Abstract
Endometriosis is an estrogen-dependent, inflammatory gynecological disorder characterized by the growth of endometrial cells in lesions outside the uterus. Bone marrow-derived cells (BMDCs) engraft lesions and increase lesion size. Do endometriosis cells regulate differentiation of engrafted BMDCs in the pathogenesis and growth of endometriosis? Here, we report endometriosis derived stromal cells promote the differentiation of BMDCs to stromal, epithelial and leukocyte cell fates through paracrine signaling. In-vitro studies demonstrated that both mRNA and protein levels of vimentin, cytokeratin and PD-1 were significantly increased in BMDCs cocultured with stromal cells from endometriosis (ENDO) patients compared to stromal cells from normal endometrium (CNTL). Increased expression of PD-1 has been reported in malignancy where it promotes T cell quiescence and immune tolerance. Increased PD-1 was also confirmed in-vivo where we showed that PD-1 expression was induced in BMDCs engrafted into endometriotic lesions in a murine model of endometriosis. AMD3100, an antagonist for CXCR4 receptor inhibited PD-1 expression in BMDCs suggesting that PD-1 induction requires CXCL12. These results suggest that endometriosis stimulated BMDC differentiation through paracrine signaling and increased T cell PD-1 expression. Increased PD-1 expression may be one mechanism by which endometriosis avoids immune surveillance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endometriosis is an estrogen dependent, chronic inflammatory gynecological disorder, common among reproductive-aged women that causes infertility and pelvic pain [1, 2]. Approximately 10 percentage of reproductive age women suffer from this disease and it is far more common in women experiencing infertility or chronic pelvic pain [1, 3]. Endometriosis is characterized by the growth of endometrial tissue outside the uterine cavity, commonly in the pelvic peritoneum [1]. While the etiology of endometriosis still remains unknown, the most widely accepted mechanism for the development of the peritoneal endometriotic lesions is through retrograde menstruation [4, 5]. In multiple organs bone marrow-derived cells (BMDCs) migrate and differentiate into tissue-specific cells [6]; in endometrium BMDCs contribute to epithelial and stroma regeneration and are likely the main source of extra-pelvic endometriosis [7, 8].
BMDCs include mesenchymal stem cells that have a vast potential for differentiation; a single cell has the potential differentiate into multiple different phenotypes and induce regeneration and repair of multiple tissues [9]. Moreover, BMDCs include hematopoietic stem cells that have been shown to regulate immune response and play a role in improving the outcome of allogeneic transplantation [10,11,12]. Patients with endometriosis have many abnormalities in the immune system such as impaired T-cell, B cell, and NK cell functions and increased levels of various proinflammatory cytokines and angiogenetic factors [13,14,15]. Recent studies demonstrate that BMDCs promote proliferation in cancer cells through inhibition of T cell immune responses via programmed cell death 1 (PD-1) and its ligand PD-L1 [16]. In patients with endometriosis, there are increased numbers of circulating lymphocytes that express PD-1 and PD-L1 [17]. PD-1 is an immunoreceptor containing 288–amino acids, expressed on all T-cells during their activation by the T-cell antigen receptor and cytokine receptors [18]. PD-1 expression causes immune tolerance by functioning as an immune checkpoint. Increased PD-1 expression on T-cell surface is known to promote cancer cells survival [19,20,21]. Furthermore, Wu et al. reported upregulated PD-1 expression on immune cells in endometriosis, implying immune dysfunction in endometriosis through PD-1/PD-L1 pathway and subsequently endometriosis development [22]. PD-1 and its ligands PD-L1 and PD-L2 (programmed death ligand 1 and 2), play key roles in the regulatory inhibition of immune homeostasis as well as peripheral tolerance. The role of the PD-1 in endometriosis has not been extensively studied. The relationship between endometriosis and the impaired immune response indicate that PD-1 may be involved in the pathogenesis of the disease.
The maintenance and remodeling of endometrium and endometriosis likely relies on the interaction of BMDCs with local cell types. The interactions between mesenchymal stem cells and resident endometrial cell populations in a given tissue are still poorly characterized. The mechanisms leading to tissue specific cell type induction of BMDCs is not well understood. We developed an in vitro model to monitor these interactions, allow us to investigate the role of paracrine cellular communication in endometriosis formation.
We demonstrated that stromal cells from endometriosis lesions induced BMDC stromal, epithelial and immune cell (T cell) differentiation in vitro. Further PD-1 expression was increased both in vitro and in engrafted BMDCs in a murine model of endometriosis.
