Abstract
Exosomes are 40- to 100- nm cell-originated vesicles derived from endocytic compartments that are released into almost all biological fluids. Exosomes are cell-created vesicles that inherit identical phospholipid membrane, explaining a wide application of electroporation as a technique for exosomes loading with exogenous cargoes. Another way of loading exosomes with therapeutic cargo is to overexpress a certain gene in exosome-donor cells or treat cell line with drug of interest that later will be gently enveloped into vesicles based on the process of EV biogenesis. Similarly, to visualize siRNA loading into exosomes as well as the exosomal product delivery to recipient cells, we have conducted an experiment where chemical-based exosome transfection was used. In this review, we discuss different ways of extracellular vesicle loading with exogenous cargoes and their advantages/limitations as well as novel alternative techniques of substance incorporation into nanoparticles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
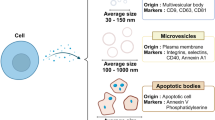
Pan and Johnstone first discovered exosomes in 1983 and in 1989 the same group defined these functional vesicles as exosomes [1, 2]. Exosomes are cell-originated vesicles of 40–100 nm in diameter derived from endocytic compartments that are released into almost all biological fluids [3]. The putative primary function of exosomes is to transmit cell-to-cell molecular messages through small noncoding ribonucleic acid (ncRNAs), messenger RNAs (mRNA), deoxyribonucleic acids (DNA), and protein [4]. To date, several different types of extracellular vesicles have been described and depending on the nature of vesicular secretion from cells, extracellular vesicles can be grouped into two classes. The first class is known as microvesicles, which are directly shed from the cellular membrane. The second class is referred to as exosomes, which involves the tightly controlled biogenesis of these vesicles, whereby exosomes are released by exocytosis when multivesicular bodies (MVBs) fuse with the plasma membrane [4, 5].
The primary components of an exosomal membrane are proteins and lipids. Very recently, Zomer et al. showed that purified exosomes contain functional microRNAs (miRNAs) and small ncRNAs, but detected little mRNA. This study also speculated that exosomes specialize in carrying small RNA including, regulatory miRNAs [5, 6]. However, since exosomes have variable compositions, they can carry different information to different cells. The type of cell, or where an exosomes originate, can determine the composition, function and the molecular message that they are carrying [7].
Role of exosomes in cardiovascular diseases, cancer, diabetes and other pathological states has been well documented. Therefore, there is a possibility of development and implementation of novel approach in the treatment of various diseases relying on exosomes as therapeutic targets [8]. Moreover, great effort has been invested in examination of exosomes as drug delivery system for cancer and autoimmune/inflammatory disease therapy. Their future potential as diagnostic biomarkers and vehicles for the application of antitumor agents has yet to be thoroughly understood and so far they are in the early stage of research specifically for breast cancer [9, 10].
The discovery of different types of extracellular vesicle cargo, as well as the transportation of these vesicles to numerous tissues in the body that allows for global cell-to-cell communication has led investigators to concentrate on the therapeutic potential of exosomes, especially as drug delivery vehicles. To date, cell-derived vesicles exhibit multiple advantages over other existing or potential new drug delivery methods, including natural composition, small size (nanoscale) and immune invisibility. In this review, we discuss different ways of extracellular vesicle loading with exogenous cargoes and their advantages/limitations as well as novel alternative techniques of substance incorporation into nanoparticles.
Electroporation
Electroporation or electropermeabilization is the process of hydrophilic pore formation due to external electric field application that increases cellular membrane permeability and allows passage of chemicals, DNA, RNA or drugs into the cell [11,12,13]. Exosomes are cell-created vesicles that inherit identical phospholipid membrane, explaining a wide application of electroporation as a technique for exosome loading with exogenous cargoes [14,15,16,17]. After drug loading into the vesicle interior through temporary pores, recovery of exosome membrane integrity shortly occurs. While small hydrophobic molecules spontaneously diffuse into exosomes, electroporation has been proposed as convenient method for hydrophilic cargo incorporation (siRNA, miRNA) [18].
