Abstract
We recently showed that α-, γ-, and δ-tocopherols (Toc) were isoform dependent in modulating an inflammatory response in differentiated human Caco-2 intestinal cells. Here, we aim to investigate the relative capacity of Toc isoforms to modify the stress-activated NfκB and Nrf-2 signaling pathways that regulate the expression of pro-inflammatory cytokines and antioxidant enzymes, respectively, in this well-established in vitro model of the small intestine The modulation of IFNγ/phorbol myristate acetate (PMA)-induced inflammatory responses, determined by the expression of IL8 mRNA and protein, corresponded to the extent by which different Toc isoforms altered intracellular oxidative status in Caco-2 cells. α Toc was more effective at suppressing IFNγ/PMA-induced NfκB activation than γ-Toc, while δ-Toc was ineffective. On the other hand, only δ-Toc and to a lesser extent γ-Toc promoted IFNγ/PMA-induced Nrf-2 activation. Up-regulation of Nrf-2 by δ-Toc coincided with a decrease in GSH/GSSG ratio, thus pointing to pro-oxidant activity of δ-Toc isoform in IFNγ/PMA-stimulated Caco-2 cells. The induction of oxidative stress in IFNγ/PMA-treated cells by δ-Toc was lowered (P < 0.05) in the presence of ascorbic acid. Ascorbic acid also enabled a greater suppression of IL8 secretion than when cells were treated with δ-Toc isoform alone. Our findings show that δ-Toc uniquely promoted oxidative stress which translated to Toc isoform-specific modulation of the stress-activated Nrf-2 and NfκB signaling pathway and an influence on IL8 expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
α-tocopherol (Toc), a vitamin E isoform, is recognized foremost for being an important lipid soluble antioxidant [1] and by extension has received considerable attention as to its role in preventing chronic diseases that are associated with oxidative stress [2, 3]. α-Toc, however, is not the main vitamin E isoform consumed in the North American diet [4, 5]. γ-Toc, and to a lesser extent δ-Toc, is typically consumed from soybean and other vegetable oils that represent important sources of vitamin E intake for North American consumers [6–8]. In general, the biological significance of non-α-Toc isoforms has been overlooked, mainly since α-Toc is commonly considered to be the primary active vitamin E isoform. The failure of many large, randomized clinical trials to demonstrate the efficacy of α-Toc to mitigate different chronic health concerns has raised the question as to whether non-α-Toc isoforms, such as γ- and δ-Toc, should also be regarded as active forms of vitamin E that contribute to health and wellness [5, 9, 10].
We have recently reported that α-, γ-, and δ-Toc elicit isoform-specific biological activity in the fetal-derived intestinal FHs 74 Int and the adult-derived Caco-2 intestinal cell line [11]. For example, δ-Toc exhibited the greatest ability to protect against peroxyl radical-induced oxidative stress in both intestinal cell lines, followed by γ- and α-Toc. This is consistent with the relative antioxidant activity of Toc isoforms observed in the chemical-based antioxidant oxygen radical absorbance capacity assay [11]. Despite exhibiting comparable antioxidant activity in both intestinal cell lines, we found that Toc isoforms, particularly γ- and δ-Toc, modulate an inflammatory response in an opposing manner in Caco-2 and FHs-74 Int cells. In the fetal-derived intestinal cell line, the γ- and δ-Toc incrementally promoted inflammatory response, which was associated with the activation of both NfkB and Nrf-2 signaling pathways [12]. Using differentiated Caco-2 cells, which are an established model of adult intestine, we observed that γ-Toc reduced IFNγ/phorbol myristate acetate (PMA)-induced IL8 secretion relative to a greater extent compared to α-Toc [11]. δ-Toc in particular was less effective than α-Toc in suppressing the IL8 secretion, despite having the greatest free radical scavenging activity of the three Toc isoforms tested. The molecular mechanism by which α, γ, and δ-Toc differentially modulate IL8 expression from IFNγ/PMA-challenged Caco-2 cells has not been explored.
The present study aims to demonstrate the relative efficacy of different Toc isoforms to modulate oxidative stress-activated cell signaling pathways, specifically NfκB, which regulates the expression of pro-inflammatory cytokines and nuclear factor erythroid-derived 2-like 2 (Nrf-2); the latter is a promoter of antioxidant enzyme expression [13, 14]. In addition, we investigated the important role of a Toc isoform–ascorbic acid interaction that can maintain antioxidant status in Caco-2 cells when exposed to a pro-inflammatory condition.
