Abstract
Nerve growth factor (NGF) is a neurotrophic factor that modulates survival and differentiation of neural stem cells (NSCs). We investigated the function of NGF in promoting growth and neuronal differentiation of NSCs isolated from mouse cochlear tissue, as well as its protective properties against gentamicin (GMC) ototoxicity. NSCs were isolated from the cochlea of mice and cultured in vitro. Effect of NGF on survival, neurosphere formation, and differentiation of the NSCs, as well as neurite outgrowth and neural excitability in the subsequent in vitro neuronal network, was examined. Mechanotransduction capacity of intact cochlea and auditory brainstem response (ABR) threshold in mice were also measured following GMC treatment to evaluate protection using NGF against GMC-induced neuronal hearing loss. NGF improved survival, neurosphere formation, and neuronal differentiation of mouse cochlear NSCs in vitro, as well as promoted neurite outgrowth and neural excitability in the NSC-differentiated neuronal culture. In addition, NGF protected mechanotransduction capacity and restored ABR threshold in gentamicin ototoxicity mouse model. Our study supports a potential therapeutic value of NGF in promoting proliferation and differentiation of NSCs into functional neurons in vitro, supporting its protective role in the treatment of neuronal hearing loss.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurotrophins were recognized as promoters crucial for neuron survival, but they were also found to modulate many other aspects of neuronal function and development, e.g., synaptic plasticity and synapse formation [1–3]. They are secreted as survival factors in order to maintain the balance between the number of innervating neurons and target organ size [4, 5]. There are four different neurotrophins found in mammals: nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4 (NT-4), all of which share similar sequences and structures and hence are thought to evolve from a single gene ancestor [6]. NGF was discovered and characterized as the earliest among all neutrophils and reported to be crucial in maintaining nociceptive sensory and sympathetic neurons in vivo [7, 8].
Neural stem cells (NSCs) are found to be multipotent, self-renewal, proliferative, and with the right cue, they can be differentiated into neurons or glial cells [9]. It was found in recent studies that NSCs become active after neuronal injury and migrate to the injured site to replace the damaged neurons, demonstrating a potential to use NSCs as therapeutic agents in the treatment of neural degeneration or damage [10, 11]. The mechanisms of how NSC self-renew or differentiate, however, remains poorly defined. Primary NSC culture as an in vitro model provides firsthand information in nervous system development. Protocols of how to isolate and expand NSCs in vitro for regenerative therapies were recently developed [9, 12]. NSCs are normally differentiated from embryonic stem cells or other pluripotent stem cells. With stimulation from mitogens, NSCs proliferate and form neurospheres and eventually develop into neural network in vitro [12].
Hair cells, which reside in vertebrate inner ear, are responsible for the functions of both auditory system and the vestibular system. Auditory hair cells in mammals locate in the cochlea. Cochlear and vestibular hair cells are innervated by neurons of auditory or vestibulocochlear nerves. Hair cell damage can cause impaired hearing sensitivity and even complete hearing loss in severe cases [13]. Loss of hearing can be caused by the primary spiral ganglion neuron degeneration or secondary neuron degeneration following hair cell loss. Therefore, the priority of restoring sensory neuronal hearing loss is to replace these neurons. It has been reported that inner ear stem cells could form neurospheres in vitro and later differentiate into both hair cells and neurons [14]. Previous studies found that in vitro NSC-derived neural progenitors could be further differentiated into bipolar neurons and facilitate the outgrowth of neurite and attachment to sensory hair cells when co-cultured with cochlear or vestibular sensory epithelial cells [15, 16]. To improve the technique of using inner ear stem cells to treat hearing loss, further studies on NSC differentiation are needed.
In this study, we investigated the role of NGF in self-renewal and differentiation of cochlear NSCs. We found that in vitro the NSCs’ survival, proliferation, and the ability to form neurosphere were significantly improved by NGF treatment. Additionally, our results suggested that NGF could promote NSCs’ differentiation into neurons and facilitate neural network formation by increasing the complexity of neurite outgrowth and more frequent calcium oscillations. Furthermore, we found in both isolated intact cochlear tissue and animal model that NGF could significantly reduce ototoxicity from gentamicin. Our findings showed the important role of NGF in cochlear NSCs differentiation into functional neurons, therefore supporting their therapeutic potential in treatment of hearing loss.
Materials and methods
Ethical statements
The protocol in this study was approved by the Committee on the Ethics of Animal Experiments and IACUC members of Eye Ear Nose and Throat Hospital, Fudan University. All procedures involving animals in this study were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Sodium pentobarbital anesthesia was used with all efforts made to minimize suffering in all surgeries performed in this study.
