Abstract
Migration and invasion are important characteristics of rheumatoid arthritis fibroblast-like synoviocytes (RA-FLSs), which are involved in joint damage and contribute to rheumatoid arthritis (RA) pathology. However, the underlying mechanisms remain unclear. Because epithelial–mesenchymal transition (EMT) is a key mechanism related to migration and invasion in cancer cells, we investigated the relationship between EMT and RA-FLSs and explored whether the transforming growth factor β1 (TGF-β1)/Smad signaling pathway is involved. In vivo, fibroblast-like synoviocytes (FLSs) were isolated from the synovium of RA or osteoarthritis (OA) patients and cultured for 4–8 passages. EMT markers were detected by immunofluorescence and Western blotting. RA-FLSs were treated with TGF-β1 or Smad2/3 small interfering RNA (siRNA), EMT markers were detected, and migration and invasion were assessed by Transwell assays. EMT markers could be detected in FLSs; when compared with osteoarthritis fibroblast-like synoviocytes (OA-FLSs), E-cadherin and vimentin decreased, while N-cadherin and α-smooth muscle actin (α-SMA) increased in RA-FLSs. Furthermore, TGF-β1 enhanced migration and invasion by inducing EMT via activating Smad2/3 in RA-FLSs. Phosphorylation of Smad2/3 was accompanied by degradation of Smad3. Silencing Smad2/3 blocked EMT and inhibited the migration and invasion induced by TGF-β1. Matrix metalloproteinase 9 (MMP9) and vimentin were not affected when cells were treated with TGF-β1 or Smad2/3 siRNA. The TGF-β1/Smad signaling pathway is involved in EMT and contributes to migration and invasion in RA-FLSs. Interestingly, vimentin decreased in RA-FLSs, but there is no correlation between vimentin and TGF-β1/Smad signaling pathway. Thus, further research on vimentin should be conducted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a complex autoimmune disease. It affects mainly the joints and is characterized by synovial hyperplasia and inflammatory cell infiltration [1]. In fact, it should be considered a syndrome that includes extra-articular manifestations, such as rheumatoid nodules, pulmonary involvement or vasculitis, and systemic comorbidities [2]. Its clinical features include chronic pain and joint destruction that usually progresses from distal to more proximal joints [3]. According to the etiology of RA, the annual incidence has been reported to be 0.5% to 1%; women (2:1 to 3:1) are more likely to be affected than men [4], and genetic admixture, environmental factors, age, gender, and smoking may contribute [5]. Treatment strategies involve traditional disease-modifying anti-rheumatic drugs and novel biologic agents. Although clinical symptoms can be improved if patients are treated early and appropriately, this disease carries a substantial burden for both the individual and society [6]. To date, considerable research has been conducted, but the exact pathogenesis remains unclear. Neumann E reported joint resident rheumatoid arthritis fibroblast-like synoviocytes (RA-FLSs) as key players in the pathogenesis of RA. RA-FLSs have similar biological properties as tumor cells. For example, they undergo tumor-like proliferation and produce matrix metalloproteinases that could in turn promote their migration and invasion, finally leading to progressive articular cartilage and bone degradation [7, 8]. RA-FLSs can also spread by migrating through the bloodstream and invade distant exposed cartilage matrix [9].
Epithelial–mesenchymal transition (EMT) is a phenomenon present in both physiological and pathological processes. It plays an important role in organ formation, tissue differentiation, and repair. Additionally, EMT contributes to organ fibrosis and promotes carcinoma progression [10]. Many studies have demonstrated that transforming growth factorβ1 (TGF-β1) could induce EMT. This process induces cell proliferation and extracellular matrix deposition, increases the expression of α-smooth muscle actin (α-SMA), fibronectin, and collagen type I (collagen I) in vitro, and leads to organ fibrosis [11]. In addition, some research also demonstrated that TGF-β1 could upregulate vimentin and fibronectin and downregulate E-cadherin, contributing to migration and invasion capabilities [12,13,14]. Some studies have demonstrated that the expression of TGF-β1 was high in the fibroblast-like synoviocytes (FLSs) and synovial fluids from RA patients [15,16,17]. All of these findings indicate that RA-FLSs may undergo a EMT process induced by TGF-β1. However, the role and molecular mechanisms of EMT in RA remain unidentified.
In this study, we demonstrated RA-FLSs undergo EMT by detecting the expression of the epithelial marker E-cadherin and the mesenchymal markers N-cadherin, vimentin, and α-SMA. We found that TGF-β1 exerts its role in EMT by activating RA-FLSs. Finally, we investigated the involvement of the TGF-β/Smad signaling pathway in the induction EMT phenotype transformation with Smad2/3 interference.