Materials and methods
Sample collection
Written consent was obtained from subjects admitted to the Yale-New Haven Hospital for laparoscopy or laparotomy with benign indications including uterine bleeding, pelvic pain, elective contraception, infertility, or endometriosis. Institutional Review Board (IRB) approval was obtained for the use of human samples (Human Investigations Committee protocol, #1,004,006,657). Inclusion criteria included women who were aged 18–49 years, had regular menstrual cycles, and used no hormonal therapy for at least 3 months prior to surgery. Exclusion criteria included post-menopausal status, previous hormone use within 3 months of surgery, hyperplasia, polyps, malignancy, autoimmune disease, cardiovascular disease, or use of anti-inflammatory medications. Twenty (20) subjects were included in each group: endometriosis patients and controls with no evidence of endometriosis or endometrial pathology. Endometrial biopsies in the secretory phase were obtained from women with moderate-to-severe disease (American Society for Reproductive Medicine stages III and IV) [23]. The endometriosis group was surgically diagnosed and histologically verified and the control group patients were visually verified to be free of endometriosis during the surgery. Controls underwent surgery for benign gynecologic disease and had no evidence of endometriosis. The most common indications for surgery in the controls were elective tubal ligation, infertility, uterine bleeding or ovarian cysts. The phase of the menstrual cycle was determined based on the subjects’ menstrual history and last menstrual period.
Cell culture
Primary stromal cells from ectopic lesions (ENDO) and normal eutopic endometrial (CNTL) tissues were processed and cultured using a protocol previously described by Ryan et al. [24] with minor modifications [25]. Briefly, endometriotic or endometrial tissue was minced finely and digested in digestion medium containing collagenase B (1 mg/ml, Roche Diagnostics, Indianapolis, IN, USA) and deoxyribonuclease I (0.1 mg/ ml, Sigma-Aldrich, St. Louis, MO, USA) in DMEM medium, at 37 °C for 30 min, and tissue was pipetted gently to disperse the cells every 10 min. Stromal cells were filtrated through a 40-μm cell strainer and cultured in DMEM/F-12 containing 10% fetal bovine serum (Millipore Sigma, St. Louis, MO, Cat.#F4135) and 1% penicillin–streptomycin. The fibroblast-like appearance of endometriosis derived stromal cells in culture under phase contrast microscopy appeared identical to that of endometrial stromal cells. Both endometriosis and normal eutopic stromal cells were used at 2 to 3 passages for all experiments. The normal human endometrial stromal cell line (HESC) was used as previously described [26]. The immortalized human endometriosis stromal cell line (endoHESC) was a gift from Prof. Gil Mor laboratory at Yale School of Medicine, New Haven, CT, USA.
BMDCs were obtained from the American Type Culture Collection (ATCC, cat. # PCS-500–012, Manassas, VA, USA). Coculture between BMDCs and stromal cells was carried out using 4-μm pore size polycarbonate membrane (Millipore, Burlington, USA). Stromal cells were seeded into a 6-well plate at a concentration of 1 × 105 cells per well. BMSCs were plated into the trans-well insert at a concentration of 1 × 104 cells per insert and the insert was placed into the 6-well plate. Each well had 3 ml growth media (ATCC CRL-11731) with a final concentration of 15% fetal bovine serum. BMDCs were treated with AMD3100 (100 ng/ml) purchased from Millipore Sigma (Cat.#A5602). Total RNA and protein were collected from the cell cultures grown for 24, 48, and 72 h. Total RNA was used for the analysis of gene expression by qRT-PCR while protein was analyzed by western blot.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated from endometriotic lesions by TRIzol reagent (Life Technologies), followed by purification using Qiagen cleaning kit (Qiagen, Valencia, CA, USA) to prepare cDNA with 50 ng RNA in a 20 μl reaction mixture by iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA). Quantitative real-time PCR was performed to quantify gene expression using specific primers for PD-1, vimentin and CK19 and SYBR Green (Bio-Rad) and optimized in the MyiQ Single Color Real-Time PCR Detection System (Bio-Rad). The specificity of the amplified transcript (39 cycles) and absence of primer-dimers was confirmed by a melting curve analysis. Gene expression was normalized to the expression of β-actin for each sample. Relative mRNA expression for each gene was calculated using the comparative cycle threshold (Ct) method, also known as the 2-ΔΔCT method [27]. All experiments were carried out three times and each experiment was performed in duplicate. Nuclease-free water was used as a negative control replacing the cDNA template.
Western blot analysis
Protein was extracted from the cells with RIPA lysis buffer containing protease inhibitors (Bio-Rad Laboratories). Total protein concentration was determined by BCA protein assay kit (Pierce, Rockford, IL, USA). Equal amounts of protein (25 μg) from lysates were separated on 4–20% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) (Bio-Rad Laboratories) and transferred on to a polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories). The membranes were blocked in 5% non-fat milk at room temperature for 1 h and incubated overnight at 4 °C with rabbit anti-CK19 (1:1000, catalog #12,434), rabbit anti-vimentin (1:400, catalog #5741), and anti-PD-1 (1:500, catalog #86,163; 1:200) and anti-GAPDH (1:1000, catalog #5174) primary antibodies purchased from Cell Signaling Technology, Beverly, MA, USA. On the following day, membranes were washed three times each for 5 min in 1% TBST, followed by incubation with a goat anti-rabbit IgG conjugated to horseradish peroxidase secondary antibody (Abcam) in 5% BSA. The protein bands on the membrane were visualized using enhanced chemiluminescence solutions A and B mix for 3 min (PerkinElmer, Inc., Waltham, MA). The density of the bands was assessed by the Image J software, and values were normalized to the densitometric values of GAPDH. Western blots were run twice with duplicate samples.
