Abstract
Cryptotanshinone (CPT) is a natural compound extracted from herbal medicine that has been previously shown to possess antitumor properties in various types of human cancer cells. In the present study, we examined the potential role of CPT in the treatment of colorectal cancer. Using SW480, HCT116, and LOVO colorectal cancer cell lines, the effects of CPT on cell viability, apoptosis, and tumorigenicity were evaluated. The results showed that CPT significantly inhibited the growth and viability of SW480, HCT116, and LOVO cell lines by inducing apoptosis and prevented anchorage dependent growth on agar. In addition, CPT inhibited the activation of Signal transducer and activator of transcription 3 (Stat3) pathways in colorectal cancer cells. Stat3 is a transcription factor that mediates the expression of various genes associated with many cellular processes, such as inflammation and cell growth, and has been shown to promote several cancer types, including colorectal cancer. These findings indicate that CPT may be a potential candidate for the treatment and prevention of colorectal cancer in part by inhibiting the activation of Stat3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer is the third most common cancer worldwide and the fourth most common cause of cancer-related mortality [1]. Despite the availability of screening test, approximately more than half of the colorectal cancer cases are diagnosed at late stages when treatment is more difficult [2]. Chemotherapy is the primary intervention for the treatment of colorectal cancer; however, the use of chemotherapy is limited due to its side effects, which significantly impact the patient quality of life. Consequently, many efforts are being made to find alternative agents with fewer side effects to complement conventional chemotherapy. The use of herbal medicine derived from traditional Chinese medicine is an alternative approach for the prevention and treatment of cancer with fewer side effects.
STAT (Signal Transducer and Activator of Transcription) proteins regulate many aspects of growth, survival, and differentiation in cells. There are seven mammalian Stat family members that have been identified: Stat1, Stat2, Stat3, Stat4, Stat5 (Stat5α and Stat5β), and Stat6. Stat3 appears to be more generally transcribed than the other members, and recent data demonstrate its role in a wide variety of physiological processes. Stat3 has been implicated in cancer, following the discovery that Stat3 is required for malignant transformation in v-SRC-transformed cells [3]. Activated Stat3 has been observed in malignant cells and is capable of inducing the expression of a large number of genes involved in tumorigenesis, thus becoming a suitable target for preventing and treating cancer [4–6]. In the case of colorectal cancer, Stat3 activation is associated with adverse clinical outcome [7].
Cryptotanshinone (CPT) is a quinoid diterpene purified from the root of medicinal plant Danshen (Salvia miltiorrhiza) that has been widely used in the clinic for various diseases, including cardiac fibrosis [8], acute lung injury [9], and arthritis [10]. CPT has been previously shown to inhibit growth and induce apoptosis in a number of human cancer cell lines [11]. In prostate cancer cells, CPT induced cell apoptosis by suppressing mammalian target of rapamycin (mTOR)-mediated cyclin D1 expression [12] and inhibiting Stat3 activity [13]. In myeloid leukemia cells, CPT induced apoptosis by inhibiting the eukaryotic initiation factor 4E (eIF4E) regulatory system [14] and through ROS-dependent activation of caspase-8 and p38 MAPK [15]. While the anticancer activities of CPT against diverse types of cancer cells have been reported, the efficacy and molecular mechanisms of CPT remain unclear in colorectal cancer cells.
The current study investigated the efficacy of CPT against human colorectal cancer in vitro. Previous research has suggested antitumor effects of CPT and its ability to inhibit Stat3 activation. We evaluated the effect of CPT on human colorectal cancer cell viability, cell apoptosis, tumorigenicity, and Stat3 signaling.
Materials and methods
Reagents
CPT (purity ≥ 99 % purity by high-performance liquid chromatography, with chemical structure as shown in Fig. 1a) was supplied by National Food and Drug Control Institute, China. CPT was dissolved in DMSO to prepare a 50 mM stock solution (with a final DMSO concentration ≤0.1 %) and stored in −20 °C. RPMI-1640, Dulbecco’s Modified Eagle’s Medium (DMEM), and 0.25 % Trypsin–EDTA were purchased from Gibco (Life Technologies, Grand Island, NY, USA). Fetal Bovine Serum (FBS) was purchased from Atlantic Biological. TACS MTT Cell Proliferation Assay kits were ordered from Trevigen (Helgerman, Gaithersburg, MD, USA). RIPA buffer was purchased from Thermo Scientific (Thermo scientific, Rockford, IL, USA). Annexin V-FITC Apoptosis Detection Kit was purchased from BD Biosciences (San Diego, CA, USA).
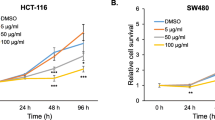
Cryptotanshinone inhibits colorectal cell viability. a Chemical structure of Cryptotanshinone. 2 × 104 cells b HCT1116, c SW480, d LOVO were seeded into 96-well plates and after 24 h were treated with 5–100 µmol/L CPT or 0.1 % DMSO. At 24, 48, and 72 h after CPT treatment, the cell viability was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay as described in “Materials and methods” section. Absorbance at 570 nm was detected with Microplate reader. The experiments were independently performed at least twice, each in triplicate. *p < 0.05 and **p < 0.01 indicate a significant difference compared with DMSO control by t test. CPT Cryptotanshinone
Antibodies
p-Stat3Tyr705, Stat3, Cyclin D1, Survivin, cleaved-caspase-3, Bcl-xl, Bcl-2, p21, p27, p-EGFR, EGFR, and goat-anti-rabbit and anti-mouse horseradish peroxidase (HRP) conjugates were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti-β-actin was ordered from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
HCT116, SW480, and LOVO colon cancer cell lines were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA) and Division of Cancer Treatment and Diagnosis (DCTD) Tumor Repository (National Cancer Institute, USA). HCT116 and LOVO cells were cultured in RPMI1640 supplemented with 10 % FBS, 1 % penicillin streptomycin, and 1 % l-Glutamine. SW480 cells were cultured in DMEM supplemented with 10 % FBS, 1 % penicillin streptomycin, and 1 % l-Glutamine. Cultures were maintained in a humidified incubator at 37 °C in 5 % CO2.
