Abstract
Different approaches have been used to study the pattern of cytokines in celiac disease (CD). Laser capture microdissection (LCM) is a powerful tool for the isolation of specific tissue compartments. We aimed to investigate the mucosal immune response that takes place in different intestinal compartments of CD patients, dissected by LCM, analyzing cytokine expression profile. Frozen section of jejunum was obtained from 15 untreated CD and 15 control. Surface epithelium and lamina propria compartment were isolated by LCM. RNA from each LCM sample was extracted and, after a retrotranscription step, messenger RNA levels for MxA, IL-15, TNF-α, IFN-γ, IL-17α, IL-21, IL-10, and TGF-β were determined by quantitative reverse transcriptase-PCR. Increased gene expression levels of MxA, IL-15, TNF-α, IL-10, and TGF-β was observed in the surface epithelium of untreated CD with respect to control. Furthermore, all the cytokines investigated were upregulated in the lamina propria of untreated CD as compared to control. Within the untreated CD group the expression of IL-15 was higher, in the surface epithelium than in the lamina propria, whereas the expression levels of IL-17 and IL-21 were higher in the lamina propria than in the surface epithelium. Finally, high levels of IL-10 and TGF-β were detected in both compartments of untreated CD biopsies. In CD, surface epithelium and lamina propria compartments, play a prominent role in determining innate and adaptive immunity, respectively. Conversely, surface epithelium and lamina propria produce high levels of anti-inflammatory cytokines, suggesting that both compartments are involved in the immunoregulatory response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Celiac disease (CD) is an immune-mediated disease, triggered by the ingestion of wheat gliadin and related prolamins from other toxic cereals such as barley and rye. An immune response against these cereal-derived proteins is mediated by cytokines produced by both innate and adaptive immune branches. In the early phase of CD, epithelial cells are probably destroyed by the presence of toxic gliadin peptides, which may activate the innate immune system up-regulating interleukin (IL)-15 secretion [1]. Recently, as another sign of innate immune activation in CD, it was shown that the human myxovirus resistance protein A (MxA), a type -1 IFN inducible gene, increased significantly in CD patients after challenge [2].
The entry of increased amounts of toxic gliadin peptides into lamina propria activates a specific immune reaction to these peptides that are deamidated by tissue transglutaminase and presented by DQ2 + or DQ8 + antigen-presenting cells to lamina propria CD4 + T cells [3]. Upon activation, CD4 + T cells polarize along the T helper (Th) 1-type pathway, as substantiated by their ability to produce large amounts of interferon (IFN)-γ, the signature cytokine of Th1 responses [4]. Therefore, the immune response to gluten in CD, may follow two complementary (and sometimes parallel) pathways, mediated by the adaptive and innate immunity.
Different approaches have been used to study the pattern of cytokines in CD, which have led sometimes to contradictory results. Most data have been found by mRNA expression in biopsy homogenates [4–6], from patients with active CD. However, the results of analysis of whole tissue samples are usually determined by the major or predominant cell type and may mask biologically relevant and important changes present either in the different tissue compartments or in a particular type of cells. Other studies analyzed cytokine mRNA expression in enterocytes, intraepithelial lymphocytes (IELs), and lamina propria mononuclear cells (LPMC), obtained from intestinal biopsy specimens of CD patients, using techniques that fail to provide an isolation of highly pure cell populations [7–9]. Therefore, it is essential to analyse specific tissue compartments or cell types to identify and define biologically important processes.
The development in the last decade of sophisticated, but generally easy to use, laser-based methods of tissue microdissection has allowed this goal to be achieved by combining microscope-based morphological methods of analysis with a diverse range of very powerful molecular technologies [10–12]. This has permitted the compartment- or cell type-specific molecular analysis of solid tissues, without contamination from surrounding cells, and has provided new insights into both normal cell biology and pathogenic mechanisms.
In this study, we combined a laser capture microdissection (LCM) technique with quantitative reverse transcriptase real-time PCR (qRT-PCR) to evaluate the mucosal immune response by analyzing cytokine expression profile in different intestinal compartments of CD patients. To this end, we compared the mRNA levels for interleukin (IL)-15, IL-17, IL-21, IL-10, transforming growth factor (TGF)-β, IFN-γ, tumor necrosis factor (TNF)-α, and mRNA level of a single IFN-stimulated gene, MxA, in both surface epithelium and lamina propria compartments of jejunum biopsy samples from adults with newly diagnosed active CD. Comparisons with gene expression levels detected in both epithelium and lamina propria compartments of jejunal biopsy samples from healthy control subject were also performed.
