Abstract
Sirtuin 3 (SIRT3) modulates mitochondria-localized processes and is implicated in the metabolic reprogramming of cancer cells, especially fatty acid (FA) synthesis. However, the relationship between SIRT3 and aberrant lipid synthesis in cervical cancer remains unclear. Here, we investigated the clinical relevance of SIRT3 expression in cervical squamous cell carcinoma (CSCC), cervical intraepithelial neoplasia (CIN), and normal tissues. To analyze the role of SIRT3 in CCSC in vitro, endogenous SIRT3 levels were up- and down-regulated in SiHa and C33a cells, respectively, via lentiviral-based transfection. Levels were quantified using qRT-PCR. Acetylation levels for acetyl-coA carboxylase (ACC1) were measured with the anti-acetyllysine antibody. Knockdown of SIRT3 reduced levels of cellular lipid content in cells. To investigate the role of SIRT3 in cell proliferation, nude mice were xenografted with SIRT3-overexpressing or SIRT3-knockdown CCSC cells. Overall, the results demonstrate that SIRT3 significantly contributed to the reprogramming of FA synthesis in CCSC by up-regulating ACC1 to promote de novo lipogenesis by SIRT3 deacetylation. Moreover, the findings show that the SIRT3-mediated regulation of FA synthesis played a critical role in the proliferation and metastasis of CCSC cells, suggesting that SIRT3 has therapeutic potential in CCSC treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cervical cancer (CC) is the most common malignancy of the female reproductive system, with more than 527,000 new cases worldwide. Approximately 50% of CC patients are already at an advanced stage at the time of diagnosis, and the 5-year survival rate is 8–12% [1, 2]. Pelvic lymph node metastasis is the most significant prognostic factor for recurrence and death in CC patients [3]. However, there is still no effective method to control lymph node metastasis in CC.
Metabolic abnormalities are a hallmark of malignant transformed cells and may provide growth advantages [4, 5]. In addition to the well-known Warburg effect, alterations in lipid metabolism have been recognized in cancer. The Warburg effect describes an increased uptake of glucose and the switch to aerobic glycolysis, which results in lactate production, and it is the most commonly observed metabolic phenotype in the early stages of cancer cells [6, 7]. However, in the case of metastatic cells, the migration and colonization of distant tissues require extra energy, including a higher demand of lipid metabolism [8]. Multiple studies have indicated that the changing of fatty acid (FA) synthesis in cancer cells may provide a selective advantage toward the metastatic process [4, 9, 10]. It has been confirmed that FAs are the major building blocks for energy metabolism, as well as the fundamental constituents of the signal transduction network of biological membranes [11]. Furthermore, the expressions of many key enzymes involved in de novo FA synthesis, such as acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS), are dysregulated and associated with poor clinical outcomes in many types of cancer [12,13,14]. Therefore, potential therapeutic strategies include the pharmacological inhibition of the FAS gene and the inhibition of the cellular uptake of exogenous FAs. A relationship between abnormal FA metabolism and the occurrence and development of tumors has been suggested. We and others have previously identified similar metabolic profile changes in the tissue and peripheral blood of patients with cervical squamous cell carcinoma (CSCC) and CIN, thus implying an enhanced catabolism of lipid, sugar, and amino acids during tumorigenesis. These are also closely related to the expression of speed-limiting enzymes in key metabolic pathways [15,16,17].
Sirtuin 3 (SIRT3) is a mitochondria-localized member of the Sirtuin family that is essential for modulating diverse processes, including FA metabolism, the tricarboxylic acid cycle, and oxidative phosphorylation by deacetylation of the targeted lysine on mitochondrial proteins [18]. SIRT3 also plays a critical role in various cellular activities, including cell proliferation, apoptosis, and stress reactions [19, 20]. In colorectal cancer, SIRT3 regulates mitochondrial metabolic functions through controlling post-translational deacetylation modifications of mitochondrial protein SHMT2, ultimately affecting cell viability [21]. In breast cancer, SIRT3 also mediates dimerization of IDH2 that linking glucose metabolism to oxidative phosphorylation directs promoted cell transformation and tumor genesis and cancer cell metabolism. [22].