Materials and methods
Materials
All reagents, including RRR-γ-Toc (99 %) and (+)-δ-Toc (≥90 %), were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise mentioned. RRR-α-Toc (99 %) was obtained from Fisher (Fairlawn, NJ, USA); Caco-2 cell line was obtained from Cedarlane (Hornby, ON), NE-PER nuclear protein extraction kit was from Pierce (Rockford, IL), and fetal bovine serum and penicillin/streptomycin were from Gibco/Invitrogen (Grand Island, NY). NfκB and Nrf-2 DNA-binding ELISA kits were purchased from Active Motif (Carlsbad, CA); IL8 single analyte ELISA kit and PCR array (CAPH10994) were obtained from Qiagen (Valencia, CA). Glutathione assay kit was purchased from Cayman Chemical (Ann Arbor, MI), while DC-Protein Assay was obtained from Bio-Rad Laboratories Inc. (Hercules, CA, USA).
Cell culture protocol
Caco-2 cells were cultured in Eagle’s minimum essential medium (MEM) containing 10 % FBS with 100 mg/mL streptomycin and 100 U/mL penicillin at 37 °C in a 5 % CO2 humidified incubator according to ATCC recommendations. Caco-2 cells were seeded in six-well plates at 4.5 × 105 cells/mL and differentiated for a total of 21 days with complete MEM media that were changed every 2–3 days. Maximum ethanol concentration in all treatments was 0.25 % (v/v).
Modulation of inflammatory response
Differentiated Caco-2 cells were incubated with α-, γ-, or δ-Toc, dissolved in ethanol at 1, 10, or 100 µM, for 24 h at 37 °C. Inflammation was induced in Caco-2 cells by treatment with IFNγ (8000 U/mL) in combination with PMA (0.1 μg/mL) and co-cultured with Toc treatments for 24 h. Following incubation at 37 °C, cell supernatants were recovered and the IL8 levels were measured by an ELISA-based assay according to manufacturer’s instruction [11].
NfκB and Nrf-2 activation
The effects of individual Toc isoforms on IFNγ/PMA-induced translocation of NfκB and Nrf-2 to the nucleus of Caco-2 cells were established at 90 min and 24 h exposure, respectively. These time points were chosen because preliminary experiments showed that NfκB nuclear translocation occurred as early as 90 min after IFNγ/PMA exposure, while Nrf-2 translocation to the nucleus was elevated between 4 and 24 h. We chose to evaluate the effect of Toc on Nrf-2 translocation after 24 h of IFNγ/PMA exposure. Supernatant was analyzed for IL8 at 24 h. Treated cells were scraped into ice-cold PBS from six-well plates, and nuclear protein was extracted using a NE-PER kit. Nrf-2 and NfκB nuclear translocations were measured using TransAM® NfκB (p65) or Nrf-2 DNA-binding ELISA kit according to manufacturer’s instructions.
Effect of Toc on gene transcription
Apparent changes in modulation of Nrf-2 and NfκB signaling pathway were confirmed by examining the effect of Toc isoforms on the mRNA level of selected NfκB and Nrf-2 target genes using real-time PCR analysis. IL8 was selected as the NfκB target gene, while a few glutathione-related antioxidant enzymes were selected as the Nrf-2 target genes. Specifically, glutathione peroxidase (GPX)-1, GPX-2, GPX-4, glutathione-S-transferase α 1, glutamate-cysteine ligase, glutathione synthetase, and glutathione reductase were included in the PCR array. Briefly, Caco-2 cells were pre-treated with Toc isoforms for 24 h and harvested after both 8 and 24 h of IFNγ/PMA challenge, in the presence of Toc isoforms. The chosen incubation period corresponds to the time point at which Nrf-2 translocation to the nucleus was maximum (8 h, data not shown) after IFNγ/PMA exposure and at the end of IFNγ/PMA and Toc exposure (24 h). cDNA was synthesized from 1 µg of mRNA that was recovered from treated Caco-2 cells as previously described [15]. A negative control consisted of cells that were not exposed to Toc or IFNγ/PMA, while a positive control consisted of cells challenged with IFNγ/PMA alone. PCR analysis was performed using ABI7500 (Applied Biosystem, Foster City, CA, USA), and data were analyzed using RT2 Profiler Data Analysis version 3.5 from the SABiosciences website [16]. Ribosomal protein L13a was used as housekeeping gene.