Induction of endometriosis in mice
C57BL/6J wild-type and ubiquitin-GFP mice were purchased from Charles River Laboratories (Wilmington, MA, USA) and The Jackson Laboratory (Bar Harbor, ME, USA), respectively. All animal experiments were conducted in accordance with an approved protocol from Institutional Animal Care and Use Committee (IACUC) of Yale University for animal care, 5-fluorouracil (5-FU) treatments, bone marrow cell injections, blood collection, and anesthesia. Briefly, wild-type mice (6-wk) received 125 mg/kg of 5-fluorouracil (5-FU) by i.p on 6 and 1 days (day -6 and -1) before bone marrow transplantation (BMT, day 0). In addition, stem cell factor (SCF, 50 mg/kg) was injected twice before BMT, as previously described [28], and fresh BM cell transplantation was performed as described previously [29].
Endometriosis in mice (N = 12) was surgically induced under aseptic conditions and anesthesia using a modified method previously described [8, 30]. Surgery was performed 30 days following BMT. Uterine horns were removed from wild-type female donor mice at diestrus, opened longitudinally, cut into fragments of 3-mm size and sutured onto the peritoneal wall of recipient mice. Three fragments were systematically transplanted into peritoneal wall of each mouse. Sham mice were subjected to the surgery, but in place of suturing uterine tissue, peritoneal tissue from the ventral midline was used.
Immunohistochemistry and Immunofluorescence
Tissue from endometriotic lesions was fixed in 4% paraformaldehyde and embedded in paraffin. Five-micrometer tissue sections were mounted on slides, steamed in sodium citrate at pH 6 for 10 min for antigen retrieval, and blocked using 10% donkey serum (Vector Laboratories, Burlingame, CA, USA). Sections on slides were incubated at 4 °C overnight with primary antibodies, rabbit anti-CD3 (catalog #ab5690; 1:250, Abcam, Cambridge, MA, USA) rabbit anti-PD-1 (catalog # 84,651; 1:200; Cell Signaling) and goat anti-GFP (catalog #ab6673; 1:400, Abcam). Secondary antibodies used were Alexa Fluor 568-conjugated donkey anti-goat (1:200, catalog #A11057, Invitrogen, CA, USA) and Alexa Fluor 488-conjugated donkey anti-rabbit (1:200, catalog #A21206, Invitrogen). Sections were mounted under coverslips using Vectashield fluorescent mounting media with 46-diamidino-2-phenylindole (DAPI) (catalog #H-1200; Vector Laboratories, Burlingame, CA). Visualization of the slides was performed using a laser scanning confocal microscope (LSM 710; Zeiss) and the ZEN software (Carl Zeiss). GFP+ cells as well as GFP+-PD-1+ double positive cells were quantified in three sections per mouse (N = 6). The average of total cells was analyzed for statistical significance between endometriosis and sham groups.
Statistical analysis
Data were analyzed using GraphPad Prism 8.0 (GraphPad Software Inc., La Jolla, CA, USA). An unpaired student’s t-test for total labeled cells, one-way analysis of variance (ANOVA) for cell counts and RT-PCR data were used to determine statistical significance. Data is expressed as means ± standard error mean (SEM).
Results
Upregulation of vimentin in BMDCs by stromal cells from endometriosis
Vimentin in BMDCs was used as a specific marker for stromal cells. Both mRNA and protein levels were determined for vimentin in BMDCs on day 1 (24 h), day 3 (72 h) and day 6 (144 h) of co-culture. Expression was compared between BMDCs cultured alone or cocultured either with primary endometriosis stromal cells (ENDO), or primary eutopic endometrial stromal cells (CNTL). Vimentin mRNA (Fig. 1a) as well as protein levels (Fig. 1b) were significantly (p < 0.05) increased in BMDCs cocultured with ENDO cells at all time points (day1, 3, & 6) compared to BMDCs alone, while coculture with CNTL cells showed significant decreases in vimentin levels on day 3 and 6. Densitometry analysis of protein bands shows an increase in protein levels to 2.0, 2.5, and 3.0 fold on day 1, 3, and 6 respectively in BMDCs cocultured with ENDO cells compared to BMDCs alone while protein levels decreased significantly by 2.0 fold on day 3 and 6 in BMDCs cocultured with CNTL cells.
Increased expression of vimentin in BMDCs. a vimentin mRNA levels were significantly increased in BMDCs on day 1, 3 and 6 when co-cultured with ENDO cells and decreased with CNTL cells on day 3 and 6. Each bar represents the mean ± SEM of three individual experiments and each experiment was performed in duplicate. b increased protein levels in BMDCs by ENDO cells on day 1,3 and 6 and decreased by CNTL cells on day 3 and 6. Each bar represents the mean ± SEM of two separate blots and each sample run in duplicate. *p < 0.05 between BMDCs alone vs coculture with ENDO or CNTL cells
Upregulation of CK19 in BMDCs by stromal cells from endometriosis
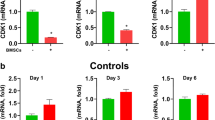
After determining the expression levels of stromal cell marker vimentin in BMDCs, we determined the status of CK19, a specific marker for an epithelial cells, under the paracrine influence of endometriosis derived cells. Figure 2a shows a significant increase (p < 0.05) in CK19 mRNA levels on day 3 and 6, but not on day 1 in BMDCs cocultured with ENDO cells compared to BMDCs alone, while CNTL cells did not have any effect on this marker of epithelial differentiation. Figure 2b shows that CK19 protein levels increased significantly (p < 0.05) on day 6 (7.5 fold) in BMDCs cocultured with ENDO cells and to twofold after coculture with CNTL cells.