Isolation of mouse cochlear NSCs
Early postnatal (P1-3) Balb/c mice were decapitated, followed by dissecting the temporal bone. The temporal bone was removed from the brain and transferred into ice-cold Hank’s balanced salt solution (Invitrogen). After separating the otic capsule from the otic bulla, membranous labyrinth of cochlea is visible. The cochlear ducts (organ of Corti, spiral ligament, and stria vascularis) were carefully separated from the spiral ganglion concentrated modiolus.
Flow cytometry
Isolated mouse cochlear NSCs were stained by antibodies against positive surface markers CD15, CD24, and CD133 and negative surface marker CD44 (eBioscience) [17]. Isotype control antibody-stained cells were used to optimize photo-multiplier tube (PMT) and compensation in the analysis using BD FACScan. Flow cytometric data were analyzed with FlowJo.
Neurosphere formation assay
Previously established neurosphere assay protocol was used [18]. Briefly, 0.125% trypsin/EDTA in phosphate-buffered saline (PBS) was used to treat spiral ganglia for 5 min at 37 °C. A cocktail of 10 mg/ml soybean trypsin inhibitor (Worthington, Lakewood, NJ) and 1 mg/ml DNase I (Worthington) in Dulbecco’s modified Eagle’s medium and Nutrient Mixture F-12 (DMEM/F12; Sigma) was used to neutralize the abovementioned trypsin solution. A homogenous single-cell suspension was obtained by firstly mechanically dissociating with a pipette tip and passing through a 70-μM cell strainer (BD Falcon). The cells were cultured in 2 ml of DMEM/F12 media supplemented with B27 (Invitrogen), N2 (Invitrogen), 50 ng/ml heparan sulfate, 50 ng/ml insulin-like growth factor-1 (IGF-1), 20 ng/ml epidermal growth factor (EGF; Sigma-Aldrich, catalog number SRP3196), 10 ng/ml basic fibroblast growth factor (bFGF) (all growth factors are from Sigma-Aldrich), and 50 μg/ml ampicillin in poly-HEMA-coated suspension culture six-well plates (Sigma). Sphere size was recorded as the maximum sphere diameter. Cells between passages 3 and 5 were used in this study.
NSC differentiation
After 3–5 days of culture, neurospheres were harvested, single-cell dissociated using Accumax (PAA Laboratories), and re-suspended to a concentration of 2 × 104 cells/ml. The cells were then differentiated in 100 μl of DMEM/F12 per well supplemented with N2 and B27, 50 ng/ml NT-3 (both from R&D Systems), 50 ng/ml brain-derived neurotrophic factor (BDNF), and 50 μg/ml ampicillin in 0.1% gelatin-coated 4-well tissue culture plates. Medium change was done every other day in a way that three quarters of the medium was removed and replaced with an equal volume of fresh and pre-warmed medium. Gene expressions of MAP-2 and Nestin were assessed after attachment of single cells to tissue culture plates. Immunofluorescence analysis and Western blot were done after 8–10 days in vitro.
NGF and GMC treatments
NGF (Catalog number SRP4304) used in this study was purchased from Sigma-Aldrich (St. Louis, MO, USA). In this study, NGF was supplemented in all media at the concentrations as previously reported; the same media without NGF were used as control. To ensure concentration of NGF and other growth factors, all media were changed daily. The in vivo ototoxicity mouse model was established by supplying gentamicin (GMC) and NGF into the inner ear according to established procedures [14]. The mice were randomly divided equally into three experimental groups (n = 12 each): (1) Control, 0.5 ml normal saline; (2) GMC, gentamicin 50 mg/kg in 0.5 ml normal saline; and (3) GMC + NGF, gentamicin 50 mg/kg + NGF 5 mg/kg in 0.5 ml normal saline. Briefly, appropriate solutions according to experimental group assignment as described above were filled into the middle ear cavity of the mice. The head of each mouse was maintained in appropriate position for 20 min, in order to make sure that the solution completely diffuses into the cochlea through the round window.
3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) assay
MTT assay was adapted from previously established procedure [19] to assess NSC viability. 5000 cells/100 μl were seeded in each well of a 96-well plate with NGF at indicated concentrations followed by 24 h incubation. 10 μl of 5 mg/ml MTT solution was added to each well to achieve a final concentration of 0.45 mg/ml, followed by approximately 4-h incubation at 37 °C in the dark until a purple precipitate was visible. After incubation, the media with MTT were removed and 100 μl DMSO was added to each well to dissolve formazan crystals. The reduction of MTT was evaluated by absorbance at 570 nm in a microplate reader.