Although SIRT3 has been studied in many tumors, the molecular mechanism underlying the relationship between SIRT3 and aberrant CC lipid synthesis remains elusive. In the present study, we systematically explored the role of SIRT3 in the reprogramming of FA synthesis in CC cells.
Methods
Ethics
120 cases of paraffin-embedded (FFPE) cervical tissue specimens and 39 cases of fresh-frozen tissues were collected from women with cervical squamous cell carcinoma (CSCC) and cervical intraepithelial neoplasia (CIN), or without cervical diseases, but treated by hysterectomy at Department of Gynecology of the First Affiliated Hospital of Xinjiang Medical University, upon written informed consent, and clinical study approval was obtained from the ethics committee of the First Affiliated Hospital of Xinjiang Medical University. The animal studies were also approved by the ethics committee of the First Affiliated Hospital of Xinjiang Medical University.
Immunohistochemistry
To examine the staining patterns of various target proteins in CSCC, CIN, and normal adjacent tissues (NATs), fixed preparations were dewaxed in dewaxing agent (Zhong Shan Goldenbridge Biotechnology Co. Ltd, Beijing, China), rehydrated in alcohol, blocked with endogenous peroxidase inhibitor (Zhong Shan Goldenbridge Biotechnology Co. Ltd, Beijing, China) at room temperature for 30 min, and then incubated with antibodies against SIRT3 (Abcam plc, Cambridge, MA, USA) overnight at 4 °C. Immunohistochemistry of clinical samples was performed as previously reported [23]. Two experienced pathologists independently scored the staining patterns. Immunostaining scores were semiquantitatively estimated according to staining intensity and distribution. Scores of 7–8 were classified as high expression, whereas scores of 3–6 and 0–2 were classified as moderate and low expression, respectively.
Cell culture and lentiviral infection
Two human CC cell lines (SiHa and C33a) and human immortalized cervical squamous epithelial (H8) cells were purchased from Shanghai Cell Collection (Shanghai, China). Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (BI, Cromwell, CT, USA), maintained in a 37 °C incubator filled with 5% CO2, and routinely passaged at a density of 90%. The culture medium was supplemented with 10% fetal bovine serum (FBS) and 100 units/mL of penicillin and streptomycin (BI, Cromwell, CT, USA). Construct lentiviral vectors and the lentivirus were produced by GENECHEM (Shanghai, China): shSIRT3 (knockdown) target -TCGATGGGC TTGAGAGAGT- and LV-SIRT3 (overexpression) target -GAGGATCCCCGGGTACCGGT CGCCACCATGGCG TTCTGGGGTTGGCG-. Infection of the cell lines was carried out in 6-well plates with serum-free DMEM. C33a cells were transduced with lenti-shSIRT3 at a multiplicity of infection (MOI) of 10, and SiHa cells were transduced with lenti-SIRT3 at an MOI of 20. Infections were performed at 37 °C with 8 μg/ml polybrene and enhanced for 72 h, according to the manufacturer’s guidelines. The medium was then removed and replaced with culture medium containing 10% FBS. Cells were continuously cultured for 6–8 days, followed by selection with flow cytometry.
Isolation of mitochondria
Mitochondria were isolated from SiHa and C33a cells by using a mitochondria isolation kit (Pierce Biotechnology, Inc., Rockford, IL, USA). Briefly, cells were harvested, pelleted, and resuspended in cold mitochondrial isolation medium (MIM). Cells were then homogenized using a glass–Teflon potter. After homogenization, samples were centrifuged at 700 g at 4 °C for 7 min. The supernatant containing mitochondria was centrifuged again at 10,000 g for 10 min. Mitochondrial pellets were washed with cold bovine serum albumin-free MIM.