Cell viability assay
Cells were seeded at a density of 2 × 104 cells per well in a flat-bottomed 96-well plate and cultured for 24 h. Cells were treated with CPT (5, 10, 20, 40, 80, and 100 µM) or 0.1 % DMSO for 24, 48, and 72 h. Subsequently, 10 µl MTT reagent was added into each well and incubated for another 4 h until purple dye is visible. 100 µl of detergent reagent was added to the plate and was incubated at room temperature in the dark for 2–4 h. Absorbance was measured at 570 nm with BIO-RAD Model 680 Microplate reader according to the instruction of the TACS MTT Cell Proliferation Assay kits (Trevigen, Helgerman, Gaithersburg, MD, USA). MTT Cell Proliferation Assay kit measures viable cells that have active NAD(P)H-dependent cellular oxidoreductase activity and not dead or quiescence cells. The relative cell viability was expressed as the ratio of the optical density of CPT-treated cells to that of DMSO control cells. The experiments were independently performed at least twice, each in triplicate.
Cell apoptosis assay
HCT116 and SW480 cells were seeded in 100-mm dishes at a density of 2 × 106 cells per dish in growth medium and grown overnight at 37 °C in a humidified incubator with 5 % CO2. Cells were treated with CPT (5, 10, 25, 50 µM) or 0.1 % DMSO for 24 h. Apoptosis was determined with the Annexin V-FITC Apoptosis Detection Kit I (BD Biosciences, San Diego, CA, USA). The experiments were independently performed at least twice, each in triplicate.
Soft agar assay
HCT116 cells and SW480 cells were trypsinized and resuspended at 6000 cells in 2x RPMI media. Cells were treated with 0.1 % DMSO or CPT (5, 10, 25, and 50 µM). The bottom layer consisted of 2 ml of 1.2 % agarose. The cell suspensions were mixed 1:1 with 0.5 % agarose (2 ml/well for a 6 well plate) and layered on top. The cells were maintained in an incubator for 10 days after which the colonies were stained with Nitrotetrazolium Blue chloride (NBT), scanned, and counted with GelCount (Oxford Optronix Ltd, Oxford United Kingdom). The experiments were independently performed at least twice, each in triplicate.
Western blotting
Colon cancer cells were seeded 2 × 104 on 100-mm plates and treated with either DMSO vehicle (0.1 %) or various concentrations of CPT (5, 10, 25, and 50 µM) for 24 h or 25 µM CPT for various times (0, 0.5, 1, 4, 8, 12,24, and 36 h). The whole-cell protein extracts were isolated using RIPA lysis buffer (Thermo scientific, Rockford, IL, USA) containing protein inhibitor cocktail (Roche, Mannheim, Germany). Protein concentration was determined by Micro BCA Protein Assay Kit (Thermo Scientific, Rockford, IL, USA). Approximately 30 μg total protein was loaded and fractionated by SDS-PAGE, transferred to nitrocellulose membrane (Bio-Rad), and probed with primary antibodies: p-Stat3Tyr705, Stat3, Cyclin D1, Survivin, cleaved-caspase-3, Bcl-xl, Bcl-2, p21, p27, and p-EGFR, EGFR. Signal was detected using Super Signal West Femto Maximum Sensitivity Substrate (Thermo Scientific, Rockford, IL, USA) and quantified by optical densitometry with Image J software. The experiments were independently performed at least twice, each in triplicate.
Statistical analyses
All data are presented as the mean ± standard deviation. The significance of the difference between groups was evaluated by one-way repeated-measures analysis of variance (ANOVA) or Student’s t test, and multiple comparisons with Prism 5.0 software. P < 0.05 was considered to be statistically significant.
Results
CPT inhibits colorectal cancer cell viability
To study the effect of CPT on cell viability in HCT116, SW480, and LOVO colorectal cancer cell lines, MTT assay was performed. Cells were treated with various concentrations of CPT (5, 10, 20, 40, 80, and 100 µM) for 24, 48, and 72 h. CPT substantially reduced the cell viability of HCT116, SW480 and LOVO colorectal cancer cell lines in a dose- and time-dependent manner with an IC50 of 11, 12, and 9 µM, respectively (Fig. 1b–d) after 72 h. CPT treatment had no effect on the cell viability of non-tumor human embryonic kidney HEK293 cells (Supplementary Fig. S1). These results show that CPT exerts cytotoxic effects on HCT116, SW480, and LOVO colorectal cell lines, but not on non-tumor cells. Based on the chemosensitivity MTT assay, further analysis was limited to HCT116 and SW480 cell lines, which displayed a similar IC50 and excluded the exquisitely sensitive LOVO cell line.