Materials and methods
Reagents
Human TGF-β1 (8915LC), polyclonal rabbit anti-Smad2/3 (8685), polyclonal rabbit anti-phospho-Smad2 (Ser465/467)/Smad3 (Ser423/425) (D27F4), and polyclonal rabbit anti-Snail (3879) were purchased from Cell Signaling Technology (USA). Monoclonal mouse anti-N-cadherin (sc-59987), anti-p5/>Smad2/3 small interfering RNA (siRNA) (sc-37238), and control siRNA (sc-36869) were purchased from Santa Cruz (USA). Polyclonal rabbit anti-E-cadherin (20874-1-AP), polyclonal rabbit anti-vimentin (10366-1-AP), polyclonal rabbit anti-α-SMA (55135-1-AP), and monoclonal mouse anti-GAPDH (60004-1-Ig) were obtained from Proteintech (Wuhan, China).
FLSs culture
Osteoarthritis (OA) is a degenerative disease. Compared with RA, the clinical symptoms and pathological manifestations of OA are milder. OA has been frequently used as control to study the pathobiology of RA. Fibroblast-like synoviocytes were obtained from the synovium of RA or OA patients undergoing knee replacement surgery. The present study was approved by the Ethics Committee of Southern Medical University (Guangzhou, China, NFEC-20120201). Written informed consent was obtained from all patients. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc.) and antibiotics (100 mg/ml streptomycin and 100 U/ml penicillin) in a humidified incubator at 37 °C with 5% CO2. The cells used for experiments were from passages 3–8. RA-FLSs were identified by immunofluorescence (phenotype: > 98% vimentin, data not shown). RA-FLSs were cultured in zero serum conditions for 12 h, and then treated with TGF-β1 in 1% serum conditions.
Immunofluorescence
RA-FLSs were seeded in confocal dishes and incubated for 24 h. Then, the culture medium was discarded, and the cells were washed three times with phosphate buffer (PBS). The cells were fixed with 4% paraformaldehyde for 20 min and permeabilized in 0.2% Triton X-100/PBS for 5 min at room temperature. After washing with PBS three times, the cells were blocked with 10% normal goat serum (Bioss) for 1 h at room temperature and incubated with specific primary antibodies at 4 °C overnight. The primary antibodies included anti-E-cadherin (1:100), anti-N-cadherin (1:100), anti-α-SMA (1:100), and anti-vimentin (1:100). Then, the cells were washed with PBS three times and incubated with goat anti-rabbit IgG/Alexa Fluor 488 antibody (1:200) (Bioss, bs-0295G-AF488), rabbit anti-mouse Kappa light chain/Alexa Fluor 488 antibody (1:200) (Bioss, bs-0330R-AF488), or goat anti-rabbit IgG/Alexa Fluor 647 antibody (1:200) (Bioss, bs-0295G-AF647) for 1 h at room temperature. Finally, fluoroshield with DAPI (Abcam, ab104139) was used to distinguish the cellular nuclei. Immunofluorescence was viewed with a laser scanning confocal microscope.
For phosphorylated-Smad2/3 detections, cells were incubated with TGF-β1 for 48 h in DMEM with 1% FBS. Triton X-100/PBS (0.5%) was used to permeabilize the cells for 20 min at room temperature. Then, the cells were blocked and incubated with phospho-Smad2/3 (1:200). The rest of the procedure was performed as described above.
Small interfering RNA transfection
To knock down Smad2/3, RA-FLSs were cultured in six-well plates (1.0 × 105 per well). Transfection with siRNA was performed using LipofectAMINE 3000 (Invitrogen) according to the manufacturer’s instructions. The cells were transfected with control siRNA or Smad2/3 siRNA (Santa Cruz Biotechnology) at a final concentration of 30 nM for 48 h.
Western blot analysis
Cells were lysed with a Minute™ total protein extraction kit (SD-001/SN-002, Invent Biotechnologies). The cells were washed with PBS and then lysed in SD-001 lysis buffer containing protease inhibitor cocktail and phosphatase inhibitors (Sigma-Aldrich) on ice. The extracts were centrifuged at 15,000 rpm for 1 min, and the protein concentrations were quantified using a BCA Protein Assay Kit (Cwbio, Beijing, China). Equal amounts (25 μg) of protein were separated by 10% SDS-PAGE and transferred onto polyvinylidene difluoride membranes. The membranes were blocked with 5% non-fat dry milk in Tris-buffered Saline-Tween (TBST) at room temperature for 1 h and incubated overnight at 4 °C with the primary antibodies. The membranes were then washed three times with TBST and then incubated with the secondary antibody. GAPDH was used as an internal control. Immunoreactive bands were detected with the chemiluminescent substrate, and the intensities of the bands were quantified by Quantity One Software (Bio-Rad).
