Abstract
Osteopontin (OPN) is involved in various physiological processes such as inflammatory and wound healing. However, little is known about the effects of OPN on these tissues. OPN is cleaved by thrombin, and cleavage of the N-terminal fragment exposes a SVVYGLR sequence on its C-terminus. In this study, we examined the effects of the thrombin-cleaved OPN fragments on fibroblasts and myocardial fibrosis, particularly the role of the SVVYGLR sequence. The recombinant thrombin-cleaved OPN fragments (N-terminal fragment [N-OPN], C-terminal fragment [C-OPN], and the N-terminal fragment lacking the SVVYGLR sequence [ΔSV N-OPN]) were added to fibroblasts, and the cellular motility, signal activity, and production of collagen were evaluated. A sustained-release gel containing an OPN fragment or SVVYGLR peptide was transplanted into a rat model of ischemic cardiomyopathy and the quantities and ratio of collagen type I (COL I) and type III (COL III) were estimated. N-OPN significantly promoted fibroblast migration. Smad signal activity, expression of smooth muscle actin (SMA), and the production of COL III were enhanced by N-OPN and SVVYGLR peptide. Conversely, ΔSV N-OPN and C-OPN had no effect. In vivo, the expression level of N-OPN was associated with COL III distribution, and the COL III/COL I ratio was significantly increased by the sustained-release gel containing N-OPN or SVVYGLR peptide. The cardiac function was also significantly improved by the N-OPN- or SVVYGLR peptide-released gel treatment. The N-terminal fragment of thrombin-cleaved OPN-induced Smad signal activation, SMA expression, and COL III production, and its SVVYGLR sequence influences this function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteopontin (OPN) is a phosphorylated glycoprotein that is present as an extracellular matrix component in various tissues. OPN has two integrin-binding sites, Arg-Gly-Asp (RGD) and Ser-Val-Val-Tyr-Gly-Leu-Arg (SVVYGLR), and it binds to the α5β1 and αvβ3 integrins via the RGD domain [1] and the α9β1 and α4β1 integrins via the SVVYGLR domain [2]. It is involved in tissue remodeling, apoptosis, and the inflammatory response [3, 4]. OPN is cleaved and fractionated by enzymes such as matrix metalloproteinase (MMP)-3, MMP-7, and thrombin [5]. The fragmented OPN exhibits different activities than the full-length OPN, and functions as an inflammatory cytokine [6]. Thrombin cleaves OPN near the RGD domain, exposing a SVVYGLR sequence on the C-terminus of the N-terminal fragment. The SVVYGLR sequence exposed by thrombin shows high avidity for integrin α9β1, unlike full-length OPN [7, 8]. Thrombin-cleaved OPN performs novel functions, and the thrombin-cleaved N-terminal fragment promotes cell adhesion, cell survival, and the inflammatory response via the RGD and SVVYGLR motifs [6, 8–10]. However, the function of the C-terminal fragment is not known in detail.
Our previous studies reported that an OPN-derived SVVYGLR peptide (SV peptide) induces angiogenesis and activates adhesion, migration, and tube formation by endothelial cells [10–13]. Furthermore, the SV peptide binds to the transforming growth factor (TGF)-β receptor and promotes the myofibroblast differentiation of fibroblasts [14]. These processes are important to the maintenance and repair of myocardial tissue. Myocardium comprises cardiomyocytes, cardiac fibroblasts, and vascular cells. Cardiac fibroblasts mediate alterations in the mechanical environment of the heart, not only maintaining cardiac homeostasis, but also playing a role in the repair and remodeling of the myocardium. Furthermore, fibroblasts respond to alterations in their microenvironment, and they undergo dramatic phenotypic changes and differentiate into myofibroblasts, depending on the cardiac pathology, to aid in tissue repair. Myofibroblasts are characterized by an increased expression of the contractile protein smooth muscle actin (SMA) and focal adhesion protein and by the secretion of extracellular matrix proteins such as collagen type I (COL I), collagen type III (COL III), and fibronectin [15, 16]. Fibroblasts and myofibroblasts provide structural and functional support and are associated with scar formation and matrix remodeling in the heart [17].
Several studies have shown that the expression level of OPN, especially the thrombin-cleaved N-terminal fragment, is enhanced in response to inflammation and tissue injury and is associated with fibrosis during remodeling [18, 19]. OPN is secreted by cardiac fibroblasts and cardiomyocytes, and it is involved in hypertrophy and fibrotic responses [20–22]. However, the precise effect of OPN on the remodeling process is not fully known. In this study, we evaluated the effects of the OPN fragments produced by thrombin cleavage on cardiac fibroblasts and cardiac fibrosis. Furthermore, we analyzed the role of the SVVYGLR sequence in these effects.
Materials and methods
Recombinant OPN
The cDNA fragments for human N-terminal OPN that mimics thrombin-cleaved OPN (N-OPN; amino acids 17-168), the SVVYGLR sequence-removed N-terminal OPN (ΔSV N-OPN; amino acids 17-161), and the C-terminal OPN that mimics thrombin-cleaved OPN (C-OPN; amino acids 169-314) were inserted into the protein expression vector pGEX-4T-2 (Fig. 1). The expression vectors were transformed into E. coli BL21 and the expressed recombinant OPN fragments were purified by affinity chromatography. Bound recombinant protein was eluted with 50 mM Tris–HCl containing 1 M NaCl. The recombinant OPN fragment preparations were more than 90 % homologous on SDS-PAGE.
