; 2 mM, 72 h) and different concentration of metformin (0–16 mM). Crocin significantly decreased the viability (p < 0.001) and metformin further decreased this response in a time- and concentration-dependent manner (Fig. 1g–i, p < 0.001). To further investigate this synergistic response, cells were cultured and treated with metformin (IC50: 16 mM, 24 h; 8 mM, 48 h; 4 mM, 72 h) and different concentrations of crocin (0–4 mM). Similarly, metformin reduced the cell viability (p < 0.001) and this response enhanced with crocin in a time- and concentration-dependent manner (Fig. 1j–l, p < 0.001).
Crocin and metformin but not their combinations delay the wound healing in 4T1 cells
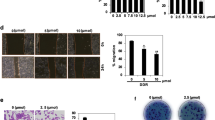
The scratch and the adhesion assays are commonly used methods to study the cells migratory and adhesive behaviors that are fundamental for different biologic processes including tumor metastasis [30]. To assess the inhibitory effects of crocin and metformin and their combinations on 4T1 cell migration, the scratch test was performed after the treatment period as explained in the section “Materials and methods”. As shown in Fig. 2a, scratch healing occurred in unchallenged cells in a time-dependent manner. Crocin (Fig. 2b, c), and metformin (Fig. 2f, g) significantly delayed in scratch healing in a concentration- and time-dependent manner (p < 0.001). To assess the impact of the combination therapy, cells were treated with crocin (IC50: 4.5 mM) in the absence and the presence of metformin (5 and 8 mM; Fig. 2d, e) as well as metformin (IC50: 16 mM) with and without Crocin (2 and 2.5 mM; Fig. 2h, i). Interestingly, metformin did not alter the effect of crocin on the wound-healing delay. Crocin also was unable to change the metformin effect on the wound healing delay.
Crocin and metformin and their combinations delay the wound healing in 4T1 cells. Crocin (b, c) and metformin (f, g) dramatically (p < 0.001, compared to non-treated cells) delayed the 4T1 cells wound healing compared to unchallenged cells (a). The breast cancer cells (4T1) cultured with crocin (2 and 4.5 mM) and metformin (8 and 16 mM) at 12 and 24 h and wound healing was assessed using scratch test. Metformin could not change the crocin effect on 4T1 wound healing (d, e; p < 0.05 compared to non-treated cells). Crocin did not also alter metformin impact of the wound healing (h, i; p < 0.05, compared to non-treated cells). Cells (4T1) treated with either crocin (IC50: 4.5 mM) with and without metformin (5 and 8 mM) or metformin (IC50: 16 mM) in the absence and presence of crocin (2 and 2.5 mM) at 12 and 24 h and wound healing was assessed using scratch test. n = 3, **p < 0.01; ***p < 0.001 all p values evaluated by comparison to non-treated cells (control group)
Crocin and metformin reduced 4T1 cells adhesion
To further examine the hypothesis, the above experiment has been extended to assess the impact of crocin and metformin on cell adhesion using cell–matrix adhesion assay as explained in the section “Materials and methods”. In comparison with a control group (100%), the adhesion of 4T1 cells to the matrix was reduced to 80.9% and 58.01% following the treatment of the cells with 2 (p < 0.05) and 4.5 mM (p < 0.001) of crocin, respectively. However, metformin did not increase this response (Fig. 3a). Metformin (8 and 16 mM) also reduced 4T1 cells adhesion to matrix to 78.37% (p < 0.01) and 69.55% (p < 0.001), respectively. Although the addition of crocin to the treatment led to a reduction in cell adhesion, this response was not significantly different (Fig. 3a).