Transwell migration and invasion assays
First RA-FLSs were treated with TGF-β1 or Smad2/3 siRNA for 48 h. For the migration assays, 2 × 104 cells in 200 μl of 1% FBS-containing medium were placed in the top chamber of the Transwell plate (pore size, 8 μm; BD Bioscience). For the invasion assays, 3 × 104 cells in 200 μl of 1% FBS-containing medium were placed in the top chamber coated with Matrigel (BD Bioscience) following the manufacturer’s protocol. The lower chamber was filled with 900 μl of 10% FBS-containing medium. After incubation at 37 °C for 24 h, the cells on the upper surface were wiped away with a cotton swab, while the cells invading the bottom of the membranes were fixed in a methanol solution for 20 min and stained with a 0.1% crystal violet solution for 20 min and counted under a microscope. Five random fields were counted. The experiments were repeated three times.
Statistical analysis
All the results are expressed as the mean ± standard deviation (SD). The results from different groups were analyzed by one-way analysis of variance (ANOVA) with Fisher’s probable least-squares difference test or Student’s t test. p < 0.05 was considered statistically significant.
Results
Comparison of EMT markers in RA-FLSs and OA synovial fibroblasts (OA-FLSs)
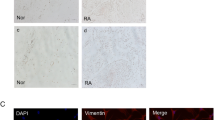
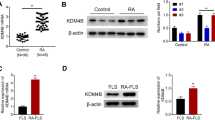
To ensure that RA-FLSs can express epithelial–mesenchymal transition markers, we cultured cells until passage 4 and then measured EMT markers (including N-cadherin, E-cadherin, vimentin, and α-SMA) by immunofluorescence. As shown in Fig. 1a, N-cadherin and E-cadherin are expressed in mainly the cell membrane, while vimentin and α-SMA are expressed in mainly the cytoplasm. To confirm that RA-FLSs had undergone EMT, we isolated FLSs from the synovium of RA and OA patients. Then, we lysed the cells, measured the total cellular protein, and finally analyzed the EMT markers by Western blotting. The results are shown in Fig. 1b. Compared with those in OA patients, N-cadherin (p < 0.01) and α-SMA (p < 0.01) protein levels were increased, and E-cadherin (p < 0.01) and vimentin (p < 0.01) protein levels were decreased in RA patients. As for vimentin, the results of immunofluorescence were consistent with western blot (Fig. 1c).
Expression analysis of EMT markers in FLSs. a Fluorescence micrographs with staining for N-cadherin (green), E-cadherin (green), vimentin (ren), α-SMA (red), and nuclei (blue) in RA-FLSs at ×400. b Western blot analyses for N-cadherin, E-cadherin, vimentin, and α-SMA expression in RA-FLSs and OA-FLSs. c Fluorescence micrographs with staining for vimentin in RA-FLSs and OA-FLSs at ×200. d Densitometric analysis of the Western blot results (n = 4) *p < 0.05, #p < 0.01. (Color figure online)
TGF-β1 induces EMT in RA-FLSs
TGF-β1 is a known factor that promotes EMT in cancer cells, and when EMT occurs, the migration and invasion capabilities of cancer cells are enhanced. The previous results indicate that RA and OA-FLSs could express EMT markers and likely RA-FLSs undergo EMT. To investigate whether TGF-β1 could induce EMT in RA-FLSs, we treated RA-FLSs with TGF-β1, and the results are shown in Fig. 2. First, RA-FLSs were treated with a control or different concentrations of TGF-β1 (0.1, 1, 10 ng/ml) for 48 h. Figure 2b indicates that compared with the PBS control, 1 ng/ml (p < 0.01) and 10 ng/ml (p < 0.01) TGF-β1 significantly increased N-cadherin, and 10 ng/ml (p < 0.01) TGF-β1 increased α-SMA and matrix metalloproteinase 2 (MMP2). However, 1 ng/ml (p < 0.05) and 10 ng/ml (p < 0.01)TGF-β1 decreased E-cadherin. Second, RA-FLSs were treated with 10 ng/ml TGF-β1 for 12 h, 24 h, and 48 h. Figure 2c indicates that compared with the PBS control, TGF-β1 increased N-cadherin (p < 0.01) and MMP2 (p < 0.01) when the cells were treated for 48 h and increased α-SMA (p < 0.01) when the cells were treated for 24 h and 48 h. At the same time, E-cadherin (p < 0.01) was decreased when the cells were treated for 48 h. However, the present study also showed that TGF-β1 had no effect on the expression of vimentin (p > 0.05) and matrix metalloproteinase 9 (MMP9) (p > 0.05) in RA-FLSs.