Increased expression of CK19 in BMDCs. a CK19 mRNA levels were significantly increased in BMDCs on day 3 and 6 when co-cultured with ENDO cells. Each bar represents the mean ± SEM of three individual experiments and each experiment was performed in duplicate. b increased CK19 protein levels in BMDCs by ENDO and CNTL cells on day 6. Each bar represents the mean ± SEM of two separate blots and each sample run in duplicate. *Denotes p < 0.05 between BMDCs vs coculture with ENDO or CNTL cells
Increased PD-1 expression in BMDCs induced by stromal cells from endometriotic lesions
We determined the effect of primary stromal cells obtained from endometriotic lesions (ENDO) from subjects with endometriosis as well as eutopic endometrium (CNTL) from subjects without endometriosis on PD-1 expression in BMDCs. As shown in Fig. 3a, PD-1 mRNA levels increased significantly (p < 0.05) on day 1 (threefold), 3 (threefold) and 6 (7.5-fold) in BMDCs cocultured with ENDO cells compared to BMDCs alone. In contrast, PD-1 mRNA levels in BMDCs decreased significantly (p < 0.05) on day 1, 3 and 6 when cocultured with CNTL cells. Figure 3b shows the western blot analysis of PD-1 protein levels mimic the mRNA levels in cocultures as well as BMDCs alone. Densitometry analysis of protein bands shows an increase in protein levels to 1.5, 2.2, and 2.2 fold on day 1, 3, and 6 respectively in BMDCs cocultured with ENDO cells compared to BMDCs alone while protein levels decreased significantly in coculture with CNTL cells. Besides primary stromal cells from endometriosis, we also tested the effect of immortalized stromal cells originated from an endometriosis cell line (endoHESC) as well as normal human stromal endometrial cell line (HESC) as a control, on PD-1 expression in BMDCs to establish the effect of endometriosis stromal cells on PD-1 expression in BMDCs. In-vitro coculture studies showed that PD-1 mRNA levels were significantly (p < 0.05) increased on day 1, 3 and 6 in BMDCs when cocultured with endoHESC cells compared to HESC cells or BMDCs alone as shown in Fig. 4. There is a dramatic increase in mRNA levels on day 6 (16-fold) compared to day 1 (2.5- fold) and 3 (threefold) compared to BMDCs alone.
Effect of primary stromal cells from endometriosis patients on PD-1 expression in BMDCs. Upregulation of PD-1 expression in BMDCs by primary stromal cells from endometriosis. a PD-1 mRNA levels significantly increased in BMDCs on day 1, 3 and 6 when co-cultured with ENDO cells while decreased with CNTL cells. Each bar represents the mean ± SEM of three individual experiments and each experiment was performed in duplicate. b increased PD-1 protein levels in BMDCs by ENDO cells on day 1, 3 and 6. Each bar represents the mean ± SEM of two separate blots and each sample run in duplicate. *Denotes p < 0.05 between BMDCs vs coculture with ENDO or CNTL
Effect of endometriosis stromal cell line on PD-1 expression in BMDCs. PD-1 mRNA levels were upregulated in BMDCs on day 1, 3 and 6 that are cocultured with endoHESC cells from a cell line compared with HESC cells or BMDCs alone. Each bar represents the mean ± SEM of three individual experiments and each experiment was performed in duplicate. *denotes statistical significance (p < 0.05) between BMDCs coculture with endoHESC cells vs BMDCs
We created murine experimental endometriosis by transplanting uterine tissue into the peritoneal cavity. Four weeks after transplantation to recipient mice the lesions were confirmed using H & E staining that demonstrated growth of glandular and stromal endometrial tissue as shown in supplemental Fig. 2. Before analyzing PD-1 expression in lesions, we localized T cells in endometriotic lesions using IHC with an anti-CD3 antibody (Suppl. Figure 1). Similarly, we used PD-1 as another T cell marker with a known immune function. In-vivo studies in a mouse model of endometriosis showed that some of the GFP-BMDCs (green) engrafted into endometriotic lesions, expressed PD-1 protein (red) as shown in Fig. 5a in the endometriosis group (right, endometriotic lesions) compared to sham mice where no GFP-BMDCs expressed PD-1 protein (left, eutopic endometrium). The number of GFP-BMDCs engrafted into lesions and the number of GFP-BMDCs that express PD-1 is significantly higher (P < 0.05) in mice with endometriosis compared to sham mice as shown in Fig. 5b.