Effect of Toc isoform on glutathione levels
The glutathione content of Caco-2 cells that were pre-treated with Toc isoforms for 24 h and subsequently co-cultured with IFNγ/PMA for additional 24 h was measured using a glutathione assay kit according to manufacturer’s instructions. Glutathione content in cells was reported as total glutathione and as a ratio between the reduced and oxidized glutathione to indicate cellular oxidative status of treated Caco-2 cells.
Effect of Toc on oxidative status of IFNγ-induced Caco-2 cells
Differentiated Caco-2 cells were cultured in 96 wells with α-, γ-, or δ-Toc isoforms at final concentrations of 1, 10, and 100 μM, respectively, at 37 °C for 24 h. Toc-containing media were removed, and IFNγ/PMA cocktail (8000 U/mL/0.1 μg/mL) was added along with fresh Toc-containing media. After 24 h of incubation, media were removed and cells were rinsed once with PBS. 2′,7′-dichlorodihydrofluorescein diacetate (DCFHDA) (5 μM) in PBS was incubated with cells at 37 °C and removed after 30 min. HBSS was added, and cells were further incubated for 1 h. At the end of incubation period, fluorescence intensity was measured at excitation wavelength of 485 nm and emission wavelength of 527 nm using a luminometer (Fluoroskan Ascent FL, Labsystem, Helsinki, Finland). Negative and positive controls consisted of cells untreated with Toc, or treated with the IFNγ/PMA cocktail only, respectively. Data were expressed according to the following equation:
where F i is the fluorescence reading of sample and F con is the fluorescence reading of control cells treated with IFNγ/PMA cocktail only.
Effect of ascorbic acid on oxidative status and inflammatory response
A separate experiment was performed to determine if ascorbic acid, with known reducing capacity for recycling oxidized Toc, could influence the Toc-induced changes observed in both oxidative status and inflammatory response in Caco-2 cells. An equimolar concentration of ascorbic acid to Toc isoforms was added during the treatment of cells. Briefly, cells were treated with Toc isoforms in the presence of ascorbic acid for 24 h, followed by IFNγ/PMA challenge in the presence of replenished Toc isoforms and ascorbic acid for additional 24 h. Oxidative status and IL8 secretion from Caco-2 cells treated with Toc isoforms co-cultured with ascorbic acid were determined as described above.
Statistics
All experiments were performed in triplicate, and samples were analyzed also in triplicate, with final results being expressed as mean ± standard deviation. Significant differences between Toc isoforms at specific concentrations were identified using one-way ANOVA at P < 0.05, followed by Tukey’s post hoc analysis using Graph-Pad Prism version 3.02 statistical software (La Jolla, CA). In addition, modulation of oxidative status and inflammatory response compared to untreated control cells was determined using t test between treatment and control group (P < 0.05). In all statistical tests used, P < 0.05 was considered statistically significant.
Results
Effect of Toc isoforms on modulating IFNγ/PMA-induced inflammatory response
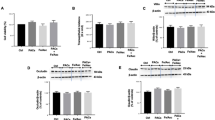
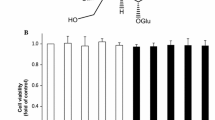
Treatment of differentiated Caco-2 cells with IFNγ/PMA resulted in a significant (P < 0.05) production of IL8, which in turn was suppressed according to the specific Toc isoform present (Fig. 1). For example, γ-Toc inhibited the production of IFNγ/PMA-induced IL8 expression at a significantly (P < 0.05) greater extent than α-Toc (P < 0.05; Fig. 1), at both 10 and 100 μM, respectively. In contrast, δ-Toc at 100 µM was the least (P < 0.05) effective of all Toc isoforms tested to reduce IL8 production from IFNγ/PMA-induced Caco-2 cells. None of the Toc isoforms were found to exert cytotoxic effects on differentiated Caco-2 cells challenged with IFNγ/PMA to promote inflammatory response (data not shown).