CPT induces apoptosis in colorectal cancer cells
CPT treatment significantly attenuated the cell viability of HCT116 and SW480 cells. To determine if the effect of CPT on cell viability is mediated by apoptosis, FITC Annexin V apoptosis detection assay was performed. Consistently, apoptosis analysis showed an increased accumulation in the number of apoptotic cells in a dose-dependent manner in both HCT116 and SW480 cell lines after being treated with CPT for 24 h (Fig. 2a, b). Treatment with 25 µM of CPT resulted in a 48 and 26 % increase in apoptotic cells in HCT116 and SW480 cells, respectively (p < 0.05). To confirm these results, the activation of caspase-3, a key molecule in the intrinsic apoptosis pathway, was evaluated by Western blot. As expected, CPT induced the cleavage of caspase-3 in a dose-dependent manner (Fig. 2c, d). Bcl-2 is a key anti-apoptosis regulator that is often over-expressed in human cancers and can result in chemotherapy resistance [16–18]. Our results show that CPT inhibited Bcl-2 level in a dose-dependent manner (Fig. 2c, d). Taken together, these results suggest that the effect of CPT on colorectal cancer cell viability is at least in part mediated by apoptosis.
Cryptotanshinone induces colorectal cell apoptosis. a HCT116 cells or b SW480 cells were grown in 100-mm dishes (2 × 106 cells per dish) and treated with indicated concentration of Cryptotanshinone (5-50 µmol/L) for 24 h. Cells were stained with Annexin V-FITC and Propidium Iodide, followed by flow cytometric analysis. Results are shown as mean ± SE (n = 3). The experiments were independently performed at least twice, each in triplicate. *p < 0.05 and **p < 0.01 indicate a significantly different compared with DMSO control. c CPT activated cleavage of caspase-3 and inhibited Bcl-2. HCT116 cells or SW480 cells were treated with 5–50 µmol/L CPT for 24 h. Cleaved caspase-3 and Bcl-2 expression were analyzed by Western blot. Blots are representatives of three independent experiments. d Densitometry analyses of Western blots were determined by Image J to quantify the relative protein levels of Bcl-2 versus β-actin and Cleaved Caspase-3 versus β-actin. Three independent experiments were analyzed. *p < 0.05 and **p < 0.01 indicate a significant difference compared with DMSO control by t test
CPT inhibits Stat3 activation in colorectal cancer cells
We show that CPT can induce apoptosis in HCT116 and SW480 cells. Previous reports show that CPT can inhibit Stat3 activity in other cell types [13, 19], and Stat3 is known to regulate gene products associated with cell cycle progression [20–22]. To determine if CPT can inhibit Stat3 activation in colorectal cancer cell lines, we evaluated Stat3 phosphorylation by Western blot. HCT116 and SW480 cells were treated with various concentrations of CPT (5, 10, 25, and 50 µM) for 24 h and treated with 25 µM CPT for various times (0, 0.5, 1, 4, 8, 12, 24, and 36 h). CPT significantly inhibited the phosphorylation of Stat3 in a dose- and time-dependent manner in both HCT116 and SW480 cell lines (Fig. 3). The inhibition of Stat3 was achieved at a much lower concentration and significantly shorter time in HCT116 cells compared to SW480 cells (Fig. 3a, b). In addition, CPT inhibited phosphorylation of EGFR (Epidermal growth factor receptor), an upstream regulator of Stat3 activation [23], in HCT116 and SW480 cell lines (Fig. 4a, b); however, EGFR inactivation was only observed at higher concentrations in both the HCT116 and SW480 cell lines. Thus, STAT3 inhibition by CPT is likely not mediated by changes in EGRF activity.
Cryptotanshinone suppresses Stat3 phosphorylation in colorectal cancer cells. HCT116 and SW480 cells were seeded as 2 × 104 in 100-mm plates and after 24 h were treated with 0.1 % DMSO, 5, 10, 25, or 50 µmol/L CPT. After 24 h of treatment, extracts were analyzed by Western blotting analysis. a, c Total and phosphorylated Stat3. Representative blots of two colon cancer cell lines HCT116 (a) SW480 (c). Blots are representative of three independent experiments. b, d Inhibition of Stat3 by CPT treatment. Time course inhibition of Stat3 b and d in HCT1116 and SW480 cells treated with 25 µmol/L CPT from 0 to 36 h and analyzed by Western blot. Phospho-Stat3 (p-Stat3). Blots are representative of three independent experiments
Cryptotanshinone inhibits phosphorylation of EGFR in colorectal cancer cells. HCT116 and SW480 cells were seeded as 2 × 104 in 100-mm plates and after 24 h were treated with 0.1 % DMSO, 5, 10, 25, or 50 µmol/L CPT. After 24 h of treatment, extracts were analyzed by Western blotting analysis. a Total and phosphorylated EGFR representative blots of two colon cancer cell lines HCT116 and SW480. Blots are representative of three independent experiments. b Densitometry analyses of Western blots were determined by Image J to quantify the relative protein levels of p-EGFR versus EGFR. Three independent experiments were analyzed. *p < 0.05 indicates a significant difference compared with DMSO control by t test
CPT inhibits CyclinD1 and survivin in colorectal cancer cells
Stat3 activity has been implicated in promoting various types of human cancers by regulating gene products involved in cell survival and proliferation [24, 25]. CyclinD1 and Survivin are two well-known examples of Stat-related survival gene products [26, 27]. CyclinD1 is a major cell cycle regulatory protein that functions as a cofactor for several transcription factors [28] and its overexpression has been linked to the development and progression of cancer [29]. Survivin is a negative regulator of apoptosis that can inhibit the activation of caspases to promote cellular survival under otherwise apoptotic conditions [30]. Our results show that treating HCT116 and SW480 cells with CPT significantly attenuated the expression of both CyclinD1 and Survivin in a dose-dependent and time-dependent manner (Fig. 5).
