Abstract
The microbial polyketide, 2, 4-diacetylphloroglucinol (DAPG), exhibited a broad-spectrum of anti-leukemic, anti-lung, and anti-breast cancer properties. The aim of the present investigation was to study the interactive potentials of DAPG with the metastatic proteins such as MMP-2, MMP-9, and NF-κB and antiapoptotic Bcl-2 family proteins such as Bcl-2, Bcl-w, and Bcl-xL through in silico interaction and in vitro studies. The in silico modeling predicted high interactions of DAPG with the metastatic proteins, especially MMP-2, MMP-9, and NF-κB with the glide score of −7.028, −6.304, and −5.231, respectively. Similarly, the DAPG had weak interactions with the antiapoptotic Bcl-2, Bcl-w, and Bcl-xL with the glide score of −4.505, −3.839, and −4.003, respectively. The interaction studies further revealed the inhibition of MMP-2, MMP-9, and NF-κB activities with the low IC50 concentration of 5.82 ± 1.6, 6.74 ± 1.2, and 10.7 ± 1.5 μM respectively, in the presence of DAPG. Similarly, DAPG inhibited the Bcl-2, Bcl-xL and Bcl-w activities with the high IC50 concentration of 29.8 ± 1.9, 85.9 ± 2.7, and 97.4 ± 1.5 μM, respectively. These results correlate with the relatively high IC50 concentration of 16.3 ± 1.76, 7.67 ± 0.78, and 10.7 ± 0.96 μM in the Bcl-2-overexpressing HL-60, K562 and Raji leukemic cells than the metastatic A549 and MDA MB-231 cancer cells with the low IC50 concentration of 0.06 ± 0.02 and 0.08 ± 0.01 μM, respectively, compared to the healthy, human embryonic kidney (HEK-293) cells with the high IC50 concentration of 54.7 ± 1.43 μM. In summary, the affinity of DAPG with proteins are in the order of MMP-2 > MMP-9 > NF-κB > Bcl-2 > Bcl-xL > Bcl-w. Results presented in this study confirmed the high interaction of DAPG with the metastatic proteins than the antiapoptotic Bcl-2 family proteins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metastatic and antiapoptotic mechanisms are the two important hallmarks of cancer, which contribute to the poor prognosis in the treatment of cancer [1–3]. Metastasis is the complex and co-ordinated process where cancer cells detach due to the lack of nutrients and space from the primary tumor sites, enter the blood stream, invade the other tissues, and establish a colony in secondary sites [1, 4]. These series of steps involve the expression of genes that mediate the survival and invasion of cancer cells. The expression of matrix metalloproteinases (MMPs) especially the MMP-2 and -9 is crucial for the invasion, which is regulated by hyperactivation of NF-κB. The activation of NF-κB also regulates the expression of other antiapoptotic proteins and protein kinases in the cancer cells [4–6]. Resistance to antigrowth stimuli is the major hallmark of cancer cells which is due the overexpression of antiapoptotic Bcl-2 family proteins such as Bcl-2, Bcl-xL, and Bcl-w. The overexpression of Bcl-2 family proteins also contributes to the resistance and recurrence of cancer [7–10]. The drugs that interfere with cancer cell survival, metastasis, inflammation, and induction of apoptosis are of great importance [10–13].
The microbial polyketide, DAPG, has been reported to exhibit antiproliferative, proapoptotic, antimetastatic, and anti-inflammatory potential in the cancer and LPS-inflamed immune cells through blockade of NF-κB activity [14]. However, the interactive potential of DAPG with metastatic proteins such as MMP-2, MMP-9, NF-κB, and antiapoptotic Bcl-2 family members such as Bcl-2, Bcl-w, and Bcl-xL is not known yet. In order to understand the mode of action, the present investigation was aimed to determine the interaction of DAPG with cancer-related proteins. In the present investigation, for the first time, we reported the high interaction of DAPG with the metastatic proteins than the antiapoptotic proteins.
Materials and methods
Production and purification of DAPG
The DAPG was extracted and purified from Pseudomonas aeruginosa FP10 as described [14], dissolved in dimethyl sulfoxide (DMSO; 20 %), and stored at −20 °C until further use.
