Abstract
High-mobility group box chromosomal protein 1 (HMGB-1), a nuclear DNA binding protein, was recently rediscovered as a new proinflammatory cytokine. The purpose of this study was to determine HMGB-1 expression in vivo and to identify the effect of extracellular HMGB-1 in inflammatory process associated with bone destruction in cholesteatoma. We investigated the expression and location of HMGB-1 in the cholesteatoma and healthy skin using an immunofluorescence assay. We also detected apoptosis and DNA fragments in the cholesteatoma by TUNEL staining. HMGB-1 concentration in apoptotic supernatants from UV light-treated cells, culture supernatants and its translocation in cholesteatoma keratinocytes stimulated by supernatants from UV light-treated cells were measured by immunoblot analysis and immunofluorescence assay. Cultures of human cholesteatoma keratinocytes were exposed to CpG-DNA, HMGB-1, or CpG-DNA complexed to HMGB-1 for 24 h. Cytokines in the culture supernatant were measured by ELISA. In addition, levels of proinflammatory cytokines released by cholesteatoma keratinocytes stimulated by supernatants from UV light-treated cells with or without anti-HMGB-1 antibodies and supernatants from UV light-treated cells with DNase 1 were measured by enzyme-linked immunosorbent assay. The expression of HMGB-1 in cholesteatoma increased and it translocated both to the cytoplasm and extracellular space. Furthermore, the HMGB-1 concentration in supernatants increased significantly after addition of supernatants from UV light-treated cells. TNF-α and IL-1β can be induced by purified HMGB-1 combined with CpG-DNA in the cholesteatoma keratinocytes. In addition, supernatants of apoptotic cells containing HMGB-1–DNA were effective in inducing TNF-α and IL-1β secretion. This study suggested that persistent expression of extracellular HMGB-1 and DNA fragments in cholesteatoma leads to TNF-α and IL-1β production, causing bone resorption and destruction. Thus, we have implicated that HMGB-1–DNA complexes might act as a key molecule involved in bone resorption associated with cholesteatoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cholesteatoma of the middle ear is characterized by invasion of the middle ear cavity by epithelium of the external auditory meatus and drum as well as resorption of the auditory ossicles and surrounding bone tissues in the invaded subepithelial region [1]. Accumulation of keratin debris is caused by the decreased migration ability of keratinized epithelial cells. Infection is thought to accelerate this process. The histopathological changes include hyperproliferation and differentiation of the epithelium and accompanying bone destruction.
Cholesteatoma may cause bone destruction, resulting in intratemporal and intracranial complications, which have high morbidity and mortality [2]. There are numerous theories to explain the destructiveness of cholesteatomas, including physical pressure due to accumulated keratin and other waste, which was initially proposed as the cause of bone destruction. A biochemical theory was also postulated, in which enzymes and cytokines released by cholesteatomas could cause bone lysis and destruction.
Recent studies have focused on the role of cytokines such as tumor necrosis factor (TNF)-α/β, interleukin (IL)-1α/β, epidermal growth factor, and transforming growth factor-β in cholesteatoma bone destruction and discussed their relative importance in bone destruction and the inflammatory process [3–7]. Akimoto et al. [8] reported that IL-1 and TNF-α levels were higher in congenital and acquired cholesteatomas compared with skin of the external auditory meatus. However, the exact source of TNF-α and IL-1 in cholesteatoma is still unknown.
High-mobility group box protein 1 (HMGB-1) is a structural nuclear protein that binds DNA and is involved in the organization of chromatin [9]. Recently, it was shown to function as a proinflammatory mediator when released from cells [10]. HMGB-1 can be actively secreted by macrophage/monocytes via inflammatory stimuli [11] or passively released from the nuclei of necrotic or damaged cells. In contrast, cells undergoing apoptosis are poor HMGB-1 secretors [12]. However, some studies have investigated the direct involvement of HMGB-1 in the induction of apoptosis. Bell et al. [13] reported HMGB-1 release occurred during the course of apoptosis and necrosis in Jurkat cells. Binding of HMGB-1 to the receptor for advanced glycation end products (RAGE) and Toll-like receptor (TLR)2 and TLR4 leads to the recruitment of inflammatory cells and the release of proinflammatory cytokines including IL-1β, TNF-α, and IL-6 [14–17].
Here, we analyzed the expression and subcellular localization of HMGB-1 in cholesteatomatous specimens and the source of extracellular HMGB-1 in cholesteatoma. In addition, we also investigated the role of HMGB-1 in inflammatory process associated with bone destruction in cholesteatoma.