(a) Crocin and metformin and their combinations regulate 4T1 cells adhesion. Crocin [2 mM (p < 0.05) and 4.5 mM (p < 0.001) compared to non-treated cells] and metformin [8 mM (p < 0.01) and 16 mM (p < 0.001) compared to non-treated cells] attenuated the 4T1 cells adhesion to the matrix in a concentration-dependent manner in comparison with the control group (100%). The response to individual agent was not potentiated in the presence another one (p > 0.05 compared to non-treated cells). The 4T1 cells were cultured and treated with crocin (2 and 4.5 mM) and metformin (8 and 16 mM) alone and their combinations, and the cell adhesion assessed using cell–matrix adhesion assay. n = 3, *p < 0.05; **p < 0.01; ***p < 0.001 compared to non-treated cells. (b) Crocin hindered mice body weight reduction induced by tumor. Crocin (p < 0.05) but not metformin and their combination (p > 0.05) restored body weight reduction (p < 0.001 compared to non-treated cells) due to the tumor development. (c) Crocin and metformin and their combination enhanced the animal survival rate. The animal survival has been increased by crocin (87.5%), metformin (75%) and their combination (75%) at the end of the study (day 40) in comparison to the tumor control (50%). (d) Crocin and metformin and their combination altered the tumor volume size. Crocin and metformin and their combination altered the tumor volume size (p < 0.001 compared to non-treated group) at the end of the study in 40 days after 4T1 cells injection. Female BALB/c mice were inoculated with 4T1 cells, injected with 200 mg/kg of crocin, 150 mg/kg metformin, their combination and normal saline as control from the 14th day of cell inoculation for 4 weeks (thrice per week). Normal mice group was just treated with normal saline as control. The animal weights were measured every 5 days. The tumor volume was measured every 5 days using a digital vernier caliper
Crocin hindered mice body weight reduction induced by tumor
To extend our in vitro studies to in vivo model, we have induced tumor in female BALB/c mice model to examine the effect of crocin and metformin on the animal weights, tumor volumes as well as their survival rate as explained in the section “Materials and methods”. As shown in Fig. 3b, the animal weight in the normal group rose steadily to reach a plateau after about 4 weeks (p < 0.001). In the tumor control group, it increased gradually up to 2 weeks, when it reached a plateau until the 5th week and decreased slightly thereafter. Crocin treated group showed that the body weight was constant until 2 weeks and then steadily elevated (p < 0.001), notably it was significantly different with that of the tumor control group (p < 0.05) and almost the same and comparable with the normal mice group (p > 0.05). Metformin group and combination group (crocin + metformin) could not alter the body weight compared to the tumor control group (p > 0.05). Moreover, the body weights were significantly lower than those of the normal mice group (p < 0.01, p < 0.001), respectively.
Crocin and metformin and their combination reduced the tumor volume size and enhanced the animal survival rate
As shown in Fig. 3d, the survival study showed that all the animals in the normal group survived until the end of the study (day 40); However, 50% of the mice in the tumor control group died during the study. While the survival in the crocin-treated group increased up to 87.5% compared to 50% in the tumor control group, these figures for metformin and crocin + metformin groups were 75%.
We have also measured the tumor volume of the animals in the different treatment groups during the studies every 5 days after tumor-bearing (the day 15) as demonstrated in the Fig. 3c, mice in tumor control group harbored the highest tumor volume size specifically at the last week of the study. While all treated groups showed a lower tumor volume size in comparison to the tumor control group (p < 0.001) at the end of the study, animals treated with crocin alone exerted the lowest volume size.
Crocin- and metformin inhibitory metastatic effects are associated with decreased VEGF and MMP9 protein contents
To better understand how crocin and metformin exert their metastatic inhibitory effects and also to elucidate the molecular mechanism involved in this regard, we analyzed the protein expression of two metastatic related proteins including MMP9 and VEGF in the tumor, breast and lung tissues for all treated groups of the animals using Western blot.
As shown in Fig. 4a, b, tumor expressed VEGF and both intact and active forms of MMP9 proteins that migrate as a 15, 92 and 58 kDa immune reactive protein, respectively (Fig. 4a, b, lane 1). Crocin, metformin, and the combination treatment resulted in a dramatic decrease in the protein content of VEGF and both MMP9 forms in tumor tissues (p < 0.001; Fig. 4a, b, lane 2–4). VEGF and both MMP9 forms were detected in normal breast and lung tissues (Fig. 4a, b, lane 5, 9); however, their expression was significantly higher in their tumor control tissue samples (p < 0.001; Fig. 4a, b, lane 6, 10). Interestingly, crocin, metformin, and their combination dramatically reduced (p < 0.001) the protein content of VEGF and both MMP9 forms to the basal levels in the breast (Fig. 4a, b, lane 7–9) and lung tissues (Fig. 4a, b, lane 11–13).
