Abstract
Phosphofructokinase-2/fructose-2, 6-bisphosphatase 3 (PFKFB3) catalyzes the synthesis of F2,6BP, which is an allosteric activator of 6-phosphofructo-1-kinase (PFK-1): the rate-limiting enzyme of glycolysis. During tumorigenesis, PFKFB3 increases glycolysis, angiogenesis, and tumor progression. In this study, our aim was to investigate the significance of PFKFB3 and Ki67 in human lung adenocarcinomas and to target PFKFB3 as a therapeutic strategy. In this study, we determined the expression levels of PFKFB3 mRNA and proteins in cancerous and normal lung adenocarcinomas by quantitative reverse transcription PCR (qRT-PCR), Western blot analysis, and tissue microarray immunohistochemistry analysis, respectively. In human adenocarcinoma tissues, PFKFB3 and Ki67 protein levels were related to the clinical characteristics and overall survival. Both PFKFB3 mRNA and protein were significantly higher in lung adenocarcinoma cells (all P < 0.05). A high expression of PFKFB3 and Ki67 were associated with the degree of differentiation, TNM staging, lymph node metastasis, and survival. A high expression of PFKFB3 protein was an independent prognostic marker in lung adenocarcinoma. Subsequently, 1-(4-pyridinyl)-3-(2-quinolinyl)-2-propen-1-one (PFK15) was used as a selective antagonist of PFKFB3. Glycolytic flux was determined by measuring glucose uptake, F2,6BP, and lactate production. Cell viability, cell cycle, cell apoptosis, cell migration, and invasion were analyzed by MTT, flow cytometry, Western blot analysis, wound healing assay, and transwell chamber assay. By targeting PFKFB3, it inhibited cell viability and glycolytic activity. It also caused apoptosis and induced cell cycle arrest. Furthermore, the migration and invasion of A549 cells was inhibited. We conclude that PFKFB3 bears an oncogene-like regulatory element in lung adenocarcinoma progression. In the treatment of lung adenocarcinoma, targeting PFKFB3 would be a promising therapeutic strategy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is one of the most frequently diagnosed malignant tumors in the entire world, and it is also the leading cause of cancer-related deaths [1]. Lung adenocarcinoma accounts for approximately 80% of non-small cell lung cancer (NSCLC), and it has become the most common subtype. By investigating major histological types of lung cancer, we found that smoking and pollution are the two primary causes of lung cancer. However, lung adenocarcinoma frequently occurs in women and non-smokers; its pathophysiology does not have a strong correlation with smoking [2]. Lung adenocarcinoma is the most prevalent form of lung cancer, and its frequency of occurrence is increasing rapidly [3]. Its somatic gene aberrations have been most extensively discussed in previous studies [4, 5]. Several evidences indicate that lung adenocarcinoma and squamous cell carcinoma respond differently to chemotherapy. The pathogenesis of lung adenocarcinoma remains unclear till date, Therefore, several research studies must be extensively investigated about the relevant molecular mechanisms causing carcinogenesis in patients with lung adenocarcinoma.
The bifunctional enzyme, 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases (PFKFBs), exhibits both kinase and phosphatase activities. There are four different genes coding different isozymes 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFKFB1, PFKFB2, PFKFB3, and PFKFB4); the kinase/phosphatase activities and the tissue expression profiles of these isozymes are completely different [6]. Among the PFKFB members, PFKFB3 exhibits greater (740-fold) kinase activity and lesser bisphosphatase activity. This increases the production of fructose 2,6-biphosphate (F2,6BP), which strictly controls the glycolysis rate under normal and pathophysiological conditions [7, 8]. Previous studies have found that PFKFB3 was ubiquitously expressed in different organs and tumor cells, such as gastric, colon, lung, breast, ovarian, and thyroid carcinomas. It brings about changes in metabolism, causing the proliferation and survival of tumor cells [9, 10]. This indicates that PFKFB3 has an oncogene-like regulatory element, and the high expression of PFKFB3 is an independent prognostic marker for the overall poor survival of patients with lung adenocarcinoma.
Recent studies have found that Ki67 is one of the most familiar and widely used markers of cell proliferation. It is expressed in various stages of the cell cycle outside the G0 phase. Because it has short half-life period, it rapidly degrades once it is out of the cell cycle; therefore, it is used to determine the tumor proliferation ratio. In many patients with malignant tumor, the expression of Ki67 is extremely high. Moreover, it is closely correlated with tumorigenesis, development, metastasis, and prognosis of carcinoma [11, 12].
By silencing or inhibiting PFKFB3 in tumor cells, the glycolysis rate and proliferation of cancer cells can be declined. Han reported about the overexpression of PFKFB3 in patients with human colorectal adenoma and adenocarcinoma. Following the knockdown of PFKFB3 by siRNAs, the proliferation and migration abilities of gastric cancer cells decreased significantly [13]. PFK15 is a potent and selective PFKFB3 inhibitor, and it causes a rapid decrease in F2,6BP, glucose uptake, and lactate secretion. This leads to a decrease in the steady-state concentration of ATP, and an arrest cell cycle progression in lung adenocarcinoma cells and Jurkat T-cell leukemia cells [14].