TGF-β1 induces EMT in RA-FLSs. a Western blot analyses for N-cadherin, E-cadherin, vimentin, α-SMA, MMP9, and MMP2 expression after RA-FLSs were treated with a vehicle control or different concentrations of TGF-β1 (0.1, 1, or 10 ng/ml) for 48 h. c Western blot analyses for N-cadherin, E-cadherin, vimentin, α-SMA, MMP9, and MMP2 expression after RA-FLSs were treated with a vehicle control or TGF-β1 (10 ng/ml) for different times (12, 24 or 48 h). b, d Densitometric analysis of the Western blot results (n = 4). *p < 0.05, #p < 0.01
Snail is involved in TGF-β1-induced EMT in a Smad2/3-dependent manner
Smad2/3 and Snail are important factors that regulate EMT through TGF-β. To confirm that TGF-β1 can activate Smad2/3 and regulate Snail in RA-FLSs, we treated cells with 10 ng/ml TGF-β1 for 48 h and then detected Phospho-Smad2/3 and Snail by Western blot and immunofluorescence assays. As shown in Fig. 3, compared with the PBS control, TGF-β1 upregulated the expression of phospho-Smad2/3 (p < 0.01) and downregulated the expression of Smad2/3 (p < 0.05), thus promoting phospho-Smad2/3 entry into the nucleus. Ultimately, the expression of Snail (p < 0.05) was increased. These results demonstrated that TGF-β1 could induce RA-FLSs to undergo EMT by activating Smad2/3 and regulating Snail.
TGF-β1 increases Snail levels via activating Smad2/3 in RA-FLSs. a Western blot analyses for Smad2/3, P-Smad2/3, and Snail after RA-FLSs were treated with TGF-β1 (10 ng/ml) for 48 h. b Fluorescence micrographs with staining for P-Smad2/3 (green) and nuclei (blue) after RA-FLSs were treated with TGF-β1 (10 ng/ml) for 48 h. c Western blot analyses for Smad2/3 after RA-FLSs were treated with TGF-β1 (10 ng/ml) for 12, 24, 48 h. d Densitometric analysis of the Western blot results (n = 4). *p < 0.05, #p < 0.01. (Color figure online)
Smad2/3 knockdown affected EMT by regulating the expression of EMT markers
Previous results indicate that Smad2/3 is a key factor in EMT and activating Smad2/3 could induce EMT in RA-FLSs. Therefore, we investigated whether RA-FLSs would undergo mesenchymal–epithelial transition (MET) when treated with Smad2/3 siRNA. As shown in Fig. 4a, Smad2/3 protein expression levels were significantly decreased after Smad2/3 siRNA transfection for 48 h. As a result, E-cadherin protein expression was increased (p < 0.05), and N-cadherin (p < 0.05) and α-SMA (p < 0.05) protein expression was decreased (Fig. 4d). However, there were no changes in the protein expression levels of MMP2 (p > 0.05), MMP9 (p > 0.05), or vimentin (p > 0.05).
Knockdown of Smad2/3 reversed EMT in RA-FLSs RA-FLSs were pretreated with scrambled siRNA (30 nM) or Smad2/3 siRNA (30 nM) for 48 h. a Western blot analyses for Smad2/3 and Snail. b Densitometric analysis of the Western blot results (n = 4). c Western blot analyses for N-cadherin, E-cadherin, vimentin, α-SMA, MMP9, and MMP2 expression. d Densitometric analysis of the Western blot results (n = 4). *p < 0.05, #p < 0.01
Smad2/3 knockdown inhibited the migration and invasion of RA-FLSs by blocking TGF-β1-induced EMT
The previous results demonstrated that the pathological changes in RA-FLSs were related to EMT. TGF-β1 could induce RA-FLSs to undergo EMT by activating Smad2/3. Smad2/3 knockdown reversed the expression of EMT markers, including N-cadherin, E-cadherin, and α-SMA. As migration and invasion are important characteristics of RA-FLSs, it is necessary to investigate whether the characteristics will be affected when Smad2/3 are knocked down. Cells were treated with Smad2/3 siRNA or control siRNA for 48 h and then treated with TGF-β1 (10 ng/ml) for another 48 h. EMT markers were detected by Western blotting, and the migration and invasion capabilities were detected by Transwell assays. As shown in Fig. 5a, compared with the TGF-β1 control, Smad2/3 knockdown significantly blocked the TGF-β1-induced expression of N-cadherin (p < 0.05), E-cadherin (p < 0.05), MMP2 (p < 0.05), and α-SMA (p < 0.05). However, the protein expression levels of MMP9 (p > 0.05) and vimentin (p > 0.05) remained unchanged. At the same time, as shown in Fig. 5c, TGFβ1-induced migration and invasion in RA-FLSs were also inhibited upon Smad2/3 knockdown. The Transwell assay results were consistent with the Western blot analysis results.