Structure of the recombinant osteopontin (OPN) fragment. OPN is cleaved between R168 and S169 by thrombin, and the N-terminal fragment exposes a SVVYGLR sequence on its C-terminus. The human N-terminal OPN mimicking thrombin-cleaved OPN (N-OPN; amino acids 17-168), SVVYGLR sequence-removed N-terminal OPN (ΔSV N-OPN; amino acids 17-161), and C-terminal OPN mimicking thrombin-cleaved OPN (C-OPN; amino acids 169-314) are shown
Cell culture
The rat dermal fibroblast (RDF) cell line was obtained from Cell Applications, San Diego, CA, USA. The cells were grown in rat fibroblast growth medium with growth supplement (Cell Application) under 5 % CO2 at 37 °C. The normal human cardiac fibroblast (NHCF) cell line was purchased from Lonza, Basel, Swiss. NHCFs were cultured in Fibroblast Basal Medium (Lonza) and incubated in a humidified incubator at 5 % CO2 at 37 °C.
Proliferation assay
The effect of the OPN fragments and SV peptide on cardiac fibroblast proliferation was evaluated using the WST-1 assay. A 96-well plate was prepared, containing 5 × 103 NHCFs in 100 μL of culture medium containing 10 % fetal bovine serum (FBS). The cells were incubated with 1 µg/mL full-OPN, 1 µg/mL N-OPN, 1 µg/mL ΔSV N-OPN, 1 µg/mL C-OPN fragment, 1 µg/mL full-OPN plus 0.12 unit thrombin, 10 ng/mL SV peptide, or 10 ng/mL SV scrambled peptide (GYRVLSV). After 24, 48, or 72 h incubation at 37 °C, WST-1 (Dojindo Molecular Technologies, Kumamoto, Japan) solution was added to each well, and after further incubation for 2 h, the absorbance of each well was measured at 415 nm, with the reference wavelength at 630 nm, using a microplate reader.
Boyden chamber assay
Initially, 1 × 104 NHCFs in media containing 0.1 % BSA without FBS were seeded on the upper chamber of a polycarbonate membrane (8 μm pore) coated with 10 μg/mL TypeI-C collagen (Nitta Gelatin, Osaka, Japan). Then, 1 µg/mL full-OPN, 1 µg/mL N-OPN, 1 µg/mL ΔSV N-OPN, 1 µg/mL C-OPN fragment, 1 µg/mL full-OPN plus 0.12 unit thrombin, 10 ng/mL SV peptide, or 10 ng/mL SV scrambled peptide in media containing 0.1 % BSA without FBS was added to the lower chamber as a chemoattractant and incubated in 5 % CO2 at 37 °C. After 3 h, the cells on the lower surface were fixed with 10 % formalin and stained with hematoxylin, and the numbers of chemotaxed cells were counted from the digital images of the stained cells.
Western blotting
The RDFs were treated with or without the TGF-β receptor type I (TβR I) inhibitor SB-505124 (Sigma, St. Louis, MO, USA). After 1 h, the cells were incubated with 5 ng/mL TGF-β, 10 ng/mL SV peptide, or 10 ng/ml SV scrambled peptide. The NHCFs were treated with or without 10 µM RGD peptide, and after 1 h they were incubated with 1 µg/mL full-OPN, 1 µg/mL N-OPN, 1 µg/mL ΔSV N-OPN, 1 µg/mL C-OPN fragment, 1 µg/mL full-OPN plus 0.12 unit thrombin, 10 ng/mL SV peptide, or 10 ng/mL SV scrambled peptide. The cells were lysed in lysis buffer (50 mM Tris at pH 8.0, 120 mM NaCl, 1 mM EDTA, 0.5 % Nonidet P-40) and centrifuged. The supernatants were resolved using SDS-PAGE and transferred to a polyvinylidene fluoride transfer membrane (Millipore, Billerica, MA, USA). The membranes were probed with primary antibodies against TβRI (phospho S165) (Abcam, Cambridge, UK), phospho-Smad3, Smad2/3 (Cell Signaling Technology, Danvers, MA, USA), ERK1/2 (Cell Signaling Technology), phospho-ERK1/2 (Cell Signaling Technology), SMA (Dako, Glostrup, Denmark), and α-tubulin, and detected using an enhanced chemiluminescence kit (GE Healthcare, Piscataway, NJ, USA).
The infarcted left ventricle samples from the infarcted area were homogenized in lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 % [wt/vol] Nonidet P-40, and 0.1 % [wt/vol] SDS). The expression levels of OPN and thrombin in the infarcted myocardium were evaluated using Western blotting, similar to the procedures described previously. Primary antibodies against full-length OPN (Immuno-Biological Laboratories, Gunma, Japan), the N-OPN fragment (Immuno-Biological Laboratories), and thrombin (Abcam) were used.