To our knowledge this study is the first example of this technique being used to analyse the cytokine network in intestinal compartments of CD; therefore, providing new insights in the involvement of the epithelium and lamina propria in the immunopathogenesis of CD.
Methods
Subjects
Biopsy specimens were obtained from the distal duodenum of 15 untreated CD patients (n. 5 male and n. 10 female patients; median age, 35 years; range 21–57). They were snap-frozen in liquid nitrogen or immediately processed. Diagnosis was based on typical mucosal lesions with crypt cell hyperplasia and total villous atrophy. All untreated CD patients were positive for serum anti-endomysial antibodies. Duodenal biopsy samples were also obtained from 15 non-celiac individuals (n. 6 male and n. 9 female patients; median age 43 years; range 29–58) with normal intestinal mucosa and negative serology for anti-endomysial. The study received approval from a local ethics committee of San G. Moscati Hospital (Avellino, Italy), in conformity to the provisions of the Declaration of Helsinki. Informed consent was obtained for each participating subject.
Microdissection
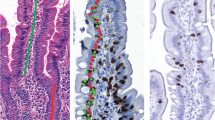
Freshly frozen duodenal mucosa samples were cut into 8 μm sections using a cryostat (Leica CM1850; Leica Microsystems, Wetzlar, Germany) and collected on RNase-free membrane slides (PEN-membrane, 1 mm glass, Carl Zeiss MicroImaging, Munich, Germany). Immediate fixation in cold acetone was performed and the slides kept on dry ice for a maximum of 2 min prior to staining. Rehydration in RNase-free water was followed by haematoxylin staining prior to dehydration in graded ethanol for 60 s and xylene for 2 min. The slides were then air dried for 3 min. Microdissection was performed using the Leica LMD 6000 microdissector. In particular, we evaluated the compartments where effector immune response to gliadin occurs in CD, that is surface epithelium and lamina propria, since the crypts of Lieberkühn represent mainly a proliferative compartment, maintained by stem cells [13]. Therefore, a first set of microdissection to obtain the surface epithelium was followed by microdissection to isolate the lamina propria compartment (Fig. 1). Nevertheless, we also evaluated cytokine profile within the crypts of Lieberkühn isolated from jejunal biopsy specimens of 5 control and 5 untreated CD patients (Fig. 1).
Selective microdissection of surface epithelium, lamina propria and crypts of Lieberkühn compartments on frozen section of jejunal biopsy from untreated celiac disease patient. The laser cuts along the line drawn by the operator, isolating the compartments. a Roadmap before microdissection (Ep indicates the surface epithelium; Lp indicates lamina propria; Cr indicates crypts). b Postmicrodissection (Ep, Lp, and Cr indicates, respectively, the lifted surface epithelium, lamina propria and crypt area). Original magnification ×200
RNA preparation and qRT-PCR
Cells derived from laser capture microdissection were immediately processed in the presence of 4 U RNasin RNase inhibitor (Promega, Italy), and total RNA was obtained using the PicoPure® RNA isolation kit according to the manufacturer’s protocol (Arcturus, Life Technologies). RNA was eluted in 12 μl DEPC water and its quality was assessed using the Experion automated electrophoresis station (BioRad). Isolated RNAs were reverse transcribed using the SuperScript® VILO cDNA Synthesis Kit (Life Technologies) with random hexamer primers according to the manufacturer’s instructions. IL-10, IL-15, IL-17, IL-21, MxA, TNF-α, TGF-β, and IFN-γ cDNAs were quantified by SYBR green (PowerSYBR Green PCR Master Mix, Applied Biosystems)-based qRT-PCR assays on an ABI-PRISM 7000 SDS instrument (Applied Biosystems). The PCR reactions were performed in triplicate in a final volume of 35 μl containing 2 μl of cDNA dilutions. Serial dilutions of cDNA containing a known quantity of each transcript were used in each quantitative PCR run to generate a standard curve. To assess the specificity of the amplification products, melting curve analysis was performed. In preliminary experiments, the optimal dilution of cDNAs to obtain a PCR product within the linear phase of the amplification was established. We included only study samples where both gene and housekeeping gene had a sigmoid shaped curve between Ct values from 15 to 36. Samples that did not meet the RNA quality and quantity requirements were excluded from the study. Primers, designed using the Primer Express Software (Applied Biosystems) according to ABI recommendations or derived from literature, were synthesized from Sigma-Aldrich. Oligonucleotides were designed to (a) generate amplicons of 50–150 bp from specific National Center for Biotechnology Information (NCBI) reference sequences, to (b) span exons and were blasted through NCBI GenBank to ensure lack of homology to other known human cDNA sequences. Gene expression was normalized to the level of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Primer sequences are reported in Table 1.