Knockdown of Smad2/3 blocked TGF-β1-induced EMT and inhibited migration and invasion in RA-FLSs. RA-FLSs were pretreated with scrambled siRNA (30 nM) or Smad2/3 siRNA (30 nM) for 48 h and then treated with TGF-β1 (10 ng/ml) for 48 h. a Western blot analyses for N-cadherin, E-cadherin, vimentin, α-SMA, MMP9, and MMP2 expression. b Densitometric analysis of the Western blot results (n = 4). c Transwell migration and Matrigel invasion assays for the migration and invasion abilities of RA-FLSs after treatment. The migrated and invaded cells were photographed (×200 magnification). d The migrated and invaded cells were counted in five random fields for each treatment (n = 3). *p < 0.05, #p < 0.01
Discussion
It has been shown that the migration and invasion of RA-FLSs are related to EMT. For example, hypoxia could induce EMT, resulting in the migration and invasion of RA-FLSs [18]. In this study, we demonstrated that TGF-β1 was involved in the EMT of RA-FLSs by activating Smad2/3, which results in their migration and invasion.
It is necessary to confirm the relationship between RA and EMT, as there are differing opinions on the issue. Some research showed that classical E-cadherin expression was scant [19], but E-cadherin was found in healthy and arthritic synovial tissue [20]. E-cadherin is an important component of extracellular connections. As a key protein in EMT, its decreased expression is accompanied by an increase in N-cadherin, and it facilitates cell migration and invasion [21]. In this study, we provided evidence that E-cadherin was present in RA and OA-FLSs. As shown in Western blots, the expression level of E-cadherin was decreased, while that of N-cadherin was increased in RA-FLSs when compared with those of OA-FLSs. Regarding α-SMA, the tumor microenvironment is rich in carcinoma-associated fibroblasts, which are characterized by the expression of α-SMA and contribute to tumor progression [22]. α-SMA-positive FLSs reportedly play a key role in the pathogenesis of RA [23, 24]. According to immunofluorescence and Western blotting assays, α-SMA could be detected in both RA and OA-FLSs. In addition, α-SMA was increased in RA-FLSs. These results indicate that RA-FLSs can express EMT markers and that phenotypic cell characteristics are related to EMT.
TGF-β1 is a multifunctional cytokine whose effects are different, even opposite, depending on the cell type and the conditions. For example, TGF-β1 can inhibit cell proliferation but also promote cell growth, enhance stem cell pluripotency but also differentiation, and regulate muscle genes in myoblasts and neural genes in neuroblasts [25, 26]. TGF-β1 is secreted by many types of cells, including FLSs. Some studies demonstrated that TGF-β1 expression was upregulated in the synovial fluid and fibroblasts of arthritis patients [15,16,17]. However, it is not clear whether TGF-β1 has an effect on RA-FLSs or contributes to the progression of RA. In this study, we evaluated the effect of TGF-β1 on RA-FLSs by treating cells for various times and with different concentrations. The results demonstrated that N-cadherin, MMP2, and α-SMA expression levels were increased, E-cadherin expression levels were decreased, and cell migration and invasion capacity were enhanced when cells were treated with 10 ng/ml TGF-β1 for 48 h. These findings confirmed that TGF-β1 had an effect on RA-FLSs, and might lead to joint damage by inducing EMT.
TGF-β1 exerts biological functions by activating downstream effectors via either a Smad-dependent pathway or a Smad-independent (TAK1-p38MAPK) signaling axis [26]. Increased P-Smad-2/3 levels with a nuclear distribution were observed in blood vessels, synovial lining cells, and mononuclear cell infiltrates of RA and OA synovial tissues compared to those in healthy controls [27]. The present study confirmed that Smad2/3 could be phosphorylated and enter the nucleus when cells were treated with TGF-β1. Smad2/3 knockdown led to mesenchymal-epithelial transition (MET). Furthermore, TGF-β1-induced EMT was blocked, and cell migration and invasion were inhibited after Smad2/3 was silenced. All these results showed that TGF-β1 enhanced migration and invasion and contributed to RA pathology by inducing EMT via activating Smad2/3 in RA-FLSs. In addition, we observed that Smd2/3, especially smad3 decreased when RA-FLSs were treated with TGF-β1. Smad ubiquitination regulatory factor 2 (SMURF2), one of the HECT (homologous with E6-APC terminus) family of E3 ubiquitin ligases, regulates ubiquitination-mediated Smad3 degradation [28]. Meanwhile, SMURF2 could be induced by TGF-β1 [29, 30]. So, we speculated that TGF-β1 might contributed to the expression of SMURF2, leading to ubiquitination-mediated Smad3 degradation RA-FLSs.