Relative effect of tocopherol isoforms to modulate IL8 secretion stimulated by IFNγ/PMA in Caco-2 cells [11]. Values are mean ± SD (n = 3) and refer to percentage of differences calculated between negative and positive controls. Negative control consists of untreated cells, while positive controls consist of cells challenged with IFNγ/PMA in the absence of Toc isoforms. Different letters indicate significant (P < 0.05) differences observed between Toc isoforms at the same concentration *Significant difference (P < 0.05) compared to Caco-2 cells treated with IFNγ/PMA only
Toc modulation of IL8 mRNA
Stimulation of Caco-2 cells with IFNγ/PMA for 8 h resulted in a significant (P < 0.05) up-regulation of IL8 gene transcription which became less pronounced after 24 h (Fig. 2). After 8 h of IFNγ/PMA challenge, we observed that only γ-Toc effectively down-regulated the IFNγ/PMA-induced IL8 gene expression (P < 0.05). IL8 mRNA recovered from cells treated with the IFNγ/PMA cocktail for 24 h was not different when pre-treated with α- and γ-Toc, respectively. In contrast, δ-Toc-treated cells expressed greater IL8 mRNA when compared to cells exposed to IFNγ/PMA (Fig. 2).
Relative effect of Toc isoforms at 100 μM on modulation of IL8 mRNA in Caco-2. Cells were challenged by IFNγ/PMA for: a 8 and b 24 h. Values are mean ± SD (n = 3) for fold differences in mRNA transcript level compared to untreated Caco-2 cells. Different letters indicate significant (P < 0.05) differences observed between treatments
Effect of Toc isoforms on modulating IFNγ/PMA-induced NfκB signaling
The α-Toc isoform was the only Toc to display a clear inhibition of IFNγ/PMA-induced NfκB activation in differentiated Caco-2 cells (Fig. 3). At 100 μM, α-Toc was the most effective Toc isoform to mitigate NfκB activation, followed by γ-Toc, while δ-Toc was the least effective.
Effect of tocopherol isoforms on modulating NfκB activation in Caco-2 cells induced by IFNγ/PMA. Values are mean ± SD (n = 3) of control. Controls consist of cells challenged with IFNγ/PMA in the absence of Toc isoforms. Means with different letters indicate significant (P < 0.05) differences observed between Toc isoforms at the same concentration *Significant difference (P < 0.05) compared to Caco-2 cells treated with IFNγ/PMA only
Effect of Toc isoforms on modulating IFNγ/PMA-induced Nrf-2 signaling
Treatment of Caco-2 cells with α-Toc at all concentrations tested had no effect on Nrf-2 signaling (Fig. 4). Only δ-Toc exhibited a significant enhancement of IFNγ/PMA-induced Nrf-2 nuclear translocation at 10 µM, thus displaying the lowest concentration threshold of this Toc isoform to evoke this effect. Increasing the concentration of Toc isoforms to 100 μM resulted in a Toc isoform-dependent promotion of Nrf-2 activation, with δ-Toc inducing the greatest Nrf-2 nuclear translocation, followed by γ-Toc, whereas α-Toc was again ineffective.
Effect of tocopherol isoforms on Nrf-2 activation in Caco-2 cells induced by IFNγ/PMA. Values are mean ± SD (n = 3) of control. Controls consist of cells challenged with IFNγ/PMA for 24 h in the absence of Toc isoforms. Means with different letters indicate significant (P < 0.05) differences observed between Toc isoforms at the same concentration. *Significant difference (P < 0.05) compared to Caco-2 cells treated with IFNγ/PMA only
Toc modulation of Nrf-2 target genes
Of all the glutathione-related antioxidant enzymes tested, only GPX-2 was significantly up-regulated by IFNγ/PMA at both 8 and 24 h of incubation period, while up-regulation of GCLC occurred at 8 h of IFNγ/PMA exposure (P < 0.05; Table 1). Treatment of Caco-2 cells with Toc isoforms did not alter GCLC mRNA expression relative to IFNγ/PMA-treated cells. On the other hand, significant (P < 0.05) changes in GPX-2 mRNA occurred but varied depending on the individual Toc isoform. For example, after a 8 h incubation, γ-Toc down-regulated GPX-2, whereas δ-Toc up-regulated GPX-2 mRNA. Nevertheless, all Toc isoforms were found to up-regulate the GPX-2 mRNA transcript level to the same extent when compared to Caco-2 cells treated with IFNγ/PMA for 24 h (Table 1).
Effect of Toc on glutathione content
Treatment of Caco-2 with IFNγ/PMA resulted in a significant (P < 0.05) decrease in both total and reduced glutathione content (Fig. 5a, b). Toc isoforms did not further lower total or reduced glutathione content of Caco-2 cells (Fig. 5a, b). Despite the significant reduction in GSH content, the GSH/GSSG ratio of IFNγ/PMA-treated cells remained unchanged, relative to control untreated cells (Fig. 5d). Similarly, incubation of cells with α or γ-Toc at 100 µM had no effect on the GSH/GSSG ratio (Fig. 5d). Nevertheless, δ-Toc at the same concentration was found to significantly (P < 0.05) increase GSSG content and reduce the GSH/GSSG ratio when compared to cells that were treated with IFNγ/PMA only (Fig. 5c, d).