Cryptotanshinone inhibits CyclinD1 and Survivin and induces p21and p27 in colorectal cancer cells. Expression levels of candidate Stat3 targets, Cyclin D1 and Survivin, were inhibited by CPT. a, c HCT116 cells or SW480 cells were treated with 0.1 % DMSO or 5–50 µmol/L CPT for 24 h. Cyclin D1, Survivin expression was analyzed by Western blot. Blots are representatives of three independent experiments. Time course inhibition of Cyclin D1 and Survivin in HCT1116 cells (b) or SW480 cells (d) treated with 25 µmol/L CPT from 0 to 36 h and analyzed by Western blot. Phospho-Stat3 (p-Stat3). Blots are representative of three independent experiments. CPT induces p21 in colorectal cancer cells. a, c HCT116 cells or SW480 cells were treated with 0.1 % DMSO or 5–50 µmol/L CPT for 24 h. p21 expression was analyzed by Western blot. Blots are representatives of three independent experiments. Time course activation of p21 in HCT1116 cells (b) or SW480 cells (d) treated with 25 µmol/L CPT from 0 to 36 h and analyzed by Western blot. Blots are representative of three independent experiments. e HCT116 cells or SW480 cells were treated with 0.1 % DMSO or 5–50 µmol/L CPT for 24 h. p27 expression was analyzed by Western blot. Blots are representatives of three independent experiments
CPT induces p21 and p27 expression in colorectal cancer cells
The cyclin-dependent kinase inhibitor p21 (also known as p21WAF1/Cip1) promotes cell cycle arrest in response to many stimuli. It can function as both a sensor and an effector of multiple anti-proliferative signals [31]. In addition to inhibiting Stat-related survival gene products, we also show that CPT can induce p21 expression in a dose- and time-dependent manner in both HCT116 and SW480 cells (Fig. 5). CPT can also induce p27 expression in colorectal cancer cells (Fig. 5e), which is frequently down-regulated in cancer and correlates with poor prognosis [32, 33].
CPT inhibits colorectal cancer cell oncogenic phenotypes
To evaluate the effects of CPT on tumor phenotypes of CRC cells, soft agar assay was performed. Soft agar assays are commonly used to evaluate the efficacy and sensitivity of chemotherapeutic agents by monitoring anchorage-independent three-dimensional colony formation in a semi-solid culture medium. This method delivers results that are comparable to those obtained when injecting tumorigenic cells into nude mice [34]. Following pre-treatment with CPT, colorectal cancer cells, HCT116 or SW480 cell lines, were placed in soft agar and colonies were counted after 10 days. As shown in Fig. 6, treatment with 5, 10, 25, and 50 µM of CPT provided significant concentration-dependent inhibition of colony formation by 79, 84, 90, and 95 percent, respectively, (p < 0.05) compared to control. These results show that CPT affected the ability of colorectal cancer cells to form colonies in soft agar, which may be contributed to the inhibition of the Stat3 pathway.
Cryptotanshinone inhibits colorectal cancer cells anchorage independent growth. a HCT116 cells or SW480 cells were treated with vehicle (0.1 % DMSO) or 5–50 µmol/L CPT, for 10 days in soft agar, and the average colony numbers were counted. b and c show the colony numbers in HCT116 cells or SW480 cells treated by CPT, Column, mean of triplicate samples, bars, standard deviation, **p < 0.01 versus DMSO control cells. Vehicle (0.1 % DMSO), CPT Crypotanshinone
Discussion
The use of Chinese herbal medicines and natural products has received considerable attention in recent years for the prevention and treatment of many health conditions. CPT is one of the active constituents extracted from the dry root and rhizome of medicinal plant Salvia miltiorrhiza (Chin. Danshen) and has been used clinically in China for centuries as an anticoagulant for ischemic syndromes and for the prevention and treatment of Alzheimer’s disease [35]. More recently, CPT has been shown to possess anticancer properties in human prostate, leukemic, melanoma, and glioma cancer cells [11, 19, 36–38]. The efficacy and mechanism of CPT in colon cancer cells has yet to be investigated. In the present study, we investigated the anticancer activity of CPT in colorectal cancer. The concentration of CPT used in the current study was based on that of a previous study in mice where a 25 mg/kg dose of CPT (human equivalent dose of 2.34 mg/kg) was used and found to be well tolerated with no reported toxicity [39]. In addition, pharmacokinetic studies of CPT carried out in rats, mice, and pigs [40, 41] revealed that CPT is readily absorbed with an appreciable bioavailability with the highest plasma concentrations found in liver, lung, brain, and heart, with the lowest concentration in spleen and kidney [42]. We show that CPT can effectively hinder the cell viability of HCT116, SW480, and LOVO human colorectal cancer cells (IC50 12, 11, and 9 µM, respectively), with no apparent effect on the viability of non-tumor cells. CPT anticancer effects appear to be mediated by cell cycle arrest and induction of apoptosis in colorectal cancer cells with no evidence of apoptosis in non-tumor HEK293 cells. These results were accompanied by a significant reduction in colony formation on agar by CPT, indicating that CPT can prevent tumorigenesis in colorectal cancer cells. Here, we also report that CPT can inhibit the activation of Signal transducer and activator of transcription 3 (Stat3).