BrdU incorporation assay
Proliferation Assay Kit (Millipore, catalog number 2750) incorporating bromodexyuridine (BrdU), adapted from previously established procedure [19], was used to assess NSC proliferation. 5000/100 μl cells were seeded in each well of a 96-well plate with NGF at indicated concentrations followed by 48 h of incubation. 11 μl of 100 μM BrdU was added to each well to achieve a final concentration of 10 μM at hour 46 of incubation. At the end of 48 h of incubation, the media were removed from the well followed by washing twice with PBS. Cells were fixed by adding 200 µl/well Fixing Solution and incubated at room temperature for 30 min, followed by washing three times with 1× Washing buffer. Cells were then incubated with 100 µl/well anti-BrdU monoclonal antibody for 1 h at room temperature, followed by washing three times with 1× Washing buffer. Cells were then incubated with 100 µl/well 1× peroxidase-labeled goat anti-mouse antibody for 30 min at room temperature, followed by washing three times with 1× Washing buffer. 100 µl of TMB peroxidase substrate was added into each well and incubated for 30 min at room temperature in the dark. The reaction was stopped by adding 100 µl of acid Stop Solution into each well. The incorporation of BrdU was evaluated by an absorbance ratio of 450/550 nm in a microplate reader.
Proliferating cell nuclear antigen (PCNA) assay
PCNA ELISA Kit (Cell Biolabs, catalog number CBA-254), adapted from previously established procedure [19], was used to assess NSC proliferation. 5000/100 μl cells were seeded in each well of a 96-well plate with NGF at indicated concentrations followed by 48 h of incubation. Cells were then washed with PBS and fixed with 3.7% paraformaldehyde for 15 min at room temperature. Cells were then permeabilized with 0.1% Triton X-100 for 10 min and incubated with 100 µl/well anti-PCNA antibody for 1 h at room temperature, followed by washing three times with PBS. Cells were then incubated with 100 µl/well HRP-conjugated secondary antibody for 1 h at room temperature, followed by washing three times with PBS. 100 µl of HRP substrate was added into each well and incubated for 30 min at room temperature in the dark. The reaction was stopped by adding 100 µl Stop Solution into each well. The PCNA staining was evaluated by absorbance at 450 nm in a microplate reader.
Quantitative RT-PCR
RNeasy MiniPrep Kit (Qiagen) was used to extract total RNA from the samples. Superscript II First-Strand Synthesis Kit (Life Technologies) was used to reverse-transcribe 1 μg of total RNA from each sample according to the manufacturer’s instructions. GAPDH was used as an internal control to normalize all data, and the results were presented as relative expression. Primers used in this study were as follows: MAP-2 forward 5′-TTGGTGCCGAGTGAGAAGA-3′, reverse 5′-GTCTGGCAGTGGTTGG-3′; Tuj1 forward 5′-TCAGCGATGAGCACGGCATA-3′, reverse 5′-CACTCTTTCCGCACGACATC-3′; Gata3 forward 5′-CCAAGGCACGATCCAGCACAG-3′, reverse 5′-TGCCGACAGCCTTCGCTTGG-3′; NeuroD1 forward 5′-AAGCCATGAATGCAGAGGAGG-3′, reverse 5′-AGCTGCAGGCAGCCGGCGACC-3′; and GAPDH forward 5′-AGGGCTGCTTTTAACTCTGGT-3′, reverse 5′-CCCCACTTGATTTTGGAGGGA-3′.
Immunofluorescence
Fixation of cells was performed at room temperature in 3.5% paraformaldehyde for 20 min. Permeabilization was performed with 0.2% Triton X-100 in PBS for 10 min, followed by incubation with blocking buffer (2% BSA in PBS, pH 7.4) for 20 min at room temperature. Cells were then stained with antibodies against Tuj1 or GAP-43 (Abcam) in blocking buffer at room temperature for 1 h. After washing, the cells were incubated with rhodamine or FITC-conjugated secondary antibodies (Santa Cruz) for 1 h and imaged using Olympus laser confocal scanning microscope.
Western blot
Sample cells were washed twice with ice-cold 1× PBS followed by lysis in RIPA buffer containing 1 mM Na3VO4, 1 mM PMSF, 150 mM NaCl, 1 mM EDTA, 1 mg/ml aprotinin, 1 mg/ml leupeptin, 1% Nonidet P-40, and 50 mM Tris–HCl (pH 7.4). Cell lysates were separated by electrophoresis on SDS-PAGE gel and transferred to nitrocellulose membrane. Anti-MAP-2 or anti-GAP-43 antibodies (Sigma) were used to immunoblot the membrane for 2 h, followed by incubation of HRP-conjugated secondary antibody (Santa Cruz) for 1 h. ECL detection reagents were used to detect specific bands on the membrane.