Quantitative real-time PCR (qRT-PCR)
Total RNA was isolated from the NHCFs and incubated with 1 µg/mL full-OPN, 1 µg/mL N-OPN, 1 µg/mL ΔSV N-OPN, 1 µg/mL C-OPN fragment, 10 ng/mL SV peptide, or 10 ng/ml SV scrambled peptide for 1 h. Quantitative PCR was performed with Power SYBR Green master mix (TOYOBO, Osaka, Japan) in a LightCycler 480 System (Roche Diagnostics, Basel, Swiss). The Ct values for each sample were obtained by subtracting the values for glyceraldehyde-phosphate dehydrogenase (GAPDH) from the target gene (COL I and COL III).
Enzyme-linked immunosorbent assay (ELISA)
The NHCFs were treated with the 10 µM RGD peptide, and after 1 h, incubated with 1 µg/mL full-OPN, 1 µg/mL N-OPN, 1 µg/mL ΔSV N-OPN, 1 µg/mL C-OPN, 10 ng/mL SV peptide, or 10 ng/mL scrambled SV peptide for 120 h. The supernatants were collected, and COL I and COL III production was analyzed using ELISA kits according to the manufacturer’s protocol (COL I, COL III; Uscn Life Science, Wuhan, China). The captured collagen was bound to a second specific monoclonal antibody. The amount of specifically bound monoclonal antibody was detected using an enzyme-labeled antibody. Following incubation with chromogenic substrates, the intensity of the color precipitate was measured.
The infarcted left ventricle samples were homogenized in lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 % [wt/vol] Nonidet P-40, and 0.1 % [wt/vol] SDS). The expression levels of COL I and COL III in the infarcted myocardium were similarly evaluated using ELISA.
Animal model
This animal investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, updated 2011), and the animal study protocols were approved by the Animal Care and Use Committee of Osaka University. The left anterior descending coronary artery (LAD) was ligated in 8-week-old Sprague–Dawley rats under inhalation anesthesia with isoflurane (2.0 %, 0.2 mL/min) administered via endotracheal intubation. The adequacy of anesthesia was monitored by electrocardiography and pulse rate. The rats were anesthetized with pentobarbital (300 mg/kg) and heparin (150 U) by intraperitoneal injection, and the myocardial tissues were removed at 1, 2, and 3 weeks after LAD ligation (normal, 1, 2, and 3 weeks models; n = 3 in each group). The removed myocardia were divided into the infarction and border areas.
Recombinant OPN fragments or SV peptide sustained-release collagen gel treatment
The myocardial infarction (MI) models were generated by ligating the LAD in 8-week-old Sprague–Dawley rats. Two weeks after LAD ligation, sustained-release collagen gels (MedGels®; MedGEL Corporation, Tokyo, Japan) were implanted on the left ventricular anterior wall of the MI model rat, as previously described [23]. The gel incorporated a 1/10 phosphate-buffered saline (PBS) solution containing the recombinant N-OPN fragment, ΔSV N-OPN fragment, SV peptide, or scrambled SV peptide (each 50 µg/gel). The implanted collagen gels were dissolved and absorbed for approximately 2 weeks in vivo, and the physiologically active substance integrated into the gel was released as the gel dissolved [24, 25]. The rats were divided into the following groups: (1) N-OPN, (2) ΔSV N-OPN, (3) SV peptide, and (4) scrambled SV peptide. As a control, a gel containing recombinant OPN or a peptide-free gel containing only 1/10 PBS solution was similarly implanted (n = 5 each group). According to the MedGels® manufacturer’s protocol, the calculated rate of sustained-release of each substance from the gel in vitro was approximately 7.5–10.0 µg/day (Supplement Fig. 1).
Histological analysis
At 4 weeks after gel treatment, the MI model rats were anesthetized with intraperitoneal pentobarbital (200 mg/kg) and heparin (150 U) and their hearts were rapidly removed. The removed myocardia were fixed with 10 % formalin and embedded in paraffin. To estimate the distribution of collagen in the infarcted and border areas, immunohistochemical staining with antibodies against COL I (Abcam), and COL III (Novus Biologicals, Littleton, CO, USA) was performed, and the ratio of COL III/COL I was quantified. Quantitative analysis for each sample was performed using the NIS Elements system (Nikon, Tokyo, Japan). The percentage of fibrosis and expression level of collagen were measured, excluding the perivascular area. The sections were incubated with biotinylated secondary antibody (Dako) and peroxidase-conjugated streptavidin (GE Healthcare). Visualization was performed with a biphenyl-3,3′,4,4′-tetramine solution (Sigma).
Assessment of cardiac function using echocardiography
The cardiac function of the gel-treated rats was monitored by echocardiography before gel implantation (baseline) and 2 and 4 weeks after gel treatment using a SONOS 5500 sonograph (Agilent Technologies, Palo Alto, California). The rats were anesthetized with isoflurane (2 %, 0.2 ml/min) by inhalation, as described above. The left ventricular diastolic (LVDd) and systolic (LVDs) dimensions were measured using M-mode tracing. The LV ejection fraction (LVEF) was calculated using the formula: (LVDd3 − LVDs3)/LVDd3 × 100 (%). Moreover, percentage fractional shortening (%FS) was estimated using the formula: (LVDd − LVDs)/LVDd × 100 (%).