Cryptotanshinone inhibits Stat3 activation
Stat3 is a transcription factor that has become a suitable molecular target for cancer prevention and therapy. Stat3 activation is associated with poor prognosis in colorectal cancer [7]. Stat3 can regulate genes involved in proliferation, survival, tumor-associated inflammation, and angiogenesis, thus promoting tumorigenesis and cancer progression [43, 44]. Blockade of Stat3 was shown to induce apoptosis in colorectal cancer cells [45, 46] and retard tumor growth in colorectal cancer xenografts [46]. In our study, we found that CPT inhibits Stat3 activation in HCT116 and SW480 colorectal cancer cells in a dose- and time-dependent manner. This is consistent with that of previous reports that demonstrates CPT ability to rapidly inhibit Stat3 activation through JAK2-independent mechanisms in glioma cancer cells [19], lung cancer cells [47], and prostate cancer cells [13]. In the case of prostate cancer cells, the mechanism involved the direct binding to the Stat3 monomer preventing downstream dimerization [13]. CPT appears to target the activation of endogenous Stat3, thus should predominately target cancer cells that have higher activation of Stat3 and minimal effect on non-tumor cells (HEK293) cells that have limited Stat3 activity unless in the presence of pro-inflammatory cytokines [6]. EGFR is an upstream regulator of Stat3 activation [48]. Consistent with the proposed mechanism of action of CPT by direct interaction with Stat3, we observed only a minor inhibition of EGFR phosphorylation at higher dosages of CPT (Fig. 4), thus suggesting that pSTAT inactivation by CPT is downstream of EGFR and independent of EGFR inactivation.
Cryptotanshinone blockade of Stat3-related targets and cell apoptosis
Stat3 signaling is involved in cancer progression by upregulating genes promoting cell cycle progression (cyclin D1 and c-myc) and/or preventing apoptosis (Bcl-xL, Bcl-2, and Survivin) [20, 49–51]. In our study, we found that CPT reduced the expression level of anti-apoptosis proteins, Bcl-2, CyclinD1, and Survivin in HCT116 and SW480 colorectal cancer cell lines. Apoptosis induction by CPT was confirmed using FITC Annexin V apoptosis detection assay and by activation of caspase-3. p21WAF1/CIP1 and p27 are important inhibitors of cell proliferation by its interactions with complexes of cyclins and cyclin-dependent kinases (CDK) [52] and are also involved in the regulation of apoptosis [53]. Our results show that CPT can also induce p21 and p27 expression in HCT116 and SW480 colorectal cancer cells. The down-regulation of Bcl-2 and Survivin may be linked with the ability of CPT to induce cell death in colorectal cancer cells. Further studies using constitutively active Stat3 cell lines are necessary to confirm that CPT-mediated Stat3 inhibition is responsible for the precise apoptotic induction seen with CPT treatment.
Possible role of CPT in combination therapies
The effect of CPT on cell growth was evaluated in the present study by performing soft agar assays. This traditional method has been widely published and delivers results that are predictive of those seen in xenograft studies [54, 55]. Notably, in the present study, incubation with 5 µM of CPT alone provided significant inhibition of colony formation by both HCT116 and SW480 colorectal cell lines. There are currently a number of drug combinations approved for the treatment of colorectal cancer; however, despite the therapeutic potential, serious adverse effects can limit the use of most drugs. CPT may be a potential agent to sensitize tumor cells to be combined with conventional therapies. CPT has been shown to suppress doxorubicin efflux in doxorubicin-resistant HEPG2 hepatic liver cells [56] and radio sensitize HeLA cervical cancer cell lines [37, 57]. In the near future, we plan to investigate whether CPT can have synergistic effects in colorectal cancer cells with conventional chemotherapy drugs.
In summary, Stat3 has emerged as one of the most attractive targets for the treatment of cancer. Our findings demonstrate the anticancer activity of CPTin colorectal cancer cell line. CPT has been previously shown to directly bind to Stat3 inhibiting its activity in prostate cancer cells [13], and our study demonstrates that CPT can attenuate the phosphorylation of Stat3 in colorectal cancer cells. CPT also inhibited stat3-related gene products, including cyclinD1, Bcl-2, and Survivin in colorectal cancer cell lines (Fig. 7). The specificity of CPT in affecting the viability of tumor cells sparing non-tumor cells together with reported low patient toxicity makes CPT a strong candidate for combination therapy for the treatment of colorectal cancer.