Crocin and metformin down-regulate VEGF and MMP9 protein contents. Crocin, metformin, and the combination treatment dramatically decreased (p < 0.001 compared to non-treated group) the protein content of VEGF (a) and both MMP9 forms (b) in tumor tissues (lane 2–4), breast (lane 7–9) and lung tissues (lane 11–13). Female BALB/c mice were inoculated with 4T1 cells, injected with 200 mg/kg of crocin, 150 mg/kg metformin, their combination and normal saline as control from the 14th day of cell inoculation for 4 weeks (thrice per week). Normal mice group was just treated with normal saline as control. The tumor and normal tissue samples were collected and VEGF and MMP9 protein contents were measured by Western blotting. No error bar on control group because of the all values was similar in this group
Discussion
Breast cancer is the most prevalent cancer among women globally, with an incidence rate of over 1.6 million instances per year [31]. Despite the significant improvement in current therapies in extending patient life, 30–40% of patients may eventually suffer from distant relapse and succumb to the disease. Consequently, a better understanding of metastasis biology is essential for developing treatment strategies and achieving long-lasting therapeutic efficacies against breast cancer [32].
We have conducted the present in vitro and in vivo studies to investigate the molecular mechanisms of crocin and metformin inhibitory effects on breast cancer metastasis. Our in vitro data shown that both crocin and metformin (a) reduced 4T1 cell viability (Fig. 1), (b) delayed scratch healing, (c) inhibited the adhesion of 4T1 cells to fibronectin, in a concentration- and time-dependent manner. In vivo study also shown that while crocin hindered the mice’s weight reduction after tumor development, crocin and metformin, and their combination significantly reduced the tumor volume size and enhanced animal survival rate. The later responses were associated with a dramatic reduction of VEGF and MMP9 protein levels in tumor, breast and lung tissues.
Our MTT findings are consistent with previous studies on crocin cytotoxicity. Although numerous studies reported the anti-proliferative effects of crocin [16, 17, 33,34,35,36] and metformin [37,38,39,40] in different cancers these components did not have any significant inhibitory effect on growth and proliferation of normal cells. However, not any relevant study reported the cytotoxic effects of metformin and crocin combination in this context. We have demonstrated that not only crocin or metformin alone but also their combination led to a dramatic decrease in cell viability of the breast cancer cells.
We have also studied the effects of crocin and metformin on migratory and the adhesive behavior of the cells and shown that both crocin and metformin but not their combination created a significant time- and concentration-dependent delay in scratch healing (Fig. 2). We also demonstrated that crocin, metformin, and combination of metformin/crocin were able to significantly inhibit the adhesion of 4T1 cells to fibronectin in a dose-dependent manner after 24 h. This evidence proved the important role of crocin and metformin in the migratory behavior of cancerous cells. However, it seems that crocin was more effective than metformin. In this context, previous studies reported the inhibitory roles of crocin and metformin on metastasis in different cancers. Rattan et al., in 2011, for the first time, indicated that in addition to inhibition of tumor cell proliferation, metformin treatment inhibited both angiogenesis and metastatic spread of ovarian cancer via modulating AMPK/mTOR pathway [41]. Esfahanian et al., in 2012 reported that metformin significantly inhibited the proliferation and migration of human umbilical vein endothelial cells and this response might be due to its suppressive effect on the mRNA levels of MMP-2 and -9 [42]. Festuccia et al., in 2014 also demonstrated that mobility and invasion features of prostate cancer cells (PC3 and 22rv1) were inhibited by crocin [43]. Our results are in line with the previous studies; however, they have not studied the effect of crocin and metformin combination in this regard.
Moreover, we performed an in vivo study to confirm the anti-tumor effects of crocin and metformin, and shown that crocin has the strongest effect on the reduction of tumor volume size and enhancing the animal survival rate.
In line with our in vitro results regarding the cell adhesion and migratory properties, the combination of crocin and metformin exerted a less suppressive effect on tumor volume size; however, these differences were not significant (Fig. 3c). Several case-control studies and cohort clinical trials have reported that systemic treatment of diabetic patients with metformin significantly decreased the risk of death due to cancer metastasis [11, 13].
Our finding demonstrated that while the animal weight in the normal group increased steadily at the end of the study, tumor induction reversed the weight gain and crocin hindered this reduction. In this context, it has been shown that crocin enhanced mice’s weight, survival rates, and decreased tumor volume size [19]. Metformin treatment did not recover the mice’s weight reduction in murine breast cancer model [44].