Cell culture
The leukemic cell lines such as acute myeloid leukemic (HL-60), chronic myeloid leukemic (K562), and acute lymphocytic leukemic (Raji) cells of passage number between 21 and 45, metastatic A549 (passage number 45–69), and MDA MB-231 (passage number 43–72) cancer cell lines and non-cancer human embryonic kidney (HEK-293) cells were obtained from National Centre for Cell Science (NCCS), India, and maintained in RPMI supplemented with 20 % FBS, 10 U ml−1 penicillin, and 10 mg l−1 streptomycin. Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized venous blood by standard Histopaque-1077 (Sigma, USA) gradient centrifugation technique. Briefly, the blood (10 ml) collected from healthy volunteers was mixed gently with heparin and carefully layered over Ficoll gradient (10 ml) and centrifuged at 2000 rpm for 30 min at room temperature. The interface buffy layer containing PBMCs was collected and washed twice with the RPMI medium without serum by centrifugation at 1500 rpm for 5 min. The isolated PBMCs were suspended in RPMI with serum and counted in hemocytometer using trypan blue staining.
In silico interactions
The ligand coordinates of DAPG (CID 16547) were retrieved from PubChem and prepared using Ligprep module of Schrodinger Maestro by applying OPLS-2005 force field at target pH 7.0 ± 2.0 with possible ionization states generated by Ionizer [15–17]. At most 32 tautomers and 10 low-energy ring confirmations were generated for DAPG retaining its chiralities. The protein coordinates for Bcl-2 (4AQ3), Bcl-xL (2YQ6), Bcl-w (2Y6W), MMP-2 (1HOV), MMP-9 (1GKC), and NF-κB p65 (1VKX) were retrieved from RCSB Protein Data Bank (PDB). A grid was generated using Receptor Grid Generation by enclosing “NWGR” sequence, which is highly conserved signature sequence of Bcl-2 family members with a default Vander Waals radius scaling factor of 1.0 and a partial charge cut-off of 0.25. For MMPs proteins, the grid generation was done based on the ligand binding. Then, the flexible docking followed by post-docking minimisation of DAPG with the proteins was performed with extra precision (XP) mode, and the protein–ligand complex with highest docking energy, glide energy, and lowest potential energy was merged and taken as the complex for further analysis. The hydrogen bond and hydrophobic interactions of complex structures were visualised by Chimera.
Protein–DAPG interactions
Fluorescence polarisation assay (FPA)
The Bid BH3, peptide II, and TAMRA-labeled 5-TAMRA-EDIIRNIARHLAQVGDSMDR were obtained from Ana Spec EGT group, USA, and FPA assay was performed as described [10]. The Bcl-2, Bcl-w, and Bcl-xL proteins of 100 nM were pre-incubated with different concentrations of DAPG (47.5 µl in PBS) in 96-well V-shaped black plates at room temperature for 15 min. Then, 2.5 µl of 50 nM of labeled peptide was added, while the control had DMSO (0.01 %) and negative controls had only proteins. Then, the mixtures were incubated at room temperature for 30 min and the polarisation potential was measured at excitation of 480 nm and emission of 520 nm in the Spectromax 190 reader. The IC50 concentration for each protein was calculated using GraphPad Prism 6.0 software.
NF-κB binding assay
The NF-κB p65 protein (100 nM; Sigma, USA) was incubated with the different concentrations of DAPG (1, 2, 5, 10, 25, and 50 µM) for 30 min at 37 °C. Then, the NF-κB binding assay was performed using NF-κB (p65) transcription assay kit (Cayman chemicals, USA) according to the instructions of manufacturer.
Measurement of MMP-2 and MMP-9 activity
MMP-2 (gelatinase-A) and MMP-9 (gelatinase-B) proteins (100 nM; Sigma, USA) were incubated with different concentrations of DAPG (1, 2, 5, 10, 25, and 50 µM) for 30 min at 37 °C. Then, the activity of MMP-2 and MMP-9 was measured using Sensolyte 520 MMP-2 and MMP-9 fluorimetric assay kit (AnaSpec, USA), respectively, according to the instructions of manufacturer.