Proposed mechanisms of action of CPT in suppressing colorectal cancer. CPT inhibits Stat3 phosphorylation and downstream targets CyclinD1, Survivin, and Bcl-2 and stimulates the cleavage of caspase-3. CPT treatment also induces p21 and p27, proteins involved in modulating cell cycle arrest. Interactions leading to activation of molecular targets are indicated by arrows; those that inhibited are indicated by a bar
References
Inoue-Choi M, Lazovich D, Prizment AE, Robien K (2013) Adherence to the World Cancer Research Fund/American Institute for Cancer Research recommendations for cancer prevention is associated with better health-related quality of life among elderly female cancer survivors. J Clin Oncol 31:1758–1766. doi:10.1200/JCO.2012.45.4462
Haggar FA, Boushey RP (2009) Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg 22:191–197. doi:10.1055/s-0029-1242458
Garcia R, Bowman TL, Niu G, Yu H, Minton S, Muro-Cacho CA, Cox CE, Falcone R, Fairclough R, Parsons S, Laudano A, Gazit A, Levitzki A, Kraker A, Jove R (2001) Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene 20:2499–2513. doi:10.1038/sj.onc.1204349
Germain D, Frank DA (2007) Targeting the cytoplasmic and nuclear functions of signal transducers and activators of transcription 3 for cancer therapy. Clin Cancer Res 13:5665–5669. doi:10.1158/1078-0432.CCR-06-2491
Lau GK, Ye D (2010) STAT3 implicated in the development of colon cancer: a step closer for targeted therapy? Gastroenterology 139:353–355. doi:10.1053/j.gastro.2010.05.030
Chen X, Du Y, Nan J, Zhang X, Qin X, Wang Y, Hou J, Wang Q, Yang J (2013) Brevilin A, a novel natural product, inhibits janus kinase activity and blocks STAT3 signaling in cancer cells. PLoS ONE 8:e63697. doi:10.1371/journal.pone.0063697
Morikawa T, Baba Y, Yamauchi M, Kuchiba A, Nosho K, Shima K, Tanaka N, Huttenhower C, Frank DA, Fuchs CS, Ogino S (2011) STAT3 expression, molecular features, inflammation patterns, and prognosis in a database of 724 colorectal cancers. Clin Cancer Res 17:1452–1462. doi:10.1158/1078-0432.CCR-10-2694
Ma Y, Li H, Yue Z, Guo J, Xu S, Xu J, Jia Y, Yu N, Zhang B, Liu S, Liu M, Shao W, Chen S, Liu P (2014) Cryptotanshinone attenuates cardiac fibrosis via downregulation of COX-2, NOX-2 and NOX-4. J Cardiovasc Pharmacol. doi:10.1097/FJC.0000000000000086
Tang Y, Chen Y, Chu Z, Yan B, Xu L (2014) Protective effect of cryptotanshinone on lipopolysaccharide-induced acute lung injury in mice. Eur J Pharmacol 723:494–500. doi:10.1016/j.ejphar.2013.10.019
Zheng FL, Chang Y, Jia XY, Huang M, Wei W (2011) Effects and mechanisms of Cryptotanshinone on rats with adjuvant arthritis. Chin Med J (Engl) 124:4293–4298
Chen W, Liu L, Luo Y, Odaka Y, Awate S, Zhou H, Shen T, Zheng S, Lu Y, Huang S (2012) Cryptotanshinone activates p38/JNK and inhibits Erk1/2 leading to caspase-independent cell death in tumor cells. Cancer Prev Res (Phila) 5:778–787. doi:10.1158/1940-6207.CAPR-11-0551
Chen W, Luo Y, Liu L, Zhou H, Xu B, Han X, Shen T, Liu Z, Lu Y, Huang S (2010) Cryptotanshinone inhibits cancer cell proliferation by suppressing Mammalian target of rapamycin-mediated cyclin D1 expression and Rb phosphorylation. Cancer Prev Res (Phila) 3:1015–1025. doi:10.1158/1940-6207.CAPR-10-0020
Shin DS, Kim HN, Shin KD, Yoon YJ, Kim SJ, Han DC, Kwon BM (2009) Cryptotanshinone inhibits constitutive signal transducer and activator of transcription 3 function through blocking the dimerization in DU145 prostate cancer cells. Cancer Res 69:193–202. doi:10.1158/0008-5472.CAN-08-2575
Ge Y, Cheng R, Zhou Y, Shen J, Peng L, Xu X, Dai Q, Liu P, Wang H, Ma X, Jia J, Chen Z (2012) Cryptotanshinone induces cell cycle arrest and apoptosis of multidrug resistant human chronic myeloid leukemia cells by inhibiting the activity of eukaryotic initiation factor 4E. Mol Cell Biochem 368:17–25. doi:10.1007/s11010-012-1338-3
Kim JH, Jeong SJ, Kwon TR, Yun SM, Jung JH, Kim M, Lee HJ, Lee MH, Ko SG, Chen CY, Kim SH (2011) Cryptotanshinone enhances TNF-alpha-induced apoptosis in chronic myeloid leukemia KBM-5 cells. Apoptosis 16:696–707. doi:10.1007/s10495-011-0605-1
Youle RJ, Strasser A (2008) The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9:47–59. doi:10.1038/nrm2308
Adams JM, Cory S (2007) The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 26:1324–1337. doi:10.1038/sj.onc.1210220
Bai L, Chen J, McEachern D, Liu L, Zhou H, Aguilar A, Wang S (2014) BM-1197: a novel and specific Bcl-2/Bcl-xL inhibitor inducing complete and long-lasting tumor regression in vivo. PLoS ONE 9:e99404. doi:10.1371/journal.pone.0099404
Lu L, Li C, Li D, Wang Y, Zhou C, Shao W, Peng J, You Y, Zhang X, Shen X (2013) Cryptotanshinone inhibits human glioma cell proliferation by suppressing STAT3 signaling. Mol Cell Biochem 381:273–282. doi:10.1007/s11010-013-1711-x
Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE Jr (1999) Stat3 as an oncogene. Cell 98:295–303