Materials and methods
Patients and tissues
All cholesteatomatous specimens including cholesteatomatous matrix, perimatrix, and contents were obtained from 10 patients with acquired primary cholesteatoma undergoing tympanoplasty in the Department of Otorhinolaryngology at Eye and ENT Hospital, Fudan University, Shanghai, China. The diagnosis was based on clinical, radiological, and intraoperative findings. Prior to surgery, written consent was obtained from all patients to obtain tissue samples of the resected cholesteatoma. The study was approved by the Institutional Ethics and Review Committee of the Faculty of Clinical Medicine, Eye and ENT Hospital, Fudan University, Shanghai, China and was performed in accordance with the 1964 Declaration of Helsinki and its later amendments. In addition, microbiopsies obtained from the external canal skin of the deep meatus in five patients undergoing myringoplasty for dry perforation served as a control group.
Tissue preparation and immunofluorescence
Specimens were obtained from 10 patients (five patients with acquired primary cholesteatoma and five healthy external canal skins) and used for immunofluorescence. Each specimen was fixed overnight in 10 % buffered formalin at room temperature and embedded in paraffin. Serial sections were cut at 7 μm thickness and then placed onto 3-aminopropyltriethoxysilane-coated glass slides. Paraffin sections were deparaffinized with toluene and rehydrated with serially graded ethanol solutions. After rinsing in PBS, the sections were preincubated with 10 % normal goat serum for 1 h to block nonspecific reactions with the primary antibody. Then, the sections were incubated overnight with rabbit monoclonal anti-HMGB-1 antibody (Abcam, Cambridge, MA, USA) at 250-fold dilution. After washing with PBS, the sections were incubated for 1 h at room temperature with Alexa 546-labeled goat anti-rabbit antibody. The slides were washed with PBS and the nuclei were counterstained with blue fluorescent 4′,6-diamidino-2-phenylindole (DAPI). Finally, the sections were examined with an Axioskop microscope (Carl Zeiss, Oberkochen, Germany).
TUNEL staining
Paraffin sections of the acquired primary cholesteatoma and healthy skin were stained for TUNEL with In situ Cell Death Detection Kit (Roche, Indianapolis, IN, USA). Briefly, each slide was deparaffinized and rehydrated, and then treated with citrate buffer (10 mM, pH 6) for 10 min. After rinsing in PBS, the sections were incubated with the TUNEL reaction mixture containing terminal deoxynucleotidyl transferase (TdT) and TMR-dUTP for 1 h. The TdT reaction was carried out in a humidified chamber at 37 °C. DAPI was used as counterstaining. For the negative control, TdT was omitted from the reaction mixture. For positive controls, some sections were treated with DNase I (2,000 U/ml; Thermo Fisher) before staining.
Cell culture, validation, and stimuli
Tissue from the acquired primary cholesteatoma was hand carried to the lab 2 h after resection and transferred into 5 ml Hanks balanced salt solution (HBSS). The tissue was then cut into small pieces with scissors, and digested with 200 U/ml collagenase IV (Sigma Aldrich, St. Louis, MO, USA) at 4 °C overnight. The digested cells were washed twice with HBSS and then centrifuged for at 1,500 rpm for 5 min. The pellet was removed, added to 10 ml Keratinocyte serum-free medium (KSFM; Invitrogen, Carlsbad, CA, USA) with 100 μl Pen Strep (Invitrogen, Carlsbad, CA, USA), and cultured in humidified CO2 at 37 °C. The KSFM media and antibiotics were changed every 3 days. Cells were passaged when they reached confluence (>50 % coverage of culture dish).
The cultured cells were validated for phenotype by immunofluorescence analysis. In this study, aliquots of the cultured cell were grown on 35-mm collagen-coated glass bottom dishes and labeled for immunofluorescent confirmation of specific marker anti-pankeratins for cholesteatoma keratinocytes.
The cholesteatoma keratinocytes were placed at a density of 2 × 104 cells per well in 96-well plates and were stimulated for 24 h with CpG oligodeoxynucleotides alone (2 μM/l, Invivogen, San Digo, CA, USA), HMGB-1 (3 uM/l, R&D systems, Minneapolis, MN, USA) alone, or HMGB-1 plus CpG-DNA. Cytokines in the supernatant were measured by ELISA according to the manufacturer’s instructions (Neobioscience, China).
Induction of cell death
The cholesteatoma keratinocytes were resuspended at 5 × 107 cells/ml in KSFM and irradiated with UV light as described previously [18]. Briefly, the UV light irradiation was conducted in 2 ml of cell suspension in 9.4-cm petri dishes (BD Falcon) using 500 mJ (GS gene linker UV chamber; Bio-Rad, Hercules, CA, USA). Cells treated with UV light were cultured for 16–24 h at 37 °C with 7 % CO2. The cells were removed by centrifugation (400×g for 5 min), the supernatants were tested for cytokine-inducing capacity, and the remaining cells were analyzed by flow cytometry for cell death by staining with FITC-labeled annexin V (BD Biosciences) and propidium iodide (PI; BD Biosciences).