Quantitative RT-PCR
Total RNA from clinical specimens and CC cells was extracted by using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and was reverse transcribed into 2 mg cDNA with the Revert Aid First Strand cDNA Synthesis Kit (Roche Diagnostics, Mannheim, Germany). Real-time PCR was performed using the SYBR Green Premix PCR Master Mix (Roche Diagnostics, Mannheim, Germany), according to the manufacturer’s protocols. Quantitative RT-PCR was carried out using the FastStart Universal SYBR Green Master (Roche, Mannheim, Germany) on an Applied Biosystems ABI 7900 RealTime PCR System (Applied Biosystems, Foster City, CA, USA). The oligonucleotide primers for human SIRT3 and glyceraldehyde-3-phosphate dehydrogenate (GAPDH) were as follows: SIRT3-F5′-CGTCTCAAAACAAAACAAAAC-3, SIRT3-R5′-AAAATCCAAAGCCAAACTG-3, GAPDH-F5′-GGACCTGACCTGCCGTCTAG-3′, and GAPDH-R5′-GTAGCCCAGGATGCCCTTGA-3′. Gene expression levels were normalized using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the internal reference. The average relative change was calculated using 3–5 determinations by relative quantification, applying the delta–delta cycle threshold method. All reactions were performed in triplicate.
Immunoprecipitation and western blot
Cells were lysed in radioimmunoprecipitation assay buffer. Then, immunoprecipitation was performed with 2 mg of protein from the lysates, using a Pierce® Crosslink IP Kit (Pierce Biotechnology, Inc., Rockford, IL, USA) and following the manufacturer’s protocol. Protein concentrations were quantified using the BCA protein assay kit (Bio-Rad, Hercules, CA, USA). Lysates were resolved by 10% SDS-PAGE and then transferred to polyvinylidene difluoride membranes. The membranes were blocked and incubated with specific antibodies against SIRT3, GAPDH (Proteintech, Rosemont, IL, USA), ACC1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and acetylated-lysine (Cell Signaling, Danvers, MA, USA). Western blotting was conducted with the WesternBreeze® Chromogenic Immunodetection System (Invitrogen, Carlsbad, CA, USA), following the manufacturer’s protocol.
Oil Red O
After washing with phosphate-buffered saline (PBS), cells were fixed for 20 min at 37 °C with paraformaldehyde fix solution. Cells were washed with PBS and subsequently washed with 60% isopropanol for 1 min, after which they were stained with a filtered 0.3% Oil Red O (Sigma, Louis, MO, USA) solution for 30 min at 37 °C. Then, cells were washed with PBS and counted under a Nikon ECLIPSE TS100 epifluorescence microscope. Twenty random fields were analyzed (× 20). To quantify lipid content, the incorporated Oil Red O was extracted in 6-well plates by shaking in 500 μl isopropanol for 10 min at room temperature. The liquids were collected and read using a spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) at 490 nm.
Migration assay
Cell migration was analyzed using the wound healing assay in 6-well plates. Briefly, 1 ml of cells (1 × 105 cells/ml) was transferred into each well and incubated at 37 °C and 5% CO2. After appropriate cell attachment was achieved (24 h), migration was assessed at various time points (0, 24, and 48 h), and images were captured using the Nikon ECLIPSE TS100 epifluorescence microscope using the NIS Elements AR 3.1 software.
Invasion assay
Transwell assays were performed as previously described [24]. Briefly, 8.0-μm Matrigel-coated transwell supports from Becton–Dickinson Canada (BD, Mississauga, Ontario, Canada) were used to evaluate cell invasion. Five thousand cells were suspended in 100 µL serum-free DMEM and seeded in the upper chamber. The bottom chamber was filled with 600 µL DMEM with 10% FBS. Cells were allowed to invade for 24 h and were then fixed in 4% paraformaldehyde fix solution. The underside of membranes was stained with Giemsa solution for 20 min, and the cells on top of the membrane were removed using a wet cotton swab. The number of migrated cells was counted under a Nikon ECLIPSE TS100 epifluorescence microscope. Ten random fields were analyzed (× 10).
Tumor xenograft mouse model
Nude mice (4 weeks old) were randomly allocated into six groups (6 mice/group): C33a, shNON (normal SIRT3 expression of C33a), shSIRT3 (low SIRT3 expression), SiHa, LV-NON (normal SIRT3 expression of SiHa), and LV-SIRT3 (high SIRT3 expression). We subcutaneously injected 5 × 106 cells into each mouse. Investigators were not blinded for the animal studies. All mice were euthanized by cervical dislocation at 8 weeks following tumor implantation. During the experiments, mice were monitored and euthanized for histopathology examination after cell inoculation for 30 days. Then, tumor weights were measured.