In vitro validations
Cytotoxicity activity
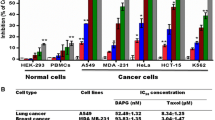
MTT reduction assay was employed to measure the cytotoxic activity of DAPG [14]. Briefly, leukemic (HL-60, Raji, and K562), lung (A549), and breast (MDA MB-231) cancer and normal cells (human peripheral blood mononuclear cells—PBMCs, isolated by Ficoll gradient centrifugation) and human embryonic kidney (HEK-293) cells were seeded at a density of 1 × 104 cells ml−1 in 96-well plates and incubated for 24 h in a CO2 incubator. After incubation, different concentrations of DAPG dissolved in DMSO (0–100 μM) were treated in octaplates. After 48 h of incubation, the cells were treated with MTT (10 µl, 5 mg ml−1) at 37 °C for 4 h. Then, the medium was aspirated after pelleting of cells by centrifugation and the intracellular formazan crystals formed were dissolved in DMSO for 15 min. The plates were read at 590 nm on a scanning multiwell spectrophotometer and IC50 values (concentration of DAPG to inhibit 50 % of cells) were determined using non-linear regression curve fit method in GraphPad Prism version 5.0 software.
Immunoblotting
The control and DAPG-treated leukemic, A549, MDA MB-231, and HEK-293 cells were pelleted, washed once with PBS, and lysed in the RIPA buffer at 4 °C for 30 min. Then, the cell lysates were centrifuged at 12,000 rpm for 15 min at 4 °C and the supernatant was collected. The protein (100 μg) was resolved in SDS-PAGE (10–12 %) and electroblotted onto the PVDF membranes. The BSA (1 %) was used as a blocking agent and incubated with the primary antibodies for 1 h at room temperature. The primary antibodies were detected by secondary antibody conjugated with alkaline phosphatase system.
In vitro invasion assay
In vitro invasion assay was performed using transwell cell culture chambers as described [14]. Briefly, the A549 and MDA MB-231 cancer cells at a density of 1 × 105 cells ml−l were cultured in 12-well plates until >98 % confluence. The cells were treated with DMSO (control), with respective IC50 concentration of DAPG, and incubated for 48 h in a CO2 incubator at 37 °C. After incubation, the cells were trypsinized, suspended in the serum-free medium, and seeded at a density of 1 × 106 cells ml−l in the upper chamber lined with 8-μm-pore-size polycarbonate filters which were pre-coated with matrigel (50 µM) and the lower chamber contained media with serum. The cells were incubated for 24 h in a CO2 incubator. After incubation, the top side of the membrane insert was scrubbed with a cotton swab to remove uninvaded cells and the bottom side was fixed with 4 % formaldehyde and stained with crystal violet (0.5 % crystal violet in 20 % methanol) for 15 min. The crystal violet dye retained on the filter was extracted with DMSO, the absorbance at 595 nm was measured in the spectrophotometer, and the results were expressed as percent inhibition of invasion.
Measurement of MMP-2 and MMP-9 activity
MMP-2 (gelatinase-A) and MMP-9 (gelatinase-B) activities in the media obtained from DMSO-treated control and the IC50 concentration of DAPG-treated A549 and MDA MB-231 cancer cells were measured using Sensolyte 520 MMP-2 and MMP-9 fluorimetric assay kit (AnaSpec, USA) according to the instructions of manufacturer.
Statistical analysis
Statistical analysis, ANOVA test with the P < 0.05 (*) levels of significance, and interpolation of results of the three independent experiments were performed using GraphPad Prism software. The graphs were plotted using Origin pro-8 software.
Results
In silico interaction
The MMP family consists of 23 structurally related zinc-dependent endopeptidases that includes hydrophobic signaling peptide, propeptide domain, highly conserved catalytic domain with zinc binding site, and a hemopexin-like C-terminal domain (PEX) linked to the catalytic domain via flexible hinge region. MMPs play crucial roles in invasion and metastasis and also regulate signaling pathways that control cell growth, survival, invasion, inflammation, and angiogenesis [3–6]. Molecular docking of DAPG with MMPs has predicted the relatively high interactions of DAPG with MMP-2 with a glide score of −7.028 and a glide energy of −30.036 kJ mol−1. The carboxyl functional group and hydroxyl group of DAPG form H-bond with ‘O’ atom of THR-143 and ‘H’ of ARG-149 with the bond distance of 2.185 and 2.075 Å, respectively. Similarly, DAPG had a glide score of −6.304 and a glide energy of −35.888 kJ mol−1 with MMP-9. In the case of MMP-9, the ‘O’ atom of LEU-188, ‘H’ atom of ALA-189, and ‘H’ atom of GLU-402 residue form H-bond with atoms ‘H,’ ‘O,’ and ‘H’ of three hydroxyl groups of DAPG with the H-bond distance of 2.132, 1.778, and 1.554 Å, respectively (Fig. 1a; Table 1).