MMP9 and vimentin were not affected in RA-FLSs treated with TGF-β1 or Smad2/3 siRNA. MMP2 and MMP9 are both type IV collagenases, which are involved in the invasion and metastasis of various tumor cells. Previous studies showed that they could be detected in synovial tissue and cells [31], and the present study confirmed these findings. Although both MMP2 and MMP9 can be induced by TGF-β1 in tumor cells [32,33,34], we provided evidence that MMP2, but not MMP9, is involved in TGF-β1/Smad signaling in RA-FLSs. Vimentin is also a marker of EMT and associated with epithelial and non-epithelial tumor spread, tumor cell migration, and angiogenesis [35]. Vimentin contributes to cell migration by regulating focal adhesion-associated proteins [36]. The loss of vimentin alters signaling through focal adhesion and mechanotransduction, resulting in reduced cancer cell adhesion and migration [37]. Therefore, we predicted that the expression of vimentin would increase when RA-FLSs undergo EMT. In fact, our study showed that the expression level of vimentin decreased in RA-FLSs. This result is consistent with those of Kim [38]. Previous observations also demonstrated that the induction of cellular vimentin synthesis in MPC-11 mouse myeloma cells was correlated with cell progression inhibition at the G1 phase of the cell cycle [24]. In addition, vimentin inhibited proliferation, while citrullinated vimentin significantly stimulated proliferation capacity in RA-FLSs [39]. As FLSs proliferation is an important characteristic of RA [40], present study demonstrated that decrease in vimentin might contribute to proliferation and provide new insight into the pathology of RA.
Conclusion
The present study demonstrated that the RA-FLS phenotype was related to EMT. TGF-β1 could induce EMT via activating Smad2/3 and contributing to migration and invasion in RA-FLSs. Silencing Smad2/3 blocked TGF-β1-induced EMT and inhibited cell migration and invasion. Vimentin and MMP9, special EMT markers in RA-FLSs, were not regulated by TGF-β1 and need further investigation.
References
Klein K, Gay S (2013) Epigenetic modifications in rheumatoid arthritis, a review. Curr Opin Pharmacol 13(3):420–425. https://doi.org/10.1016/j.coph.2013.01.007
Scott DL, Wolfe F, Huizinga TW (2010) Rheumatoid arthritis. Lancet 376(9746):1094–1108. https://doi.org/10.1016/S0140-6736(10)60826-4
Kourilovitch M, Galarza-Maldonado C, Ortiz-Prado E (2014) Diagnosis and classification of rheumatoid arthritis. J Autoimmun 48–49:26–30. https://doi.org/10.1016/j.jaut.2014.01.027
Smolen JS, Aletaha D, McInnes IB (2016) Rheumatoid arthritis. Lancet 388(10055):1984
Carmona L, Cross M, Williams B, Lassere M, March L (2010) Rheumatoid arthritis. Best Pract Res Clin Rheumatol 24(6):733–745. https://doi.org/10.1016/j.berh.2010.10.001
Klareskog L, Catrina AI, Paget S (2009) Rheumatoid arthritis. Lancet 373(9664):659–672. https://doi.org/10.1016/S0140-6736(09)60008-8
Nakamura H, Shimamura S, Yasuda S, Kono M, Kono M, Fujieda Y, Kato M, Oku K, Bohgaki T, Shimizu T, Iwasaki N, Atsumi T (2018) Ectopic RASGRP2 (CalDAG-GEFI) expression in rheumatoid synovium contributes to the development of destructive arthritis. Ann Rheum Dis 77(12):1765–1772. https://doi.org/10.1136/annrheumdis-2018-213588
Neumann E, Lefèvre S, Zimmermann B, Gay S, Müller-Ladner U (2010) Rheumatoid arthritis progression mediated by activated synovial fibroblasts. Trends Mol Med 16(10):458–468. https://doi.org/10.1016/j.molmed.2010.07.004
Lefèvre S, Knedla A, Tennie C, Kampmann A, Wunrau C, Dinser R, Korb A, Schnäker E, Tarner IH, Robbins PD, Evans CH, Stürz H, Steinmeyer J, Gay S, Schölmerich J, Pap T, Müller-Ladner U, Neumann E (2009) Synovial fibroblasts spread rheumatoid arthritis to unaffected joints. Nat Med 15(12):1414–1420. https://doi.org/10.1038/nm.2050
Thiery JP, Acloque H, Huang RYJ, Nieto MA (2009) Epithelial–mesenchymal transitions in development and disease. Cell 139(5):871–890. https://doi.org/10.1016/j.cell.2009.11.007
Shi L, Dong N, Fang X, Wang X (2016) Regulatory mechanisms of TGF-β1-induced fibrogenesis of human alveolar epithelial cells. J Cell Mol Med 20(11):2183–2193. https://doi.org/10.1111/jcmm.12918