Statistical analyses
All statistical analyses were performed using SPSS software (version 17.0; SPSS, Inc. Chicago, IL, USA) and Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA). Data are presented as the mean ± standard deviation (SD) of at least three independent experiments. The Mann–Whitney test was used to test continuous variables for differences in SIRT3 immunohistochemistry scores between tumors, CIN, and normal tissues. The relationships between SIRT3 expression and clinicopathological characteristics were tested by the Chi-square test or Fisher’s exact test, as appropriate. Correlations between SIRT3 and ACC1 were analyzed by Spearman rank correlation analysis. Results were considered statistically significant at P < 0.05.
Result
SIRT3 is up-regulated in human CC
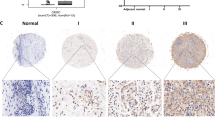
SIRT3 expression levels were assessed in CSCC tissues, CIN, and NATs in a fresh cohort of 13 cases each. SIRT3 mRNA levels in CC tissues were higher than those in CIN and NATs (Fig. 1a). To further investigate the clinical significance of SIRT3 expression in CSCC, we performed immunohistochemistry on tissues from 120 cases of CSCC, CIN, and NATs. SIRT3 was significantly up-regulated in CSCC tissues compared with CIN and NATs (Fig. 1b). To further understand the clinicopathologic significance of SIRT3 expression in CSCC, the relationship between SIRT3 expression and its clinicopathologic characteristics was analyzed (Table 1). Up-regulation of SIRT3 expression was significantly and positively related to clinical stage (P < 0.001), lymph node metastasis (P = 0.002), and the degree of cell differentiation (P = 0.039). No significant correlations between SIRT3 expression and other clinical parameters, including age and tumor volume, were observed.
Sirtuin 3 (SIRT3) is overexpressed in cervical cancer in humans. a Quantitative RT-PCR methods were used to detect mRNA levels of SIRT3 in human cervical cancer tissues, cervical intraepithelial neoplasia (CIN), and normal cervical tissues. b The protein expression of SIRT3 was detected by immunohistochemistry in cervical cancer tissues, CIN, normal cervical tissues, and lymph nodes. c SIRT3 mRNA levels in cervical cancer cell lines (C33a, SiHa) and immortalized H8 cells were assessed by quantitative RT-PCR. d Western blotting was performed to detect SIRT3 protein expression in C33a, SiHa, and H8 cells. Data are expressed as mean ± standard error of the mean (SEM) for (a), (c), and (d). **P < 0.01
To determine whether SIRT3 expression exhibited similar patterns in CC cells, we detected the expression of SIRT3 in SiHa, C33a, and immortalized cervical (H8) cells. As expected, the expression level of SIRT3 was significantly higher in SiHa and C33a cells than in immortalized H8 cells (Fig. 1c). Additionally, compared to the SiHa cell line, SIRT3 protein levels were elevated in C33a cell lines, and the mRNA levels of SIRT3 in these cell lines were in accordance with their protein levels (Fig. 1c and d). These results further supported the up-regulation of SIRT3 expression in CC.
SIRT3 promotes lipid metabolism in CC cells
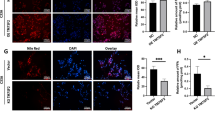
To determine whether SIRT3 regulates lipid metabolism in CC cells, we first generated stable SIRT3-overexpressing CC cells that originally expressed low levels of SIRT3 (LV-SIRT3) and also generated stable SIRT3-knockdown CC cells that originally expressed high levels of SIRT3 (shSIRT3). Western blot analysis confirmed the lentiviral infection efficiency of SIRT3 (Fig. 2a) and the successful alteration in SIRT3 expression. As SIRT3 is localized to the mitochondria [25], we next detected variations in mitochondrial SIRT3 mRNA. The lentiviral infections resulted in significant changes in mitochondrial SIRT3 mRNA (Fig. 2b). Subsequently, findings from Oil Red O staining showed that levels of lipids were reduced in shSIRT3 cells, whereas the lipid content was significantly increased in LV-SIRT3 cells (Fig. 2c and d). To better explain the mechanisms by which SIRT3 regulates lipid metabolism, we analyzed the levels of a lipogenesis-related enzyme (ACC1) in cells expressing different levels of SIRT3. Knockdown of SIRT3 decreased ACC1 expression. In contrast, overexpression of SIRT3 increased ACC1 levels compared with control (Fig. 2a). Overall, SIRT3 exerted significant effects on ACC1 in cervical cells, and the findings suggest that SIRT3 can regulate FA synthesis of cervical cells.