Alvarez-Erviti et al. performed loading of small interfering RNA (siRNA) into dendritic cell exosomes by electroporation [19]. To endow targeting quality, exosomes were engineered to express central nervous system-specific rabies viral glycoprotein (RVG) peptide that was fused with exosomal membrane protein Lamp2b. This group not only demonstrated efficient delivery of siRNA with targeted exosomes into neuronal cell line (Neuro2A) in vitro, but also investigated targeted exosome capabilities for systemic siRNA delivery. GAPDH siRNA was successfully delivered specifically to neurons, microglia and oligodendrocytes with RVG-targeted exosomes that were intravenously administered. Significant functional knockdown of BACE1 gene that contributes to cleavage of amyloid precursor protein and plays role in beta amyloid plaque formation in Alzheimer’s disease was achieved applying RVG-targeted exosomes loaded with BACE1 siRNA in vivo and in vitro. In a study by Banizs et al. luciferase transfected primary endothelial cells were treated with luciferase siRNA encapsulated with endothelial exosomes (exosome/siRNA Luc) by electroporation [7]. The luciferase gene expression in endothelial cells decreased by 40% with exosomes/siRNA Luc treatment, confirming the effective exosomal delivery of siRNA to endothelial cells. Multiple studies have exhibited successful loading of siRNA into exosomes via electroporation as well as delivery of an exosomal product to target organ [20,21,22], however, there are only few reported attempts to demonstrate effective incorporation of DNA into extracellular vesicles (EVs). Lamichhane et al. have shown that nucleic acids larger than miRNA or siRNA can be inserted into EVs applying electroporation, approximately 100 molecules per vesicle [23]. This group also demonstrated transfer of exogenous DNA to recipient cells by EVs, however, functional gene transfer was not observed. Despite the fact that electroporation is well known and widely used technique, Kooijmans et al. described downsides of electropermeabilization as it favors extensive siRNA aggregation, which overestimates the amount of loaded siRNA into EVs [24].
This brings a great demand for further exploration of novel effective concepts of exosomes loading.
Transfection of exosome-secreting cells
Another way of loading exosomes with therapeutic cargo is to overexpress a certain gene in exosome-donor cells or treat cell line with drug of interest that later will be gently enveloped into vesicles based on the process of EV biogenesis. Several reports demonstrated endogenous insertion of siRNA, miRNA, mRNA and protein into EVs that was accomplished via prior vesicle-secreting cell transfection. Kanada et al. performed loading of plasmid DNA/mRNA or siRNA into EVs by prior HEK293FT donor-cell transfection followed by EV isolation [25]. The study was focused on two types of EVs: exosomes and microvesicles (MV) and their ability to encapsulate and deliver cargo of interest. It was confirmed that reporter mRNA was successfully loaded and transferred by both types of EVs, however, MVs only could encapsulate and deliver plasmid DNA to recipient cells. In vivo experiment was also conducted where plasmid DNA-encoding Cre recombinase was effectively transported to tissue in transgenic Crelox reporter mice via MVs. In a study by Mizrak et al. HEK293T cells were transfected with plasmids to create EVs containing suicide gene mRNA and cytosine deaminase anchored to phosphoribosyltransferase to treat pre-established schwannoma [26]. Local injection of drug containing vesicles into schwannoma induced tumor growth regression. Ohno et al. engineered targeted exosomes via overexpression of the transmembrane domain of platelet derived growth factor receptor in donor cells for breast cancer therapeutics [27]. Bellavia et al. designed IL3-Lamp2b containing exosomes that targeted IL3 receptors overexpressed on hematopoietic cells in chronic myelogenous leukemia (CML) [28]. To obtain targeted exosomes that would incorporate anti-CML drug Imatinib, IL3L-HEK293T donor cells were simply treated with Imatinib followed by exosome isolation. Silencing of BCR-ABL gene that is overexpressed in CML was conducted using BCR-ABL siRNA incorporated into exosomes via IL3L-HEK293T donor cells siRNA transfection. It was confirmed that IL3L targeted exosomes containing Imatinib attenuated tumor growth more efficiently than Imatinib alone. Similarly, targeted exosomes loaded with BCR-ABL siRNA produced effective BCR-ABL gene silencing that inhibited tumor growth.