To better understand the molecular mechanism of the protective effect of crocin and metformin on metastasis, Western blot analysis was done to detect VEGF and MMP9 protein contents. As expected, the results showed that crocin, metformin, and their combination caused a dramatic reduction of VEGF and both intact and active MMP9 protein levels. Up to now, several studies approved the essential role of MMP9 in the metastasis process. An in vivo study performed by Mehner et al. showed that MMP9 knockdown in triple-negative breast tumors led to complete suppression of pulmonary metastasis [45]. VEGF facilities tumor metastasis and anti-VEGF monoclonal antibody (bevacizumab) have been used clinically to reduce metastasis [46]. Many studies approved the therapeutic impact of anti-VEGF agents. For instance, Lui et al. reported that the VEGF-VEGFR1 signaling pathway is crucial for tumor metastasis in lung tumor bearing-mice and blocking this pathway significantly suppressed metastasis [46]. Also, Yang et al. indicated that VEGF increased cancer metastasis via the remodeling of tumor micro vasculature and targeting this protein could be an efficient therapeutic approach [47]. Other study provide pre-clinical evidences for the vascular mechanism of metformin-induced metastasis inhibition [48]. The finding of the present study is in line with the above-mentioned studies.
Conclusion
Altogether, according to our results, both crocin and metformin have a significant suppressive effect on tumor cell invasion and metastasis that is likely through reducing VEGF and MMP9. Combination of crocin and metformin could serve as a novel therapeutic approach to enhance the effectiveness of metastatic breast cancer therapy.
Data availability
All data generated or analyzed during this study are included in this published article. Additional information is available from the corresponding author on reasonable request.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Lambert W, Pattabiraman DR, Weinberg RA (2017) Emerging biological principles of metastasis. Cell 168(4):670–691
Massagué J, Batlle E, Gomis RR (2017) Understanding the molecular mechanisms driving metastasis. Mol Oncol 11(1):3–4
de Ruijter TC, Veeck J, de Hoon JP, van Engeland M, Tjan-Heijnen VC (2011) Characteristics of triple-negative breast cancer. J Cancer Res Clin Oncol 137(2):183–192
Seyfried TN, Huysentruyt LC (2013) On the origin of cancer metastasis. Crit Rev Oncog 18(1–2):43–73
Yao H, Ashihara E, Maekawa T (2011) Targeting the Wnt/β-catenin signaling pathway in human cancers. Expert Opin Ther Targets 15(7):873–887
Dey N, Barwick BG, Moreno CS, Ordanic-Kodani M, Chen Z, Oprea-Ilies G, Tang W, Catzavelos C, Kerstann KF, Sledge GW (2013) Wnt signaling in triple negative breast cancer is associated with metastasis. BMC Cancer 13(1):537
Ferrara N, Gerber H-P, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9(6):669
Jabłońska-Trypuć A, Matejczyk M, Rosochacki S (2016) Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. Enzyme Inhib Med Chem 31(1):177–183
McArthur HL, Hudis CA (2007) Breast cancer chemotherapy. Cancer J 13(3):141–147
Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD (2005) Metformin and reduced risk of cancer in diabetic patients. BMJ 330(7503):1304–1305
Kasznicki J, Sliwinska A, Drzewoski J (2014) Metformin in cancer prevention and therapy. Ann Transl Med 2(6):57
DeCensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, Gandini S (2010) Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res 3:1451–1461. https://doi.org/10.1158/1940-6207.CAPR-10-0157
Abdullaev FI (2002) Cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus L.). Exp Biol Med 227(1):20–25
Abdullaev F, Espinosa-Aguirre J (2004) Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detect Prev 28(6):426–432
Hoshyar R, Mollaei H (2017) A comprehensive review on anticancer mechanisms of the main carotenoid of saffron, crocin. J Pharm Pharmacol 69(11):1419–1427