SIRT3 promotes lipid metabolism via acetyl-carboxylase 1 (ACC1) in cervical cancer cells. a Western blotting was performed to detect ACC1 expression in cells. The efficiency of lentiviral infection was also assessed. b The efficiencies of knockdown or overexpression of SIRT3 in the mitochondria of cervical cells were assessed by quantitative RT-PCR. c C33a and SiHa were infected with lentivirus constructs to knock down or overexpress SIRT3, respectively. d Oil Red O was used to stain lipids in cells (left), and the mean optical density (OD) of Oil Red O staining was quantified (right). Original magnification, × 200. e Immunoprecipitation (IP) assay analysis of the acetylation levels of ACC1, followed by SIRT3 overexpression and knockdown. Data are expressed as mean ± SEM. **P < 0.01
SIRT3 is the major deacetylase for ACC1
We examined whether SIRT3 could deacetylate ACC1 and affect their function. Acetylation levels for ACC1 were measured after immunoprecipitation by western blotting with the anti-acetyllysine antibody (Fig. 2e). Afterwards, we further analyzed the correlation between acetylation and protein expression levels. The results showed that the acetylation levels of ACC1 were significantly decreased under high SIRT3 expression. The acetylation level of ACC1 was inversely correlated with the protein expression of ACC1 in human CC cells. This suggests that SIRT3 played a role in ACC1 deacetylation by activating its enzymatic activity [26, 27].
SIRT3 improves the migration and invasion of CC cells
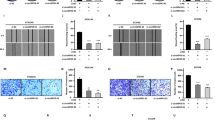
To investigate the role of SIRT3 on cell migration and invasion, we conducted wound healing and transwell invasion assays. SIRT3 knockdown resulted in a markedly decreased migration capacity in a time-dependent manner (Fig. 3a). A significant reduction in cell invasion was also observed (Fig. 3b and c). However, SIRT3 overexpression clearly increased migration and invasion capacity in cells. These results suggest that lipid metabolism may accelerate the metastasis of CC cells.
SIRT3 enhances the ability of migration and invasion in cervical cancer cells. a Representative photographs of cell migration. b Representative photographs of the staining intensity of cell invasion (left). The bar graph summarizes the number of invasions per field (right). Data are expressed as mean ± SEM. **P < 0.01
SIRT3 knockdown inhibits tumor growth in a xenograft nude mouse model
To further investigate the influence of SIRT3 on proliferation in vivo, nude mice were xenografted with SIRT3-overexpressed SiHa cells or SIRT3-knockdown C33a cells. Mice that were xenografted with SIRT3-overexpressing SiHa cells exhibited significantly larger tumors compared with control mice (Fig. 4 and 5a). In contrast, mice that were xenografted with SIRT3-knockdown C33a cells exhibited significantly smaller tumors compared with control mice (Figs. 4 and 5a). This suggests that SIRT3 can improve proliferation in vivo. In these experiments, mice were individually followed, and those with tumors measuring 20 mm in the largest dimension were withdrawn and sacrificed. SIRT3 knockdown resulted in a significant decrease in average tumor volume, which was measured on a weekly basis (Fig. 5a). Average tumor weight was also decreased with SIRT3 knockdown (Fig. 5b). Importantly, the incidence of lymph metastasis dramatically increased in mice that were xenografted with SIRT3-overexpressing SiHa cells, and necrosis was enhanced (Fig. 5c). These results were pathologically confirmed with hematoxylin and eosin staining. Overall, these data suggest that SIRT3 may facilitate lymph node metastasis by modulating FA metabolism.