Immunoblot analysis of HMGB-1 in supernatants from UV light-treated cells
Supernatants from UV light-treated cells were fractionated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and HMGB-1 levels were determined by immunoblot analysis with reference to the standard curve for purified human-recombinant HMGB-1 (Prospec, East Brunswick, NJ, USA), obtained by serial dilutions. The concentrations of purified human-recombinant HMGB-1 standard ranged from 0 to 400 ng/ml. Supernatants from UV light-treated cells were fractionated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then transferred to a polyvinylidene difluoride membrane (PVDF membrane) for Western blotting analysis.
Anti-HMGB-1 antibodies neutralize HMGB-1 in the supernatants of UV light-treated cells and enzyme treatments in vitro
Anti-HMGB-1 neutralizing antibody (mouse anti-HMGB-1 monoclonal antibody) was obtained from Biolegend Corporation (San Diego, USA). A solution of the supernatants of UV light-treated cells was mixed with equal volume of the anti-HMGB-1 antibody and incubated at 37 °C for 12 h. Neutralization was confirmed by electrophoresis on a 12 % SDS-PAGE. RNase-free DNase I (Thermo Fisher) was used at a final concentration of 20 Kunitz units/ml. This enzyme was added to the supernatants of UV light-treated cell and incubated at 37 °C for 1 h. Then the neutralized supernatants and supernatants treated with enzyme were added to the cultures of the cholesteatoma keratinocytes. After being stimulated for 0, 1, 4, 8, 12, 24, or 48 h, the culture supernatants were collected. IL-1β and TNF-α levels in culture supernatants were assayed using commercial human IL-1β and TNF-α enzyme-linked immunosorbent assay (ELISA) test kits according to the manufacturer’s instructions (Neobioscience, China).
Western blotting for HMGB-1 detection in culture supernatant
Cholesteatoma keratinocytes were washed twice with HBSS and 1.0 ml was introduced into each well of a six-well culture plate at a concentration of 1 × 106 cells/ml. The supernatant from UV light-treated cells was added into each well at a final volume of 1.3 ml. After the plates were stimulated for 0, 4, 8, 12, 24, 36, or 48 h, the conditioned media was collected and the cells were obtained and prepared for extraction of total cellular protein. Conditioned media from cells were centrifuged through Centricon (Millipore, Billerica, MA, USA) 10-kDa filter devices for 1 h at 3,000×g. Supernatants were collected from the top reservoir of the filter device and spun through an Ultrafree-MC (Millipore) 100-kDa filter device for 15 min at 4,000×g. Equal volumes of elution were fractioned by SDS-PAGE and transferred to a polyvinylidene difluoride membrane (PVDF membrane) for Western blotting analysis. Total cellular protein extracts were prepared using Whole Cell Lysis/Extraction Buffer (WCLB, Applygen, Beijing, China) according to the manufacturer’s instructions.
Measurement of IL-1β and TNF-α in culture supernatants and preparation of cytoplasmic extracts
Using the same method as described above, the cholesteatoma keratinocytes were cultured and stimulated in six-well culture plates. After being stimulated for 0, 1, 4, 8, 12, 24, 36, or 48 h, the culture supernatants were collected and the cells were obtained and stored at −80 °C until used. IL-1β and TNF-α levels in culture supernatants were assayed using commercial human IL-1β and TNF-α enzyme-linked immunosorbent assay (ELISA) test kits according to the manufacturer’s instructions (Neobioscience, China). To prepare the cytoplasmic fraction, cells were lysed using a nuclear and cytoplasmic protein extraction kit according to the manufacturer’s instructions (Beyotime Institute of Biotechnology, China). Briefly, cells were washed in ice-cold phosphate-buffered saline (PBS) and resuspended by pipetting up and down 10 times in 100 μl of ice-cold cell lysis buffer (10 mM HEPES, pH 7.9; 10 mM KCl; 0.1 mM EDTA; 1 mM DTT, 0.4 % IGEPAL with the following protease inhibitors: Aprotinin 2 μg/ml, PMSF 1 mM, benzamidine 250 μg/ml, leupeptin 2 μg/ml). After being placed on ice for 15 min, cell lysates were spun in a microcentrifuge at 12,000 rpm for 5 min at 4 °C and supernatants were collected as the cytoplasmic fraction and stored at −80 °C for Western blot analysis.