In the present study, we determined both mRNA and protein expression of PFKFB3 in lung adenocarcinoma cells; then, the results were correlated with the clinical characteristics and overall survival of patients. Moreover, we utilized PFK15 to investigate the contribution of PFKFB3 in the development of lung adenocarcinoma. By targeting PFKFB3, we elicited both anti-metabolic effects and anti-tumor progression.
Materials and methods
Patients and tissue samples
Human lung adenocarcinoma tissue samples were obtained from the Affiliated Hospital of Nantong University in Nantong, China, with written consent of patients and ethical approval from the Human Research Ethics Committee. Fresh tissues were collected by surgical resection, and they were immediately stored at − 80 °C. For histological analysis, we collected samples from 263 patients who had undergone lung resection at the Affiliated Hospital of Nantong University from 2010 to 2015. Chemotherapy was not provided to these patients. Among the 263 patients included in this study, there were 129 male patients and 134 female patients; the average age of these patients was 62 years (range 39–80). The tumors of these patients were classified as follows: well differentiated (grade I; n = 28), moderately differentiated (grade II; n = 122), or poorly (grade III; n = 113) differentiated. All the specimens were fixed in formalin and embedded in paraffin. Table 1 presents the main clinicopathological data of patients included in this study. The follow-up of patients was conducted until December 31, 2016. The primary end point was the overall survival period, which extended from the date of surgery until death.
Quantitative reverse transcription PCR (qRT-PCR)
Total RNA was extracted by following the manufacturer’s protocol of TRIZOL reagent (Life Technologies, Carlsbad, USA). The amount and quality of RNA were determined by spectrophotometry after immediate separation. Quantitative real-time PCR was performed using the Bio-Rad CFX96 system (Bio-Rad, Hercules, USA). The primer sequences were as follows: PFKFB3 forward primer (5′-AGC CCG GAT TAC AAA GAC TGC-3′) and PFKFB3 reverse primer (5′-GGT AGC TGG CTT CAT AGC AAC-3′). All the experiments were performed in triplicate.
Western blot analysis
Tissue and cell samples were lysed in lysis buffer, and protein concentrations were determined by performing bicinchoninic acid (BCA) protein assay (Biosharp, Guangzhou, China). Then, 20 μg protein samples were separated by performing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The separated samples were electroblotted onto PVDF membranes (Millipore Corporation, Bedford, MA, USA). After blocking the membranes with 5% skimmed milk in Tris-buffered saline Tween-20 (TBST) for 2 h, we incubated the membranes with primary antibodies overnight at 4 °C. Primary antibodies were diluted for PFKFB3 (1:1000), cyclin D1 (1:500), cleaved caspase-3 (1:1000), and β-Actin (1:10,000). Then, they were incubated at room temperature for 1 h with the corresponding secondary antibody (1:1000). Immunoreactive bands were visualized by chemiluminescence detection system (Pierce, Waltham, MA). The experiments were performed in triplicates.
Immunohistochemistry (IHC)
In this experiment, slices of 4 μm thickness were dewaxed in xylene. Then, they were rehydrated in grade ethanol. Endogenous peroxidase activity was blocked for 10 min. Then, these slices were processed in 10 mmol/L citrate buffer (pH 6.0). To obtain the tumor antigen, these slices were heated to 121 °C for 20 min in an autoclave. After washing these slices in phosphate-buffered saline (PBS) (pH 7.2), they were incubated with mouse anti-human PFKFB3 antibodies (diluted 1:200, Santa Cruz Biotechnology, Dallas, Texas) at room temperature for 2 h. After washing the slices with PBS, we treated the specimens with peroxidase reagent (DAKO, Hamburg, Germany) according to the manufacturer’s instructions.
Immunohistochemical evaluation
Three pathologists evaluated all the stained sections in a blind manner; the clinical and pathological parameters of patients were not considered by the three pathologists. This method was used to assess the expression of PFKFB3 and Ki-67 in the stained sections [15].
Cell culture and PFK15 treatment
The human lung adenocarcinoma cell line (A549) and human bronchial epithelioid cells (16HBE) were obtained from the Shanghai Institute of Cell Biology, Shanghai, China. They were kept in an incubator at 37 °C with 5% carbon dioxide, and they were maintained in high glucose DMEM (Gibco, Grand Island, NY, USA) supplemented with 10% FBS and 100 U/mL penicillin–streptomycin mixture (Gibco, Grand Island, NY, USA). The cells were then exposed to specific concentration of PFK15 (Advanced Cancer Therapeutics, Louisville, KY, USA) for 24 h. Then, we measured the glycolytic activity, cell cycle, apoptosis, migration, and invasion of cells. The experiments were performed for 3 or 4 h, and the statistical significance was assessed by performing unpaired two-tail T test.