SIRT3 facilitates cell growth in vivo. a Subcutaneous tumor growth curves of mice in different groups are shown. b The average tumor weights at the time the animals were sacrificed in experimental groups are depicted. c Representative pictures of hematoxylin and eosin staining of lymph nodes (LN) and necrosis in different parts of mice. d A proposed schematic model summarizing the promotion of tumor metastasis by SIRT3 in cervical cancer, as illustrated in this study. Data are expressed as mean ± SEM for (a) and (b). *P < 0.05, **P < 0.01
Discussion
There is increasing evidence that lipid metabolism is a key player in tumor growth, metastasis, and resistance. Importantly, SIRT3 has been shown to be involved in regulating FA synthesis [13, 27,28,29,30,31,32,33,34]. In this study, we demonstrate for the first time that lipid metabolism in cancer cells was regulated by SIRT3 and was essential for the progression of CSCC. First, we detected the expression of SIRT3 in CC tissues, normal cervical tissues, and CC cell lines. Staining of SIRT3 was significantly higher in CC tissues compared with CIN and normal tissues. Furthermore, there was a significant association between SIRT3 expression and clinical stage, lymph node metastasis, tumor volume, or poor overall prognosis, which suggests that SIRT3 plays important roles in CC. Rapid proliferation is a driving force for malignant cells to accommodate the massive energy requirement by metabolic modifications or reprogramming. In addition, we explored the role of SIRT3 in the regulation of lipid synthesis. Our findings show that SIRT3 remarkably promoted de novo FA synthesis by up-regulating ACC1 (Fig. 3e).
Several studies have explored the tumorigenesis and prognostic value of SIRT3 in various cancers, but the results were inconsistent. SIRT3 has been reported to play a tumor-suppressor role in hepatocellular carcinoma and gastric cancer, but it acts as an oncogene in breast cancer, lung cancer, and bladder cancer [30]. In our study, SIRT3 played an oncogenic role in CC via the deacetylation of ACC1, which resulted in increased ACC1 protein expression and elevated de novo lipogenesis in tumor cells. Moreover, our clinical analysis shows that high expression levels of SIRT3 were correlated with lymph node metastasis in patients with CC.
De novo lipogenesis has been recognized as a central stage in the field of cancer metabolism. It provides large amounts of lipids for the synthesis of membranes, signaling molecules, and lipoproteins in cancer cells. A few reports have shown that neoplastic lipogenesis is always accompanied by significantly increased activity and the simultaneous expression of lipogenic enzymes in tumor cells. Recently, the expression and activity of ACC and FAS have been associated with poor clinical outcomes in human epithelial cancers [13, 31]. Consistently, our results demonstrated that SIRT3 promoted FA synthesis in CSCC cells through the up-regulation of ACC1. Findings from in vitro experiments suggest that SIRT3 overexpression promotes CC cell invasion and migration. Furthermore, SIRT3 overexpression accelerated CC cell proliferation in vivo. These data show that high SIRT3 expression was associated with an increase in invasion and migration, thus emphasizing its potential as a prognostic biomarker for CC. Accordingly, these findings suggests that high SIRT3 expression creates a favorable environment for metastasis in CC. Moreover, we identified SIRT3 as a pivotal regulator of lipid metabolism and showed that it elevated the intracellular lipid content of CC cells. SIRT3 was also positively associated with the expression of the de novo lipogenesis key enzyme, ACC1, thus supporting its role in cellular lipid accumulation via enhanced lipid synthesis. Previous studies have shown that the inhibition of different enzymes within the FA biosynthesis pathway can block cancer cell growth [32].
SIRT3 is an important mitochondrial protein that may function as a primary mitochondrial protein deacetylase [27, 33]. SIRT3-dependent deacetylation has been shown to increase the enzymatic activity of acetyl-CoA synthetase 2 and glutamate dehydrogenase [27, 34]. In our study, we found that SIRT3 was responsible for ACC1 deacetylation and activation. Our findings suggest that SIRT3 functions as a lipid metabolism regulator that significantly elevates lipid content via affecting the expression of lipid synthetic enzymes.
Overall, results from this study demonstrate that SIRT3 played a key regulatory role in reprogramming FA synthesis in CSCC cells. This provided an alternative mechanism by which SIRT3 mediates CSCC. Our study provides novel insights to help understand the mechanisms of SIRT3 in CSCC progression and to find new strategies for future drug development in SIRT3 treatment.