Effect of 100 µM Toc on IFNγ/PMA-treated Caco-2 cells on cellular glutathione content. After 24 h of co-incubation of Toc isoforms with IFNg/PMA, total glutathione content (a), reduced glutathione (b), oxidized glutathione (c), and reduced to oxidized glutathione (GSH/GSSG) ratio (d) of Caco-2 cells were assessed
Effect of Toc isoforms on oxidative status of Caco-2 cells
A comparison of the different abilities of Toc isoforms to modulate the oxidative status induced by IFNγ/PMA-treatment in differentiated Caco-2 cells is shown in Fig. 6. While α and γ-Toc isoforms did not alter oxidative status in IFNγ/PMA-challenged Caco-2 cells, δ-Toc was found to promote oxidative stress in IFNγ/PMA-stimulated Caco-2 cells.
Effect of tocopherol isoforms on the oxidative status of Caco-2 Cells stimulated with IFNγ/PMA. Values are mean ± SD (n = 3) referring to oxidative status measured in Caco-2 cells using a DCFHDA probe. Caco-2 cells were incubated with Toc isoforms and then co-cultured with IFNγ/PMA. Control cells were stimulated with only IFNγ/PMA. Different letters denote significant difference at P < 0.05 between treatments
Effect of ascorbic acid and Toc isoforms on oxidative status and IL8
Combining an equimolar ratio of ascorbic acid with δ-Toc reversed the oxidative stress induced by δ-Toc in IFNγ/PMA-challenged Caco-2 cells (Fig. 7). In contrast, the addition of ascorbic acid in the presence of α and γ-Toc isoforms, respectively, had no effect in reducing the oxidative stress observed in Caco-2 cells (Fig. 7).
Effect of ascorbic acid to modulate tocopherol-induced changes in oxidative status of IFNγ/PMA-stimulated Caco-2 cells. Panels a α-Toc, b γ-Toc, c δ-Toc. Values are mean ± SD (n = 3). *Significant P < 0.05 difference between Toc-treated cells in the presence or absence of ascorbic acid at specified Toc concentrations
The finding that the pro-oxidant activity of δ-Toc can be neutralized by the presence of ascorbic acid was extended to a similar ability to modulate IFNγ/PMA-induced IL8 expression. Ascorbic acid had no effect on the induction of IL8 secretion observed in the presence of both α and γ-Toc in IFNγ/PMA-challenged Caco-2 cells (Fig. 8). However, co-culturing equimolar ascorbic acid with δ-Toc significantly (P < 0.05) reduced IL8 expression in IFNγ/PMA-induced Caco-2 cells when compared to a treatment of δ-Toc alone (Fig. 8).
Discussion
Our results have confirmed that vitamin E isoforms α-, γ-, and δ-Toc will exhibit anti-inflammatory properties when tested in the IFNγ/PMA-stimulated Caco-2 cell model system [11]. The degree by which IL8 expression was suppressed, however, was shown herein to be specific to the isoform tested. For example, at the highest concentration tested, γ-Toc was the most effective, followed by α-Toc, while δ-Toc was the least effective at reducing IL8 secretion. The effect of different Toc isoforms to modulate IL8 expression was confirmed at the transcriptional level. It should be noted that this isoform-specific modulation of inflammatory response cannot be attributed to differences in cell viability since all Toc isoforms were not cytotoxic to the differentiated Caco-2 cells at concentration ranges tested in this study [11].
We also have previously shown that Toc is taken up by Caco-2 cells in a concentration and isoform-dependent manner [11]. For example, the more polar Toc isoform, δ-Toc, had relatively greater uptake, followed by γ and α-Toc, respectively. Based on the cellular content of Toc isoforms, δ-Toc was therefore expected to be potentially more bioactive than γ and α-Toc in modulating an inflammatory response. Nevertheless, the anti-inflammatory effect of Toc isoform did not correspond to the cellular uptake of Toc. We conclude therefore that the relative amount of Toc in cells was not the primary determining factor contributing to the inflammatory response elicited by individual Toc isoforms.