Cell viability assay
Cell viability was determined by performing MTT assay. Briefly, cells were seeded into 96-well plates and incubated with PFK15 for 24 h to perform dose-dependent analysis.
Measurement of 2-DG glucose uptake, F2,6BP, and lactate
2-DG glucose uptake and F2,6BP were measured with a kit (Abcam, Cambridge, UK) according to the manufacturer’s instructions. Cells were grown to 80% confluence in 96-well plates and added with PFK15 for 24 h. The lactate concentration in the medium was determined with a lactic acid assay kit (Shanghai Juchuang Biotechnology Corporation, China). The amount of lactate release was calculated by subtraction of lactate concentrations between the cell-plated wells and the blank wells.
Cell cycle analysis
In this experiment, A549 cells were cultured in six-well plates at 5 × 105 per well and incubated with PFK15 (10 μmol/L) for 24 h. Then, cells were collected and fixed overnight in 70% ethanol at 4 °C. The fixed cells were washed and re-suspended in phosphate-buffered saline (PBS). Then, the cells were incubated with 800 μL propidium iodide (PI) solution (50 μg/mL) in the dark for 30 min at 37 °C. The stained cells were analyzed and cell cycle distributions were calculated by flow cytometry (BD Biosciences).
Apoptosis analysis
Cells were cultured in six-well plates at 5 × 105 per well and incubated with PFK15 (10 μmol/L) for 24 h. Harvested cells were washed with PBS and stained with Annexin V and propidium iodide (Beyotime, Beijing, China).
Cell migration and invasion assay
Wound healing assay was performed as follows: A549 cells were seeded into a six-well plate. When cell confluence reached 90%, wounds were created by scraping the cells with a 100 μL pipette tip. After 24 h, a microscope (Olympus, Japan) was used to observe the migrated distance of cells.
Transwell cell invasion assay was performed as follows: about 2.0 × 105 cells were cultured in 0.1 mL serum-free medium. Then, they were treated with 10 μmol/L PFK15 at the top of Matrigel-coated chambers (24-well insert, 8-μm pore size; Corning, NY, USA). The lower chambers were completely filled with 10% FBS. After 24 h, we removed the non-invasive cells from the upper surface of the filter using the swab. Then, the cells were stained quantitatively and the five randomly selected areas were observed under a microscope at ×200 magnification.
Statistics
Statistical analyses were performed using SPSS 17.0 software. All values were expressed as means ± SEM. Clinicopathological features, PFKFB3 expression, and Ki67 expression were analyzed by χ2 test. Kaplan–Meier curves were constructed. Multivariate analysis was performed by Cox’s proportional hazards model, and the risk ratio and its 95% confidence interval were recorded. P < 0.05 was considered to be statistically significant.
Results
High expressions of PFKFB3 mRNA and protein in human lung adenocarcinoma tissue
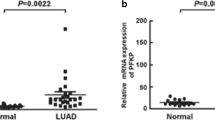
Data indicate that PFKFB3 mRNA expression was significantly higher (P < 0.001) in the human lung adenocarcinoma tissue than in the corresponding normal tissue counterparts of 20 patients (Fig. 1a). By performing Western blot analysis of PFKFB3 proteins, we found that they were clearly up-regulated in lung adenocarcinoma tissues (Fig. 1b).
The expression of PFKFB3 was detected in lung adenocarcinoma and normal lung tissues. a PFKFB3 mRNA expression was determined by qRT-PCR analysis. Lung adenocarcinoma and corresponding normal tissues from 20 patients. b PFKFB3 protein expression in two representative paired samples of lung adenocarcinoma tissue (T) and normal lung tissues (N). β-actin was used as a loading control. **P < 0.01, compared with normal
Expression of PFKFB3 and Ki67 in lung adenocarcinoma tissue and the correlation with clinical pathologic features
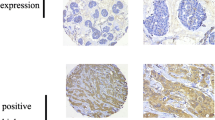
We determined the expression of PFKFB3 and Ki67 by immunohistochemistry (IHC) in 263 cases of lung adenocarcinoma. In these patients, PFKFB3 was mainly expressed in the cytoplasm of the tumor but Ki67 was mainly distributed in cell nucleus. In lung adenocarcinoma tissue, the expression of PFKFB3 and Ki67 was much higher than that in normal lung tissue (Fig. 2). Table 1 summarizes the correlation of clinicopathological features with the expression of PFKFB3 and Ki67. We found that the expression intensity of PFKFB3 and Ki67 were related to the following parameters: histological differentiation of lung adenocarcinoma, tumor-node metastasis (TNM) staging, lymph node metastasis, and lifetime. The positive rates of well, moderately, and poorly differentiated groups are as follows: well-differentiated group (17.9 and 7.1%), moderately differentiated group (38.5 and 46.7%), and poorly differentiated group (73.5 and 80.5%) (P < 0.001). The various TNM stages are as follows: stage 1 (24.6 and 26.9%), stage 2 (78.6 and 82.1%), stage 3 (78.8 and 92.4%), and stage 4 (63.6 and 72.7%) (P < 0.001). Lymph node metastasis positive group (78.6 and 87.0%) was larger than lymph node metastasis negative group (24.2 and 27.3%) (P < 0.001).