Western blot analysis
Protein concentration was measured using a BCA protein assay kit (Beyotime Institute of Biotechnology, China). For Western blot assay, the cytoplasmic fraction, concentrated culture supernatants, and total cellular protein extracts were added to Laemmli sample buffer and boiled for 5 min. Subsequently, protein from each sample was subjected to 12 % SDS-PAGE and transferred onto a PVDF membrane. The membrane was washed with Tris-Buffered Saline/Tween-20 (TBST; 20 mM Tris, 500 mM NaCl, and 0.1 % Tween-20) and blocked with 5 % milk in TBST for 1 h. After washing with TBST three times, the membrane was incubated with primary antibody overnight at 4 °C. The following antibodies were used: monoclonal anti-HMGB-1 antibody was purchased from Abcam and monoclonal anti-β-actin antibody was from Sigma. The membrane was washed three times with TBST and incubated with HRP-conjugated secondary antibody in blocking buffer for 1 h. After washing three times with TBST, immunoreactive bands were visualized by incubation with ECL plus detection reagents (GE Healthcare) for 5 min and exposure to ECL Hyperfilm (GE Healthcare). The densitometry of bands was quantified with NIH Image 1.63 software. Protein expression was normalized to the quantity of β-actin. The gray values were expressed in relation to that of control and presented as mean ± standard deviation (M ± SD).
Immunofluorescence assay for HMGB-1 translocation
To investigate the translocation of HMGB-1 in the cholesteatoma, cholesteatoma keratinocytes were cultured in a Lab-Tech chamber slide (Nalge Nunc International, Cambridge, MA, USA) and then stimulated with supernatants from UV light-treated cells. After stimulation, cells were fixed with 4 % paraformaldehyde solution for 30 min. The chamber slide was then incubated with a blocking buffer containing 1 % BSA and PBS-0.02 % Tween (PBST) for 1 h at 25 °C and incubated with anti-HMGB-1 rabbit monoclonal antibody (Abcam) at 250-fold dilution overnight at 4 °C. The chamber slide was then washed with PBST, followed by 1 h incubation with Alexa 546-labeled anti-rabbit IgG diluted 1:200 with PBST. Finally, the chamber was examined using an Axioskop microscope (Carl Zeiss, Oberkochen, Germany) after washing three times. Nuclear counterstaining was performed with DAPI.
Quantitative RT-PCR
To examine the influence of supernatants from UV light-treated cells on mRNA expression for HMGB-1, IL-1β, and TNF-α, the cholesteatoma keratinocytes were cultured and stimulated in six-well plates for 0, 4, 8, 12, 24, 36, or 48 h. After stimulation, the cells were used for total RNA extraction using Trizol (Invitrogen) according to the manufacturer’s instructions. After purification, complementary DNA (cDNA) was synthesized from 1 μg total RNA using the first-strand cDNA synthesis kit (Takara, Dalian, China) according to the manufacturer’s instructions. HMGB-1, TNF-α, IL-1β, and GAPDH were then amplified by quantitative real-time PCR (qRT-PCR) using the primer sequences and PCR parameters shown in Table 1. qRT-PCR was performed using the SYBR® Premix Ex Taq™ GC (Takara, Dalian, China) and run in a LightCycler (Roche Diagnostics, Indianapolis, IN, USA). Individual RT-PCR tests were repeated on at least three independent occasions. Melting curve analysis was performed to verify that only one product was amplified. Data were analyzed for fold change (2−△△CT) value calculation using GADPH for normalization.
Statistical analysis
Statistical analysis was performed using the statistical software package SPSS (Version 11.5) and were evaluated using one-way ANOVA followed by a LSD multiple comparison test for comparison of three or more groups. Statistical significance was defined as a p value less than 0.05 for all analyses and the data were presented as mean ± standard deviation (M ± SD).
Results
Expression of HMGB-1 in cholesteatomatous tissue
HMGB-1 expression was evaluated in acquired primary cholesteatoma and normal skin by immunofluorescence. Nuclear HMGB-1 was expressed in both primary cholesteatomatous specimens (Fig. 1d–f) and normal external canal skin (Fig. 1a–c). Control staining with irrelevant isotype-matched control antibody was negative (data not shown). Translocation of HMGB-1 to the cytosol was detected in primary cholesteatoma (Fig. 1e). Staining without obvious restriction by cell membranes, suggestive of extracellular presence of HMGB-1, was also detected in acquired primary cholesteatoma (Fig. 1e). In normal external canal skin, no cytosol and extracellular staining for HMGB-1 was observed.
Localization of HMGB-1 in normal skin (a–c) and primary cholesteatoma (d–f). Immunofluorescence analysis demonstrated HMGB-1 that was localized in the cytoplasm and nucleus of epithelial cells situated mostly in the metrical layer (e). The sections were incubated with anti-HMGB-1 rabbit polyclonal antibody. Skin tissues were used as controls. The nucleus was labeled with DAPI (blue, a, d). Blue arrows depict nuclear HMGB-1. White arrows depict cytoplasmic HMGB-1 and extracellular HMGB-1. (Color figure online)
Apoptosis and DNA fragment is present in cholesteatoma
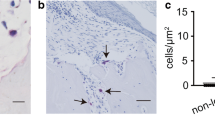
To detect apoptosis and DNA fragments in cholesteatoma, we stained paraffin sections using terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL). A great number of apoptotic cells was observed in the cholesteatoma epithelium (Fig. 2d–f) but not in healthy skin (Fig. 2a–c). At the same time, DNA fragments were also detectable in the cytoplasm and extracellular space of keratinocytes in all patients with cholesteatoma (Fig. 2g–i).