NfκB and Nrf-2 signaling pathways are modulated by oxidative stress, which is a known factor to promote IL8 expression [13, 17, 18]. Activation of NfκB signaling in particular is indispensable for IL8 expression [18]. On the other hand, activation of Nrf-2 signaling by oxidative stress generally results in the expression of antioxidant enzymes which protect against inflammation [17, 19]. Possible cross-talk between NfκB and Nrf-2 signaling has also been proposed, which could collectively influence the oxidative status of cells and IL8 expression [19, 20]. In this study, the extent to which Toc isoforms modulated both IFNγ/PMA-activated NfκB and Nrf-2 signaling pathway was shown to be isoform dependent. At the highest concentration for each Toc isoform, α-Toc was the most effective at suppressing IFNγ/PMA-induced Caco-2 cell NfκB signaling, while δ-Toc had no effect. This outcome differed from the Toc modulation of IL8 expression in Caco-2 cells which were challenged with IFNγ/PMA. To our surprise, it also did not correspond to the relative free radical scavenging capacity described in our former study, where α-Toc was shown to be the least effective isoform to scavenge peroxyl radicals (2,2′-azobis (2-amidinopropane) dihydrochloride)-induced oxidative stress in cell membranes, while γ- and δ-Toc were incrementally more effective [11]. Based on the finding that α-Toc was the least effective free radical scavenger, we expected that α-Toc would also be relatively less potent to reduce NfκB activation compared to δ-Toc. Our results also show that δ-Toc, despite its greatest efficacy as a free radical scavenger, exacerbated IFNγ/PMA-induced Nrf-2 nuclear translocation the most. It is therefore important to recognize that the pattern by which Toc isoform modulates NfκB and Nrf-2 activation does not completely correspond to the ability of Toc isoforms to scavenge free radicals.
Since increased Nrf-2 activation promotes the expression of antioxidant enzymes that can protect against oxidative stress and modulate IL8 expression, we evaluated the gene expression of a number of antioxidant enzymes, with a focus on those that are involved in maintaining intracellular glutathione levels. Of the seven antioxidant enzymes tested, GPX-2 and glutamate-cysteine ligase were observed to be up-regulated by IFNγ/PMA. Nevertheless, only the gene transcription of GPX-2, a known Nrf-2 target gene in Caco-2 cells, was transiently down-regulated by γ-Toc and up-regulated by δ-Toc. To confirm the differential modulation of Toc on GPX-2, an enzyme that utilizes glutathione to neutralize hydrogen peroxide, we also assessed the effect of Toc on total glutathione content and the ratio between the reduced glutathione (GSH) and oxidized glutathione (GSSG), as an indicator of cellular oxidative status. Corresponding to the up-regulation of GPX-2 and GCLC by IFNγ/PMA challenge, we observed a down-regulation of both reduced and oxidized glutathione content that resulted in an unchanged GSH/GSSG ratio, when compared to untreated cells. It was thus evident that the up-regulation of GCLC mRNA expression was not sufficient to maintain the total intracellular glutathione pool.
While treatment of cells with α- or γ-Toc had no effect on total glutathione or the GSH/GSSG ratio, the presence of δ-Toc increased oxidized glutathione which lowered the GSH/GSSG ratio. δ-Toc therefore promoted oxidative stress in Caco-2 cells, which is consistent with the observed enhanced Nrf-2 nuclear translocation. The finding that non-α-Toc isoforms promoted Nrf-2 signaling is a novel finding, because the activation of Nrf-2 signaling is generally associated with oxidative stress [17, 21]. We thus hypothesized that δ-Toc at higher concentrations acts as a pro-oxidant which in turn promotes Nrf-2 activation and leads to increase the GSH/GSSG ratio; a compensatory mechanism to neutralize the pro-oxidant state generated by δ-Toc treatment. Using the non-fluorescent DCFHDA probe, which turns fluorescent when the probe is taken up by cells and oxidized by a reactive species [15], we show that δ-Toc, but not α and γ-Toc, promote oxidative stress. While we have previously shown that δ-Toc was the most effective antioxidant against peroxyl radical, here, we report for the first time that δ-Toc promotes oxidative stress in IFNγ/PMA-treated differentiated cells.
The proposed mechanism by which Toc promotes oxidative stress can be related to the capacity of different Toc isoforms to act as a free radical scavenger in the cell membrane. Upon acting as antioxidants, Toc isoforms are oxidized to more water-soluble Toc-derived radicals, which if not neutralized will express cytosolic pro-oxidant activity [22]. α-Toc, which we previously demonstrated to be the most effective antioxidant against induced peroxyl radical formation at the Caco-2 cell membrane level, is also the most polar isoform [22–24]. Thus, δ-Toc is likely partitioned to a greater extent in the cytoplasm, and therefore is the first to encounter reactive species generated in the cytoplasm. Upon acting as antioxidant in the membrane, δ-Toc would therefore be oxidized to a radical form more effectively than other relatively less polar Toc isoforms. The accumulation of this Toc-derived radical may further propagate a free radical chain reaction that produced the observed pro-oxidant activity observed for δ-Toc.