PFKFB3 and Ki67 have a positive correlation
Lung adenocarcinoma tissues obtained from 263 cases were stained simultaneously with PFKFB3 and Ki67. Both PFKFB3 and Ki67 showed positive expression in the lung adenocarcinoma tissues of 118 cases. In the lung adenocarcinoma tissues of 17 cases, PFKFB3 showed positive expression but Ki67 showed negative expression. In lung adenocarcinoma tissues of 96 cases, both PFKFB3 and Ki67 showed negative expression. In lung adenocarcinoma tissues of 32 cases, PFKFB3 showed negative expression but Ki67 showed positive expression. According to Spearman correlation analysis, there was positive correlation in the expressions of PFKFB3 and Ki67 in lung adenocarcinoma tissues of 263 cases (r = − 0.630, P < 0.001) (Table 2).
PFKFB3 and Ki67 are significantly associated with the survival of lung adenocarcinoma patients
Survival statistics analysis was performed by Kaplan–Meier method. We found that the median of the survival rate of positive group was lower than that of negative group (Fig. 3). Furthermore, the factors influencing the prognosis of lung adenocarcinoma were determined by Cox proportional hazards model: a high expression of PFKFB3 and Ki67 was found to increase the risk of death in lung adenocarcinoma patients (P < 0.05, Table 3); therefore, PFKFB3 and Ki67 may be used considered as independent prognostic factors.
PFK15 abolished the proliferation and viability of A549 cell
Previous studies have reported that PFK15 is a specific small-molecule antagonist of PFKFB3, which inhibits the proliferation and metabolism of various cancer cells at a relatively low concentration [14]. Since PFKFB3 was related to the expression of Ki-67 and histological differentiation in lung adenocarcinoma specimens, we proposed that PFKFB3 might also have an influence on cell proliferation and viability of lung adenocarcinoma cells. We found that the expression of PFKFB3 was clearly down-regulated in A549 cells, which were treated with different concentrations of PFK15 for 24 h (Fig. 4a), and we observed that cell viability of A549 lung adenocarcinoma cells was inhibited by PFK15 in a dose-dependent manner (Fig. 4B). Moreover, PFK15 decreased the viability of normal human bronchial epithelioid cells (16HBE) by < 10%. In these cells, PFK15 also inhibited the expression of PFKFB3 protein by < 10%. These findings suggest that PFK15 induces little cytotoxicity in normal cells.
PFK15 inhibits glycolytic activity in A549
Previous studies have reported that PFKFB3 regulates high glycolytic activity in individual tumor cells [16]. Therefore, we examined the metabolic effects of PFK15 on 2DG glucose uptake, F2,6BP, and lactate production in A549 cells. The results indicate that 2DG glucose uptake, intercellular level of F2,6BP, and lactate production decreased in A549 cells, which were exposed to PFK15 (10 μmol/L) for 24 h (Fig. 5).
PFK15 causes apoptosis in A549 lung adenocarcinoma cells
PFKFB3 expression is quite important for maintaining an anti-apoptotic state [17]. After treating A549 cells with PFK15, we analyzed them by flow cytometry. The results indicated that PFK15 induced apoptosis in A549 cells (Fig. 6a). Following PFK15 treatment, we observed changes in apoptosis-associated proteins in A549 cells.. For example, caspase 3 protein levels were down-regulated, and cleaved caspase-3 protein levels were up-regulated in A549 cells, which were treated with PFK15 (Fig. 6b).
Role of PFK15 on cell apoptosis. A549 cells were exposed to the 10 μmol/L PFK15, after 24 h. a Cells were analyzed by flow cytometry after stained with Annexin V and PI. The apoptotic cells (Annexin V+/PI+) were quantified. b The apoptosis-associated proteins were analyzed by Western blot. *P < 0.05, compared with controls
PFK15 induces cell cycle arrest and reduces the expression of cyclin D1 protein
Previous studies have established that PFKFB3 expression plays an important role in cell cycle progression [17, 18]. For example, cells with knockdown PFKFB3 gene showed higher cell percentage during the S phase of the cell cycle [19]. Therefore, we performed flow cytometry to investigate whether PFK15 induced cell cycle changes in A549 cells. By treating A549 cells with PFK15 for 24 h, we observed cell cycle arrest at the G0/G1 phase: the percentage of cells in the G0/G1 phase significantly increased to 49.34% in A549 cells treated with PFK15. In contrast, the percentage of cells in the G0/G1 phase was only 33.91% in the control group (P < 0.05) (Fig. 7a). Simultaneously, we determined the expression level of G0/G1 regulatory protein. We observed that the expression level of cyclin D1 was significantly reduced in A549 cells treated with PFK15 for 24 h (Fig. 7b).