Lin L, Liu A, Peng Z, Lin HJ, Li PK, Li C, Lin J (2011) STAT3 is necessary for proliferation and survival in colon cancer-initiating cells. Cancer Res 71:7226–7237. doi:10.1158/0008-5472.CAN-10-4660
Lin L, Fuchs J, Li C, Olson V, Bekaii-Saab T, Lin J (2011) STAT3 signaling pathway is necessary for cell survival and tumorsphere forming capacity in ALDH(+)/CD133(+) stem cell-like human colon cancer cells. Biochem Biophys Res Commun 416:246–251. doi:10.1016/j.bbrc.2011.10.112
Zheng Q, Han L, Dong Y, Tian J, Huang W, Liu Z, Jia X, Jiang T, Zhang J, Li X, Kang C, Ren H (2014) JAK2/STAT3 targeted therapy suppresses tumor invasion via disruption of the EGFRvIII/JAK2/STAT3 axis and associated focal adhesion in EGFRvIII-expressing glioblastoma. Neuro Oncol 16:1229–1243. doi:10.1093/neuonc/nou046
Klampfer L (2006) Signal transducers and activators of transcription (STATs): novel targets of chemopreventive and chemotherapeutic drugs. Curr Cancer Drug Targets 6:107–121
Kusaba T, Nakayama T, Yamazumi K, Yakata Y, Yoshizaki A, Inoue K, Nagayasu T, Sekine I (2006) Activation of STAT3 is a marker of poor prognosis in human colorectal cancer. Oncol Rep 15:1445–1451
Aoki Y, Feldman GM, Tosato G (2003) Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood 101:1535–1542. doi:10.1182/blood-2002-07-2130
Li H, Xiao W, Ma J, Zhang Y, Li R, Ye J, Wang X, Zhong X, Wang S (2014) Dual high expression of STAT3 and cyclinD1 is associated with poor prognosis after curative resection of esophageal squamous cell carcinoma. Int J Clin Exp Pathol 7:7989–7998
Krecicki T, Smigiel R, Fraczek M, Kowalczyk M, Sasiadek MM (2004) Studies of the cell cycle regulatory proteins P16, cyclin D1 and retinoblastoma protein in laryngeal carcinoma tissue. J Laryngol Otol 118:676–680. doi:10.1258/0022215042244769
Alao JP (2007) The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer 6:24. doi:10.1186/1476-4598-6-24
Altieri DC (2008) Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer 8:61–70. doi:10.1038/nrc2293
Abbas T, Dutta A (2009) p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer 9:400–414. doi:10.1038/nrc2657
Collado M, Serrano M (2010) Senescence in tumours: evidence from mice and humans. Nat Rev Cancer 10:51–57. doi:10.1038/nrc2772
Majumder PK, Grisanzio C, O’Connell F, Barry M, Brito JM, Xu Q, Guney I, Berger R, Herman P, Bikoff R, Fedele G, Baek WK, Wang S, Ellwood-Yen K, Wu H, Sawyers CL, Signoretti S, Hahn WC, Loda M, Sellers WR (2008) A prostatic intraepithelial neoplasia-dependent p27 Kip1 checkpoint induces senescence and inhibits cell proliferation and cancer progression. Cancer Cell 14:146–155. doi:10.1016/j.ccr.2008.06.002
Freedman VH, Shin SI (1974) Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell 3:355–359
Zhou L, Zuo Z, Chow MS (2005) Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol 45:1345–1359. doi:10.1177/0091270005282630
Lee HJ, Jung DB, Sohn EJ, Kim HH, Park MN, Lew JH, Lee SG, Kim B, Kim SH (2012) Inhibition of hypoxia inducible factor alpha and astrocyte-elevated gene-1 mediates cryptotanshinone exerted antitumor activity in hypoxic PC-3 cells. Evid Based Complement Alternat Med 2012:390957. doi:10.1155/2012/390957
Park IJ, Kim MJ, Park OJ, Park MG, Choe W, Kang I, Kim SS, Ha J (2010) Cryptotanshinone sensitizes DU145 prostate cancer cells to Fas(APO1/CD95)-mediated apoptosis through Bcl-2 and MAPK regulation. Cancer Lett 298:88–98. doi:10.1016/j.canlet.2010.06.006
Chen L, Zheng SZ, Sun ZG, Wang AY, Huang CH, Punchard NA, Huang SL, Gao X, Lu Y (2011) Cryptotanshinone has diverse effects on cell cycle events in melanoma cell lines with different metastatic capacity. Cancer Chemother Pharmacol 68:17–27. doi:10.1007/s00280-010-1440-8
Xu D, Lin TH, Li S, Da J, Wen XQ, Ding J, Chang C, Yeh S (2012) Cryptotanshinone suppresses androgen receptor-mediated growth in androgen dependent and castration resistant prostate cancer cells. Cancer Lett 316:11–22. doi:10.1016/j.canlet.2011.10.006
Song M, Hang TJ, Zhang Z, Chen HY (2007) Effects of the coexisting diterpenoid tanshinones on the pharmacokinetics of cryptotanshinone and tanshinone IIA in rat. Eur J Pharm Sci 32:247–253. doi:10.1016/j.ejps.2007.07.007
Liu Y, Li X, Li Y, Wang L, Xue M (2010) Simultaneous determination of danshensu, rosmarinic acid, cryptotanshinone, tanshinone IIA, tanshinone I and dihydrotanshinone I by liquid chromatographic-mass spectrometry and the application to pharmacokinetics in rats. J Pharm Biomed Anal 53:698–704. doi:10.1016/j.jpba.2010.03.041
Xie MZ, Shen ZF (1983) Absorption, distribution, excretion and metabolism of cryptotanshinone. Yao Xue Xue Bao 18:90–96
Aggarwal BB, Kunnumakkara AB, Harikumar KB, Gupta SR, Tharakan ST, Koca C, Dey S, Sung B (2009) Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann NY Acad Sci 1171:59–76. doi:10.1111/j.1749-6632.2009.04911.x