Data and statistical analysis
Relative expression of transcripts was calculated by the ΔΔCT method using the Data Assist Software v3.0 (Applied Biosystems). Data were organized in Excel (Microsoft) for later statistical analyses that were performed in GraphPad Prism (GraphPad Software, CA, USA). Results are presented as normalized mean expression ± standard error (SEM). Differences between groups were compared using unpaired Student’s t tests and one-way analysis of variance (ANOVA) tests. When ANOVA tests indicated significant effects, further individual comparisons were calculated with post hoc analysis using the Bonferroni multiple comparison test. Statistical significance was achieved when p < 0.05. Non-parametric tests (Kruskal–Wallis) were also applied and the results are concordant with those obtained using parametric tests.
Results
Active CD is characterized by increased MxA, pro- and anti-inflammatory cytokine production from both surface epithelium and lamina propria
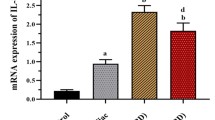
qRT-PCR analyses of surface epithelium revealed a significant increase (p < 0.05) of mRNA levels of IL-15, MxA, IFN-γ, and TNF-α in active CD mucosa as compared with control jejunal biopsies (Fig. 2). In contrast, no significant differences were noted in the transcripts of IL-17 and IL-21 pro-inflammatory cytokines, in the surface epithelium of active CD mucosa as compared to the surface epithelium isolated from control jejunal biopsies (Fig. 2). Additionally, significant increases (p < 0.05) of both down-inflammatory cytokines TGF-β and IL-10 mRNA levels were seen in the surface epithelium isolated from active CD mucosa with respect to the surface epithelium isolated from control jejunal biopsies (Fig. 2).
Selected mRNA transcripts analysed by quantitative reverse transcription-polymerase chain reaction in the surface epithelium (Ep) and lamina propria (Lp) compartments isolated by LCM in jejunal biopsies from untreated celiac disease (n = 15) and normal controls (n = 15). Fold change (y axis) represents messenger RNA expression normalized to GAPDH. Intergroup (Epceliacs vs Epcontrols and Lpceliacs vs Lpcontrols; solid lines) and intragroup (Epceliacs vs Lpceliacs and Epcontrols vs Lpcontrols; dashed lines) differences associated with statistical significance are marked by respective symbols. * = p < 0.05, ** = p < 0.005. Unless otherwise specified differences are not significant
qRT-PCR analyses of lamina propria compartment isolated by LCM from active CD mucosa, showed a significant increase (p < 0.05) of mRNA levels of IL-15, IFN-γ and ΤNF-α, pro-inflammatory cytokines, compared with the lamina propria compartment isolated from jejunum biopsy samples of control subjects (Fig. 2). Moreover, we found highly significant differences (p < 0.005) of mRNA expression for IL-17 and IL-21 pro-inflammatory cytokines, in lamina propria of active CD mucosa in comparison with lamina propria of control jejunal biopsies (Fig. 2). Similarly, as obtained in the surface epithelium of active CD mucosa compared with control, even mRNA levels of TGF-β and IL-10 regulatory cytokines produced in the lamina propria of active CD mucosa were significantly higher compared with lamina propria compartment of control jejunal biopsies (Fig. 2).
Finally, we analyzed also cytokine mRNA levels in the crypts of Lieberkühn isolated from jejunal biopsy specimens of 5 control and 5 untreated CD patients. No increased gene expression levels of all cytokines investigated were noted in the crypts of Lieberkühn of both group of patients (data not shown).