NF-κB family exist as p65/p50 hetero-dimer in association with IKβα in inactive state. Thus, nuclear translocation of p65/p50 is the marker of NF-κB activation in the cells. These NF-κB/Rel transcription factors recognise highly conserved sequence of 5′-GGGrNYYYCC-3′ called as κB DNA binding sequence through Rel homology domain (RHD). The docking of DAPG with the p65 RHD domain showed the glide score of −5.231 and the glide energy of −23.155 kJ mol−1. The hydroxyl group of DAPG forms H-bonds with the ‘O’ of ARG-35 and ARG-33 with the H-bond distance of 1.64 and 1.96 Å, respectively, present in the RH domain.
Bcl-2 family proteins are a group of evolutionarily conserved regulatory factors in apoptosis. Based on the function and structure, the Bcl-2 family members are of two types: antiapoptotic proteins (Group-I) such as Bcl-xL, Mcl-1, and Bcl-w that inhibit proapoptotic proteins; all of these proteins have four short Bcl-2 homology (BH) domains—BH1–4 and proapoptotic proteins (Group-II) such as Bad, Bax, Bak, Bcl-xS, Bid Bik, and Bim that induce apoptosis; all of these proteins have only BH3 domains. Mutation/altered expression of pro- and antiapoptotic proteins can drastically alter drug response in the experimental systems. Many members of Bcl-2 family proteins have a conserved C-terminal transmembrane region that localise proteins to the overleaflet of nuclear envelope, outer mitochondrial membrane, and endoplasmic reticulum. An important feature of the Bcl-2 family proteins is that they can form homo/heterodimers, suggesting the neutralization of competition between these proteins, and they function either independently or together in the regulation of apoptosis. In the absence of death stimuli, the proapoptotic members can change their location within cells and undergo various pre- and post-translational modifications. In response to death signals, the cytosolic proapoptotic proteins (Bax, Bad, and Bid) change conformations, integrate into the outer mitochondrial membrane, and neutralize the antiapoptotic family members (Bcl-2, Bcl-xL, and Mcl-1). In mitochondria, antiapoptotic proteins form ion channels and help maintain mitochondrial space. In addition, Bcl-2 and Bcl-xL block apoptosis by preventing cytochrome-C release through a direct effect on mitochondrial channel such as voltage-dependent anion channel (VDAC) or through permeability transmembrane pore complex (PTPC). Structural studies of Bcl-2 family proteins have revealed two central predominant hydrophobic 7-α-helices amphiphatic in nature. The BH3 domain is responsible for mediating the interactions with antiapoptotic proteins and the ability of the proteins to promote programmed cell death [9–11]. The signature ‘‘NWGR’’ sequence, which is highly conserved among Bcl-2 family members, directly precedes α5 helices. Molecular docking studies of DAPG with the protein and ‘‘NWGR’’ sequence of BH3 domain revealed that the DAPG lies in the hydrophobic groove of Bcl-2 and interacts with BH3 binding domain, which mediates the interaction with programmed cell death promoting proteins. In this BH3 domain, the ‘O’ atom of ALA-59 residue forms H-bond with ‘H’ of hydroxyl group in DAPG, showing a bond distance of 2.011 Å. The glide score and glide energy of Bcl-2 and DAPG complex were −4.505 and −21.686 kJ mol−1, respectively.
Similarly, the glide score and glide energy of, respectively, −4.003 and −18.820 kJ mol−1 were observed in case of Bcl-xL and DAPG complex. In this complex ‘O’ atom of ALA-93, ‘HH’ atom of TYR-101, and ‘O’ atom of GLY-138 shows hydrogen bond with ‘H,’ ‘O,’ and ‘H’ atoms of three hydroxyl groups present in DAPG with a bond distance of 2.133, 2.337, and 1.856 Å, respectively. In the same way, three H-bonds were shown by Bcl-w and DAPG complex, between the ‘HH22′ atom of ARG-56, ‘O’ atom of GLY-94, ‘OH’ atom of TYR-151, and ‘H,’ ‘O,’ and ‘H’ atoms of hydroxyl group present in DAPG with a bond distance of 2.271, 2.173, and 1.822 Å, respectively. The glide score was −3.839, the whereas glide energy was −22.994 kJ mol−1 (Fig. 2; Table 1). The inhibition of metastatic and antiapoptotic proteins might have contributed to the antimetastatic and apoptosis-inducing effects of DAPG. Further, the predictions were validated through protein–DAPG interaction and in vitro studies.