Statistical analyses
Data are expressed as the mean ± standard deviation. To assess the significance of the differences between individual groups, Student t-tests were used. Probability (P) values <0.05 were considered significant.
Results
Effects of the OPN fragment on the proliferation and migration of cardiac fibroblasts
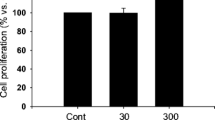
In the WST-1 assays, all OPN fragments and the SV peptide had no effect on the proliferation of NHCFs (Fig. 2a). In the Boyden chamber assay, the N-OPN fragment and SV peptide significantly enhanced the migratory activity of NHCFs (Fig. 2b). In contrast, the ΔSV N-OPN, C-OPN fragment, and scrambled SV peptide had no influence on cell migration activity.
The effects of the thrombin-cleaved osteopontin (OPN) fragment and the SVVYGLR (SV) peptide on motility of the normal human cardiac fibroblast (NHCF) cell line. a Cardiac fibroblast proliferation assessed by the WST-1 assay (three independent tests using different cell culture passages, n = 9 wells). b Migration activity assessed by the Boyden chamber assay (three independent tests using different cell culture passages, n = 9 wells). *P < 0.05 versus the control lacking the SV peptide
TGF-β/Smad signal activation and SMA expression induced by the SV peptide
Phosphorylation of TβRI and Smad3 and the expression level of SMA were enhanced in RDFs with the addition of TGF-β or the SV peptide (Fig. 3a). Furthermore, the addition of the TβRI inhibitor SB-5050124 reduced these increases in the phosphorylation of Smad3 and the expression of SMA by the TGF-β or SV peptide (Fig. 3b).
Analysis of TGF-β/Smad signaling in rat dermal fibroblasts (RDFs) exposed to the SVVYGLR (SV) peptide (a, b). The expression of phospho-TGF-β receptor type I (TβR I) and phospho-Smad3 were evaluated 1 h after the addition of the SV peptide, and the expression of smooth muscle actin (SMA) was evaluated 48 h after. (a, b three independent experiments using different cell lysates). The expression level of SMA after the addition of the thrombin-cleaved osteopontin (OPN) fragments and SV peptide in normal human cardiac fibroblasts (NHCFs). Immunoblotting of the myofibroblast differentiation marker SMA (c) and its quantitative assessment (d). (c, d three independent experiments using different cell lysates). The effects of the thrombin-cleaved OPN fragment and SV peptide on intracellular signal activity. MAPK pathway (e) and Smad signal (f) activation by the addition of the thrombin-cleaved OPN fragment and SV peptide in the presence or absence of the RGD peptide. MAPK pathway and Smad signal activation were estimated 30 min after addition of the thrombin-cleaved OPN fragments and SV peptide, and expression level of SMA was quantified 72 h after. (e, f three independent experiments using different cell lysates)
SMA expression and signaling activity influenced by thrombin-cleaved OPN fragments
The expression level of SMA was increased upon the addition of OPN combined with thrombin, the N-OPN fragment, or the SV peptide (Fig. 3c, d). The expression level of phosphorylated Erk was increased by the addition of OPN, OPN combined with thrombin, N-OPN, or the SV peptide, and the addition of the RGD peptide inhibited these increases in phosphorylated Erk (Fig. 3e). The phosphorylation of Smad3 was also enhanced by the addition of OPN, OPN combined with thrombin, N-OPN, or the SV peptide, and in contrast, the RGD peptide had no effect on Smad3 phosphorylation by these fragments and peptide (Fig. 3f). Increased expression of SMA and signal activation were not seen with the addition of ΔSV N-OPN, the C-OPN fragments, or the scrambled SV peptide.
The effects of the OPN fragments on collagen production
To investigate the effect of the OPN fragments or SV peptide on collagen production in NHCFs, the expression of COL I and COL III mRNA was detected using real-time qRT-PCR after treatment with each OPN fragment or the SV peptide for 1 h. With the addition of N-OPN, the COL I mRNA level was significantly higher than the no-additions control (Fig. 4a). In contrast, the amount of COL III mRNA was significantly increased, compared with the control, with the addition of N-OPN or the SV peptide (Fig. 4b). The ΔSV N-OPN, C-OPN fragment, and scrambled SV peptide had little influence on the amount of collagen mRNA expression (Fig. 4a, b).
The effects of thrombin-cleaved osteopontin (OPN) fragments and the SVVYGLR (SV) peptide on collagen production by normal human cardiac fibroblasts (NHCFs). mRNA expression level of collagen type I (a) and type III (b) after the addition of the thrombin-cleaved OPN fragment or the SV peptide, using quantitative RT-PCR. (a, b five independent experiments using different total RNA amounts). The production quantification of collagen type I and type III using ELISA in the presence (c, e) or absence (d, f) of the RGD peptide. *P < 0.05, **P < 0.01 versus the control with nothing added. (c–f four independent measurements using different cell culture media)
The amount of collagen in the culture media of NHCFs that were incubated with each of the OPN fragments or the SV peptide was measured using ELISA. The amount of COL I was similar among all culture media containing any OPN fragment or the SV peptide (Fig. 4c). COL III production was significantly increased by the addition of the N-OPN fragment and SV peptide compared to the OPN, ΔSV N-OPN, and C-OPN fragments (Fig. 4e). Addition of the RGD peptide had no effect on the collagen production by the OPN fragments and SV peptide (Fig. 4d, f).