TUNEL staining in normal skin (a–c), acquired secondary cholesteatoma (d–i). Detection of large numbers of apoptotic cells (d–f) and DNA fragments in cytoplasm and extracellular space of keratinocytes in the cholesteatoma (g–i). (j–l) is for the negative control. DNase I-treated cholesteatoma tissue served as a positive control (m–o). White arrows depict apoptotic cells. Blue arrows depict DNA fragments in cytoplasm and extracellular space. (Color figure online)
Validation of cholesteatoma keratinocytes
To validate the primary cultures, the expression of pancytokeratins (CKs) which was widely used as positive marker for keratinocytes was analyzed. The immunofluorescence analysis showed that the cultured cells were characterized by a positive staining for cytokeratins (Fig. 3).
HMGB-1 levels in the supernatants of UV light-treated cells treated with or without anti-HMGB-1 antibodies
Using immunoblot analysis, we measured the HMGB-1 concentrations in the supernatants of UV light-treated cells treated with or without anti-HMGB-1 antibodies. We could detect expression of HMGB-1, a 25-kd protein, in the supernatants of UV light-treated cells, but not in that treated with anti-HMGB-1 antibodies (Fig. 4).
High-mobility group box chromosomal protein 1 (HMGB-1) concentrations in the supernatants of UV light-treated cells treated with or without antibodies. HMGB-1 concentrations were determined by immunoblot analysis. HMGB-1, a 25-kd protein, was expressed in the supernatants of UV light-treated cells (a) but not in supernatants of cells treated with anti-HMGB-1 antibodies (b). Lanes on the left indicate the range of standard HMGB-1 concentrations (0–400 ng/ml)
HMGB-1 levels change in culture supernatants over time
HMGB-1 concentrations in culture supernatants of cholesteatoma keratinocytes stimulated with supernatants from UV light-treated cells for 0, 4, 8, 12, 24, 36, or 48 h were measured by immunoblot analysis. Combined PI and annexin V staining and flow cytometric analysis of UV light-treated cells revealed a pattern consistent with late apoptosis (Fig. 5). The expression of HMGB-1, a 25-kd protein, was detected in culture supernatants and the supernatants from UV light-treated cells. This indicated that cholesteatoma keratinocytes could release HMGB-1 during the course of apoptosis (Fig. 6). Concurrently, HMGB-1 concentrations in culture supernatants were high at 4 h, had slightly declined by 12 h, and then slightly increased, reaching a peak at 48 h (Fig. 6), suggesting UV light-treated cells can release an unknown substance that induces HMGB-1 release. Although the nature of the substance is unknown, we demonstrated that it could be released into the culture medium.
Translocation and expression of HMGB-1 in cholesteatoma keratinocytes
To examine the translocation of HMGB-1, cholesteatoma keratinocytes were cultured in a Lab-Tech chamber slide and stimulated with supernatants from UV light-treated cells for 1 and 24 h. Immunofluorescence assay demonstrated that in the control group without stimulation, HMGB-1 staining was observed only in the nucleus (Fig. 7a–c). However, translocation of HMGB-1 from the nucleus to the cytosol was observed in cholesteatoma keratinocytes at 1 h (Fig. 7d–f) and it was maintained for 24 h (Fig. 7g–i).
Translocation of high-mobility group box chromosomal protein 1 (HMGB-1) in cholesteatoma keratinocytes after stimulation with supernatants from UV light-treated cells. Immunofluorescence assay indicated that HMGB-1 was translocated from the nucleus to the cytosol following stimulation. Cholesteatoma keratinocytes were stimulated for 1 h (d–f) or 24 h (g–i) and then compared with controls (a–c). The control group only exhibited HMGB-1 staining in the nucleus (a–c). Cholesteatoma keratinocytes were incubated with anti-HMGB-1 rabbit polyclonal antibody after stimulation. Nuclei were stained with DAPI (blue). Blue arrows depict nuclear HMGB-1. White arrows depict cytoplasmic HMGB-1. (Color figure online)
We quantified the expression of HMGB-1 in cytosol of cholesteatoma keratinocytes by immunoblot analysis. Treatment with supernatants from UV light-treated cells for 0, 1, 4, 8, 12, 24, 36, and 48 h demonstrated the levels of HMGB-1 in the cytoplasm of cholesteatoma keratinocytes was time-dependent. HMGB-1 concentrations reached a first peak at 1 h, slightly declined at 24 h and then sharply increased, reaching a second peak at 48 h (Fig. 8).