Calcium assays
Sample cells were washed twice with standard solutions containing 5 mM KCl, 10 mM HEPES, 150 mM NaCl, 1mM MgCl2, 2 mM CaCl, and 10 mM D-glucose with pH 7.3, followed by adding Pluronic F-127 (Sigma) and 2.5 μM Fluo-4-AM (Dojindo Laboratories) for 45 min of incubation. The Fluo-4-AM solution was then removed and standard solution was added and incubated for another 30 min. Cells were visualized and imaged using a Carl Zeiss scanning confocal microscope. The wavelengths of excitation and emission for Fluo-4-AM were 488 and 510 nm, respectively. The frequency of calcium oscillations was recorded as the number of spikes per minute. The relative fluorescence amplitudes of the calcium spikes were calculated by normalizing the fluorescence of each cell (∆F) to the average fluorescence intensity (F) as ∆F/F.
Cochlear tissue isolation and in vitro culture
Early postnatal (P1-3) Balb/c mice were sacrificed to obtain the cochlear duct as described above. Cochlear tissue isolation was performed according to previously published procedures [20, 21], in which a flat cochlear surface preparation was obtained by removing most basal cochlear segment. The cochlear tissue was then cultured on SPI filter membranes (SPI Supplies) in DMEM-F12 (Invitrogen) basal media supplemented with B27 (Invitrogen), 5 ng/ml EGF (Sigma-Aldrich, catalog number SRP3196), 2.5 ng/ml FGF2 (Sigma-Aldrich, catalog number SRP4038), and 100 U/ml Penicillin (Sigma). All cell cultures were incubated in a 5% CO2/20% O2 humidified incubator.
Hair cell mechanotransduction measurement
The hair cell mechanotransduction measurements were done according to previously published protocol [22, 23]. In brief, cochlear tissues were treated with PBS, 2.5 mM gentamicin, or 2.5 mM gentamicin + 10 ng/ml NGF accordingly for 24 h. Utricles and cochleae were separated and mounted on glass coverslips, and viewed using Axioskop FS upright microscope (Zeiss) equipped with a 633 water immersion objective and differential interference contrast optics. Electrophysiological recordings were performed in solutions containing 5.6 mM D-glucose, 5.8 mM KCl, 0.9 mM MgCl2, 137 mM NaCl, 0.7 mM NaH2PO4, 10 mM HEPES, and 1.3 mM CaCl2, with vitamins (1:100) and amino acids (1:50) as in MEM (Invitrogen) and a pH of 7.4 (311 mOsm/kg). Recording electrodes (2–4 MΩ) were pulled from R-6 glass (King Precision Glass) and filled with a solution containing 0.1 mM CaCl2, 5 mM EGTA-KOH, 2.5 mM MgCl2, 2.5 mM Na2ATP, 5 mM HEPES, and 135 mM KCl, pH 7.4 (284 mOsm/kg). Axopatch 200B (Molecular Devices) was used for utricles, and MultiClamp 700 A amplifier (Molecular Devices) was used for cochleae in patch clamp experiment. Cells were held at a physiologically relevant holding potential of 75 mV. The currents were filtered using a low-pass Bessel filter at 2–5 kHz and digitized using a 12-bit acquisition board (Digidata 1322A or 1440A) at above 20 kHz, and the data were recorded with pClamp 8.2 software (Molecular Devices). A stiff glass probe mounted on a PICMA chip piezo actuator was used to deflect inner hair bundles. The actuator is driven by a 400-mA ENV400 amplifier (Piezosystem Jena) filtered at 10–40 kHz with an 8-pole Bessel filter in order to eliminate residual pipette resonance.
Auditory brainstem response (ABR) threshold measurement
ABR threshold measurements were carried out according to previously published methods [24]. The investigator performing the measurements was blinded to the treatments. Animals were anesthetized using a mixture of ketamine (40 mg/kg) and xylazine (10 mg/kg) on a heating pad before ABR measurement. The reference, ground, and active needle electrodes were inserted beneath the post-measured auricle, the sacrococcygeal region, and the calvaria, respectively. After amplification and filtration, the responses to 1024 click presentations were synchronously averaged. Each stimulus was presented at 100 dB SPL initially. A decreasing stimulus intensity at 5-dB per step was used till ABR waveform becomes not visually discernible. The lowest level of the stimulus that produced a visually detectable response was defined as ABR threshold.
Statistical analysis
Data were presented as mean ± SD. One-way ANOVA was performed for statistical analysis of Figs. 1, 2, 3, and 7 followed by Tukey’s post hoc test, and unpaired Student’s t test was performed for analysis between groups as shown in Figs. 4, 5, and 6; the tests were considered significant when p values are <0.05.