Protein–DAPG interactions
In addition, several clinical correlative studies have provided support that high level of antiapoptotic Bcl-2 protein expression confers clinically important chemo-resistant phenotype on cancer cells including AML, ALL, CLL, multiple myeloma, prostate cancer, brain tumor, and neuroblastoma [7, 8]. Likewise, a decrease in Bax level has been associated with poor responses to chemotherapy and shorter overall survival in breast or colorectal cancer. The FPA results confirmed a dose-dependent inhibition of antiapoptotic Bcl-2 family proteins—Bcl-2, Bcl-w, and Bcl-xL—with IC50 concentrations of 29.78 ± 1.89, 85.89 ± 2.45, and 97.32 ± 1.33 µM, respectively, in the presence of DAPG (Fig. 3a; Table 2). These results showed the interactions of DAPG with antiapoptotic Bcl-2, Bcl-w, and Bcl-xL proteins correlating with the results of in silico studies.
Inhibitory activity of DAPG. a Dose-dependent inhibition of antiapoptotic Bcl-2 family proteins in the presence of DAPG b Dose-dependent inhibition of metastatic proteins in the presence of DAPG c Dose-dependent inhibition of Bcl-2-overexpressing leukemic cells treated with DAPG for 48 h and d Dose-dependent inhibition of MMP-2- and MMP-9-overexpressing metastatic cancer and normal (human embryonic kidney HEK-293) cells treated with DAPG for 48 h
NF-κB is the transcriptional factor in nucleus of B cells expressed in the cytosol of all cell types but as an inactive form with inhibitory protein (IκB). NF-κB is activated and translocates to the nucleus where it regulates >200 genes that control infection and cell growth. In many cancer cells, NF-κB is constitutively activated. The NF-κB signaling has been observed in cancer and inflammatory disorders, by production of cytokines, inhibitors of apoptosis, MMPs, and adhesion molecules for metastasis of tumor cells. Based on the NF-κB binding assay, the DAPG directly inhibited the binding of NF-κB in a dose-dependent manner with the IC50 concentration of 10.82 ± 1.5 μM (Fig. 3b).
MMP-2 and MMP-9 are the most important gelatinases involved in the basement membrane collagen-IV degradation, which are frequently observed in cancers. MMP-2 (72 kDa; gelatinase-A) is constitutively expressed by most cells including endothelial, epithelial, and cancer cells, while MMP-9 (92 kDa; gelatinase-B) is produced by cancer cells as well as inflammatory cells including blood neutrophils and tissue macrophages and stimulated connective tissue cells. The DAPG directly inhibited the activity of MMP-2 and MMP-9 in a dose-dependent manner with the IC50 concentration of 5.82 ± 1.6 and 6.75 ± 1.2 μM, respectively (Fig. 3b). These results also correlated with the high interactions of DAPG with MMPs by in silico interaction studies.
In vitro validations
The cytotoxic activity results showed that DAPG inhibited the A549, MDA MB-231, HL-60, Raji, and K562 cancer cells in a dose-dependent manner with the IC50 concentration of 0.06 ± 0.02, 0.08 ± 0.01, 16.31 ± 1.82, 10.72 ± 0.92, and 7.67 ± 0.73 μM, respectively, compared to the non-cancer HEK-293 cells with the IC50 concentration of 54.7 ± 1.43 μM (Fig. 3c–d). However, DAPG showed no inhibitory effect on normal blood PBMCs even up to 100 μM concentrations.