Representative images of PD-1 expressing cells by immunofluorescence studies after GFP BM transplant. Tissue sections from uterus of sham mice and lesions from mice with endometriosis were immuno-stained by anti-GFP, anti-PD-1, and DAPI for nuclear stain. a lesions from endometriosis (right) showed some of the GFP-BMDCs (green) expressing PD-1 (red) compared to sham mice (left). b shows that PD-1 expressing cells are significantly higher (p < 0.05) in endometriosis compared to sham mice. Scale bar: 100 μm
AMD3100 inhibited PD-1 expression in BMDCs cocultured with primary stromal cells from endometriosis patients
Previously we showed that AMD3100, an antagonist for CXCR4 receptor inhibited CXCR4-CXCL12 signaling axis [31] and lesion growth [7] in endometriosis. To determine the effect of AMD3100 on PD-1 expression, we treated BMDCs with AMD3100 in coculture with primary stromal cells from patients with endometriosis. AMD3100 inhibited the effect of primary stromal cells on PD-1 expression at both mRNA (Fig. 6a) as well as protein levels (Fig. 6b) in BMDCs in coculture condition on day 1, 3 and 6 as shown in Fig. 6.
Effect of AMD3100 on PD-1 expression in BMDCs. AMD3100 inhibited PD-1 expression in BMDCs. a PD-1 mRNA levels were significantly inhibited in BMDCs treated with AMD3100 on day 1, 3 and 6 when co-cultured with ENDO cells while decreased with CNTL cells. Each bar represents the mean ± SEM of three individual experiments and each experiment was performed in duplicate. b PD-1 protein levels in BMDCs were significantly decreased in BMDCs treated with AMD3100 on day 1, 3 and 6. Each bar represents the mean ± SEM of two separate blots and each sample run in duplicate. *p < 0.05 between BMDCs vs BMDCs treated with AMD3100
Discussion
We previously reported that endometrial stromal cells in endometrium provided a local microenvironment for BMDCs to differentiate and engraft endometriosis lesions [29]. Further, we have also shown that engrafted BMDCs in endometriotic lesions are responsible for the development of endometriosis in a murine model of endometriosis [29]. Others have described that BMDCs can differentiate in the direction of endometrial epithelial cells in certain microenvironments in mice [32,33,34]. To investigate the effect of endometrial stromal cells from tissue collected from endometriotic lesions from patients with endometriosis (ENDO cells), on BMDCs differentiation, we used an in-vitro coculture system that allowed the biological factors secreted by endometrial stromal cells to pass freely through a membrane to the BMDCs microenvironment. Bone marrow (BM) contains both hematopoietic and mesenchymal stem cells.
Here we observed that stromal cells from endometriosis lesions can trigger BMDCs differentiation toward vimentin expressing stromal cells, cytokeratin expressing epithelial cells and PD-1 expressing T cells. Paracrine factors drive stem cell differentiation preferentially in endometriosis. We have previously shown that endometriosis recruits stem cells to a far greater degree than eutopic endometrium [8]. While we have identified the chemokine CXCL12 as necessary for recruitment [31, 35, 36], the role of stromal cells in directing stem cell differentiation has not been previously explored. Here we conclude that stromal derived factors lead to increased cell differentiation as well as the previously described chemotaxis.
PD-1 is express by T-cells. We showed previously that some of the BMDCs engrafted into endometriotic lesions are immune cells [29, 37,38,39]. We confirmed that endometriotic lesions contain T-cells and that many of these cells expressed PD-1. T cell immune responses are inhibited through the PD-1 pathway [16, 40] which plays a role in maintaining peripheral T-lymphocyte tolerance and regulating inflammation [41]. Our in-vitro results from primary cells as well as cell lines revealed that cells from endometriosis upregulate the mRNA and protein levels of PD-1 in BMDCs compared to the normal control cells. Our in-vivo study in a murine model of endometriosis demonstrated that more of the engrafted BMDCs expressed PD-1 in endometriotic lesions from mice with endometriosis compared to sham mice controls. When PD-1 is bound to PD-1 ligand, it prevents cytotoxic T cells from killing other cells; this strategy is used by cancers to avoid immune surveillance. Similarly, endometriosis cells may use this approach; the increased PD-1 levels detected here may potentiate the immunoprotective effect enabling survival of endometriosis. Immune checkpoint inhibitors that antagonize PD-1 are an effective cancer treatment strategy; they may also have an effect on endometriosis, allowing reversal of the PD-1 mediated immunosuppression. While the paracrine effect on T cells demonstrated here may allow for local immune privilege, it is unlikely to block the systemic inflammation seen in endometriosis; endometriosis generates an inflammatory environment that is mediated by secretion of cytokines derived from macrophages and other immune cells [42]. The role of PD-1 in repressing local T cell mediated cytotoxicity but not inflammatory cytokines from other immune cells may help to explain the paradox of lost immune surveillance of endometriosis within an otherwise inflammatory milieu. C-X-C motifs containing chemokine 12 (CXCL12), also known as stromal cell-derived factor-1 (SDF-1) is also highly expressed in endometriotic lesions from patients with endometriosis compared to eutopic endometrium from normal subjects [43,44,45]. CXCL12, like PD-1, also functions in the modulation of the immune system via lymphocyte migration, development, or survival [46,47,48], and mediates the mobilization and homing of bone marrow stem/progenitor cells to injured microenvironments [48, 49]. It will be important to study the cross talk between signaling pathways that are regulated by CXCL12 and PD-1 in endometriosis. We have previously shown that CXCL12 recruits stem and immune cells to endometriosis, however PD-1 may repress T cell function, allowing endometriosis to evade immune rejection. Here we show that blockade of CXCL12 signaling with AMD3100 prevents PD-1 expression suggesting that the CXCL12 / CXCR4 axis is necessary for BM T cell differentiation. Further studies are needed to dissect the cross-talk between PD-1 and CXCR4-CXCL12 signaling pathways and the direct effects of PD-1 inhibitors.