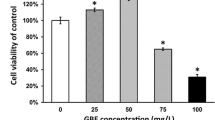
NGF improved viability and proliferation of mouse cochlear NSCs in a dose-dependent manner. Cell viability and proliferation of the mouse cochlear NSCs, followed by increasing concentrations of NGF treatment (0, 0.1, 1, 10, and 50 ng/ml), were measured by MTT (a), BrdU incorporation (b), and PCNA staining (c) assays, respectively. Data were presented as mean ± SD as percentage of control (0 ng/ml NGF). *p < 0.05, **p < 0.01, compared to 0 ng/ml NGF as control
NGF improved the capability of neurosphere formation. Mouse cochlear NSCs were incubated in the presence or absence of 10 ng/ml NGF treatment, and the number of neurospheres/104 cells (a) and sphere diameter (b) were measured at indicated time points. Data were presented as mean ± SD as percentage of control (0 ng/ml NGF at day 1). *p < 0.05, **p < 0.01, compared to control at respective time points
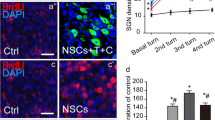
NGF improved the differentiation of mouse cochlear NSCs into neurons in a dose-dependent manner. a Density of neurons in culture was measured after 2 weeks of NGF treatment (0, 0.1, 1, 10, and 50 ng/ml) and normalized to 0 ng/ml NGF control. b Representative images of neuron culture after 2 weeks of NGF treatment (0 and 10 ng/ml), with Tuj1 stained in red and DNA stained by DAPI in blue; scale bar 100 μM. c, d Expressions of MAP-2 mRNA (c) and protein (d) were measured by RT-PCR and Western blot, respectively, after 2 weeks of NGF treatment (0, 0.1, 1, 10, and 50 ng/ml). Data were presented as mean ± SD as percentage of control (0 ng/ml NGF). ns not significant, *p < 0.05, **p < 0.01, compared to 0 ng/ml NGF as control
NGF promoted neurite outgrowth. a Differentiated mouse cochlear NSCs were treated for 2 weeks in the presence or absence of 10 ng/ml NGF treatment, and the expression of GAP-43 protein was examined by Western blot. b Representative images of neurons after 2 weeks of NGF treatment (0 and 10 ng/ml), with GAP-43 stained in red and DNA stained by DAPI in blue; scale bar 50 μM. c–e Number of primary dendrites per cell (c), number of dendritic end tips (d), and neurite length (e) were also measured. Data were presented as mean ± SD as percentage of control (0 ng/ml NGF). ns not significant, *p < 0.05, **p < 0.01, compared to 0 ng/ml NGF as control
NGF enhanced neural excitability. Following 2 weeks of neuronal differentiation of mouse cochlear NSCs in the presence or absence of 10 ng/ml NGF treatment, change in Ca2+ concentration (a), percentage of cells with Ca2+ oscillations (b), and frequency of the oscillations (c) were measured. Data were presented as mean ± SD. *p < 0.05, **p < 0.01, compared to 0 ng/ml NGF as control at respective time points
NGF protected mechanotransduction capacity of isolated mouse cochlear tissue against GMC challenged ototoxicity. Mechanotransduction currents were recorded from the mid apex segment of cochlear tissue bathed in 1.3 mM Ca2+, after treatments with control (0 ng/ml NGF), GMC (2.5 mM gentamicin), and GMC + NGF (2.5 mM gentamicin + 10 ng/ml NGF), respectively
Results
NGF improved viability and proliferation of mouse cochlear NSCs in vitro
First of all, we isolated NSCs from mouse cochlea and confirmed its neural stem cell identity using flow cytometry (Fig. S1). Next, we investigated the effect of NGF on the NSC culture in vitro. The NSCs were treated with increasing concentrations of NGF (0, 0.1, 1, 10, and 50 ng/ml) for 24 h, followed by examination of their viability by MTT assay, as well as proliferation capacity by BrdU incorporation and PCNA assays (Fig. 1), respectively. As indicated in Fig. 1a, viability of the isolated NSCs was steadily and significantly increased by NGF treatment from 0 to 10 ng/ml, whereas 50 ng/ml NGF caused a slight decrease than 10 ng/ml. In the BrdU incorporation and PCNA assays (Fig. 1b, c), we found that the proliferative capacity of the cultured NSCs exhibited a very similar pattern as that in MTT assay. 10 ng/ml NGF displayed the highest effect in improving proliferation of NSCs, whereby 50 ng/ml NGF also resulted in a slight reduction, nevertheless still higher than that of untreated control culture. The above results suggested that NGF improved viability and proliferation of mouse cochlear NSCs in a dose-dependent manner, with the exception of 50 ng/ml; therefore, the optimal dosage of NGF seemed to be 10 ng/ml in our experimental setup.
NGF improved neurosphere formation capacity
We next explored if NSC neurosphere formation is affected by 10 ng/ml NGF. Neurosphere formation assay was carried out for a period of 7 days in neural stem cell basal medium supplemented with all other growth factors, in the presence or absence of 10 ng/ml NGF, respectively. In this study, we found that neurospheres treated with NGF showed significantly more cell number compared with non-NGF-treated control since day 1 of incubation, and this difference continued to widen with prolonged culture (Fig. 2a). We also found that the diameter of NGF-treated neurospheres increased faster compared to non-NGF-treated control, especially after longer exposure (Fig. 2b). Together, our results suggested that the capability of neurosphere growth and formation was improved by NGF treatment.