HMGB-1 complexed to CpG-DNA induces expression of cytokine in cholesteatoma keratinocytes
HMGB-1 and DNA were thought to form a complex to induce cytokines release in pDCs [16]. We next evaluated whether the presence of HMGB-1 could influence cytokines expression in cholesteatoma keratinocytes. Cultures of human cholesteatoma keratinocytes were exposed to CpG-DNA, HMGB-1, or CpG-DNA complexed to HMGB-1 for 24 h. Keratinocytes cultured with CpG-DNA complexed toHMGB-1, but not CpG DNA alone or LL-37 alone, led to the release of IL-1β (Fig. 9b) and TNF-α (Fig. 9a).
Induction of cytokines by substance released from UV light-treated cells
To determine whether the supernatant from UV light-treated cells had a proinflammatory effect, we measured the concentration of IL-1β and TNF-α in culture supernatants by ELISA. Supernatants from UV light-treated cells were potential TNF-α and IL-1β inducers. However, the secretion patterns for these cytokines were different. The peak TNF-α level after stimulation with supernatants occurred at 4 h and then tended to decrease (Fig. 10a). However, except at 12 h, IL-1β levels increased in a time-dependent manner (Fig. 10b).
Secretion patterns of TNF-α (a) and IL-1β (b). Keratinocytes were stimulated with supernatants from UV light-treated cells, and levels of TNF-α and IL-1β released into culture medium were measured by enzyme-linked immunosorbent assay. Values are the mean ± SD. TNF-α levels peaked at 4 h and tended to decrease (*P < 0.05 vs. 0 h). IL-1β levels increased in a time-dependent manner, except at 12 h, and were all significantly higher than those at 0 h (*P < 0.05 vs. 0 h)
To determine whether HMGB-1 is a mediator of cytokine production, neutralizing anti-HMGB-1 antibodies were added to the cultures of the cholesteatoma keratinocytes. Treatment with anti-HMGB-1 antibodies eliminated the cytokine-inducing capacity of supernatants from UV light-treated cells, indicating that the induction of increased IL-1β (Fig. 11b) and TNF-α (Fig. 11a) synthesis by supernatants from UV light-treated cells requires HMGB-1.
Effects of anti-HMGB-1 antibodies on the cytokine-inducing capacity of supernatants from UV light-treated cells. Supernatants were obtained 16–24 h after UV light treatment. They were incubated for 12 h at 37 °C with or without anti-HMGB-1 antibodies and then tested for the ability to induce TNF-α (a) and IL-1β (b) production in cultures of the cholesteatoma keratinocytes
To examine whether treatment of apoptotic cell material with DNase affected its ability to induce cytokine production, apoptotic supernatants from UV light-treated cells treated with DNase were added to the cultures of the cholesteatoma keratinocytes. Treatment with DNase decreased the cytokines-inducing ability of supernatants from UV light-treated cells (Fig. 12). This study demonstrated the importance of DNA fragments in the apoptotic cell material for induction of IL-1β (Fig. 12b) and TNF-α (Fig. 12a).
Effects of DNase on the cytokines-inducing capacity of supernatants from UV light-treated cells. Supernatants were obtained 16–24 h after UV light treatment. They were incubated for 1 h at 37 °C with or without DNase and then tested for the ability to induce TNF-α (a) and IL-1β (b) production in cultures of the cholesteatoma keratinocytes
HMGB-1, TNF-α, and IL-1β mRNA expression levels in cholesteatoma keratinocytes
The effects of UV light-treated cell supernatants on HMGB-1, TNF-α, and IL-1β mRNA expression were analyzed by real-time PCR after exposing cholesteatoma keratinocytes to supernatants from UV light-treated cells for 0, 4, 8, 12, 24, 36, or 48 h. Supernatants from UV light-treated cells induced HMGB-1, TNF-α, and IL-1β release by cholesteatoma keratinocytes via increased gene transcription. qRT-PCR analysis revealed that HMGB-1 was up-regulated, as shown by the level of mRNA transcription after stimulation for 4 h and 8 h (Fig. 13a). TNF-α was also up-regulated at the mRNA level after stimulation for 4, 8, and 48 h (Fig. 13b). Up-regulation of IL-1β was also observed after stimulation for 4 h (Fig. 13c).