In CD the surface epithelium and lamina propria are mainly involved in innate and adaptive immune response, respectively
In patients with CD, immune responses to gliadin fractions are mediated by both the adaptive and the innate immune systems. Therefore, first we compared the profile of the dominant cytokines with a key role in the innate immune activation, between the surface epithelium and lamina propria within the patient groups. Interestingly, our results indicate that in active CD the surface epithelium might constitute the main source of the cytokines that are mostly correlated with the innate immunity. Particularly, as shown in Fig. 2, in active CD patients the mRNA levels of IL-15, were significantly higher in the surface epithelium as compared with lamina propria. We also observed an increase of MxA transcript in the surface epithelium as well as in lamina propria areas of active CD mucosa with levels that were approximately the same (Fig. 2).
We next explored in the patients group the profile of dominant cytokines with a key role in the activation of the innate immune response, at the level of both surface epithelium and lamina propria. We found that in active CD mucosa, the mRNA levels of IL-17 and IL-21, were significantly higher in the lamina propria compartment in comparison with the surface epithelium (Fig. 2). We also observed a higher expression of IFN-γ and TNF-α transcripts in both lamina propria and surface epithelium compartments of active CD mucosa, with levels that were approximately the same (Fig. 2).
There were no statistically significant differences within controls groups between, the surface epithelium and lamina propria intestinal areas, when the mRNA levels of IL-15, MxA, IFN-γ, TNF-α, IL-17, and IL-21 were compared (Fig. 2).
This finding suggests that in CD, the lamina propria compartment most likely represents the main source of adaptive inflammatory cytokines.
Surface epithelium and lamina propria are both involved in the immunoregulatory response in CD
It is known that both TGF-β and IL-10 play an important role in the regulation of the inflammatory cascade in the intestinal mucosa. Therefore, in order to define the immunosuppressive function of surface epithelium and lamina propria in CD jejunal mucosa, we compared mRNA levels of TGF-β and IL-10 produced in both areas. We found that mRNAs of either immunoregulatory cytokines were highly expressed in both surface epithelium and lamina propria intestinal compartments of active CD (Fig. 2). No significant differences were found between the two areas when TGF-β and IL-10 levels were compared (Fig. 2). This finding showed that in CD, surface epithelium and lamina propria are both involved in the immunoregulatory response.
Moreover, it is interesting to note that in control subjects, either TGF-β or IL-10 regulatory cytokines produced by the surface epithelium were significantly higher compared with those produced in the lamina propria compartment (Fig. 2).
Discussion
In patients with CD, immune response to gliadins takes place at epithelial and lamina propria levels, therefore both compartments, with their distinct subsets of effector and suppressor cell subpopulations, play an important role in CD pathogenesis. Previous reports on cytokine profile between cells belonging to the epithelial and lamina propria compartments in CD mucosa, have led sometimes to contradictory results [7–9]. Furthermore, the techniques used in these reports to isolate specific cell populations did not exclude completely the risk of contamination by unwanted cells between the two compartments, (i.e., contamination of epithelial layer with cells from the lamina propria and vice versa). In the light of this problem, we used LCM technology which can isolate specific tissue compartments, with their distinct subsets of effector cell subpopulations, excluding unwanted cells. Since the crypts of Lieberkühn represent mainly a proliferative compartment, maintained by stem cells [13], we focused our study on the compartments where it has been shown that effector immune response to gliadin occurs in CD, that is surface epithelium and lamina propria. To our knowledge this study is the first example of this technique being used to analyse the cytokine network in intestinal compartments of CD, and therefore this approach will simplify the interpretation of results providing new insights in the involvement of the epithelium and lamina propria in the immunopathogenesis of CD.
Our data firstly confirmed that pro- and anti-inflammatory cytokines are overexpressed in both surface epithelium and lamina propria, of active CD mucosa as compared with jejunum biopsy samples of control subjects. Importantly, we found in CD that the surface epithelium and lamina propria compartments play an important role in directing innate and adaptive immune response, respectively. Furthermore, we found in CD that both mucosal areas analyzed release cytokines with dominant suppressive activity. Finally, within controls group, we have shown that regulatory cytokines analyzed were significantly higher in the surface epithelium than in the lamina propria intestinal compartment.