PFK15 inhibits cell migration and invasion of A549 cells
More than 90% of cancer-related deaths occur due to invasive and metastatic complications; however, cancer metastasis is one of the major challenges in cancer treatment [20]. Therefore, wound healing assays and a transwell chamber system were performed to confirm the anti-migration and anti-invasion properties of PFK15 in lung adenocarcinoma cells, respectively. We found that the migratory and invasive ability of A549 cells was significantly reduced when they were treated with PFK15 (10 μmol/L) for 24 h. This indicates that tumor metastasis in A549 cells can be halted by blocking PFKFB3 (Fig. 8).
PFK15 inhibits the migratory and invasion abilities of A549 cells. A549 cells were exposed to the 10 μmol/L PFK15, after 24 h. a The effects of PFK15 on the migratory of A549 cells were tested by wound healing assays. b The quantitative data of the wound healing assays. c The effects of PFK15 on the invasion of A549 cells were tested by transwell chamber system. d The quantitative data of the transwell chamber system. *P < 0.05, compared with controls
Discussion
Presently, NSCLC accounts for approximately 80% of all lung cancer cases. Lung adenocarcinoma has become the most common subtype, accounting for 75% of NSCLC cases [21]. There have been many advancements in the treatment of lung cancer over the years, but the prognosis of NSCLC has not improved significantly till date. Even today, the 5-year survival rate is only 15% in lung cancer patients [22]. Previous studies have found that lung adenocarcinoma inclined to grow and spread faster than lung squamous cell carcinoma (SqCC), accounting for nearly half of all lung cancer cases [23]. However, the molecular mechanisms involved in the carcinogenesis and progression of lung adenocarcinoma remain unclear till date. Therefore, some new biomarkers of lung adenocarcinoma must be identified such that they accurately identify biological characteristics of lung adenocarcinoma. These new biomarkers would be beneficial for the diagnosis, treatment, and prognosis of lung adenocarcinoma.
Among the four isoforms of PFKFB family, the most important one is PFKFB3 because it has several pharmaceutical applications. In the pharmaceutical industry, scientists have found that the levels of PFKFB3 mRNA and proteins are significantly greater in tumors than in normal tissues [24]. Recent studies have shown that PFKFB3 is associated with the migration and growth of breast cancer [25] and bladder cancer [26]. Although scientists have not been able to completely elucidate the precise mechanism through which a high expression of PFKFB3 is elicited in human cancer cells, they have proved that HIF-1α promotes the transcription of PFKFB3 mRNA in human cancer cells [27, 28]. Scientists have proved that cancer cell glucose metabolism is reduced with the knockout of PFKFB3 gene. Thus, this enzyme serves as a promising target for anti-cancer therapy in tumors. Recently, molecular modeling has been used to develop novel small-molecule inhibitors, which are capable of competitively inhibiting PFKFB3 enzyme activity [29].
In previous studies, scientists have presented data to prove that PFKFB3 plays an important role in accelerating glycolysis, angiogenesis, and tumor progression [6]. Some studies have reported that PFKFB3 influences glucose metabolism by participating in glycolysis. Moreover, PFKFB3 has a significant control of the cell cycle, apoptosis, tumor growth, and invasiveness in gastric cancer [16]. Moreover, apoptosis of cancer cells can be achieved by suppressing the expression of PFKFB3. This would also help us to suspend cell cycles. Interestingly, the selective antagonist of PFKFB3 inhibits the growth of transplanted tumor in some animal models [14].
In this work, we determined the expression of PFKFB3 mRNA and proteins in both malignant and normal lung adenocarcinoma tissues. We found that PFKFB3 mRNA level was significantly higher in lung adenocarcinoma tissues. Similarly, PFKFB3 protein level was higher in lung adenocarcinoma tissues than in normal lung tissues. Next, we detected the expression of PFKFB3 and Ki67 in lung adenocarcinoma specimens of 263 cases. We found that the positive rate of PFKFB3 and Ki67 was significantly related to the following parameters: differentiation of tumors, TNM staging, lymph node metastasis, and 5-year survival rate; however, it was not related to the age, gender, and size of tumor. The findings of survival analysis were as follows: in the group showing positive expression of PFKFB3 and Ki67, the median of survival time of patients was lower than that of patients in the negative expression group. This implies that the level of malignancy of lung adenocarcinoma and its prognosis can be understood by clinically detecting the expression of PFKFB3 and Ki67 in patients. Therefore, high expression of PFKFB3 and Ki67 is an independent prognostic marker for poor overall survival in lung adenocarcinoma.