OPN and collagen expression in infarcted myocardium
The expression levels of OPN and N-OPN were increased in the infarcted fibrotic area 1 week after LAD ligation (Fig. 5a–c), and thrombin expression was also increased 1 week after ligation (Fig. 5a, d). N-OPN and thrombin expression levels gradually decreased 2 and 3 weeks after ligation (Fig. 5a, c, d).
The interaction between the progression of fibrosis in myocardial infarction and osteopontin (OPN) expression. The expression level of OPN and thrombin in the myocardial infarction rat model by Western blotting analysis (a) and its quantitation of b OPN, c N-OPN, and d Thrombin expression normalized by the corresponding α-tubulin expression levels. The quantification of e collagen type I (normal, 1, 2, and 3 weeks; n = 3 for each model) and f type III (normal, 1, 2, and 3 weeks; n = 3 for each model) in the infarcted wall using ELISA. *P < 0.05, **P < 0.01 versus normal
The amount of collagen in the infarcted wall was analyzed using ELISA. The quantities of COL I and COL III were increased after LAD ligation. The amount of COL I was comparable at 1, 2, and 3 weeks after LAD ligation (Fig. 5e). In contrast, the expression of COL III was highest 1 week after ligation and gradually decreased after 2 and 3 weeks (Fig. 5f). An association was observed between COL III and the N-OPN fragment expression level.
Quantitative estimation of COL I and COL III after gel treatment in a rat model of ischemic cardiomyopathy
The quantity of COL I in the infarcted area 4 weeks after gel treatment was similar among all groups (Fig. 6a). However, the quantity of COL III and the ratio of COL III/COL I in the infarcted area were significantly increased in the N-OPN and SV peptide groups compared with those in the PBS group (Fig. 6b, c). Additionally, in the infarcted border area, immunohistochemical staining showed that the quantities of COL I were significantly smaller in the N-OPN and SV peptide groups than in the PBS group (Fig. 7a, c), and in contrast, the quantity of COL III was significantly higher in the N-OPN and SV peptide groups than in the PBS group (Fig. 7b, d). The COL III/COL I ratio was significantly higher in the N-OPN and SV peptide groups than in the PBS group (Fig. 7e). The expression levels of COL I and COL III in the ΔSV N-OPN and scrambled SV groups were comparable to the PBS groups.
Quantitative estimation of collagen type I and type III in the infarcted area after gel treatment. a Expression level of collagen type I (n = 5/group). b Expression level of collagen type III. *P < 0.05, **P < 0.01 versus the PBS group (n = 5/group). c The ratio of collagen type III/collagen type I. *P < 0.05, **P < 0.01 versus the PBS group (n = 5/group). OPN Osteopontin, SV SVVYGLR, PBS phosphate-buffered saline
The histological assessment in the infarcted border area after gel treatment. a Gel-treated infarcted border area section stained with anti-collagen type I antibody (n = 5/group). Scale bar 100 µm. b Gel-treated infarcted border area section stained with anti-collagen type III antibody (n = 5/group). Scale bar 100 µm. c Expression level of collagen type I. **P < 0.01 versus the PBS group (n = 5/group). d Expression level of collagen type III. *P < 0.05, **P < 0.01 versus the PBS group (n = 5/group). e The ratio of collagen type III/collagen type I. **P < 0.01 versus the PBS group (n = 5/group). OPN Osteopontin, SV SVVYGLR, PBS phosphate-buffered saline
Evaluation of cardiac function after gel treatment
Echocardiography revealed a significant improvement in LVEF and %FS in the N-OPN and SV groups, compared with the PBS, ΔSV N-OPN, and random SV groups 2 and 4 weeks after gel treatment (Fig. 8a, b). Although the values of LVEF and %FS also demonstrated significantly better preserved cardiac function in the ΔSV N-OPN group compared to the PBS and random SV groups, the improvement effects of the N-OPN and SV groups were significantly higher than that of the ΔSV N-OPN group.
Echocardiographic evaluation of left ventricular (LV) function after sheet transplantation a ejection fraction (EF); b % fractional shortening (%FS). **P < 0.01 versus the PBS group, ## P < 0.01 versus the random group, † P < 0.05, †† P < 0.01 versus the ΔSV N-OPN group (n = 5/group). OPN Osteopontin, SV SVVYGLR, PBS phosphate-buffered saline
Discussion
OPN is a multifunctional protein that contains an integrin-binding RGD sequence and a CD44-binding site, and it participates in inflammation, remodeling, and repair responses [3, 4, 6]. OPN is cleaved by thrombin, and a SVVYGLR sequence is exposed by its cleavage. The SVVYGLR sequence binds strongly with integrin α9β1 [2, 7, 8]. Thrombin-cleaved OPN gains new functions, such as cell adhesion, migration, and tube formation via the SVVYGLR motif [10–13]. In this study, we evaluated the effects of the thrombin-cleaved OPN fragment on cardiac fibroblasts and myocardial fibrosis, and the involvement of the SVVYGLR motif in these effects.