We conclude that local paracrine factors promote BMDCs cell differentiation into stromal, epithelial and immune cells. Further, stromal cells from endometriosis upregulate PD-1 expression in BMDCs engrafted into endometriotic lesions suppressing immune mediated endometriosis rejection. Therapeutics to target and inhibit PD-1 may be helpful in treating endometriosis.
References
Giudice LC (2010) Clinical practice. Endometriosis N Engl J Med 362:2389–2398. https://doi.org/10.1056/NEJMcp1000274
Johnson NP, Hummelshoj L (2013) Consensus on current management of endometriosis. Hum Reprod 28:1552–1568. https://doi.org/10.1093/humrep/det050
Mounsey AL, Wilgus A, Slawson DC (2006) Diagnosis and management of endometriosis. Am Fam Physician 74:594–600
Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM (1984) Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol 64:151–154
Tal A, Tal R, Pluchino N, Taylor HS (2019) Endometrial cells contribute to preexisting endometriosis lesions in a mouse model of retrograde menstruationdagger. Biol Reprod 100:1453–1460. https://doi.org/10.1093/biolre/ioz039
Polymeri A, Giannobile WV, Kaigler D (2016) Bone marrow stromal stem cells in tissue engineering and regenerative medicine. Horm Metab Res 48:700–713. https://doi.org/10.1055/s-0042-118458
Pluchino N, Taylor HS (2016) Endometriosis and stem cell trafficking. Reprod Sci 23:1616–1619. https://doi.org/10.1177/1933719116671219
Sakr S, Naqvi H, Komm B, Taylor HS (2014) Endometriosis impairs bone marrow-derived stem cell recruitment to the uterus Whereas Bazedoxifene treatment leads to endometriosis regression and improved uterine stem cell engraftment. Endocrinology 155:1489–1497. https://doi.org/10.1210/en.2013-1977
Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, Kon E, Marcacci M (2001) Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med 344:385–386. https://doi.org/10.1056/nejm200102013440516
Ishida T, Inaba M, Hisha H, Sugiura K, Adachi Y, Nagata N, Ogawa R, Good RA, Ikehara S (1994) Requirement of donor-derived stromal cells in the bone marrow for successful allogeneic bone marrow transplantation. Complete prevention of recurrence of autoimmune diseases in MRL/MP-Ipr/Ipr mice by transplantation of bone marrow plus bones (stromal cells) from the same donor. J Immunol 152:3119–3127
Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O (2004) Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 363:1439–1441. https://doi.org/10.1016/s0140-6736(04)16104-7
Ikehara S (2001) Successful allogeneic bone marrow transplantation. Crucial roles of stromal cells in prevention of graft rejection. Acta Haematol 105:172–178. https://doi.org/10.1159/000046561
Herington JL, Bruner-Tran KL, Lucas JA, Osteen KG (2011) Immune interactions in endometriosis. Expert Rev Clin Immunol 7:611–626. https://doi.org/10.1586/eci.11.53
Agostinis C, Zorzet S, De Leo R, Zauli G, De Seta F, Bulla R (2015) The combination of N-acetyl cysteine, alpha-lipoic acid, and bromelain shows high anti-inflammatory properties in novel in vivo and in vitro models of endometriosis. Mediators Inflamm 2015:918089. https://doi.org/10.1155/2015/918089
Bedaiwy MA, Falcone T, Sharma RK, Goldberg JM, Attaran M, Nelson DR, Agarwal A (2002) Prediction of endometriosis with serum and peritoneal fluid markers: a prospective controlled trial. Hum Reprod 17:426–431. https://doi.org/10.1093/humrep/17.2.426
Chen D, Tang P, Liu L, Wang F, Xing H, Sun L, Jiang Z (2018) Bone marrow-derived mesenchymal stem cells promote cell proliferation of multiple myeloma through inhibiting T cell immune responses via PD-1/PD-L1 pathway. Cell Cycle 17:858–867. https://doi.org/10.1080/15384101.2018.1442624
Walankiewicz M, Grywalska E, Polak G, Korona-Glowniak I, Witt E, Surdacka A, Kotarski J, Rolinski J (2018) The increase of circulating PD-1- and PD-L1-expressing lymphocytes in endometriosis: correlation with clinical and laboratory parameters. Mediators Inflamm 2018:7041342. https://doi.org/10.1155/2018/7041342
Sharpe AH, Pauken KE (2018) The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol 18:153–167. https://doi.org/10.1038/nri.2017.108
Chen X, Fosco D, Kline DE, Meng L, Nishi S, Savage PA, Kline J (2014) PD-1 regulates extrathymic regulatory T-cell differentiation. Eur J Immunol 44:2603–2616. https://doi.org/10.1002/eji.201344423