Abbreviations
- CCSC:
-
Cervical squamous cell carcinoma
- CIN:
-
Cervical intraepithelial neoplasia
- SIRT3:
-
Sirtuin 3
- ACC1:
-
Acetyl-coA carboxylase 1
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics. CA Cancer J Clin 65:87–108
Li J, Kang LN, Qiao YL (2011) Review of the cervical cancer disease burden in mainland China. Asian Pac J Cancer Prev 12:1149–1153
Obrzut B, Semczuk A, Narnog M, Obrzut M, Król P (2017) Prognostic parameters for patients with cervical cancer FIGO stages IA2-IIB: along-term follow-up. Oncology 93:106–114
Pascual G, Avgustinova A, Mejetta S, Martín M, Castellanos A, Attolini CS, Berenguer A, Prats N, Toll A, Hueto JA, Bescós C, Di Croce L, Benitah SA (2017) Targeting metastasis initiating cells through the fatty acid receptor CD36. Nature 541:41–45
Currie E, Schulze A, Zechner R, Walther TC, Farese RV Jr (2013) Cellular fatty acid metabolism and cancer. Cell Metab 18:153–161
Warburg O (1956) On the origin of cancer cells. Science 123:309–314
Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC (2008) The M2 splice isoform and pyruvate kinase is important for cancer metabolism and tumour growth. Nature 452:230–234
Sant’Anna-Silva ACB, Santos GC, Campos SPC, Oliveira Gomes AM, Pérez-Valencia JA, Rumjanek FD (2018) Metabolic profile of oral squamous carcinoma cell lines relies on a higher demand of lipid metabolism in metastatic cells. Front Oncol 8:13
Corbet C, Feron O (2017) Emerging roles of lipid metabolism in cancer progression. Curr Opin Clin Nutr Metab Care 20(4):254–260
Nath A, Chan C (2016) Genetic alterations in fatty acid transport and metabolism genes are associated with metastatic progression and poor prognosis of human cancers. Sci Rep 6:18669
Kolusheva S, Wachtel E, Jelinek R (2003) Biomimetic lipid/polymer colorimetric membranes: molecular and cooperative properties. J Lipid Res 44(1):65–71
Kuhajda FP (2000) Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition 16:202–208
Menendez JA, Lupu R (2007) Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 7:763–777
Setoyama D, Fujimura Y, Miura D (2013) Metabolomics reveals that carnitine palmitoyltransferase-1 is a novel target for oxidative inactivation in human cells. Genes Cells 18:1107–1119
Hasim A, Ali M, Mamtimin B, Ma JQ, Li QZ, Abudula A (2012) Metabonomic signature analysis of cervical carcinoma and precancerous lesions in women by (1)H NMR spectroscopy. Exp Ther Med 3(6):945–951
Ye N, Liu C, Shi P (2015) Metabolomics analysis of cervical cancer, cervical intraepithelial neoplasia and chronic cervicitis by 1H NMR spectroscopy. Eur J Gynaecol Oncol 36(2):174–180
Hasim A, Nuermangu R, Lixiu X (2018) Analyze the metabolic changes and metabolic pathways in tumor tissues of cervical squamous cell carcinoma. J Xinjiang Med Univ 5(41):521–526
Hebert AS, Dittenhafer-Reed KE, Yu W, Bailey DJ, Selen ES, Boersma MD, Carson JJ, Tonelli M, Balloon AJ, Higbee AJ, Westphall MS, Pagliarini DJ, Prolla TA, Assadi-Porter F, Roy S, Denu JM, Coon JJ (2013) Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol Cell 49(1):186–199
Hong QL, Feng Z, Wu W, Li J, Zhang J, Xia T (2013) SIRT3 regulates cell proliferation and apoptosis related to energy metabolism in non-small cell lung cancer cells through deacetylation of NMNAT2. Int J Oncol 43:1420–1430
Qiao Y, Xu L, Tao X, Yin L, Qi Y, Xu Y, Han X, Tang Z, Ma X, Liu K, Peng J (2018) Protective effects of dioscin against fructose-induced renal damage via adjusting Sirt3-mediated oxidative stress, fibrosis, lipid metabolism and inflammation. Toxicol Lett 1(284):37–45