During tissue remodeling, fibroblasts migrate into the injured area, where they synthesize and remodel the newly created extracellular matrix. In this process, fibroblasts differentiate into α-SMA-expressing myofibroblasts, which are more contractile and synthetic. In this study, the thrombin cleaved N-OPN fragment and SV peptide induced Smad signal activation, SMA expression, and COL III production in cardiac fibroblasts. In contrast, the N-terminal OPN fragment with the SVVYGLR sequence removed (ΔSV N-OPN) and the C-terminal fragment (C-OPN) did not produce these effects. These data demonstrate that the N-OPN fragment, particularly the SVVYGLR sequence of the N-OPN fragment, has an important effect on these functions. Furthermore, the addition of the RGD peptide had no effect on Smad signal activation or increased collagen production. Integrin signaling is not involved in these functions of the N-OPN fragment or SV peptide, and signaling via other receptors expressed on the fibroblasts likely induced these activities.
TGF-β/Smad signaling is an important pathway associated with fibroblast–myofibroblast differentiation [26, 27], and it regulates collagen production and α-SMA expression in a number of tissue fibrosis conditions in the liver, kidney, and lung [28–30]. In the heart, TGF-β has been identified as a primary mediator of myofibroblast transformation, and it activates a pro-fibrotic and matrix-preserving program in infarct fibroblasts via Smad-dependent signaling [17, 26, 27]. The differentiation of fibroblasts into myofibroblasts is important in the myocardial remodeling process, and myofibroblasts have contractile properties that could provide elasticity to the stiff myocardium of an infarct [31]. Our previous study demonstrated that the OPN-derived peptide SVVYGLR binds with the TGF-β receptor and induces myofibroblast differentiation via TGF-β/Smad signaling [14]. Therefore, in this study, the SVVYGLR sequence of the N-OPN fragment could have also exerted its effects through the TGF-β receptor. In addition, when examining fibroblast motility, the thrombin-cleaved OPN fragments had no effect on proliferation, but fibroblast migration was significantly enhanced by the N-OPN fragment and SV peptide. The added N-OPN fragment would have induced the migration of the fibroblasts and also bound to the TGF-β receptor on the fibroblasts via the SVVYGLR motif. As a result, the fibroblasts would show enhanced SMA expression and differentiation into the myofibroblasts.
The myocardial extracellular matrix is mainly composed of COL I and COL III [32]. The amount and distribution of these collagens in the myocardium are important to cardiac function, and changes in the amount and distribution of collagen can affect the function. The relationship between COL I and COL III has important effects on the mechanical properties of the heart tissue in normal and pathologic conditions [33]. In liver and renal fibrosis, COL III reflects the degree of fibrosis [34, 35]. In the myocardial extracellular environment, the COL III/COL I ratio impacts the diastolic and systolic functions of the heart [36–38]. COL III forms an elastic environment, providing kinetic properties. In contrast, COL I is a stiff fibrillar protein that gives tensile force, and increases in its expression lead to increasing myocardial stiffness, impairing diastolic and systolic function. Furthermore, a decreased COL III/COL I ratio indicates a worsening physiological condition [36, 37, 39, 40]. In this study, the N-OPN and SV peptide enhanced the production of COL III mRNA and protein in cardiac fibroblasts, while they had no influence on the production of COL I proteins. The activation of the TGF-β/Smad pathway triggers both COL I and COL III production. In this study, the expression of the COL III protein was only increased by the addition of the N-OPN and SV peptide to the NHCFs, although the amount of COL I mRNA was increased by the N-OPN. This may be related to the extracellular matrix environment in the MI model of this study, or to differences in the functional mechanisms, via the TGF-β receptor ligand, between TGF-β and the SVVYGLR motif of the N-OPN fragment and the SV peptide. Due to the nature of fibrosis after MI, the infarcted area contains many COL type I fibers, thus the influence of the N-OPN and SV peptide on the COL I fibers may have been less apparent.
In the infarcted myocardium, the expression of N-OPN and COL III in the infarcted area increased 1 week after LAD ligation, and an association between N-OPN and COL III expression level was observed. The increased COL III expression level gradually decreased, and returned to its normal level 2 and 3 weeks after LAD ligation. The amount of COL I was similar at 1, 2, and 3 weeks after LAD ligation. Because COL I has a long half-life, the COL I produced at 1 week could persist through the following weeks. In contrast, COL III is less stable and becomes degraded after the first week. The expression level of thrombin was increased 1 week after LAD ligation. This enhancement was caused by the inflammation due to LAD ligation, and the increased thrombin would cleave the OPN and result in an increase in N-OPN expression at the infarcted area. We prepared an infarction rat model 2 weeks after LAD ligation, which reversed the expression level of COL III, and examined the effects of N-OPN or the SV peptide on collagen production. The transplantation of an N-OPN or SV peptide sustained-release gel into the fibrotic myocardium also increased the quantity of COL III and the ratio of COL III/COL I in both the infarcted area and the infarcted border area. However, implantation of ΔSV N-OPN sustained-release gel did not show these increases. The echocardiography data revealed that cardiac function, measured by the LVEF and %FS, was significantly improved by the N-OPN and SV peptide sustained-release gel treatment, and the increased quantity of COL III and the ratio of COL III/COL I produced by the N-OPN fragment or SV peptide could have caused this improvement in cardiac function. Moreover, the N-OPN fragment or SV peptide could have enhanced the elasticity of the stiffened LV wall by increasing the ratio of COL III/COL I in the infarcted tissue and the border area. The SVVYGLR motif appears to have a central role in the N-OPN-mediated improvement in cardiac function. However, the implantation of ΔSV N-OPN-releasing gel also significantly improved cardiac function compared with the PBS and random SV groups. Therefore, a functional domain on the N-fragment aside from the SVVYGLR motif might also be involved in the improvement in cardiac function by N-OPN.