Lim TS, Chew V, Sieow JL, Goh S, Yeong JP, Soon AL, Ricciardi-Castagnoli P (2016) PD-1 expression on dendritic cells suppresses CD8(+) T cell function and antitumor immunity. Oncoimmunology 5:e1085146. https://doi.org/10.1080/2162402x.2015.1085146
McKay JT, Egan RP, Yammani RD, Chen L, Shin T, Yagita H, Haas KM (2015) PD-1 suppresses protective immunity to Streptococcus pneumoniae through a B cell-intrinsic mechanism. J Immunol 194:2289–2299. https://doi.org/10.4049/jimmunol.1401673
Wu L, Lv C, Su Y, Li C, Zhang H, Zhao X, Li M (2019) Expression of programmed death-1 (PD-1) and its ligand PD-L1 is upregulated in endometriosis and promoted by 17beta-estradiol. Gynecol Endocrinol 35:251–256. https://doi.org/10.1080/09513590.2018.1519787
(1997) Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril 67:817–21. https://doi.org/10.1016/s0015-0282(97)81391-x
Ryan IP, Schriock ED, Taylor RN (1994) Isolation, characterization, and comparison of human endometrial and endometriosis cells in vitro. J Clin Endocrinol Metab 78:642–649. https://doi.org/10.1210/jcem.78.3.8126136
Noble LS, Takayama K, Zeitoun KM, Putman JM, Johns DA, Hinshelwood MM, Agarwal VR, Zhao Y, Carr BR, Bulun SE (1997) Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J Clin Endocrinol Metab 82:600–606. https://doi.org/10.1210/jcem.82.2.3783
Krikun G, Mor G, Alvero A, Guller S, Schatz F, Sapi E, Rahman M, Caze R, Qumsiyeh M, Lockwood CJ (2004) A novel immortalized human endometrial stromal cell line with normal progestational response. Endocrinology 145:2291–2296. https://doi.org/10.1210/en.2003-1606
Barr A, Manning D (1999) G proteins techniques of analysis. CRC Press Inc, Boca Raton, pp 227–245
Tal R, Liu Y, Pluchino N, Shaikh S, Mamillapalli R, Taylor HS (2016) A murine 5-fluorouracil-based submyeloablation model for the study of bone marrow-derived cell trafficking in reproduction. Endocrinology 157:3749–3759. https://doi.org/10.1210/en.2016-1418
Du H, Taylor HS (2007) Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells 25:2082–2086. https://doi.org/10.1634/stemcells.2006-0828
Lee B, Du H, Taylor HS (2009) Experimental murine endometriosis induces DNA methylation and altered gene expression in eutopic endometrium. Biol Reprod 80:79–85. https://doi.org/10.1095/biolreprod.108.070391
Wang X, Mamillapalli R, Mutlu L, Du H, Taylor HS (2015) Chemoattraction of bone marrow-derived stem cells towards human endometrial stromal cells is mediated by estradiol regulated CXCL12 and CXCR4 expression. Stem Cell Res 15:14–22. https://doi.org/10.1016/j.scr.2015.04.004
Zhang WB, Cheng MJ, Huang YT, Jiang W, Cong Q, Zheng YF, Xu CJ (2012) A study in vitro on differentiation of bone marrow mesenchymal stem cells into endometrial epithelial cells in mice. Eur J Obstet Gynecol Reprod Biol 160:185–190. https://doi.org/10.1016/j.ejogrb.2011.10.012
Morelli SS, Rameshwar P, Goldsmith LT (2013) Experimental evidence for bone marrow as a source of nonhematopoietic endometrial stromal and epithelial compartment cells in a murine model. Biol Reprod 89:7. https://doi.org/10.1095/biolreprod.113.107987
Yin M, Zhou HJ, Lin C, Long L, Yang X, Zhang H, Taylor H, Min W (2019) CD34(+)KLF4(+) stromal stem cells contribute to endometrial regeneration and repair. Cell Rep 27:2709-2724.e3. https://doi.org/10.1016/j.celrep.2019.04.088
Moridi I, Mamillapalli R, Cosar E, Ersoy GS, Taylor HS (2016) Bone marrow stem cell chemotactic activity is induced by elevated CXCl12 in endometriosis. Reprod Sci. https://doi.org/10.1177/1933719116672587
Pluchino N, Mamillapalli R, Shaikh S, Habata S, Tal A, Gaye M, Taylor HS (2020) CXCR4 or CXCR7 antagonists treat endometriosis by reducing bone marrow cell trafficking. J Cell Mol Med 24:2464–2474. https://doi.org/10.1111/jcmm.14933
Du H, Naqvi H, Taylor HS (2012) Ischemia/reperfusion injury promotes and granulocyte-colony stimulating factor inhibits migration of bone marrow-derived stem cells to endometrium. Stem Cells Dev 21:3324–3331. https://doi.org/10.1089/scd.2011.0193
Tal R, Shaikh S, Pallavi P, Tal A, Lopez-Giraldez F, Lyu F, Fang YY, Chinchanikar S, Liu Y, Kliman HJ, Alderman M 3rd, Pluchino N, Kayani J, Mamillapalli R, Krause DS, Taylor HS (2019) Adult bone marrow progenitors become decidual cells and contribute to embryo implantation and pregnancy. PLoS Biol 17:e3000421. https://doi.org/10.1371/journal.pbio.3000421