It is plausible that the pro-oxidant activity of δ-Toc counterbalanced the antioxidant activity of this isoform, thus reducing efficacy of δ-Toc to suppress IFNγ/PMA-induced IL8 secretion in Caco-2 cells. An alternative explanation for our current finding is based on a prior report that IL8 mRNA was stabilized by increased Nrf-2 expression, thus contributing to a greater IL8 expression [25]. This indeed was evident in this study when we found that the IL8 mRNA level in δ-Toc-treated cells was higher than that observed with α- or γ-Toc-treated cells.
Another important finding of our study that confirmed the pro-oxidant mechanism of δ-Toc was the observation that ascorbic acid reversed the promotion of oxidative stress and reduced IL8 response in δ-Toc-treated cells. Vitamin C, a water-soluble antioxidant, reduces the formation of Toc-derived radicals that contribute to the generation of oxidative stress in cells incubated with δ-Toc. The hypothesis that the pro-oxidant activity of δ-Toc is involved in modulating an inflammatory response was confirmed by the finding that ascorbic acid also lowered IL8 expression from δ-Toc-treated Caco-2 cells. Taken together, we conclude that δ-Toc in the absence of vitamin C will elicit a pro-oxidant activity that can trigger the activation of Nrf-2 and NfκB signaling, thereby promoting IL8 expression. We therefore suggest that ascorbic acid has a critical role in preventing the potential pro-oxidant activity of δ-Toc. This finding highlights the importance of considering the potential interaction between nutrients rather than single nutrients in characterizing redox status.
Our finding that γ-Toc when compared to α-Toc was the most effective Toc isoform to reduce IL8, but less effective to suppress NfkB, while also showing moderate enhancement of Nrf-2 activation, is evidence that these two signaling pathways only partially explain the anti-inflammatory properties of γ-Toc in Caco-2 cells. Other workers have shown that activation of NfkB signaling pathway, along with p38 and JNK, is required for a maximum IL8 expression in response to a pro-inflammatory stimuli [18]. It is therefore important to recognize that the effect of γ-Toc on IL8 expression should not be limited to NfkB signaling alone in explaining the potent anti-inflammatory response. Further research that investigates the efficacy of Toc isoforms on other stress-activated signaling pathways in inflammatory response is warranted.
Conclusion
In this study, we demonstrate for the first time that non-α-Toc isoforms including γ- and δ-Toc, commonly present in North American diet, can mitigate an inflammatory response specifically involving the Nrf-2 and NfκB signaling pathways, more so than α-Toc when examined in our Caco-2 intestinal cell model. Suppression of IL8 expression by different Toc isoforms resulted in a variable ability to modulate Nrf-2 and NfκB signaling pathways, which did not correspond directly to the relative capacity to act as free radical scavengers. δ-Toc in particular increased GSH/GSSG ratio which suggests promotion of oxidative stress. This event was associated with activation of Nrf-2 signaling and to an increased IL8 mRNA expression. The presence of ascorbic acid neutralized the pro-oxidant activity of δ-Toc, thereby further showing the important interactive role that these two vitamins have in regulating not only the redox status in intestinal cells but also the associated cell signaling which ultimately reduced inflammation.