Mollaei H, Safaralizadeh R, Babaei E, Abedini MR, Hoshyar R (2017) The anti-proliferative and apoptotic effects of crocin on chemosensitive and chemoresistant cervical cancer cells. Biomed Pharmacother 94:307–316
Arzi L, Riazi G, Sadeghizadeh M, Hoshyar R, Jafarzadeh N (2018) A comparative study on anti-invasion, antimigration, and antiadhesion effects of the bioactive carotenoids of saffron on 4T1 breast cancer cells through their effects on Wnt/β-catenin pathway genes. DNA Cell Biol 37(8):697–707
Arzi L, Farahi A, Jafarzadeh N, Riazi G, Sadeghizadeh M, Hoshyar R (2018) Inhibitory effect of crocin on metastasis of triple-negative breast cancer by interfering with Wnt/β-catenin pathway in murine model. DNA Cell Biol 37(12):1068–1075
Vazquez-Martin A, Oliveras-Ferraros C, Cufí S, Del Barco S, Martin-Castillo B, Lopez-Bonet E, Menendez JA (2011) The anti-diabetic drug metformin suppresses the metastasis-associated protein CD24 in MDA-MB-468 triple-negative breast cancer cells. Oncol Rep 25(1):135–140
Rattan R, Ali Fehmi R, Munkarah A (2012) Metformin: an emerging new therapeutic option for targeting cancer stem cells and metastasis. J Oncol 2012:928127
Bolhasani A, Bathaie SZ, Yavari I, Moosavi-Movahedi AA, Ghaffari M (2005) Separation and purification of some components of Iranian saffron. Asian J Chem 17:725
Abedini MR, Wang P-W, Huang Y-F, Cao M, Chou C-Y, Shieh D-B, Tsang BK (2014) Cell fate regulation by gelsolin in human gynecologic cancers. Proc Natl Acad Sci 111(40):14442–14447
Wang PW, Abedini MR, Yang LX, Ding AA, Figeys D, Chang JY, Tsang BK, Shieh DB (2014) Gelsolin regulates cisplatin sensitivity in human head-and-neck cancer. Int J Cancer 135(12):2760–2769
Abedini MR, Erfanian N, Nazem H, Jamali S, Hoshyar R (2016) Anti-proliferative and apoptotic effects of Ziziphus Jujube on cervical and breast cancer cells. Avicenna J Phytomed 6(2):142
Rodriguez LG, Wu X, Guan JL (2005) Wound-healing assay. Methods Mol Biol 294:23–29. https://doi.org/10.1385/1-59259-860-9:023
Dastpeyman M, Motamed N, Azadmanesh K, Mostafavi E, Kia V, Jahanian-Najafabadi A, Shokrgozar MA (2012) Inhibition of silibinin on migration and adhesion capacity of human highly metastatic breast cancer cell line, MDA-MB-231, by evaluation of β1-integrin and downstream molecules, Cdc42, Raf-1 and D4GDI. Med Oncol 29(4):2512–2518
Cai Z, Teng L, Zhou J, Yan Y, Zhang Y, Lv G, Chen J (2019) Design and synthesis of a native heparin disaccharide grafted poly-2-aminoethyl methacrylate glycopolymer for inhibition of melanoma cell metastasis. Int J Biol Macromol 126:612–619
Zhang Y, Zhang GL, Sun X, Cao KX, Ma C, Nan N, Yang GW, Yu MW, Wang XM (2018) Establishment of a murine breast tumor model by subcutaneous or orthotopic implantation. Oncol Lett 15(5):6233–6240
Farhangi B, Alizadeh AM, Khodayari H, Khodayari S, Dehghan MJ, Khori V, Heidarzadeh A, Khaniki M, Sadeghiezadeh M, Najafi F (2015) Protective effects of dendrosomal curcumin on an animal metastatic breast tumor. Eur J Pharmacol 758:188–196. https://doi.org/10.1016/j.ejphar.2015.03.076
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65(2):87–108. https://doi.org/10.3322/caac.21262
Redig AJ, McAllister SS (2013) Breast cancer as a systemic disease: a view of metastasis. J Intern Med 274(2):113–126. https://doi.org/10.1111/joim.12084
Rahaiee S, Hashemi M, Shojaosadati SA, Moini S, Razavi SH (2017) Nanoparticles based on crocin loaded chitosan-alginate biopolymers: antioxidant activities, bioavailability and anticancer properties. Int J Biol Macromol 99:401–408
Ashrafi M, Bathaie S, Taghikhani M, Moosavi-Movahedi A (2005) The effect of carotenoids obtained from saffron on histone H1 structure and H1–DNA interaction. Int J Biol Macromol 36(4):246–252
Hoshyar R, Bathaie SZ, Sadeghizadeh M (2013) Crocin triggers the apoptosis through increasing the Bax/Bcl-2 ratio and caspase activation in human gastric adenocarcinoma, AGS, cells. DNA Cell Biol 32(2):50–57