NGF improved neuronal differentiation of mouse cochlear NSCs
To investigate the effects of NGF on the neuronal differentiation of isolated NSCs, we differentiated the NSCs in the presence of increasing concentrations of NGF (0, 0.1, 1, 10, and 50 ng/ml). Following 2 weeks of differentiation, density of derived neurons was measured using the neuron-specific cytoskeletal protein microtubule-associated protein 2 (MAP-2) as a marker. As expected, we observed a steadily increasing neuronal density, with NGF treatment from 0 up to 10 ng/ml in the culture (Fig. 3a, b). This observation was confirmed by examination of both mRNA and protein expressions of MAP-2 (Fig. 3c, d), whose levels exhibited the exact trend with the increasing neuronal density. In addition, the identity of the differentiated neurons was further indicated by the expression of markers such as Tuj1, Gata3, and NeuroD1, all of which were also increased by NGF treatment (Fig. S2). Of note, in all of the above experiments, 50 ng/ml NGF treatment consistently caused a slight decline in terms of neuronal density and MAP-2 expression, but it was still significantly higher than that for untreated cells. Therefore, 10 ng/ml NGF was chosen as an optimal dose for the rest of our in vitro investigations.
NGF promoted neurite outgrowth in NSC-differentiated neuronal culture
We then investigated whether NGF could also affect neurite outgrowth by assessing dendritic tree formation. In this context, we started by examining a growth-associated protein 43 (GAP-43) which is associated with neurite outgrowth as well as a typical marker for the presence of neuronal growth cones [25]. GAP-43 protein level was found to be greatly increased in the NSC-derived neuron culture following NGF treatment, compared to control-treated culture (Fig. 4a, b), indicating that NGF treatment was likely to promote neurite outgrowth. To confirm this observation, we next examined the morphological characteristics of the NSC-derived neuron culture, in terms of the number of primary dendrites, dendritic end tips, and neurite length. Although the primary dendrite number was essentially the same between control and NGF-treated neurons (Fig. 4c), the number of dendritic end tips and the average length of the neurite were indeed significantly increased by NGF treatment than those of the control group (Fig. 4d, e), which confirmed our earlier speculation that NGF treatment was able to promote neurite outgrowth.
NGF enhanced neural excitability in NSC-differentiated neuronal culture
Ca2+ is essential in receiving and transmitting neural signals, as well as in regulating excitability and the changes that underlie synaptic plasticity. In order to explore how NGF regulates calcium signaling in NSC-derived neuronal culture, calcium oscillations were examined by Fluo-4-AM staining, and the baseline spike was recorded by rhythmic phenomenon of spontaneous Ca2+ oscillation [26]. Overall concentration changes of Ca2+ were assessed, and we found that NGF-treated neuronal culture showed significantly higher changes than control (Fig. 5a). A significantly higher percentage of neurons exhibiting Ca2+ (Fig. 5b) and higher frequency of spontaneous calcium spikes (Fig. 5c) in NGF group could be the reasons underlying the higher concentration change of Ca2+. Overall, we found that NGF could increase the neural excitability in NSC-differentiated neuronal culture.
NGF protected mechanotransduction capacity of isolated mouse cochlear tissue against gentamicin ototoxicity
To further investigate whether NGF exerts protective effect against ototoxicity, we isolated intact mouse cochlea and divided them into three experimental groups: control, GMC, and GMC + NGF (see “Materials and methods” section), followed by measuring their mechanosensory functions. As shown in Fig. 6a, the isolated mouse cochlear tissue exhibited prominent mechanotransduction response currents, which was significantly attenuated by GMC treatment (Fig. 6b). Importantly, the cochlea simultaneously treated with GMC + NGF exhibited transduction response currents at similar levels as that of the control, which clearly demonstrated the role of NGF to protect mechanotransduction capacity of ex vivo cultured mouse cochlea from gentamicin ototoxicity.
NGF restored ABR threshold in gentamicin ototoxicity mouse model
Auditory brainstem response (ABR) threshold is an in vivo quantitative measure used for evaluating conductive hearing loss from sensorineural impairment [24], and the measurement was performed in our study to assess the effect of NGF on protecting gentamicin-induced hearing loss in the mouse model. Mice were divided into three experimental groups: control, GMC, and GMC + NGF (see “Materials and methods” section, and the investigator performing the measurements was were blinded to treatments), and their respective ABR thresholds were measured. We found that the ABR threshold in GMC group was significantly reduced compared to the control group, whereas in GMC + NGF group it was restored back to the level comparable to the control animals (Fig. 7). This result further demonstrated that NGF was able to protect against gentamicin-induced hearing loss in an in vivo animal model.