Xu Q, Briggs J, Park S, Niu G, Kortylewski M, Zhang S, Gritsko T, Turkson J, Kay H, Semenza GL, Cheng JQ, Jove R, Yu H (2005) Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene 24:5552–5560. doi:10.1038/sj.onc.1208719
Xu JH, Zhang C, Tang B, Hao YX, Chen J, Liu T, Cui H (2010) Effect of JAK2/STAT3/vimentin signaling pathway on proliferation and migration of human colon cancer cells. Zhonghua Wei Chang Wai Ke Za Zhi 13:282–285
Corvinus FM, Orth C, Moriggl R, Tsareva SA, Wagner S, Pfitzner EB, Baus D, Kaufmann R, Huber LA, Zatloukal K, Beug H, Ohlschlager P, Schutz A, Halbhuber KJ, Friedrich K (2005) Persistent Stat3 activation in colon cancer is associated with enhanced cell proliferation and tumor growth. Neoplasia 7:545–555
Chen L, Wang HJ, Xie W, Yao Y, Zhang YS, Wang H (2014) Cryptotanshinone inhibits lung tumorigenesis and induces apoptosis in cancer cells in vitro and in vivo. Mol Med Rep 9:2447–2452. doi:10.3892/mmr.2014.2093
Harada D, Takigawa N, Kiura K (2014) The role of STAT3 in non-small cell lung cancer. Cancers (Basel) 6:708–722. doi:10.3390/cancers6020708
Epling-Burnette PK, Liu JH, Catlett-Falcone R, Turkson J, Oshiro M, Kothapalli R, Li Y, Wang JM, Yang-Yen HF, Karras J, Jove R, Loughran TP Jr (2001) Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. J Clin Invest 107:351–362. doi:10.1172/JCI9940
Shen Y, Devgan G, Darnell JE Jr, Bromberg JF (2001) Constitutively activated Stat3 protects fibroblasts from serum withdrawal and UV-induced apoptosis and antagonizes the proapoptotic effects of activated Stat1. Proc Natl Acad Sci USA 98:1543–1548. doi:10.1073/pnas.041588198
Darnell JE Jr (2002) Transcription factors as targets for cancer therapy. Nat Rev Cancer 2:740–749. doi:10.1038/nrc906
Kan WL, Yin C, Xu HX, Xu G, To KK, Cho CH, Rudd JA, Lin G (2013) Antitumor effects of novel compound, guttiferone K, on colon cancer by p21Waf1/Cip1-mediated G(0)/G(1) cell cycle arrest and apoptosis. Int J Cancer 132:707–716. doi:10.1002/ijc.27694
Russo A, Esposito D, Catillo M, Pietropaolo C, Crescenzi E, Russo G (2013) Human rpL3 induces G(1)/S arrest or apoptosis by modulating p21 (waf1/cip1) levels in a p53-independent manner. Cell Cycle 12:76–787. doi:10.4161/cc.22963
Zhang M, Truscott J, Davie J (2013) Loss of MEF2D expression inhibits differentiation and contributes to oncogenesis in rhabdomyosarcoma cells. Mol Cancer 12:150. doi:10.1186/1476-4598-12-150
Huang L, Wang HY, Li JD, Wang JH, Zhou Y, Luo RZ, Yun JP, Zhang Y, Jia WH, Zheng M (2013) KPNA2 promotes cell proliferation and tumorigenicity in epithelial ovarian carcinoma through upregulation of c-Myc and downregulation of FOXO3a. Cell Death Dis 4:e745. doi:10.1038/cddis.2013.256
Lee WY, Cheung CC, Liu KW, Fung KP, Wong J, Lai PB, Yeung JH (2010) Cytotoxic effects of tanshinones from Salvia miltiorrhiza on doxorubicin-resistant human liver cancer cells. J Nat Prod 73:854–859. doi:10.1021/np900792p
Ye Y, Xu W, Zhong W (2010) Effects of cryptotanshinone on proliferation and apoptosis of Hela cell line of cervical cancer. Zhongguo Zhong Yao Za Zhi 35:118–121
Acknowledgments
The authors thank Dr. Jeffrey D. White and Dr. Libin Jia from Office of Cancer Complementary and Alternative Medicine, National Cancer Institute, National Institutes of Health for support of this study. We thank Dr. Yinling Hu from Cancer and Inflammation Program, Center for Cancer Research, National Cancer Institute, National Institutes of Health for support of this study. This work was supported by National Cancer Institute, National Institutes of Health intramural funding (ZIA BC 011159), National Natural Science Foundation of China (No.81273718 and No.81102587), and China Postdoctoral Science Foundation (No. 2012T50199).
Conflict of interests
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Weidong Li and Shakir M. Saud have contributed equally to the work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11010_2015_2424_MOESM1_ESM.tif
Fig. S1 Cryptotanshinone does not inhibit non-tumor HEK293 cell viability 2 × 104 HEK293 cells were seeded into 96-well plates and after 24 h were treated with 5–100 µmol/L CPT or 0.1 % DMSO. At 24, 48, and 72 h after CPT treatment, the cell viability was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay as described in “Materials and methods” section. Absorbance at 570 nm was detected with Microplate reader. The experiments were independently performed at least twice, each in triplicate. Crypotanshinone (CPT). Supplementary material 1 (TIFF 187 kb)
Rights and permissions
About this article
Cite this article
Li, W., Saud, S.M., Young, M.R. et al. Cryptotanshinone, a Stat3 inhibitor, suppresses colorectal cancer proliferation and growth in vitro. Mol Cell Biochem 406, 63–73 (2015). https://doi.org/10.1007/s11010-015-2424-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2424-0