PFK15 is a novel small-molecule antagonist of PFKFB3. By pharmacologically inhibiting PFKFB3 via PFK15, it suppressed tumor growth, cell proliferation, apoptosis, and glycolytic flux in head and neck SCC. Moreover, metastasis of head and neck SCC was alleviated with this strategy [9]. The kinase activity of PFKFB3 was inhibited by treating it with PFK15. This reduced the activation and aggressive capacity of rheumatoid arthritis in vitro, and it suppressed experimental arthritis in vivo [30]. Moreover, AML cell proliferation was synergistically blunted by rapamycin and PFK15 [31]. The directional migration of endothelial cells also decreased following the inhibition of PFKFB3 [32, 33]. We treated A549 cells with PFK15 for 24 h, and we found that the expression of PFKFB3 was clearly down-regulated. Furthermore, it inhibited cell viability and glycolytic activity, caused apoptosis, induced cell cycle arrest, inhibited migration, and invasion of A549 cells.
We chose PFKFB3 as our target for several reasons. Firstly, PFKFB3 might be a biomarker for the early detection of lung adenocarcinoma. However, the expression and clinical significances of PFKFB3 have not been reported previously in a large sample of patients with lung adenocarcinoma. Secondly, PFKFB3 is one of the novel kinases involved in cell metabolism in recent years. Research studies have confirmed that glycolysis, angiogenesis, and tumor progression increase whenever there is an overexpression of PFKFB3. Scientists have not been able to completely elucidate the mechanism through which the expression of PFKFB3 is increased significantly in human lung adenocarcinoma. Our study has yet very clearly elucidated what is the mechanism in which PFKFB3 and Ki67 interact to lead to the malignant progression of tumors. Reports about the interaction between PFKFB3 and Ki67 in human lung adenocarcinoma are very limited at home and abroad.
In conclusion, tumor formation, development, and metastasis of human adenocarcinoma are significantly increased due to the expression of PFKFB3. Consequently, the malignant degree of human adenocarcinoma increases, leading to poor prognosis of patients showing an overexpression of PFKFB3. Therefore, an overexpressed PFKFB3 may serve as a preventive, therapeutic, and prognostic biomarker for lung adenocarcinoma. But we need to further investigate the specific mechanism responsible for the overexpression of PFKFB3 in human adenocarcinoma tissues. Interestingly, we confirmed that PFK15 exhibits anti-glycolytic effect. In addition, we established that the anti-tumor activities of PFK15 were associated with cell apoptosis, cell cycle block, cell migration, and invasion in lung adenocarcinoma cells. Our findings suggest that PFK15 is a promising anti-cancer drug molecule, which can be used for treating lung adenocarcinoma. These results provide rationale for the development of agents like PFK15, which selectively inhibit the PFKFB3 and act as antineoplastic agents. The findings of this study need to be further investigated in further studies.
References
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63:11–30. https://doi.org/10.3322/caac.21166
Saito M, Shiraishi K, Kunitoh H, Takenoshita S, Yokota J, Kohno T (2016) Gene aberrations for precision medicine against lung adenocarcinoma. Cancer Sci 107:713–720. https://doi.org/10.1111/cas.12941
Lee YS, Bae SC (2016) How do K-RAS-activated cells evade cellular defense mechanisms? Oncogene 35:827–832. https://doi.org/10.1038/onc.2015.153
Pasche B, Grant SC (2014) Non-small cell lung cancer and precision medicine: a model for the incorporation of genomic features into clinical trial design. JAMA 311:1975–1976. https://doi.org/10.1001/jama.2014.3742
Kohno T, Tsuta K, Tsuchihara K, Nakaoku T, Yoh K, Goto K (2013) RET fusion gene: translation to personalized lung cancer therapy. Cancer Sci 104:1396–1400. https://doi.org/10.1111/cas.12275
Bando H, Atsumi T, Nishio T, Niwa H, Mishima S, Shimizu C, Yoshioka N, Bucala R, Koike T (2005) Phosphorylation of the 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatase/PFKFB3 family of glycolytic regulators in human cancer. Clin Cancer Res 11:5784–5792. https://doi.org/10.1158/1078-0432.CCR-05-0149