Mostafavinia SE, Khorashadizadeh M, Hoshyar R (2016) Antiproliferative and proapoptotic effects of crocin combined with hyperthermia on human breast cancer cells. DNA Cell Biol 35(7):340–347
Ganjali M, Ganjali H (2013) Anticancer effect of metformin, an anti-diabetic drug, on breast cancer cells. J Novel Appl Sci 2:796–791
Ko JC, Huang YC, Chen HJ, Tseng SC, Chiu HC, Wo TY, Huang YJ, Weng SH, Chiou RY, Lin YW (2013) Metformin induces cytotoxicity by down-regulating thymidine phosphorylase and excision repair cross-complementation 1 expression in non-small cell lung cancer cells. Basic Clin Pharmacol Toxicol 113(1):56–65
Mu Q, Jiang M, Zhang Y, Wu F, Li H, Zhang W, Wang F, Liu J, Li L, Wang D (2018) Metformin inhibits proliferation and cytotoxicity and induces apoptosis via AMPK pathway in CD19-chimeric antigen receptor-modified T cells. Onco Targets Ther 11:1767
Chung Y-G, Tak E, Hwang S, Lee J-Y, Kim J-Y, Kim Y-Y, Song G-W, Lee K-J, Kim N (2018) Synergistic effect of metformin on sorafenib in in vitro study using hepatocellular carcinoma cell lines. Ann Hepato-biliary-pancreatic Surg 22(3):179–184
Rattan R, Graham RP, Maguire JL, Giri S, Shridhar V (2011) Metformin suppresses ovarian cancer growth and metastasis with enhancement of cisplatin cytotoxicity in vivo. Neoplasia (New York, NY) 13(5):483
Esfahanian N, Shakiba Y, Nikbin B, Soraya H, Maleki-Dizaji N, Ghazi-Khansari M, Garjani A (2012) Effect of metformin on the proliferation, migration, and MMP-2 and-9 expression of human umbilical vein endothelial cells. Mol Med Rep 5(4):1068–1074
Festuccia C, Mancini A, Gravina GL, Scarsella L, Llorens S, Alonso GL, Tatone C, Di Cesare E, Jannini EA, Lenzi A (2014) Antitumor effects of saffron-derived carotenoids in prostate cancer cell models. Biomed Res Int 2014:135048
Giles ED, Jindal S, Wellberg EA, Schedin T, Anderson SM, Thor AD, Edwards DP, MacLean PS, Schedin P (2018) Metformin inhibits stromal aromatase expression and tumor progression in a rodent model of postmenopausal breast cancer. Breast Cancer Res 20(1):50
Mehner C, Hockla A, Miller E, Ran S, Radisky DC, Radisky ES (2014) Tumor cell-produced matrix metalloproteinase 9 (MMP-9) drives malignant progression and metastasis of basal-like triple negative breast cancer. Oncotarget 5(9):2736
Liu W, Xu J, Wang M, Wang Q, Bi Y, Han M (2011) Tumor-derived vascular endothelial growth factor (VEGF)-a facilitates tumor metastasis through the VEGF-VEGFR1 signaling pathway. Int J Oncol 39(5):1213–1220
Yang X, Zhang Y, Hosaka K, Andersson P, Wang J, Tholander F, Cao Z, Morikawa H, Tegnér J, Yang Y (2015) VEGF-B promotes cancer metastasis through a VEGF-A–independent mechanism and serves as a marker of poor prognosis for cancer patients. Proc Natl Acad Sci 112(22):E2900–E2909
Wang J-C, Li G-Y, Bo W, Han S-X, Sun X, Jiang Y-N, Shen Y-W, Zhou C, Feng J, Shao-Ying L, Liu J-L, Wang M-D, Liu P-J (2019) Metformin inhibits metastatic breast cancer progression and improves chemosensitivity by inducing vessel normalization via PDGF-B downregulation. J Exp Clin Cancer Res 38(235):1–17
Acknowledgements
The authors would like to thank the laboratory in Birjand University of Medical Sciences.
Funding
This study was made possible by a Grant (ir.bums.REC.1396.268) from the Birjand University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Authors declared no conflict of interest.
Ethical approval
This study was made possible by ethical code (ir.bums.REC.1396.268) from the Birjand University of Medical Sciences.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Farahi, A., Abedini, M.R., Javdani, H. et al. Crocin and Metformin suppress metastatic breast cancer progression via VEGF and MMP9 downregulations: in vitro and in vivo studies. Mol Cell Biochem 476, 3341–3351 (2021). https://doi.org/10.1007/s11010-020-04043-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-04043-8