Several reports have demonstrated that simple incubation or treatment of exosome-parental cells with drug of interest is resulted in substance incorporation into a vesicle during EV biogenesis [28]. Kalani et al. performed mouse brain endothelial cell treatment with curcumin, a substance that has anti-oxidative as well as anti-inflammatory properties to obtain curcumin-containing exosomes [29]. Curcumin incorporated into endothelial exosomes was administered to homocysteine-treated mouse brain endothelial cells and resulted in alleviation of homocysteine-mediated oxidative stress and cell permeability.
Chemical-based exosome incorporation with exogenous cargoes
Chemical-based exosome transfection that utilizes commercially available transfection reagents have been shown to effectively incorporate and deliver siRNA to target cells via exosomes. Wahlgren et al. performed exosome loading with mitogen-activated protein kinase 1 (MAPK1) siRNA applying HiPerFect transfection reagent [30]. In a study by Shtam et al. RAD51, RAD52 siRNAs were incorporated into exosomes with Lipofectamine via chemical-based transfection [31]. The authors confirmed a successful administration of RAD51 siRNA via exosomes that produced RAD51 gene silencing in cancer cells.
Similarly, to visualize siRNA loading into exosomes as well as the exosomal product delivery to recipient cells, we have conducted an experiment where chemical-based exosome transfection was used. Endothelial exosomes were collected via differential ultracentrifugation [32] of mouse aortic endothelial cell (MAEC) culture-conditioned media and labeled with PKH67 green dye. Figure 1 shows MAEC that acquired PKH67-tagged exosomes. Fluorescently labeled exosomes were loaded with red fluorescent-tagged siRNA (Alexa Flour 647), using Lipofectamine or ExoFectin transfection reagents. For unincorporated siRNA clearance, Exosome Spin Columns were used. The transfected endothelial exosomes were administered to MAEC followed by 2 h incubation. Figure 2 illustrates successful delivery of siRNA to recipient MAEC via exosomes. Exosome size and protein content were characterized by emission electron microscopy and by western blotting (Fig. 3). It was confirmed that siRNA loading did not affect endothelial exosome integrity. The effective loading and introduction of siRNA to recipient cells via exosomes was achieved with both transfection reagents and no difference was found. Therefore, we have visualized both: successful incorporation of siRNA into exosomes using chemical-based transfection, as well as an effective delivery of siRNA to recipient cells via exosomes within 2 h. However, it was reported that chemical-based transfection carries several limitations including inability to separate exosome vesicles from micelles of chemical transfection that questions whether the exosomes or the micelles deliver exogenous nucleic acids to recipient cells [33, 34]. Thus, further investigation is required to explore novel advanced techniques for exosome sample purification as well as alternative loading tools.
a Representation of successful delivery of siRNA to recipient MAEC via exosomes; a siRNA alone (Lipofectamin); b Fluorescently labeled exosomes loaded with red fluorescent-tagged siRNA using ExoFectin; c Fluorescently labeled exosomes loaded with red fluorescent-tagged siRNA using Lipofectamine; MAEC—mouse aortic endothelial cell. (Color figure online)
Alternative exosome-loading techniques
Simple incubation of exosomes with cargo of interest has been widely described as one of the alternative exosome-loading technique [16, 35,36,37]. In a study by Sun et al., a natural polyphenol (curcumin) that possess anti-inflammatory, antioxidant and antineoplastic properties was simply incubated with exosomes for substance incorporation [38]. Curcumin loading into exosomes improved its solubility, stability and bioavailability.
Sonication is based on sonoporation phenomenon and is one of the alternative methods of exosome loading. In sonoporation, the application of low-frequency ultrasound induces cavitation bubble formation [34]. Microbubble burst produces cellular membrane pores that allow crossing of RNA/DNA into the cell. Exosome is a cellular descendant that has an identical lipid bilayer membrane allowing the application of sonication for the purpose of exosome transfection. In a study by Lamichhane et al., the sonication was applied for siRNA loading into EVs [39]. EVs successfully delivered siRNA to recipient cells for HER2 gene silencing in breast cancer.
O’Loughlin et al. described a novel approach of siRNA modification to allow passive incorporation into EVs [40]. Cholesterol-conjugated siRNA due to its lipophilic nature could be passively encapsulated into EVs by simple co-incubation. With this novel approach, the authors accomplished human antigen R (HuR) gene silencing to reduce tumor growth in EV-treated cells.