Discussion
In this study, we found that keratinocytes in cholesteatomatous samples exhibited an increased amount of cytoplasmic and suggestive extracellular secreted HMGB-1 compared with normal skin. Previously, it was reported that HMGB-1 was not released during apoptosis. However, Bell et al. reported that the release of HMGB-1 occurs during the course of apoptosis as well as necrosis in Jurkat cells [13]. Our study also showed that the induction of apoptosis by UV light irradiation leads to the extracellular release of HMGB-1. It was reported that proliferation and apoptosis are both present in cholesteatoma [19]. In this study, we observed that apoptotic cells were found in the cholesteatoma epithelium. This finding was in agreement with previous reports [20]. Since apoptosis is typically seen in cholesteatoma, the presence of extracellular HMGB-1 staining indicates the release of cytoplasmic HMGB-1 from apoptotic cells. In the current study, we also observed increased levels of HMGB-1 in supernatants after stimulation with supernatant from UV light-treated cells. Therefore, HMGB-1 may be actively secreted by cholesteatoma keratinocytes by stimulation of apoptotic material and may be passively released during apoptosis. Thus, it may constitute a source of extracellular HMGB-1 in cholesteatoma.
In the current study, we found that apoptotic cells released material that induced HMGB-1 translocation and release. Furthermore, such apoptotic material appeared late during the cultures, at 16–24 h after UV irradiation. This delayed release may be due to the retained cell membrane integrity in early apoptotic cells, which is eventually lost during the later stages of apoptosis. In normal in vivo situations, apoptotic cells are phagocytosed rapidly, while the membrane integrity is still intact. Consequently, there is less time for the release of material that can induce HMGB-1 release. However, patients with cholesteatomatous chronic otitis media have both increased apoptosis and decreased clearance of apoptotic cells and this may allow the continuous release of apoptotic material that induces extracellular HMGB-1 production in this disease.
HMGB-1 appears to have two distinct functions in cellular systems, first as an intracellular regulator of transcription, and second, it has an extracellular role where it promotes tumor metastasis and inflammation [21, 22]. An early suggestion was that HMGB-1 causes inflammatory cells to secrete TNF, IL-1a, IL-1b, IL-1RA, IL-6, IL-8, macrophage inflammatory protein (MIP)-1a, and MIP-1b, but not IL- 10 or IL-12 (14). Thus, HMGB-1 might participate in a ‘cytokine storm.’ But several recent studies have found that carefully purified HMGB-1 caused little secretion of any cytokine from mouse or human macrophages [16, 17, 23]. In this study, we found that the production of TNF-α and IL-1β can be induced by purified HMGB-1 combined with CpG-DNA but not by purified HMGB-1 alone or CpG-DNA alone in the cholesteatoma keratinocytes. In addition, supernatants of apoptotic cells containing HMGB-1 were effective in inducing cytokine secretion, suggesting that the activity was due to the combined action of HMGB-1 with another material. Moreover, we also observed that DNase or anti-HMGB-1 antibody treatment of supernatants from apoptotic cells decreased their cytokine-inducing activity. Therefore, we concluded that apoptotic cells release HMGB-1 that induced TNF-α and IL-1β production when combined with DNA fragments.
Furthermore, cholesteatoma keratinocytes released TNF-α and IL-1β after stimulation with apoptotic material containing HMGB-1−DNA complexes. However, the source of DNA fragments in cholesteatoma tissues remains unknown. It is reported that host-derived DNA can be released into extracellular environment during apoptotic cell death [24]. In this study, we observed that a larger number of apoptotic cells and DNA fragments was detectable in the cholesteatoma epithelium. These results demonstrated that extracellular DNA fragments were derived from surrounding apoptotic cells.
It is well established that the production of TNF-α could play an important role in bone resorption in primary cholesteatoma [25, 26]. Akimoto et al. [8] reported increased levels of TNF-α in primary cholesteatoma compared with normal external ear canal skin. TNF-α is produced by activated macrophages and mast cells [27]. However, few mononuclear cells could be detected in primary cholesteatoma. Therefore, the source of TNF-α in primary cholesteatomas is unclear. In our study, we found that keratinocytes stimulated by apoptotic material containing HMGB-1−DNA complexes could induce both TNF-α and IL-1β. Thus, HMGB-1−DNA complexes might act as a key molecule involved in bone resorption associated with cholesteatoma by inducing the production of TNF-α and IL-1β.
In conclusion, the activation of cholesteatomatous keratinocytes by apoptotic material containing HMGB-1−DNA complexes greatly induced the production of TNF-α and IL-1β. In addition, extracellular HMGB-1 and DNA fragments were present in the cholesteatomatous epithelium. These events suggest the persistent expression of extracellular HMGB-1 and DNA fragments in cholesteatoma leads to the production of TNF-α and IL-1β, which leads to cause bone resorption and destruction in cholesteatoma.