References
Traber MG, Atkinson J (2007) Vitamin E, antioxidant and nothing more. Free Rad Biol Med 43:4–15
Honarbakhsh S, Schachter M (2009) Vitamins and cardiovascular disease. Brit J Nut 101:1113–1131
Ju J, Picinich SC, Yang Z, Zhao Y, Suh N, Kong AN, Yang CS (2010) Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis 31:533–542
Wagner KH, Kamal-Eldin A, Elmadfa I (2004) Gamma-tocopherol–an underestimated vitamin? Ann Nutr Metab 48:169–188
Jiang Q, Christen S, Shigenaga MK, Ames BN (2001) γ-Tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr 74:714–722
Elisia I, Young JW, Yuan YV, Kitts DD (2013) Association between tocopherol isoform composition and lipid oxidation in selected multiple edible oils. Food Res Int 52:508–514
Institute of Medicine (2000) DRI dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. National Academy Press, Washington
United States Census Bureau (2011) M311 K-fats and oils: production, consumption, and stocks. http://www.census.gov/manufacturing/cir/historical_data/m311k. Accessed 19 Sep 2014
Hensley K, Benaksas EJ, Bolli R, Comp P, Grammas P, Hamdheydari L, Mou S, Pye QN, Stoddard MF, Wallis G, Williamson KS, West M, Wechter WJ, Floyd RA (2004) New perspectives on vitamin E: γ-tocopherol and carboxyethylhydroxychroman metabolites in biology and medicine. Free Rad Biol Med 36:1–15
Devaraj S, Jialal I (2008) Failure of vitamin E in clinical trials: is gamma-tocopherol the answer. Nutr Rev 63:290–293
Elisia I, Kitts DD (2013) Different tocopherol isoforms vary in capacity to scavenge free radicals, prevent inflammatory response and induce apoptosis in both adult- and fetal-derived intestinal epithelial cells. BioFactors 39:663–671
Elisia I, Kitts DD (2013) Modulation of NF-κB and Nrf2 control of inflammatory responses in FHs 74 Int cell line is tocopherol isoform-specific. Am J Physiol Gastrointest Liver Physiol 305:G940–G949
Schreck R, Albermann K, Baeuerle PA (1992) Nuclear factor κb: an oxidative stress-responsive transcription factor of eukaryotic cells (a review). Free Radic Res Commun 17:221–237
Singh S, Vrishni S, Singh BK, Rahman I, Kakkar P (2010) Nrf2-ARE stress response mechanism: a control point in oxidative stress-mediated dysfunctions and chronic inflammatory diseases. Free Rad Res 44:1267–1288
Elisia I, Tsopmo A, Friel JK, Diehl-Jones W, Kitts DD (2011) Tryptophan from human milk induces oxidative stress and upregulates the Nrf-2-mediated stress response in human intestinal cell lines. J Nut 141:1417–1423
SABiosciences (2012) RT2 profiler PCR array data analysis. http://pcrdataanalysis.sabiosciences.com/pcr/arrayanalysis.php. Accessed 19 Sep 2014
Nguyen T, Nioi P, Pickett CB (2009) The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem 284:13291–13295
Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M (2002) Multiple control of interleukin-8 gene expression. J Leukoc Biol 72:847–855
Li W, Khor TO, Xu C, Shen G, Jeong WS, Yu S, Kong AN (2008) Activation of Nrf2-antioxidant signaling attenuates NFκB-inflammatory response and elicits apoptosis. Biochem Pharmacol 76:1485–1489
Bellezza I, Mierla AL, Minelli A (2010) Nrf2 and NF-kB and their concerted modulation in cancer pathogenesis and progression. Cancers 2:483–497
Chang SW, Lee SI, Bae WJ, Min KS, Shin ES, Oh GS, Pae HO, Kim EC (2009) Heat stress activates interleukin-8 and the antioxidant system via Nrf2 pathways in human dental pulp cells. J Endod 35:1222–1228
Kim HJ, Min DB (2008) Prooxidant mechanisms of oxidized tocopherols in lipids in lipids. In: Akoh CC, Min DB (eds) Food lipids: chemistry, nutrition and biotechnology. CRC Press, New York, pp 435–448
Yoshida Y, Niki E (2002) Antioxidant effects of α- and γ-carboxyethyl-6-hydroxychromans. BioFactors 16:93–103
U.S. Environmental Protection Agency (2013) Estimation program interface (EPI) Suite™ for Microsoft® Windows, version 4.10 [computer program]. U.S. Environmental Protection Agency, Washington, DC
Zhang X, Chen X, Song H, Chen HZ, Rovin B (2005) Activation of the Nrf2/antioxidant response pathway increases IL-8 expression. Eur J Immunol 35:3258–3267
Acknowledgments
This work was funded by a research Grants from the Natural Science and Engineering Council of Canada (NSERC) and the Food Nutrition and Health Vitamin Research Fund (DDK). IE was a recipient of a Four Year Fellowship UBC Doctoral scholarship.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elisia, I., Kitts, D.D. Tocopherol isoforms (α-, γ-, and δ-) show distinct capacities to control Nrf-2 and NfκB signaling pathways that modulate inflammatory response in Caco-2 intestinal cells. Mol Cell Biochem 404, 123–131 (2015). https://doi.org/10.1007/s11010-015-2372-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-015-2372-8