In a study by Yim et al. a novel tool for intracellular administration of target proteins, named “exosomes for protein loading via optically reversible protein–protein interactions” (EXPLORs) was developed [41, 42]. To accomplish protein loading into exosomes via EXPLOR, cargo protein was fused with photoreceptor cryptochrome 2 (CRY2) protein and CRY-interacting basic-helix-loop-helix1 (CIB1) protein was anchored to a membrane-associated tetraspanin protein CD9. Blue light illumination promotes reversible protein–protein interaction of CRY2 and CIB1 that allows attachment of cargo protein to membrane and cargo incorporation into exosomes through endogenous biogenesis. Removal of the illumination source disrupts CRY2-CIB1 interaction that releases protein cargo into the intraluminal space of the exosomes enabling further administration of cargo to cytosol of recipient cells via exocytosis.
Future directions
Extracellular vesicles are cell-created vehicles that constantly transport cargoes from one cell to another contributing to cell signaling that become a promising model for effective drug delivery. The great number of studies confirmed the loading possibilities of exosomes with exogenous molecules as well as successful delivery to recipient cells, however, several limitations need to be solved to fully adapt this approach in a clinical field. For example, exosome isolation and purification is still a time-consuming process with a low outcome, thus, a novel technology is required for efficient isolation of purified extracellular vesicles in large quantities. In addition, loading techniques require a novel approach that will be effective enough to overcome recently addressed shortcomings. A few questions need to be addressed: (1) weather the process of exosomal loading with exogenous cargo produces a disturbance of endogenous matter of the vesicle; (2) weather there is an interaction between incorporated exogenous cargo end preexisting exosomal content after vesicular transfection. Nevertheless, nanomedicine is a rapidly developing field that in the near future will grant a solution to resolve existing challenges and will confer a perspective for personalized therapy application.
Abbreviations
- ncRNAs:
-
Noncoding ribonucleic acid
- mRNA:
-
Messenger RNAs
- DNA:
-
Deoxyribonucleic acids
- MVBs:
-
Multivesicular bodies
- miRNAs:
-
MicroRNAs
- siRNA:
-
Small (or short) interfering RNA
- RVG:
-
Rabies viral glycoprotein
- EVs:
-
Extracellular vesicles
- MV:
-
Microvesicles
- CML:
-
Chronic myelogenous leukemia
- MAPK1:
-
Mitogen-activated protein kinase 1
- MAEC:
-
Mouse-aortic endothelial cell
- HuR:
-
Human antigen R
- EXPLORs:
-
Exosomes for protein loading via optically reversible protein–protein interactions
- CRY2:
-
Cryptochrome 2
- CIB1:
-
CRY-interacting basic-helix-loop-helix1
References
Pan BT, Johnstone R (1984) Selective externalization of the transferrin receptor by sheep reticulocytes in vitro. Response to ligands and inhibitors of endocytosis. J Biol Chem 259:9776–9782
Pan BT, Johnstone RM (1983) Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 33:967–978
Zhang J, Li S, Li L, Li M, Guo C, Yao J, Mi S (2015) Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteom Bioinform 13:17–24. https://doi.org/10.1016/j.gpb.2015.02.001
Lotvall J, Valadi H (2007) Cell to cell signalling via exosomes through esRNA. Cell Adhes Migr 1:156–158
Zomer A, Vendrig T, Hopmans ES, van Eijndhoven M, Middeldorp JM, Pegtel DM (2010) Exosomes: fit to deliver small RNA. Commun Integr Biol 3:447–450. https://doi.org/10.4161/cib.3.5.12339
Mathivanan S, Ji H, Simpson RJ (2010) Exosomes: extracellular organelles important in intercellular communication. J Proteomics 73:1907–1920. https://doi.org/10.1016/j.jprot.2010.06.006