In the past decade, several studies have highlighted the central role of innate immunity in the induction of tissue damage in CD mucosa. The toxic peptides, such as peptide 31–43, trigger an innate immune response [1], characterized by the production of IL-15 by epithelial cells and lamina propria dendritic cells [8]. IL-15 act on IELs promoting IFN-γ production as well as a potent cytotoxic activity particularly by NKG2D + cells [14]. Moreover, as a sign of innate immune activation in CD it also has been shown an increase of IFN-α in untreated CD [15]. IFN-α synthesis is normally a transient phenomenon [16, 17]. Another way of exploring IFN-α signature is to examine MxA. Accordingly, MxA gene expression, in some systemic autoimmune disease, was proposed as a biomarker for type I IFN bioactivity [18, 19]. In a recent study, Brottveit et al. [2] found an increased expression of MxA in treated CD patients after short-term gluten challenge, suggesting that IFN-α is activated upon gluten challenge. Furthermore, the authors found that the expression levels of IFN-α/β-related genes for CD patients were not increased suggesting that the IFN-α response is probably transient and decreases rapidly. Therefore, in the current study, we analyzed MxA gene expression to examine IFN-α-induced responses.
We showed that mRNA levels of both IL-15 and MxA were significantly higher in the surface epithelium isolated from untreated CD mucosa in comparison with control. Moreover, we found an increase of IL-15 and not of MxA transcript in the lamina propria isolated from active CD mucosa as compared with control. Within CD group, we observed higher production of IL-15 in the surface epithelium in comparison with lamina propria. We also detected an increase of MxA transcript in the surface epithelium as compared with lamina propria compartment of active CD mucosa, but this trend did not reach statistical significance. Therefore, our study confirms previous observations, of increased expression of IL-15, particularly in the surface epithelium of the small-intestinal biopsies from patients with active CD [20–22]. New in this study is the finding of MxA in both compartments analyzed of untreated CD mucosa, with an increased production of MxA transcript in the surface epithelium with respect to lamina propria, as further confirmation that IFN-α may be involved in CD pathogenesis. Taken together these data suggest that in CD the major changes related to innate immunity mainly might take place in the surface epithelium, as indicated by increased production of IL-15 and MxA in surface epithelium with respect to lamina propria.
We analyzed also cytokine mRNA levels in the crypts of Lieberkühn isolated from jejunal biopsy specimens of 5 control and 5 untreated CD patients. No increased gene expression levels of all cytokines investigated were noted in the crypts of Lieberkühn of both group of patients (data not shown). This finding showed the absence of an immune response to gluten in this particular compartment of active CD mucosa.
In CD, innate immunity may also play a role in directing an adaptive response of gluten-specific T cells toward a Th1 cytokine profile. It has been reported in active CD that IL-15 and IFN-α drives secretion of proinflammatory cytokines IFN-γ and TNF-α, by activated intestinal T cells [8, 23]. Importantly to note that in CD the inflammatory response consists of increased numbers of activated lymphocytes in the lamina propria and in the surface layer of the epithelium. In this study, we showed higher levels of IFN-γ and TNF-α transcripts in both epithelium and lamina propria of active CD mucosa in comparison with control. Within CD group, we found that the production of IFN-γ and TNF-α was approximately the same in either intestinal compartments. Therefore, our results suggest that IFN-γ and TNF-α overexpressed in surface epithelium and lamina propria of active CD mucosa could be related to the major expansion of highly activated T cells within both intestinal compartments. Nevertheless, to address this hypothesis, cell-type-specific gene expression profiles by immuno-LCM, which involves immunohistochemical staining of tissue before LCM, enhancing the ability to identify cells of interest according to their immunophenotype, are now in progress.
The recent discovery and characterization of T helper 17 cells (Th17) in CD and their signature cytokines IL-21 and IL-17 provide a new distinctive pathway for the communication between adaptive and innate immunity [24]. Particularly, it was shown that IL-21 is strongly increased in intestinal biopsies from active CD [25] and that gluten-specific T cells are major sources of this cytokine [26]. Recently, we reported that IL-17 is highly produced in the inflamed gut of patients with CD [27]. Our finding of IL-17 upregulation in CD patients is in agreement with other studies [28, 29]. Moreover, it was found that IL-17 is produced by cells that also make IFN-γ and that IL-21 positively controls IL-17 expression [28]. In this study, we confirms previous demonstrations of increased production of pro-inflammatory cytokines IL-17 and IL-21 in active CD with respect to controls but we found that this increase take place in the lamina propria compartment. Also, within CD groups, we demonstrate that the mRNA levels of IL-21 and IL-17, were significantly higher in the lamina propria compartment in comparison to the surface epithelium. Taken together these data may suggest that lamina propria represents the main compartment involved in the adaptive immune response in CD.