Discussion
In the current study, using in vitro cultured NSC model, we found that NGF was able to improve viability and proliferation of the cultured cochlear NSCs in a dose-dependent manner. Furthermore, NGF was also found to significantly improve the capacity of neurosphere formation, defined as free-floating clusters of in vitro cultured NSCs [27], throughout the duration of a week-long culture. Stem cells, and particularly NSCs in our study, possess not only the ability to self-renew but also the potential to differentiate into functional neurons [28]. In line with this, we also assessed the effect of NGF on the neuronal differentiation of the in vitro cultured mouse cochlear NSCs. As expected, NGF treatment markedly enhanced the differentiation potential of NSCs into mature neurons, as indicated by increased number of neuron cells in the culture under differentiating conditions, and elevated expression of neuronal marker MAP-2, dose dependently. Following differentiation, continued treatment with NGF further promoted neurite outgrowth, as evidenced by elevated expression of neuronal growth marker protein GAP-43 [25], as well as increased complexity of the dendritic tree in terms of primary dendrite number, dendritic end tips, and neurite length [29]. Apart from the above morphological changes, we also evaluated calcium signaling as the indicator of the overall excitability in the in vitro cultured neural network, and found that NGF greatly enhanced cellular calcium concentration and oscillation.
Neurotrophins have been previously implicated in the prevention of ototoxicity. In cultured organ of Corti isolated from deaf mice, exogenous neurotrophins could affect the synaptogenesis of inner hair cells [30]. In the same report, of particular interest to our current study, NGF was found to promote stable neurosensory interactions, which is consistent with our result that NGF protected mechanotransduction capacity of isolated mouse cochlear tissue ex vivo from gentamicin-induced ototoxicity. Involvement of NGF in auditory neuronal degeneration has been reported previously, where its serum level is reduced in patients with sensorineural hearing impairment [31]. In our current study, using a mouse model of gentamicin-induced ototoxicity, simultaneous treatments of NGF together with gentamicin almost completely restored conductive hearing capacity. This result confirmed our hypothesis that NGF possessed the potential to protect from gentamicin-induced hearing loss in vivo.
To summarize, the results in our study demonstrated that NGF exhibited significant effect on promoting viability and neuronal differentiation capacity of NSCs isolated from mouse cochlea in vitro culture. Most importantly, data using three different models consistently supported the role of NGF in enhancing the excitability of NSC-derived neuronal network in vitro, protected mechanotransduction of isolated mouse cochlea ex vivo, and prevented hearing loss from gentamicin ototoxicity in vivo. In conclusion, our study provided the first report on the potential of exogenous NGF as a therapeutic agent, in the form of external administration, to treat aminoglycoside-induced hearing loss. In conclusion, our study reveals for the first time the potential of using exogenous NGF as a therapeutic agent in the treatment of aminoglycoside-induced hearing loss.
References
Korsching S (1993) The neurotrophic factor concept: a reexamination. J Neurosci 13:2739–2748
Lewin GR, Barde YA (1996) Physiology of the neurotrophins. Annu Rev Neurosci 19:289–317. doi:10.1146/annurev.ne.19.030196.001445
Sofroniew MV, Howe CL, Mobley WC (2001) Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci 24:1217–1281. doi:10.1146/annurev.neuro.24.1.1217
Huang EJ, Reichardt LF (2001) Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24:677–736. doi:10.1146/annurev.neuro.24.1.677
Reichardt LF (2006) Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci 361:1545–1564. doi:10.1098/rstb.2006.1894
Hallbook F (1999) Evolution of the vertebrate neurotrophin and Trk receptor gene families. Curr Opin Neurobiol 9:616–621. doi:10.1016/S0959-4388(99)00011-2
Levi-Montalcini R (1987) The nerve growth factor 35 years later. Science 237:1154–1162
Northcutt RG (1989) Body and Brain. A Trophic Theory of Neural Connections. Dale Purves. Harvard University Press, Cambridge, MA, 1988. viii, 231 pp., illus. $35. Science 244:993. doi:10.1126/science.244.4907.993
Gage FH (2000) Mammalian neural stem cells. Science 287:1433–1438
Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M (2002) Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell 110:429–441
Russo I, Barlati S, Bosetti F (2011) Effects of neuroinflammation on the regenerative capacity of brain stem cells. J Neurochem 116:947–956