Zhang C, Hao Y, Wang Y, Xu J, Teng Y, Yang X (2018) TGF-β/SMAD4-regulated LncRNA-LINP1 inhibits epithelial–mesenchymal transition in lung cancer. Int J Biol Sci 14(12):1715–1723. https://doi.org/10.7150/ijbs.27197
Wu X, Zhao J, Ruan Y, Sun L, Xu C, Jiang H (2018) Sialyltransferase ST3GAL1 promotes cell migration, invasion, and TGF-β1-induced EMT and confers paclitaxel resistance in ovarian cancer. Cell Death Dis 9(11):1102. https://doi.org/10.1038/s41419-018-1101-0
Tian X, Guan W, Zhang L, Sun W, Zhou D, Lin Q, Ren W, Nadeem L, Xu G (2018) Physical interaction of STAT1 isoforms with TGF-β receptors leads to functional crosstalk between two signaling pathways in epithelial ovarian cancer. J Exp Clin Cancer Res 37(1):103. https://doi.org/10.1186/s13046-018-0773-8
Pohlers D, Beyer A, Koczan D, Wilhelm T, Thiesen H, Kinne RW (2007) Constitutive upregulation of the transforming growth factor-β pathway in rheumatoid arthritis synovial fibroblasts. Arthritis Res Ther 9(3):R59. https://doi.org/10.1186/ar2217
Song HY, Kim MY, Kim KH, Lee IH, Shin SH, Lee JS, Kim JH (2010) Synovial fluid of patients with rheumatoid arthritis induces α-smooth muscle actin in human adipose tissue-derived mesenchymal stem cells through a TGF-β1-dependent mechanism. Exp Mol Med 42(8):565. https://doi.org/10.3858/emm.2010.42.8.057
Eliçabe RJ, Silva JE, Dave MN, Lacoste MG, Tamashiro H, Blas R, Munarriz A, Rabinovich GA, Di Genaro MS (2017) Association between IL-17 and IgA in the joints of patients with inflammatory arthropathies. BMC Immunol 18(1):8. https://doi.org/10.1186/s12865-017-0189-9
Li G, Zhang Y, Liu D, Qian Y, Zhang H, Guo S, Sunagawa M, Hisamitsu T, Liu Y (2013) PI3 kinase/Akt/HIF-1α pathway is associated with hypoxia-induced epithelial–mesenchymal transition in fibroblast-like synoviocytes of rheumatoid arthritis. Mol Cell Biochem 372(1–2):221–231. https://doi.org/10.1007/s11010-012-1463-z
Zvaifler NJ (2006) Relevance of the stroma and epithelial–mesenchymal transition (EMT) for the rheumatic diseases. Arthritis Res Ther 8(3):210. https://doi.org/10.1186/ar1963
Steenvoorden MM, Tolboom TC, van der Pluijm G, Löwik C, Visser CP, DeGroot J, Gittenberger-DeGroot AC, DeRuiter MC, Wisse BJ, Huizinga TW, Toes RE (2006) Transition of healthy to diseased synovial tissue in rheumatoid arthritis is associated with gain of mesenchymal/fibrotic characteristics. Arthritis Res Ther 8(6):R165. https://doi.org/10.1186/ar2073
Dhawan U, Sue M, Lan K, Buddhakosai W, Huang PH, Chen YC, Chen P, Chen WL (2018) Nanochip-induced epithelial-to-mesenchymal transition: impact of physical microenvironment on cancer metastasis. ACS Appl Mater Interfaces 10(14):11474–11485. https://doi.org/10.1021/acsami.7b19467
Wang J, Guan X, Zhang Y, Ge S, Zhang L, Li H, Wang X, Liu R, Ning T, Deng T, Zhang H, Jiang X, Ba Y, Huang D (2018) Exosomal miR-27a derived from gastric cancer cells regulates the transformation of fibroblasts into cancer-associated fibroblasts. Cell Physiol Biochem 49(3):869–883. https://doi.org/10.1159/000493218
Chen S, Shiau A, Li Y, Lin C, Jou I, Liu M, Wu C, Wang C (2015) Transcription factor snail regulates tumor necrosis factor α-mediated synovial fibroblast activation in the rheumatoid joint. Arthritis Rheumatol 67(1):39–50. https://doi.org/10.1002/art.38899
Giese G, Kubbies M, Traub P (1992) Cell cycle-dependent vimentin expression in elutriator-synchronized, TPA-treated MPC-11 mouse plasmacytoma cells. Exp Cell Res 200(1):118
Filer A, Ward LSC, Kemble S, Davies CS, Munir H, Rogers R, Raza K, Buckley CD, Nash GB, McGettrick HM (2017) Identification of a transitional fibroblast function in very early rheumatoid arthritis. Ann Rheum Dis 76(12):2105–2112. https://doi.org/10.1136/annrheumdis-2017-211286
Massagué J (2012) TGFβ signalling in context. Nat Rev Mol Cell Biol 13(10):616–630. https://doi.org/10.1038/nrm3434
Gonzalo-Gil E, Criado G, Santiago B, Dotor J, Pablos JL, Galindo M (2013) Transforming growth factor (TGF)-beta signalling is increased in rheumatoid synovium but TGF-beta blockade does not modify experimental arthritis. Clin Exp Immunol 174(2):245–255. https://doi.org/10.1111/cei.12179