Banizs AB, Huang T, Dryden K, Berr SS, Stone JR, Nakamoto RK, Shi W, He J (2014) In vitro evaluation of endothelial exosomes as carriers for small interfering ribonucleic acid delivery. Int J Nanomed 9:4223–4230. https://doi.org/10.2147/IJN.S64267
Yamashita T, Takahashi Y, Takakura Y (2018) Possibility of exosome-based therapeutics and challenges in production of exosomes eligible for therapeutic application. Biol Pharm Bull 41:835–842. https://doi.org/10.1248/bpb.b18-00133
Huang T, Deng CX (2019) Current progresses of exosomes as cancer diagnostic and prognostic biomarkers. Int J Biol Sci 15:1–11. https://doi.org/10.7150/ijbs.27796
Suntres ZE, Smith MG, Momen-Heravi F, Hu J, Zhang X, Wu Y, Zhu H, Wang J, Zhou J, Kuo PW (2013) Therapeutic uses of exosomes. J Exosomes Microvesicles 1:1–8
Asadirad A, Hashemi SM, Baghaei K, Ghanbarian H, Mortaz E, Zali MR, Amani D (2019) Phenotypical and functional evaluation of dendritic cells after exosomal delivery of miRNA-155. Life Sci 219:152–162
Kyuno D, Zhao K, Bauer N, Ryschich E, Zoller M (2019) Therapeutic targeting cancer-initiating cell markers by exosome miRNA: efficacy and functional consequences exemplified for claudin7 and EpCAM. Transl Oncol 12:191–199
Pomatto MAC, Bussolati B, D’Antico S, Ghiotto S, Tetta C, Brizzi MF, Camussi G (2019) Improved loading of plasma-derived extracellular vesicles to encapsulate antitumor miRNAs. Mol Ther Methods Clin Dev 13:133–144
Faruqu FN, Xu L, Al-Jamal KT (2018) Preparation of exosomes for siRNA delivery to cancer cells. J Vis Exp 142:e58814
Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, Lee JJ, Kalluri R (2017) Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 546:498–503
Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, Inskoe E, Piroyan A, Sokolsky M, Okolie O, Hingtgen SD, Kabanov AV, Batrakova EV (2016) Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed Nanotechnol Biol Med 12:655–664
Ma T, Chen Y (2018) MicroRNA-132, delivered by mesenchymal stem cell-derived exosomes, promote angiogenesis in myocardial infarction. Stem cells Int 2018:3290372
Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D (2017) Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin 38:754–763. https://doi.org/10.1038/aps.2017.12
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29:341–345. https://doi.org/10.1038/nbt.1807
Cooper JM, Wiklander PB, Nordin JZ, Al-Shawi R, Wood MJ, Vithlani M, Schapira AH, Simons JP, El-Andaloussi S, Alvarez-Erviti L (2014) Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice. Mov Disord 29:1476–1485. https://doi.org/10.1002/mds.25978
Lunavat TR, Jang SC, Nilsson L, Park HT, Repiska G, Lasser C, Nilsson JA, Gho YS, Lotvall J (2016) RNAi delivery by exosome-mimetic nanovesicles—implications for targeting c-Myc in cancer. Biomaterials 102:231–238. https://doi.org/10.1016/j.biomaterials.2016.06.024
Greco KA, Franzen CA, Foreman KE, Flanigan RC, Kuo PC, Gupta GN (2016) PLK-1 silencing in bladder cancer by siRNA delivered with exosomes. Urology 91(241):e1–e7. https://doi.org/10.1016/j.urology.2016.01.028
Lamichhane TN, Raiker RS, Jay SM (2015) Exogenous DNA loading into extracellular vesicles via electroporation is size-dependent and enables limited gene delivery. Mol Pharm 12:3650–3657. https://doi.org/10.1021/acs.molpharmaceut.5b00364
Kooijmans SA, Stremersch S, Braeckmans K, de Smedt SC, Hendrix A, Wood MJ, Schiffelers RM, Raemdonck K, Vader P (2013) Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J Control Release 172:229–238. https://doi.org/10.1016/j.jconrel.2013.08.014
Kanada M, Bachmann MH, Hardy JW, Frimannson DO, Bronsart L, Wang A, Sylvester MD, Schmidt TL, Kaspar RL, Butte MJ, Matin AC, Contag CH (2015) Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc Natl Acad Sci USA 112:E1433–E1442. https://doi.org/10.1073/pnas.1418401112