Concomitantly with the pro-inflammatory response, high amounts of the anti-inflammatory cytokines IL-10 and TGF-β are also produced in the untreated intestinal mucosa [30–32]. This apparent paradoxical milieu of both pro-inflammatory and suppressive cytokines strongly suggests that regulatory mechanisms might operate to counterbalance the gluten-triggered, abnormal immune activation in untreated mucosa [33]. We have previously reported that celiac intestinal mucosa harbours a subset of regulatory T cells (Treg), the Tr1, that through the release of both IL-10 and TGF- inhibit the pathogenic response to in vitro gluten challenge [34].
In this study, we confirm that IL-10 and TGF-β were upregulated in untreated CD compared with normal, non CD, mucosa. Moreover, we found that mRNA levels of both down-regulatory cytokines were expressed at high level in the surface epithelium and lamina propria areas of active CD mucosa, suggesting the involvement of both compartments in the immunoregulatory response. Finally, it is noteworthy to note that in control jejunal biopsies, either IL-10 or TGF-β regulatory cytokines produced in epithelial layer were significantly higher compared with those produced in lamina propria compartment. It is well known that the intestinal epithelium is at the front line when it comes to identifying commensal flora, fighting potential pathogens, and preserving mucosal immune homeostasis. Several cytokines contributing to the local homeostasis, for instance the down-regulatory cytokines TGF-β and IL-10, are produced not only by T cells and APCs, but also by epithelial cells. Therefore, we hypothesize that the increased production of IL-10 and TGF-β that we found in the epithelial layer from jejunal biopsies of both control subjects and active CD patients, could be essential for maintaining epithelial barrier integrity in physiological conditions and recovery to gut homeostasis in active inflammation, respectively.
In conclusion considering the dual involvement of the innate and adaptive branch in the mucosal immune response in the pathogenesis of CD, our results suggest that the surface epithelium and lamina propria compartments play a prominent role in determining innate and adaptive mucosal immunity, respectively.
Finally, we showed that the surface epithelium and lamina propria produce cytokines with dominant suppressive activity, suggesting that immune cells of both compartments are actively trying to downregulate ongoing inflammation.
This work underlines the importance of LCM as a valuable tool to determine potential inflammatory components involved in CD pathogenesis.
References
Maiuri L, Ciacci C, Ricciardelli I, Vacca L, Vacca L, Raia V, Auricchio S, Picard J, Osman M, Quaratino S, Londei M (2003) Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease. Lancet 362:30–37
Brottveit M, Beitnes AC, Tollefsen S, Bratlie JE, Jahnsen FL, Johansen FE, Sollid LM, Lundin KE (2013) Mucosal cytokine response after short-term gluten challenge in celiac disease and non-celiac gluten sensitivity. Am J Gastroenterol 108(5):842–850
Koning F, Schuppan D, Cerf-Bensussan N, Sollid LM (2005) Pathomechanisms in celiac disease. Best Pract Res Clin Gastroenterol 19:373–387
Nilsen EM, Jahnsen FL, Lundin KE, Johansen FE, Fausa O, Sollid LM, Jahnsen J, Scott H, Brandtzaeg P (1998) Gluten induces an intestinal cytokine response strongly dominated by interferon gamma in patients with celiac disease. Gastroenterology 115:551–563
Troncone R, Gianfrani C, Mazzarella G, Greco L, Guardiola J, Auricchio S, De Berardinis P (1998) Majority of gliadin-specific T-cell clones from celiac small intestinal mucosa produce interferon-gamma and interleukin-4. Dig Dis Sci 43(1):156–161