Xu Z, Greenblatt MB, Yan G, Feng H, Sun J, Lotinun S, Brady N, Baron R, Glimcher LH, Zou W (2017) SMURF2 regulates bone homeostasis by disrupting SMAD3 interaction with vitamin D receptor in osteoblasts. Nat Commun 8:14570. https://doi.org/10.1038/ncomms14570
Xiao L, Peng X, Liu F, Tang C, Hu C, Xu X, Wang M, Luo Y, Yang S, Song P, Xiao P, Kanwar YS, Sun L (2015) AKT regulation of mesothelial-to-mesenchymal transition in peritoneal dialysis is modulated by smurf2 and deubiquitinating enzyme USP4. BMC Cell Biol 16(1):7. https://doi.org/10.1186/s12860-015-0055-7
Zhang Z, Finnerty CC, He J, Herndon DN (2012) Smad ubiquitination regulatory factor 2 expression is enhanced in hypertrophic scar fibroblasts from burned children. Burns 38(2):236–246. https://doi.org/10.1016/j.burns.2011.08.012
Wang Y, Wan D, Zhou R, Zhong W, Lu S, Chai Y (2017) Geraniin inhibits migration and invasion of human osteosarcoma cancer cells through regulation of PI3K/Akt and ERK1/2 signaling pathways. Anti-cancer Drug 28(9):959–966. https://doi.org/10.1097/CAD.0000000000000535
Kim ES, Kim MS, Moon A (2004) TGF-beta-induced upregulation of MMP-2 and MMP-9 depends on p38 MAPK, but not ERK signaling in MCF10A human breast epithelial cells. Int J Oncol 25(5):1375–1382
Hsieh HL, Wang HH, Wu WB, Chu PJ, Yang CM (2010) Transforming growth factor-beta1 induces matrix metalloproteinase-9 and cell migration in astrocytes: roles of ROS-dependent ERK- and JNK-NF-kappaB pathways. J Neuroinflammation 7:88. https://doi.org/10.1186/1742-2094-7-88
Okamoto T, Takahashi S, Nakamura E, Nagaya K, Hayashi T, Fujieda K (2009) Transforming growth factor-beta1 induces matrix metalloproteinase-9 expression in human meningeal cells via ERK and Smad pathways. Biochem Biophys Res Commun 383(4):475–479. https://doi.org/10.1016/j.bbrc.2009.04.038
Etienne-Manneville S (2018) Cytoplasmic intermediate filaments in cell biology. Annu Rev Cell Dev Biol 34(1):1–28. https://doi.org/10.1146/annurev-cellbio-100617-062534
Leube RE, Moch M, Windoffer R (2015) Intermediate filaments and the regulation of focal adhesion. Curr Opin Cell Biol 32:13–20. https://doi.org/10.1016/j.ceb.2014.09.011
Dmello C, Sawant S, Alam H, Gangadaran P, Tiwari R, Dongre H, Rana N, Barve S, Costea DE, Chaukar D, Kane S, Pant H, Vaidya M (2016) Vimentin-mediated regulation of cell motility through modulation of beta4 integrin protein levels in oral tumor derived cells. Int J Biochem Cell Biol 70:161–172. https://doi.org/10.1016/j.biocel.2015.11.015
Kim CW, Cho EH, Lee YJ, Kim YH, Hah Y, Kim DR (2006) Disease-specific proteins from rheumatoid arthritis patients. J Korean Med Sci 21(3):478. https://doi.org/10.3346/jkms.2006.21.3.478
Fan LY, He DY, Wang Q, Zong M, Zhang H, Yang L, Sun LS (2012) Citrullinated vimentin stimulates proliferation, pro-inflammatory cytokine secretion, and PADI4 and RANKL expression of fibroblast-like synoviocytes in rheumatoid arthritis. Scand J Rheumatol 41(5):354–358. https://doi.org/10.3109/03009742.2012.670263
Connor AM, Mahomed N, Gandhi R, Keystone EC, Berger SA (2012) TNFα modulates protein degradation pathways in rheumatoid arthritis synovial fibroblasts. Arthritis Res Ther 14(2):R62. https://doi.org/10.1186/ar3778
Acknowledgements
The present study was supported by National Natural Science Foundation of China (Grant Nos. 81771747, 81801624) and Natural Science Foundation of Guangdong Province (Grant No. 2017A030313475).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared that no conflicts of interest exist.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of Southern Medical University and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, D., Zhao, J., Lou, A. et al. Transforming growth factor β1 promotes fibroblast-like synoviocytes migration and invasion via TGF-β1/Smad signaling in rheumatoid arthritis. Mol Cell Biochem 459, 141–150 (2019). https://doi.org/10.1007/s11010-019-03557-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-019-03557-0