Metastasis is a multi-step cell-biological process and involves dissemination of cancer cells from primary tumor site to distant organs and subsequent adaptation to foreign tissue microenvironment, with series of discrete steps, which have been modeled into “invasion–metastatic–cascade.” Degradation of basement membrane is a crucial event in invasion. Basement membranes and connective tissues consist of four major groups of molecules such as collagens, elastin, glycoproteins, and proteoglycans. These ECM constituents are stabilized and organized by various protein–protein and polysaccharide–protein interactions that can be destabilized by degrading enzymes. MMPs are zinc-dependent proteases, which are secreted in inactive proenzymatic forms. MMPs are divided into five types on the basis of preferential extracellular matrix substrate such as interstitial collagenases, stromelysins, gelatinases, matrilysins, and membrane-type MMPs. MMP-2 and MMP-9 are the gelatinases, which are over expressed in the metastatic cancer cells and contribute to the poor prognosis in the cancer patients [2–6]. The respective IC50 concentration of DAPG-treated A549 and MDA MB-231 cancer cells significantly inhibited the invasion of cancer cells through matrigel-coated transwell inserts compared to the DMSO-treated cancer cells (Fig. 4a, b). The MMP-2 and MMP-9 activities were also significantly inhibited in the respective IC50 concentration of DAPG-treated A549 and MDA MB-231 cancer cells (Fig. 4c).
In vitro validation of expression of metastatic and antiapoptotic Bcl-2 family proteins. a Inhibition of invasion in the respective IC50 concentration of DAPG-treated A549 and MDA MB-231 cancer cells. b Microscopic observations of crystal violet-stained invaded cancer cells treated with the respective IC50 concentration of DAPG-treated A549 and MDA MB-231 cancer cells with respect to DMSO-treated cancer cells. c Inhibition of MMP-2 and MMP-9 activities in the respective IC50 concentration of DAPG-treated A549 and MDA MB-231 cancer cells. d Immunoblot showing the down-regulation of antiapoptotic Bcl-2 family proteins (Bcl-2, Bcl-w, and Bcl-xL) and up-regulation of proapoptotic Bax in the respective IC50 concentration of DAPG-treated HL-60, K562, and Raji leukemic cells. e Relative gene expression data quantified from immunoblot using ImageJ software. f Immunoblot showing the down-regulation of antiapoptotic Bcl-2 family proteins (Bcl-2 and Bcl-xL) and up-regulation of proapoptotic Bax in the respective IC50 concentration of DAPG-treated HEK-293, A549, and MDA MB-231 cancer cells. g Relative gene expression data quantified from immunoblot using ImageJ software. C DMSO-treated control cells and T DAPG-treated cells. Scale bars-10 μm
The Bcl-2, Bcl-w, and Bcl-xL are the contributors for the antiapoptotic mechanism in cancer cells. The immunoblotting results of the respective IC50 concentration of DAPG-treated leukemic, A549, and MDA MB-231 cancer cells showed the down-regulation of antiapoptotic members of Bcl-2 family proteins such as Bcl-2, Bcl-w, and Bcl-xL and up-regulation of Bax, a proapoptotic Bcl-2 family protein, compared to non-cancer HEK-293 cells (Fig. 4d–g). These results suggested that NF-κB was suppressed by the DAPG, which might have down-regulated the antiapoptotic Bcl-2 proteins, which correlated with the in silico and interaction studies.