Wei Z, Song J, Wang G, Cui X, Zheng J, Tang Y, Chen X, Li J, Cui L, Liu CY, Yu W (2019) Deacetylation of serine hydroxymethyl-transferase 2 by SIRT3 promotes colorectal carcinogenesis. Nat Commun 10:774
Zou X, Zhu Y, Park SH, Liu G, O’Brien J, Jiang H, Gius D (2017) SIRT3-mediated dimerization of IDH2 directs cancer cell metabolism and tumor growth. Cancer Res 77:3990–3999
Yan SM, Han X, Han PJ, Chen HM, Huang LY, Li Y (2014) SIRT3 is a novel prognostic biomarker for esophageal squamous cell carcinoma. Med Oncol 31(8):103
Ma JQ, Tuersun H, Jiao SJ, Zheng JH, Xiao JB, Hasim A (2015) Functional role of NRF2 in cervical carcinogenesis. PLoS ONE 10(8):e0133876
Schwer B, North BJ, Frye RA, Ott M, Verdin E (2002) The human silent information regulator (Sir) 2 homologue hSIRT3 is a mitochondrial nicotinamide adenine dinucleotide-dependent deacetylase. J Cell Biol 158:647–657
Sol EM, Wagner SA, Weinert BT, Kumar A, Kim HS, Deng CX, Choudhary C (2012) Proteomic investigations of lysine acetylation identify diverse substrates of mitochondrial deacetylase Sirt3. PLoS ONE 7(12):e50545
Yao C-H, Liu G-Y, Wang R, Moon SH, Gross RW, Patti GJ (2018) Identifying off-target Effects of etomoxir reveals that carnitine palmitoyl transferase I is essential for cancer cell proliferation in dependent of β-oxidation. PLoS Biol 16(3):e2003782
Baenke F, Peck B, Miess H, Schulze A (2013) Hooked on fat: the role of lipid synthesis in cancer metabolism and tumour development. Dis Model Mech 6:1353–1363
Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV Jr, Alt FW, Kahn CR, Verdin E (2010) SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464(7285):121–125
Ansari A, Rahman MS, Saha SK, Saikot FK, Deep A, Kim KH (2017) Function of the SIRT3 mitochondrial deacetylase in cellular physiology, cancer, and neurodegenerative disease. Aging Cell 16(1):4–16
Wang C, Rajput S, Watabe K, Liao DF, Cao D (2010) Acetyl-CoA carboxylase-a as a novel target for cancer therapy. Front Biosci 2:515–526
Abramson HN (2011) The lipogenesis pathway as a cancer target. J Med Chem 54:5615–5638
Balaban S, Lee LS, Varney B, Aishah A, Gao Q, Shearer RF, Saunders DN, Grewal T, Hoy AJ (2018) Heterogeneity of fatty acid metabolism in breast cancer cells underlies differential sensitivity to palmitate-induced apoptosis. Mol Oncol 12(9):1623–1638
Gugiatti E, Tenca C, Ravera S, Fabbi M, Ghiotto F, Mazzarello AN, Bagnara D, Reverberi D, Zarcone D, Cutrona G, Ibatici A, Ciccone E, Darzynkiewicz Z, Fais F, Bruno S (2018) A reversible carnitine palmitoyltransferase (CPT1) inhibitor offsets the proliferation of chronic lymphocytic leukemia cells. Haematologica 103(11):e531–e536
Acknowledgements
This work was supported by the State Key Laboratory of Pathogenesis, Prevention, Treatment of High Incidence Diseases in Central Asia Fund (SKL-HIDCA-2017-3), Xinjiang Uygur Autonomous Region University Research Innovation Team Fund (Proj.No.XJEDU2017T006), and Program for Innovative Research Teams (99-11091107202).
Author information
Authors and Affiliations
Contributions
LXX and A. H. performed most experiments, analyzed the data, wrote the manuscript; LXX, LJH, and JQL participated in the in vivo study; LXX and JQM analyzed the data and revised the manuscript; and A. H. designed the overall study, supervised the experiments, and analyzed the results.
Corresponding author
Ethics declarations
Conflict of interest
The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, L.X., Hao, L.J., Ma, J.Q. et al. SIRT3 promotes the invasion and metastasis of cervical cancer cells by regulating fatty acid synthase. Mol Cell Biochem 464, 11–20 (2020). https://doi.org/10.1007/s11010-019-03644-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-019-03644-2