Chaddah R, Arntfield M, Runciman S, Clarke L, van der Kooy D (2012) Clonal neural stem cells from human embryonic stem cell colonies. J Neurosci 32:7771–7781
Yuan Y, Wang Y, Chi F (2014) Reinnervation of hair cells by neural stem cell-derived neurons. Chin Med J (Engl) 127:2972–2976
Hu Z, Wei D, Johansson CB, Holmstrom N, Duan M, Frisen J, Ulfendahl M (2005) Survival and neural differentiation of adult neural stem cells transplanted into the mature inner ear. Exp Cell Res 302:40–47
Coleman B, Fallon JB, Pettingill LN, de Silva MG, Shepherd RK (2007) Auditory hair cell explant co-cultures promote the differentiation of stem cells into bipolar neurons. Exp Cell Res 313:232–243
Matsumoto M, Nakagawa T, Higashi T, Kim TS, Kojima K, Kita T, Sakamoto T, Ito J (2005) Innervation of stem cell-derived neurons into auditory epithelia of mice. Neuroreport 16:787–790
Panchision DM, Chen HL, Pistollato F, Papini D, Ni HT, Hawley TS (2007) Optimized flow cytometric analysis of central nervous system tissue reveals novel functional relationships among cells expressing CD133, CD15, and CD24. Stem Cells 25:1560–1570. doi:10.1634/stemcells.2006-0260
Oshima K, Senn P, Heller S (2009) Isolation of sphere-forming stem cells from the mouse inner ear. Methods Mol Biol 493:141–162. doi:10.1007/978-1-59745-523-7_9
Yadav K, Singhal N, Rishi V, Yadav H (2001) Cell Proliferation Assays. In: eLS. Wiley, Hoboken. doi:10.1002/9780470015902.a0002566
Chen P, Johnson JE, Zoghbi HY, Segil N (2002) The role of Math1 in inner ear development: uncoupling the establishment of the sensory primordium from hair cell fate determination. Development 129:2495–2505
Doetzlhofer A, Basch ML, Ohyama T, Gessler M, Groves AK, Segil N (2009) Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti. Dev Cell 16:58–69. doi:10.1016/j.devcel.2008.11.008
Kawashima Y, Geleoc GS, Kurima K, Labay V, Lelli A, Asai Y, Makishima T, Wu DK, Della Santina CC, Holt JR, Griffith AJ (2011) Mechanotransduction in mouse inner ear hair cells requires transmembrane channel-like genes. J Clin Invest 121:4796–4809. doi:10.1172/JCI60405
Pan BF, Geleoc GS, Asai Y, Horwitz GC, Kurima K, Ishikawa K, Kawashima Y, Griffith AJ, Holt JR (2013) TMC1 and TMC2 are components of the mechanotransduction channel in hair cells of the mammalian inner ear. Neuron 79:504–515. doi:DOI:10.1016/j.neuron.2013.06.019
Qin Z, Wood M, Rosowski JJ (2010) Measurement of conductive hearing loss in mice. Hear Res 263:93–103. doi:10.1016/j.heares.2009.10.002
Meiri KF, Pfenninger KH, Willard MB (1986) Growth-associated protein, GAP-43, a polypeptide that is induced when neurons extend axons, is a component of growth cones and corresponds to pp46, a major polypeptide of a subcellular fraction enriched in growth cones. Proc Natl Acad Sci USA 83:3537–3541
Thomas AP, Bird GS, Hajnoczky G, Robb-Gaspers LD, Putney JW Jr (1996) Spatial and temporal aspects of cellular calcium signaling. FASEB J 10:1505–1517
Pacey L, Stead S, Gleave J, Tomczyk K, Doering L (2006) Neural stem cell culture: neurosphere generation, microscopical analysis and cryopreservation. Protocol Exchange. doi:10.1038/nprot.2006.215
Hu Z, Ulfendahl M, Olivius NP (2005) NGF stimulates extensive neurite outgrowth from implanted dorsal root ganglion neurons following transplantation into the adult rat inner ear. Neurobiol Dis 18:184–192
Urbanska M, Blazejczyk M, Jaworski J (2008) Molecular basis of dendritic arborization. Acta Neurobiol Exp 68:264–288
Sobkowicz HM, August BK, Slapnick SM (2002) Influence of neurotrophins on the synaptogenesis of inner hair cells in the deaf Bronx waltzer (bv) mouse organ of Corti in culture. Int J Dev Neurosci 20:537–554
Salvinelli F, Casale M, Greco F, Trivelli M, Di Peco V, Amendola T, Antonelli A, Stampachiacchiere B, Aloe L (2002) Nerve growth factor serum level is reduced in patients with sensorineural hearing impairment: possible clinical implications. J Biol Regul Homeost Agents 16:176–180
Funding
This work was supported by grant from the National Natural Science Foundation of China (NSFC, 81371093) to Zhao Han.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving animal rights
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed consent
Not applicable.
Additional information
Zhao Han and Cong-Pin Wang have contributed equally to this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Han, Z., Wang, CP., Cong, N. et al. Therapeutic value of nerve growth factor in promoting neural stem cell survival and differentiation and protecting against neuronal hearing loss. Mol Cell Biochem 428, 149–159 (2017). https://doi.org/10.1007/s11010-016-2925-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2925-5