Discussion
The polyketide metabolite DAPG has been reported for its antimicrobial [18–25], antiviral [22], anticancer [14], and anti-helminthic properties [20]. Recently, we have demonstrated the antiproliferative, proapoptotic, antimetastatic, and anti-inflammatory potential of DAPG in cancer and LPS-inflamed immune cells through blockade of NF-κB activity [14]. The present investigation reports the interaction of DAPG with the important metastatic and antiapoptotic Bcl-2 family proteins. As per the results, DAPG had the high interaction with the metastatic proteins, especially MMP-2 and significant inhibition of MMP-2 followed by MMP-9 and NF-κB activities. The antiproliferative activity of DAPG showed its differential and dose-dependent inhibition of MMP-2-, MMP-9-, NF-κB-, and Bcl-2 in overexpressing cancer cells. The NF-κB plays a major role in the development, maintenance, and progression of cancer and controls the expression of genes that are involved in the physiological processes such as inflammation, stress, cell adhesion, differentiation, and apoptosis [26, 27]. The antiapoptotic Bcl-2 family proteins (Bcl-2, Bcl-w, and Bcl-xL) that are over expressed in leukemic cells are associated with resistance to therapies, cell survival, and evade apoptosis and metastases [28–30]. The molecular docking studies of DAPG with metastatic and antiapoptotic Bcl-2 family proteins confirmed its ability to interact with MMP-2, MMP-9, and NF-κB than the Bcl-2, Bcl-w, and Bcl-xL proteins, which correlated the in vitro interaction studies. Results further confirmed the potential of DAPG to inhibit the expression of NF-κB and antiapoptotic family members of Bcl-2 proteins, thereby inducing the apoptosis in leukemic cells with the activation of Bax, a proapoptotic Bcl-2 protein. The inhibition of ROS and NF-κB in cancer cells by DAPG is directly supporting the evidence of inhibition of inflammation, which are responsible for proliferation and survival of cancer cells. The DAPG selectively inhibits the leukemic stem cells, which are the major contributors of cancer recurrence and resistance to the conventional therapy. Further results confirmed that DAPG had high interactions with the metastatic proteins than the antiapoptotic Bcl-2 family proteins. In summary, the affinity of DAPG with proteins is in the order of MMP-2 > MMP-9 > NF-κB > Bcl-2 > Bcl-xL > Bcl-w. Considering its innate anticancer and anti-inflammatory properties, the DAPG could be utilised as a potent anticancer therapeutic metabolite.
Abbreviations
- Bax:
-
Bcl-2-associated X-protein
- Bcl-2:
-
B-cell lymphoma 2
- DCF-2, 7:
-
Dichlorofluorescein
- DMEM:
-
Dulbecco’s modified eagle medium
- FBS:
-
Fetal bovine serum
- LPS:
-
Lipopolysaccharide
- NF-κB:
-
Nuclear factor κB
- RPMI:
-
Roswell Park Memorial Institute
- MMP:
-
Matrix metalloproteinase
- PVDF:
-
Polyvinylidene fluoride
- BSA:
-
Bovine serum albumin
References
Brooks SA, Lomax-Browne HJ, Carter TM, Kinch CE, Hall DMS (2010) Molecular interactions in cancer cell metastasis. Acta Histochem 112:3–25
Gupta GP, Massagué J (2006) Cancer metastasis: building a framework. Cell 127:679–695
Ramaswamy S, Ross KN, Lander ES, Golub TR (2003) A molecular signature of metastasis in primary solid tumors. Nat Genet 33(1):49–54
Deryugina EI, Quigley JP (2006) Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev 25:9–34
Tse JC, Kalluri R (2007) Mechanisms of metastasis: epithelial-to-mesenchymal transition and contribution of tumor microenvironment. J Cell Biochem 101:816–829
Feller L, Kramer B, Lemmer J (2012) Pathobiology of cancer metastasis: a short account. Cancer Cell Int 12:1–14
Kang MH, Reynolds CP (2009) Bcl-2 Inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res 15(4):1126–1132
Zhang L, Ming L, Yu J (2007) BH3 mimetics to improve cancer therapy; mechanisms and examples. Drug Resist Updates 10:207–217
Zhang M, Ling Y, Yang C, Liu H, Wang R, Wu X et al (2007) A novel Bcl-2 small molecule inhibitor 4-(3-methoxy-phenylsulfannyl)-7-nitro-benzofurazan-3-oxide(MNB)-induced apoptosis in leukemia cells. Ann Hematol 86:471–481
Zhai D, Jin C, Shiau C, Kitada S, Satterthwait AC, Reed JC (2008) Gambogic acid is an antagonist of antiapoptotic Bcl-2 family proteins. Mol Cancer Ther 7(6):1639–1646
Oliver L, Mah´e B, Gr´e´e R, Vallette FM, Juin P (2007) HA14-1, a small molecule inhibitor of Bcl-2, bypasses chemoresistance in leukaemia cells. Leuk Res 31:859–863