Clem B, Telang S, Clem A, Yalcin A, Meier J, Simmons A, Rasku MA, Arumugam S, Dean WL, Eaton J, Lane A, Trent JO, Chesney J (2008) Small-molecule inhibition of 6-phosphofructo-2-kinase activity suppresses glycolytic flux and tumor growth. Mol Cancer Ther 7:110–120. https://doi.org/10.1158/1535-7163.MCT-07-0482
Chesney J, Telang S, Yalcin A, Clem A, Wallis N, Bucala R (2005) Targeted disruption of inducible 6-phosphofructo-2-kinase results in embryonic lethality. Biochem Biophys Res Commun 331:139–146. https://doi.org/10.1016/j.bbrc.2005.02.193
Li HM, Yang JG, Liu ZJ, Wang WM, Yu ZL, Ren JG, Chen G, Zhang W, Jia J (2017) Blockage of glycolysis by targeting PFKFB3 suppresses tumor growth and metastasis in head and neck squamous cell carcinoma. J Exp Clin Cancer Res 36:7. https://doi.org/10.1186/s13046-016-0481-1
Minchenko OH, Tsuchihara K, Minchenko DO, Bikfalvi A, Esumi H (2014) Mechanisms of regulation of PFKFB expression in pancreatic and gastric cancer cells. World J Gastroenterol 20:13705–13717. https://doi.org/10.3748/wjg.v20.i38.13705
Wang JX, Zhang YY, Yu XM, Jin T, Pan XL (2012) Role of centromere protein H and Ki67 in relapse-free survival of patients after primary surgery for hypopharyngeal cancer. Asian Pac J Cancer Prev 13:821–825
Liu HB, Gao XX, Zhang Q, Liu J, Cui Y, Zhu Y, Liu YF (2015) Expression and prognostic implications of FOXO3a and Ki67 in lung adenocarcinomas. Asian Pac J Cancer Prev 16:1443–1448
Han J, Meng Q, Xi Q, Wang H, Wu G (2017) PFKFB3 was overexpressed in gastric cancer patients and promoted the proliferation and migration of gastric cancer cells. Cancer Biomark 18:249–256. https://doi.org/10.3233/CBM-160143
Clem BF, O’Neal J, Tapolsky G, Clem AL, Imbert-Fernandez Y, Kerr DA, 2nd, Klarer AC, Redman R, Miller DM, Trent JO, Telang S, Chesney J (2013) Targeting 6-phosphofructo-2-kinase (PFKFB3) as a therapeutic strategy against cancer. Mol Cancer Ther 12:1461–1470. https://doi.org/10.1158/1535-7163.MCT-13-0097
Liu Y, Lv L, Xue Q, Wan C, Ni T, Chen B, Liu Y, Zhou Y, Ni R, Mao G (2013) Vacuolar protein sorting 4B, an ATPase protein positively regulates the progression of NSCLC via promoting cell division. Mol Cell Biochem 381:163–171. https://doi.org/10.1007/s11010-013-1699-2
Zhu W, Ye L, Zhang J, Yu P, Wang H, Ye Z, Tian J (2016) PFK15, a small molecule inhibitor of PFKFB3, induces cell cycle arrest, apoptosis and inhibits invasion in gastric cancer. PLoS ONE 11:e0163768. https://doi.org/10.1371/journal.pone.0163768
Yalcin A, Clem BF, Imbert-Fernandez Y, Ozcan SC, Peker S, O’Neal J, Klarer AC, Clem AL, Telang S, Chesney J (2014) 6-Phosphofructo-2-kinase (PFKFB3) promotes cell cycle progression and suppresses apoptosis via Cdk1-mediated phosphorylation of p27. Cell Death Dis 5:e1337. https://doi.org/10.1038/cddis.2014.292
Yalcin A, Clem BF, Simmons A, Lane A, Nelson K, Clem AL, Brock E, Siow D, Wattenberg B, Telang S, Chesney J (2009) Nuclear targeting of 6-phosphofructo-2-kinase (PFKFB3) increases proliferation via cyclin-dependent kinases. J Biol Chem 284:24223–24232. https://doi.org/10.1074/jbc.M109.016816
Xu Y, An X, Guo X, Habtetsion TG, Wang Y, Xu X, Kandala S, Li Q, Li H, Zhang C, Caldwell RB, Fulton DJ, Su Y, Hoda MN, Zhou G, Wu C, Huo Y (2014) Endothelial PFKFB3 plays a critical role in angiogenesis. Arterioscler Thromb Vasc Biol 34:1231–1239. https://doi.org/10.1161/ATVBAHA.113.303041
Venning FA, Wullkopf L, Erler JT (2015) Targeting ECM disrupts cancer progression. Front Oncol 5:224. https://doi.org/10.3389/fonc.2015.00224
Bian T, Jiang D, Liu J, Yuan X, Feng J, Li Q, Zhang Q, Li X, Liu Y, Zhang J (2017) miR-1236-3p suppresses the migration and invasion by targeting KLF8 in lung adenocarcinoma A549 cells. Biochem Biophys Res Commun 492:461–467. https://doi.org/10.1016/j.bbrc.2017.08.074
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60:277–300. https://doi.org/10.3322/caac.20073
Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger K, Yatabe Y, Powell CA, Beer D, Riely G, Garg K, Austin JH, Rusch VW, Hirsch FR, Jett J, Yang PC, Gould M, American Thoracic Society (2011) International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma: executive summary. Proc Am Thorac Soc 8:381–385. https://doi.org/10.1513/pats.201107-042ST
Atsumi T, Chesney J, Metz C, Leng L, Donnelly S, Makita Z, Mitchell R, Bucala R (2002) High expression of inducible 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (iPFK-2; PFKFB3) in human cancers. Cancer Res 62:5881–5887
Ge X, Lyu P, Cao Z, Li J, Guo G, Xia W, Gu Y (2015) Overexpression of miR-206 suppresses glycolysis, proliferation and migration in breast cancer cells via PFKFB3 targeting. Biochem Biophys Res Commun 463:1115–1121. https://doi.org/10.1016/j.bbrc.2015.06.068
Sun CM, Xiong DB, Yan Y, Geng J, Liu M, Yao XD (2016) Genetic alteration in phosphofructokinase family promotes growth of muscle-invasive bladder cancer. Int J Biol Markers 31:e286–e293. https://doi.org/10.5301/jbm.5000189
Minchenko A, Leshchinsky I, Opentanova I, Sang N, Srinivas V, Armstead V, Caro J (2002) Hypoxia-inducible factor-1-mediated expression of the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 (PFKFB3) gene. Its possible role in the Warburg effect. J Biol Chem 277:6183–6187. https://doi.org/10.1074/jbc.M110978200
Obach M, Navarro-Sabate A, Caro J, Kong X, Duran J, Gomez M, Perales JC, Ventura F, Rosa JL, Bartrons R (2004) 6-Phosphofructo-2-kinase (pfkfb3) gene promoter contains hypoxia-inducible factor-1 binding sites necessary for transactivation in response to hypoxia. J Biol Chem 279:53562–53570. https://doi.org/10.1074/jbc.M406096200
Klarer AC, O’Neal J, Imbert-Fernandez Y, Clem A, Ellis SR, Clark J, Clem B, Chesney J, Telang S (2014) Inhibition of 6-phosphofructo-2-kinase (PFKFB3) induces autophagy as a survival mechanism. Cancer Metab 2:2. https://doi.org/10.1186/2049-3002-2-2
Zou Y, Zeng S, Huang M, Qiu Q, Xiao Y, Shi M, Zhan Z, Liang L, Yang X, Xu H (2017) Inhibition of 6-phosphofructo-2-kinase suppresses fibroblast-like synoviocytes-mediated synovial inflammation and joint destruction in rheumatoid arthritis. Br J Pharmacol. https://doi.org/10.1111/bph.13762
Feng Y, Wu L (2017) mTOR up-regulation of PFKFB3 is essential for acute myeloid leukemia cell survival. Biochem Biophys Res Commun 483:897–903. https://doi.org/10.1016/j.bbrc.2017.01.031
Schoors S, De Bock K, Cantelmo AR, Georgiadou M, Ghesquiere B, Cauwenberghs S, Kuchnio A, Wong BW, Quaegebeur A, Goveia J, Bifari F, Wang X, Blanco R, Tembuyser B, Cornelissen I, Bouche A, Vinckier S, Diaz-Moralli S, Gerhardt H, Telang S, Cascante M, Chesney J, Dewerchin M, Carmeliet P (2014) Partial and transient reduction of glycolysis by PFKFB3 blockade reduces pathological angiogenesis. Cell Metab 19:37–48. https://doi.org/10.1016/j.cmet.2013.11.008
De Bock K, Georgiadou M, Schoors S, Kuchnio A, Wong BW, Cantelmo AR, Quaegebeur A, Ghesquiere B, Cauwenberghs S, Eelen G, Phng LK, Betz I, Tembuyser B, Brepoels K, Welti J, Geudens I, Segura I, Cruys B, Bifari F, Decimo I, Blanco R, Wyns S, Vangindertael J, Rocha S, Collins RT, Munck S, Daelemans D, Imamura H, Devlieger R, Rider M, Van Veldhoven PP, Schuit F, Bartrons R, Hofkens J, Fraisl P, Telang S, Deberardinis RJ, Schoonjans L, Vinckier S, Chesney J, Gerhardt H, Dewerchin M, Carmeliet P (2013) Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 154:651–663. https://doi.org/10.1016/j.cell.2013.06.037
Acknowledgements
This study was funded by grants from Six talent peaks project in Jiangsu Province, China (No. WSN-059), the Science Foundation of Nantong City, Jiangsu, China (No. MS12015007), Scientific research topic of Jiangsu provincial health and Family Planning Commission, China (No. H201626), and Key talents of Medical Science in Jiangsu Province, China (No. QNRC2016682).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, X., Liu, J., Qian, L. et al. Expression of PFKFB3 and Ki67 in lung adenocarcinomas and targeting PFKFB3 as a therapeutic strategy. Mol Cell Biochem 445, 123–134 (2018). https://doi.org/10.1007/s11010-017-3258-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-3258-8