Lahat N, Shapiro S, Karban A, Gerstein R, Kinarty A, Lerner A (1999) Cytokine profile in coeliac disease. Scand J Immunol 49(4):441–446
Forsberg G, Hernell O, Melgar S, Israelsson A, Hammarström S, Hammarström ML (2002) Paradoxical coexpression of proinflamatory and down-regulatory cytokines in intestinal T cells in childhood celiac disease. Gastroenterology 123(3):667–678
Di Sabatino A, Ciccocioppo R, Cupelli F, Cinque B, Millimaggi D, Clarkson MM, Paulli M, Cifone MG, Corazza GR (2006) Epithelium derived interleukin 15 regulates intraepithelial lymphocyte Th1 cytokine production, cytotoxicity, and survival in coeliac disease. Gut 55(4):469–477
Fosberg G, Hernell O, Hammarström S, Hammarström ML (2007) Concomitant increase of IL-10 and pro-inflammatory cytokines in intraepithelial lymphocyte subsets in celiac disease. Int Immunol 19(8):993–1001
Simone NL, Bonner RF, Gillespie Emmert-Buck MR, Liotta LA (1998) Laser-capture microdissection: opening the microscopic frontier to molecular analysis. Trends Genet 14(7):272–276
Curran S, McKay JA, McLeod HL, Murray GI (2000) Laser-capture microscopy. Mol Pathol 53(2):64–68
Lawrie LC, Curran S (2005) Laser-capture microdissection and colorectal cancer proteomics. Methods Mol Biol 293:245–253
Yen TH, Wright NA (2006) The gastrointestinal tract stem cell niche. Stem Cell Rev 2(3):203–212
Hüe S, Mention JJ, Monteiro RC, Zhang S, Cellier C, Schmitz J, Verkarre V, Fodil N, Bahram S, Cerf-Bensussan N, Caillat-Zucman S (2004) A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity 21:367–377
Monteleone G, Pender SL, Alstead E, Hauer AC, Lionetti P, McKenzie C, MacDonald TT (2001) Role of interferon alpha in promoting T helper cell type 1 responses in the small intestine in coeliac disease. Gut 48(3):425–429
Weissmann C, Nagata S, Boll W, Fountoulakis M, Fujisawa A, Fujisawa JI, Haynes J, Henco K, Mantei N, Ragg H, Schein C, Schmid J, Shaw G, Streuli M, Taira H, Todokoro K, Weidle U (1094) Structure and expression of human IFN-alpha genes. Philos Trans R Soc Lond B Biol Sci 24(299):7–28
Feldman E, Ahmed T, Lutton JD, Farley T, Tani K, Freund M, Asano S, Abraham NG (1997) Adenovirus mediated alpha interferon (IFN-alpha) gene transfer into CD34 + cells and CML mononuclear cells. Stem Cells 15(5):386–395
Airò P, Ghidini C, Zanotti C, Scarsi M, Cattaneo R, Caimi L, Imberti L (2008) Upregulation of myxovirus-resistance protein A: a possible marker of type I interferon induction in systemic sclerosis. Rheumatology 35(11):2192–2200
van der Voort LF, Vennegoor A, Visser A, Knol DL, Uitdehaag BM, Barkhof F, Oudejans CB, Polman CH, Killestein J (2010) Spontaneous MxA mRNA level predicts relapses in patients with recently diagnosed MS. Neurology 75(14):1228–1233
Maiuri L, Ciacci C, Auricchio S, Brown V, Quaratino S, Londei M (2000) Interleukin 15 mediates epithelial changes in celiac disease. Gastroenterology 119:996–1006
Mention JJ, Ben Ahmed M, Begue B, Barbe U, Verkarre V, Asnafi V, Colombel JF, Cugnenc PH, Ruemmele FM, McIntyre E, Brousse N, Cellier C, Cerf-Bensussan N (2003) Interleukin 15: a key to disrupted intraepithelial lymphocyte homeostasis and lymphomagenesis in celiac disease. Gastroenterology 125:730–745
Bernardo D, Garrote JA, Allegretti Y, León A, Gómez E, Bermejo-Martin JF, Calvo C, Riestra S, Fernández-Salazar L, Blanco-Quirós A, Chirdo F, Arranz E (2008) Higher constitutive IL15Ra expression and lower IL-15 response threshold in coeliac disease patients. Clin Exp Immunol 154(1):64–73