Mizrak A, Bolukbasi MF, Ozdener GB, Brenner GJ, Madlener S, Erkan EP, Strobel T, Breakefield XO, Saydam O (2013) Genetically engineered microvesicles carrying suicide mRNA/protein inhibit schwannoma tumor growth. Mol Ther 21:101–108. https://doi.org/10.1038/mt.2012.161
Ohno SI, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T, Gotoh N, Kuroda M (2013) Systemically injected exosomes targeted to EGFR deliver antitumor MicroRNA to breast cancer cells. Mol Ther 21:185–191. https://doi.org/10.1038/mt.2012.180
Bellavia D, Raimondo S, Calabrese G, Forte S, Cristaldi M, Patinella A, Memeo L, Manno M, Raccosta S, Diana P, Cirrincione G, Giavaresi G, Monteleone F, Fontana S, De Leo G, Alessandro R (2017) Interleukin 3-receptor targeted exosomes inhibit in vitro and in vivo chronic myelogenous leukemia cell growth. Theranostics 7:1333–1345. https://doi.org/10.7150/thno.17092
Kalani A, Kamat PK, Chaturvedi P, Tyagi SC, Tyagi N (2014) Curcumin-primed exosomes mitigate endothelial cell dysfunction during hyperhomocysteinemia. Life Sci 107:1–7. https://doi.org/10.1016/j.lfs.2014.04.018
Wahlgren J, De LKT, Brisslert M, Vaziri Sani F, Telemo E, Sunnerhagen P, Valadi H (2012) Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res 40:e130
Shtam TA, Kovalev RA, Varfolomeeva EY, Makarov EM, Kil YV, Filatov MV (2013) Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun Signal 11:88. https://doi.org/10.1186/1478-811X-11-88
Thery C, Amigorena S, Raposo G, Clayton A (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol 3:22. https://doi.org/10.1002/0471143030.cb0322s30
Antimisiaris S, Mourtas S, Papadia K (2017) Targeted si-RNA with liposomes and exosomes (extracellular vesicles): how to unlock the potential. Int J Pharm 525:293–312. https://doi.org/10.1016/j.ijpharm.2017.01.056
Tomizawa M, Shinozaki F, Motoyoshi Y, Sugiyama T, Yamamoto S, Sueishi M (2013) Sonoporation: gene transfer using ultrasound. World J Methodol 3:39–44. https://doi.org/10.5662/wjm.v3.i4.39
Agrawal AK, Aqil F, Jeyabalan J, Spencer WA, Beck J, Gachuki BW, Alhakeem SS, Oben K, Munagala R, Bondada S, Gupta RC (2017) Milk-derived exosomes for oral delivery of paclitaxel. Nanomed Nanotechnol Biol Med 13:1627–1636
Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Patel T, Piroyan A, Sokolsky M, Kabanov AV, Batrakova EV (2015) Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Controll Release 207:18–30
Liu Y, Li D, Liu Z, Zhou Y, Chu D, Li X, Jiang X, Hou D, Chen X, Chen Y, Yang Z, Jin L, Jiang W, Tian C, Zhou G, Zen K, Zhang J, Zhang Y, Li J, Zhang CY (2015) Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci Rep 5:17543
Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang HG (2010) A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther 18:1606–1614
Lamichhane TN, Jeyaram A, Patel DB, Parajuli B, Livingston NK, Arumugasaamy N, Schardt JS, Jay SM (2016) Oncogene knockdown via active loading of small RNAs into extracellular vesicles by sonication. Cell Mol Bioeng 9:315–324
O’Loughlin AJ, Mager I, de Jong OG, Varela MA, Schiffelers RM, El Andaloussi S, Wood MJA, Vader P (2017) Functional delivery of lipid-conjugated siRNA by extracellular vesicles. Mol Ther 25:1580–1587. https://doi.org/10.1016/j.ymthe.2017.03.021
Yim N, Choi C (2016) Extracellular vesicles as novel carriers for therapeutic molecules. BMB Rep 49:585–586
Yim N, Ryu SW, Choi K, Lee KR, Lee S, Choi H, Kim J, Shaker MR, Sun W, Park JH, Kim D, Heo WD, Choi C (2016) Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat Commun 7:12277. https://doi.org/10.1038/ncomms12277
Acknowledgements
This work was supported by National Institute of Health Grants: HL74185, HL139047 and AR71789.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Familtseva, A., Jeremic, N. & Tyagi, S.C. Exosomes: cell-created drug delivery systems. Mol Cell Biochem 459, 1–6 (2019). https://doi.org/10.1007/s11010-019-03545-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-019-03545-4