In summary, we believe that in the process of myocardial fibrosis, OPN is cleaved by thrombin, the generated N-terminal fragment promotes the migration of cardiac fibroblasts to the fibrotic area, and the migrated cardiac fibroblasts differentiate into myofibroblasts and produce collagen, especially COL III via TGF-β/Smad signaling (Fig. 9). The increased distribution of COL III provides elasticity to the fibrotic myocardium and inhibits myocardial remodeling. The N-OPN fragment is an important mediator of the fibrosis process. The SVVYGLR sequence of the N-fragment induced the activation of the Smad signal, SMA expression, and COL III production in cardiac fibroblasts. The N-OPN fragment and SV peptide may provide a new approach for treating cardiac disease.
A working model of the SVVYGLR motif of the thrombin-cleaved N-terminal osteopontin (N-OPN) fragment in cardiac fibroblasts. The SVVYGLR motif of N-OPN binds to the TGF-β receptor and phosphorylates Smad2/3. The phosphorylated Smad2/3 associates with Smad4 and translocates to the nucleus to regulate gene expression, including that of α-SMA. Thus, the SVVYGLR motif of N-OPN induces the differentiation of an increased number of fibroblasts into active myofibroblasts via the TGF-β/Smad pathway. Activated myofibroblasts promote the production of collagen proteins, especially collagen type III, in the myocardial fibrosis process. The increased collagen type III provides elasticity to the fibrotic myocardium, and as a result, cardiac function is improved. TGF-β: transforming growth factor, α-SMA, α-smooth muscle actin
References
Barry ST, Ludbrook SB, Murrison E, Horgan CM (2000) A regulated interaction between alpha5beta1 integrin and osteopontin. Biochem Biophys Res Commun 267:764–769
Ito K, Kon S, Nakayama Y, Kurotaki D, Saito Y, Kanayama M et al (2009) The differential amino acid requirement within osteopontin in alpha4 and alpha9 integrin-mediated cell binding and migration. Matrix Biol 28:11–19
Denhardt DT, Noda M, O’Regan AW, Pavlin D, Berman JS (2001) Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest 107:1055–1061
Subramanian V, Krishnamurthy P, Singh K, Singh M (2007) Lack of osteopontin improves cardiac function in streptozotocin-induced diabetic mice. Am J Physiol Heart Circ Physiol 292:H673–H683
Agnihotri R, Crawford HC, Haro H, Matrisian LM, Havrda MC, Liaw L (2001) Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin). J Biol Chem 276:28261–28267
O’Regan A, Berman JS (2000) Osteopontin: a key cytokine in cell-mediated and granulomatous inflammation. Int J Exp Pathol 81:373–390
Yokosaki Y, Matsuura N, Sasaki T, Murakami I, Schneider H, Higashiyama S et al (1999) The integrin alpha(9)beta(1) binds to a novel recognition sequence (SVVYGLR) in the thrombin-cleaved amino-terminal fragment of osteopontin. J Biol Chem 274:36328–36334
Smith LL, Cheung HK, Ling LE, Chen J, Sheppard D, Pytela R et al (1996) Osteopontin N-terminal domain contains a cryptic adhesive sequence recognized by alpha9beta1 integrin. J Biol Chem 271:28485–28491
Lenga Y, Koh A, Perera AS, McCulloch CA, Sodek J, Zohar R (2008) Osteopontin expression is required for myofibroblast differentiation. Circ Res 102:319–327
Hamada Y, Nokihara K, Okazaki M, Fujitani W, Matsumoto T, Matsuo M et al (2003) Angiogenic activity of osteopontin-derived peptide SVVYGLR. Biochem Biophys Res Commun 310:153–157
Hamada Y, Yuki K, Okazaki M, Fujitani W, Matsumoto T, Hashida MK et al (2004) Osteopontin-derived peptide SVVYGLR induces angiogenesis in vivo. Dent Mater J 23:650–655
Hamada Y, Egusa H, Kaneda Y, Hirata I, Kawaguchi N, Hirao T et al (2007) Synthetic osteopontin-derived peptide SVVYGLR can induce neovascularization in artificial bone marrow scaffold biomaterials. Dent Mater J 26:487–492
Egusa H, Kaneda Y, Akashi Y, Hamada Y, Matsumoto T, Saeki M et al (2009) Enhanced bone regeneration via multimodal actions of synthetic peptide SVVYGLR on osteoprogenitors and osteoclasts. Biomaterials 30:4676–4686
Uchinaka A, Kawaguchi N, Hamada Y, Mori S, Miyagawa S, Saito A et al (2013) Transplantation of myoblast sheets that secrete the novel peptide SVVYGLR improves cardiac function in failing hearts. Cardiovasc Res 99:102–110
Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C (2001) Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell 12:2730–2741
Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G (2001) Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol 159:1009–1020
Dobaczewski M, Bujak M, Li N, Gonzalez-Quesada C, Mendoza LH, Wang XF et al (2010) Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res 107:418–428
Collins AR, Schnee J, Wang W, Kim S, Fishbein MC, Bruemmer D et al (2004) Osteopontin modulates angiotensin II-induced fibrosis in the intact murine heart. J Am Coll Cardiol 43:1698–1705
López B, González A, Lindner D, Westermann D, Ravassa S, Beaumont J et al (2013) Osteopontin-mediated myocardial fibrosis in heart failure: a role for lysyl oxidase? Cardiovasc Res 99:111–120
Xie Z, Singh M, Singh K (2004) Osteopontin modulates myocardial hypertrophy in response to chronic pressure overload in mice. Hypertension 44:826–831
Renault MA, Robbesyn F, Réant P, Douin V, Daret D, Allières C et al (2010) Osteopontin expression in cardiomyocytes induces dilated cardiomyopathy. Circ Heart Fail 3:431–439
Duerr GD, Mesenholl B, Heinemann JC, Zoerlein M, Huebener P, Schneider P et al (2014) Cardioprotective effects of osteopontin-1 during development of murine ischemic cardiomyopathy. Biomed Res Int 2014:124063
Uchinaka A, Kawaguchi N, Mori S, Hamada Y, Miyagawa S, Saito A et al (2014) Tissue inhibitor of metalloproteinase-1 and -3 improves cardiac function in an ischemic cardiomyopathy model rat. Tissue Eng Part A 20(21–22):3073–3084
Yamamoto M, Takahashi Y, Tabata Y (2003) Controlled release by biodegradable hydrogels enhances the ectopic bone formation of bone morphogenetic protein. Biomaterials 24:4375–4383
Tabata Y, Nagano A, Ikada Y (1999) Biodegradation of hydrogel carrier incorporating fibroblast growth factor. Tissue Eng 5:127–138
Leask A, Abraham DJ (2004) TGF-beta signaling and the fibrotic response. FASEB J 18:816–827
Dobaczewski M, Chen W, Frangogiannis NG (2011) Transforming growth factor (TGF)-β signaling in cardiac remodeling. J Mol Cell Cardiol 51:600–606
Pardo A, Gibson K, Cisneros J, Richards TJ, Yang Y, Becerril C et al (2005) Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. PLoS Med 2:e251
Hinz B, Gabbiani G (2010) Fibrosis: recent advances in myofibroblast biology and new therapeutic perspectives. F1000 Biol Rep 2:78
Bataller R, Brenner DA (2005) Liver fibrosis. J Clin Invest 115:209–218
Weber KT, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC (2013) Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol 10:15–26
Weber KT (1989) Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol 13:1637–1652
Lapiere CM, Nusgens B, Pierard GE (1977) Interaction between collagen type I and type III in conditioning bundles organization. Connect Tissue Res 5:21–29
Tsukui T, Ueha S, Abe J, Hashimoto S, Shichino S, Shimaoka T et al (2013) Qualitative rather than quantitative changes are hallmarks of fibroblasts in bleomycin-induced pulmonary fibrosis. Am J Pathol 183:758–773
Wolak T, Kim H, Ren Y, Kim J, Vaziri ND, Nicholas SB (2009) Osteopontin modulates angiotensin II-induced inflammation, oxidative stress, and fibrosis of the kidney. Kidney Int 76:32–43
Pauschinger M, Knopf D, Petschauer S, Doerner A, Poller W, Schwimmbeck PL et al (1999) Dilated cardiomyopathy is associated with significant changes in collagen type I/III ratio. Circulation 99:2750–2756
Marijianowski MM, Teeling P, Mann J, Becker AE (1995) Dilated cardiomyopathy is associated with an increase in the type I/type III collagen ratio: a quantitative assessment. J Am Coll Cardiol 25:1263–1272
Kitamura M, Shimizu M, Ino H, Okeie K, Yamaguchi M, Funjno N et al (2001) Collagen remodeling and cardiac dysfunction in patients with hypertrophic cardiomyopathy: the significance of type III and VI collagens. Clin Cardiol 24:325–329
Yoshikane H, Honda M, Goto Y, Morioka S, Ooshima A, Moriyama K (1992) Collagen in dilated cardiomyopathy–scanning electron microscopic and immunohistochemical observations. Jpn Circ J 56:899–910
Bishop JE, Greenbaum R, Gibson DG, Yacoub M, Laurent GJ (1990) Enhanced deposition of predominantly type I collagen in myocardial disease. J Mol Cell Cardiol 22:1157–1165
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Uchinaka, A., Hamada, Y., Mori, S. et al. SVVYGLR motif of the thrombin-cleaved N-terminal osteopontin fragment enhances the synthesis of collagen type III in myocardial fibrosis. Mol Cell Biochem 408, 191–203 (2015). https://doi.org/10.1007/s11010-015-2495-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2495-y