Monteleone G, Pender SL, Wathen NC, MacDonald TT (2001) Interferon-alphadrives T cell-mediated immunopathology in the intestine. Eur J Immunol 31:2247–2255
Fernandez S, Molina IJ, Romero P, González R, Peña J, Sánchez F, Reynoso FR, Pérez-Navero JL, Estevez O, Ortega C, Santamaría M (2011) Characterization of gliadin-specific Th17 cells from the mucosa of celiac disease patients. Am J Gastroenterol 106:528–538
Fina D, Sarra M, Caruso R, Del Vecchio Blanco G, Pallone F, MacDonald TT, Monteleone G (2008) Interleukin 21 contributes to the mucosal T helper cell type 1 response in coeliac disease. Gut 57(7):887–892
Bodd M, Ráki M, Tollefsen S, Fallang LE, Bergseng E, Lundin KE, Sollid LM (2010) HLA-DQ2-restricted gluten-reactive T cells produce IL-21 but not IL-17 or IL-22. Mucosal Immunol 3(6):594–601
Sapone A, Lammers KM, Mazzarella G, Mikhailenko I, Cartenì M, Casolaro V, Fasano A (2010) Differential mucosal IL-17 expression in two gliadin-induced disorders: gluten sensitivity and the autoimmune enteropathy celiac disease. Int Arch Allergy Immunol 152(1):75–80
Monteleone I, Sarra M, Del Vecchio Blanco G, Paoluzi OA, Franzè E, Fina D, Fabrizi A, MacDonald TT, Pallone F, Monteleone G (2010) Characterization of IL-17A-producing cells in celiac disease mucosa. J Immunol 184(4):2211–2218
Lahdenperä AI, Hölttä V, Ruohtula T, Salo HM, Orivuori L, Westerholm-Ormio M, Savilahti E, Fälth-Magnusson K, Högberg L, Ludvigsson J, Vaarala O (2012) Up-regulation of small intestinal interleukin-17 immunity in untreated coeliac disease but not in potential coeliac disease or in type 1 diabetes. Clin Exp Immunol 167(2):226–234
Lahat N, Shapiro S, Karban R, Gerstein R, Kinarty A, Lerner A (1999) Cytokine profile in coeliac disease. Scand J Immunol 49:441–446
Hansson T, Ulfgren AK, Lindroos E, DannAEus A, Dahlbom I, Klareskog L (2002) Transforming growth factor-beta (TGF-beta) and tissue transglutaminase expression in the small intestine in children with coeliac disease. Scand J Immunol 56(5):530–537
Salvati V, Mazzarella G, Gianfrani C, Levings MK, Stefanile R, De Giulio B, Iaquinto G, Giardullo N, Auricchio S, Roncarolo MG, Troncone R (2005) Recombinant human IL-10 suppresses gliadindependent T-cell activation in ex vivo cultured celiac intestinal mucosa. Gut 54:46–53
Forsberg G, Hernell O, Melgar S, Israelsson A, Hammarström S, Hammarström ML (2002) Paradoxical coexpression of proinflammatory and down-regulatory cytokines in intestinal T-cells in childhood celiac disease. Gastroenterology 123:667–678
Gianfrani C, Leving M, Sartirana C, Mazzarella G, Barba G, Zanzi D, Camarca A, Iaquinto G, Giardullo N, Auricchio S, Troncone R, Roncarolo MG (2006) Gliadin-specific type-1 regulatory T-cells from intestinal mucosa of treated celiac patients inhibit pathogenic T-cells. J Immunol 177:4178–4186
Acknowledgments
We thank Rosa Maria Bevilacqua, Maria Cristina Bruno, and Maria Rosaria Volpe (Gastroenterology and Digestive Endoscopy Service, San G. Moscati Hospital, Avellino, Italy) for their help in the collection of mucosal samples. The authors are grateful to Raffaella Mastantuoni and Clemente Meccariello for their technical help. This study was funded by Department of Translational Medicine-Pediatric Section and European Laboratory for the Investigation of Food-Induced Disease (ELFID), University of Naples “Federico II”, via Pansini, 80131 Naples, Italy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Iacomino, G., Marano, A., Stillitano, I. et al. Celiac disease: role of intestinal compartments in the mucosal immune response. Mol Cell Biochem 411, 341–349 (2016). https://doi.org/10.1007/s11010-015-2596-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2596-7