Yang C, Wang S (2011) Hydrophobic binding hot spots of Bcl-xL protein–protein interfaces by cosolvent molecular dynamics simulation. ACS Med Chem Lett 2:280–284
Manero F, Gautier F, Gallenne T, Cauquil N, Gre´e D, Cartron P et al (2006) The small organic compound HA14-1 prevents Bcl-2 interaction with Bax to sensitize malignant glioma cells to induction of cell death. Cancer Res 66(5):2757–2764
Veena VK, Popavath RN, Kennedy K, Sakthivel N (2015) In vitro antiproliferative, pro-apoptotic, antimetastatic and anti-inflammatory potential of 2, 4-diacteylphloroglucinol (DAPG) by Pseudomonas aeruginosa strain FP10. Apoptosis 20(10):1281–1295
Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ et al (2004) Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem 47:1739–1749
Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren T et al (2006) Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein–ligand complexes. J Med Chem 49:6177–6196
Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL et al (2004) Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J Med Chem 47:1750–1759
Kamei Y, Isnansetyo A (2003) Lysis of methicillin-resistant Staphylococcus aureus by 2, 4-diacetylphloroglucinol produced by Pseudomonas sp. AMSN isolated from a marine alga. Int J Antimicrob Agents 21:71–74
Ayyadurai N, Ravindra P, Sreehari RM, Sunish KR, Samrat SK, Manohar M, Sakthivel N (2006) Isolation and characterization of a novel banana rhizosphere bacterium as fungal antagonist and microbial adjuvant in micropropagation of banana. J Appl Microbiol 100:926–937
Kidarsa TA, Goebel NC, Zabriskie TM, Loper JE (2011) Phloroglucinol mediates cross-talk between the pyoluteorin and 2, 4-diacetylphloroglucinol biosynthetic pathways in Pseudomonas fluorescens Pf-5. Mol Microbiol 81:395–414
Couillerot O, Meynet EC, Pothier JF, Bellvert F, Challita E, Poirier M et al (2011) The role of the antimicrobial compound 2, 4-diacetylphloroglucinol in the impact of biocontrol Pseudomonas fluorescens F113 on Azospirillum brasilense phytostimulators. Microbiology 157:1694–1705
Tada M, Takakuwa T, Nagai M, Yoshii T (1990) Antiviral and antimicrobial activity of 2, 4-Diacetylphloroglucinols, 2-Acycyclohexane-1, 3-diones and 2-Carboxamidocyclo-hexane-1,3-diones. Agric Biol Chem 54:3061–3063
Isnansetyo A (2003) Antibacterial activity of 2, 4-diacetylphloroglucinol produced by Pseudomonas sp. AMSN isolated from a marine alga, against vancomycin-resistant Staphylococcus aureus. Int J Anti Agents 22:545–547
Isnansetyo A, Horikawa M, Kamei Y (2001) In vitro anti-methicillin-resistant Staphylococcus aureus activity of 2, 4-diacetylphloroglucinol produced by Pseudomonas sp. AMSN isolated from a marine alga. J Antimicrob Chemother 47:719–730
Brazelton JN, Pfeufer EE, Sweat TA, Gardener BBM, Coenen C (2008) 2, 4-Diacetylphloroglucinol alters plant root development. MPMI 21:1349–1358
Ellerbroek SM, Stack MS (1999) Membrane associated matrix metalloproteinases in metastasis. BioEssays 21:940–949
Pavelic SK, Sedic M, Bosnjak H, Spaventi S, Pavelic K (2011) Metastasis: new perspectives on an old problem. Mol Cancer 10:22
Becattini B, Kitada S, Leone M, Monosov E, Chandler S, Zhai D et al (2004) Rational design and real time, in-cell detection of the proapoptotic activity of a novel compound targeting Bcl-xL. Chem Biol 11:389–395
Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ (2002) Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2:183–192
Petros AM, Olejniczak ET, Fesik SW (2004) Structural biology of the Bcl-2 family of proteins. Biochim Biophys Acta 1644:83–94
Acknowledgments
The joint University Grant Commission-Council for Scientific and Industrial Research (joint UGC-CSIR), Government of India, New Delhi, through a research fellowship to Ms. V. Veena, the financial support from the Department of Biotechnology (DBT), New Delhi, and the University Grants Commission-Special Assistance Programme (UGC-SAP) co-ordinated by Prof. Dr. N. Sakthivel are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Veena, V.K., Kennedy, K., Lakshmi, P. et al. Anti-leukemic, anti-lung, and anti-breast cancer potential of the microbial polyketide 2, 4-diacetylphloroglucinol (DAPG) and its interaction with the metastatic proteins than the antiapoptotic Bcl-2 proteins. Mol Cell Biochem 414, 47–56 (2016). https://doi.org/10.1007/s11010-016-2657-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2657-6