References
Bujia J, Holly A, Antoli-Candela F, Tapia MG, Kastenbauer E (1996) Immunobiological peculiarities of cholesteatoma in children: quantification of epithelial proliferation by MIB1. Laryngoscope 106:865–868
Chole RA (1997) The molecular biology of bone resorption due to chronic otitis media. Ann N Y Acad Sci 830:95–109
Kurihama A, Toshima M, Yuasa R, Takasaka T (1991) Bone resorption mechanisms in chronic otitis media with cholesteatoma: specific production by cholesteatoma tissue in culture of bone-resorbing activity attributable to interleukin-1 alpha. Ann Otol Rhinol Laryngol 100:989–998
Yan SD, Huang CC (1991) The role of tumor necrosis factor-alpha in bone resorption of cholesteatoma. Am J Otolaryngol 12:83–89
Kinoshita T (1994) The roles of interleukin-1, tumor necrosis factor-alpha and parathyroid hormone in bone resorption of cholesteatoma otitis. Nihon Jibiinkoka Gakkai Kaiho 97:1472–1480
Ahn JM, Huang CC, Abramson M (1990) Localization of interleukin-1 in human cholesteatoma. Am J Otolaryngol 11:71–77
Amar MS, Wishahi HF, Zakhary MM (1996) Clinical and biochemical studies of bone destruction in cholesteatoma. J Laryngol Otol 110:534–539
Akimoto R, Pawankar R, Yagi T, Baba S (2000) Acquired and congenital cholesteatoma: determination of tumor necrosis factor-alpha, intercellular adhesion molecule-1, interleukin-1-alpha and lymphocyte functional antigen-1 in the inflammatory process. ORL J Otorhinolaryngol Relat Spec 62:257–265
Goodwin GH, Sanders C, Johns EW (1973) A new group of chromatin associated proteins with a high content of acidic and basic amino acids. Eur J Biochem 38:14–19
Andersson U, Erlandsson-Harris H, Yang H, Yang H, Tracey KJ (2002) HMGB1 as a DNA-binding cytokine. J Leukoc Biol 72:1084–1091
Wang H, Bloom O, Zhang M et al (1999) HMG-1 as a late mediator of endotoxin lethality in mice. Science 285:248–251
Scaffidi P, Misteli T, Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418:191–195
Bell CW, Jiang W, Reich CF, Pisetsky DS (2006) The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol 291:1318–1325
Andersson U, Wang H, Palmblad K et al (2000) High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med 192:565–570
Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E (2004) Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem 279:7370–7377
Tian J, Avalos AM, Mao SY et al (2007) Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol 8:487–496
Ivanov S, Dragoi AM, Wang X et al (2007) A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood 110:1970–1981
Lövgren T, Eloranta ML, Båve U, Alm GV, Ronnblom L (2004) Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum 50:1861–1872
Shinoda H, Huang CC (1995) Expressions of c-jun and p53 proteins in human middle ear cholesteatoma: relationship to keratinocyte proliferation, differentiation, and programmed cell death. Laryngoscope 105:1232–1237
Olszewska E, Chodynicki S, Chyczewski L (2006) Apoptosis in the pathogenesis of cholesteatoma in adults. Eur Arch Otorhinolaryngol 263:409–413
Yang H, Wang H, Tracey KJ (2001) HMG-1 rediscovered as a cytokine. Shock 15:247–253
Czura CJ, Tracey KJ (2003) Targeting high mobility group box 1 as a late-acting mediator of inflammation. Crit Care Med 31:46–50
Rouhiainen A, Tumova S, Valmu L, Kalkkinen N, Rauvala H (2007) Pivotal advance: analysis of proinflammatory activity of highly purified eukaryotic recombinant HMGB1 (amphoterin). J Leukoc Biol 81:49–58
Pisetsky DS, Fairhurst AM (2007) The origin of extracellular DNA during the clearance of dead and dying cells. Autoimmunity 40:281–284
Felix R, Fleisch H, Elford PR (1989) Bone-resorbing cytokines enhance release of macrophage colony stimulating activity by the osteoblastic cell MC3T3-E1. Calcif Tissue Int 44:356–3560
Robinson DR, Tashjian AH Jr, Levine L (1975) Prostaglandin stimulated bone resorption by rheumatoid synovia: a possible mechanism for bone destruction in rheumatoid arthritis. J Clin Invest 56:1181–1188
Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B (1975) An endotoxin-induced serum factor that causes necrosis of tumor. Proc Natl Acad Sci 72:3666–3670
Acknowledgments
This work was supported by the Shanghai Shen-kang Medical Science Project SHDC12010119, Natural Science Foundation Project of CQ CSTC and Ministry Clinical Disciplines Project 2010-439.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chi, Z., Wang, Z., Liang, Q. et al. Induction of cytokine production in cholesteatoma keratinocytes by extracellular high-mobility group box chromosomal protein 1 combined with DNA released by apoptotic cholesteatoma keratinocytes. Mol Cell Biochem 400, 189–200 (2015). https://doi.org/10.1007/s11010-014-2275-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-014-2275-0