Taylor HS (2004) Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA 292:81–85. https://doi.org/10.1001/jama.292.1.81
Augello A, Tasso R, Negrini SM, Amateis A, Indiveri F, Cancedda R, Pennesi G (2005) Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol 35:1482–1490. https://doi.org/10.1002/eji.200425405
Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL (2005) CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol 25:9543–9553. https://doi.org/10.1128/mcb.25.21.9543-9553.2005
Nematian SE, Mamillapalli R, Kadakia TS, Majidi Zolbin M, Moustafa S, Taylor HS (2018) Systemic inflammation induced by microRNAs: endometriosis-derived alterations in circulating microRNA 125b–5p and Let-7b-5p regulate macrophage cytokine production. J Clin Endocrinol Metab 103:64–74. https://doi.org/10.1210/jc.2017-01199
Bellelis P, Barbeiro DF, Rizzo LV, Baracat EC, Abrao MS, Podgaec S (2013) Transcriptional changes in the expression of chemokines related to natural killer and T-regulatory cells in patients with deep infiltrative endometriosis. Fertil Steril 99:1987–1993. https://doi.org/10.1016/j.fertnstert.2013.02.038
Kitaya K, Nakayama T, Daikoku N, Fushiki S, Honjo H (2004) Spatial and temporal expression of ligands for CXCR3 and CXCR4 in human endometrium. J Clin Endocrinol Metab 89:2470–2476. https://doi.org/10.1210/jc.2003-031293
Leconte M, Chouzenoux S, Nicco C, Chereau C, Arkwright S, Santulli P, Weill B, Chapron C, Dousset B, Batteux F (2014) Role of the CXCL12-CXCR4 axis in the development of deep rectal endometriosis. J Reprod Immunol 103:45–52. https://doi.org/10.1016/j.jri.2013.12.121
Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA (1996) A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). J Exp Med 184:1101–1109. https://doi.org/10.1084/jem.184.3.1101
Hattori K, Heissig B, Tashiro K, Honjo T, Tateno M, Shieh JH, Hackett NR, Quitoriano MS, Crystal RG, Rafii S, Moore MA (2001) Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood 97:3354–3360. https://doi.org/10.1182/blood.v97.11.3354
Cheng JW, Sadeghi Z, Levine AD, Penn MS, von Recum HA, Caplan AI, Hijaz A (2014) The role of CXCL12 and CCL7 chemokines in immune regulation, embryonic development, and tissue regeneration. Cytokine 69:277–283. https://doi.org/10.1016/j.cyto.2014.06.007
Kim CH, Broxmeyer HE (1998) In vitro behavior of hematopoietic progenitor cells under the influence of chemoattractants: stromal cell-derived factor-1, steel factor, and the bone marrow environment. Blood 91:100–110
Acknowledgements
We thank Aya Tal for anti-CD3 staining and Marie Gaye for proofreading the manuscript.
Funding
This work was supported by the Endometriosis Foundation of America AWD0003567.
Author information
Authors and Affiliations
Contributions
PC performed experiments, analyzed the data and drafted the manuscript. RM designed the study, study implementation, analyzed the data, prepared the figures, drafted and revised the manuscript. SH participated in the experiments and read the manuscript. HT conceived, design study, analyzed the data and finalized the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for publication
All authors have read the manuscript and authorized the submission for publication.
Ethical approval
All procedures performed in this study involving patients and animals were in accordance with the ethical standards of the Ethical Committee of Yale University. Appropriate guidelines have been followed for the use of animals. Written informed consent was signed by all patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Suppl Fig. 1
Immunostaining of lesions showing T-cells. Representative image of lesion section showing CD3 expression, a marker for T-cells. Murine lesions were stained with anti-CD3 antibody (brown). Scale bar: 100 μm. (PDF 713 kb)
Suppl. Fig. 2
Gross morphology of endometriotic lesions: Circles (yellow) indicate endometriotic lesions (left). H & E staining of lesion tissue section showing glandular uterine structure (right). (TIF 630 kb)
Rights and permissions
About this article
Cite this article
Chen, P., Mamillapalli, R., Habata, S. et al. Endometriosis stromal cells induce bone marrow mesenchymal stem cell differentiation and PD-1 expression through paracrine signaling. Mol Cell Biochem 476, 1717–1727 (2021). https://doi.org/10.1007/s11010-020-04012-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-04012-1