Abstract
microRNAs (miRNAs) are small non-coding RNAs that have been suggested to play an essential role in tumorigenesis. Reduced expression of miR-338 has been reported in several types of cancers; however, the role of miR-338 in oral squamous cell carcinoma (OSCC) has not been elucidated. In this study, we demonstrated that miR-338 was dramatically downregulated in OSCC tissues and cell lines. Overexpression of miR-338 significantly inhibited proliferation, colony formation, migration, and invasion of OSCC cells. In addition, neuropilin1 (NRP1) was identified as a target of miR-338 in OSCC cells and inversely correlated with miR-338 in OSCC tissues. Furthermore, restoration of NRP1 attenuated the tumor-suppressive effects of miR-338. Taken together, miR-338 might inhibit growth and metastasis of OSCC cells by targeting NRP1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral squamous cell carcinoma (OSCC) accounts for approximately 90 % of all oral cancer with over 145,500 deaths worldwide annually [1]. Despite of therapeutical advances in cancers, mortality and morbidity rates have remained very high, and the 5-year survival rate of OSCC patients is about 50 % for several decades [2]. In the early stages, the tumor responds well to the combination therapy, with 80 % 5-year survival rate in these patients. However, half of the patients present regional or distant metastases in advanced stage OSCC [2]. Therefore, the development of novel biomarkers for the early detection of OSCC is urgently needed to improve the viability of patients with OSCC. Although previous studies have identified that oral carcinogenesis arises from the oncogenes activation or tumor suppressor genes inactivation [3], the molecular mechanism of OSCC remains unclear.
microRNAs (miRNAs) are small non-coding RNAs, with approximately 22 nucleotides in length, which post-transcriptionally regulate gene expression via binding to the 3′-untranslated regions (3′-UTRs) of target mRNA, leading to mRNA degradation or translational inhibition [4]. Aberrant miRNA expression, acting as oncogenes or tumor suppressors, has also been frequently reported in many cancers, including gastric cancer [5], prostate cancer [6], ovarian cancer [7], and lung cancer [8]. Recently, accumulating evidence have indicated that miRNAs play essential roles in the processes of tumor cell biology, involving cell proliferation [9], differentiation [10], migration as well as invasion [5].
miR-338 has been reported to be aberrantly overexpressed in several cancers. Guo et al. [11] reported that miR-338 was significantly downregulated in both gastric cancer tissue and its cell lines, while overexpression of miR-338 suppressed the growth of gastric cancer cells through PTEN-PI3K signaling pathway by targeting P-Rex2a. In addition, miR-338 was epigenetically silenced in gastric cancer, miR-338 overexpression in gastric cancer cells inhibited proliferation, migration, invasion, and tumorigenicity by targeting to SSX2IP both in vitro and in vivo. However, Scapoli L et al. [12] found that miR-338 was significantly overexpressed in 15 OSCC samples using microarray analysis. However, the detailed mechanism of miR-338 in the progression of carcinogenesis of OSCC remains elusive.
In the present study, we found that the expression of miR-338 was decreased in OSCC clinical tissue samples and its cell lines. Overexpression of miR-338 inhibited the proliferation, migration, and invasion of OSCC cells. Finally, we further identified neuropilin1 (NRP1) as a direct target of miR-338 in OSCC cells.
Materials and methods
Tissue samples, cell lines, and transfection
Twenty-four-paired OSCC and matched adjacent nontumor tissues were obtained from patients undergoing resection of OSCC at Department of Stomatology, Xi’an No.4 Hospital, China. The samples were immediately snap-frozen into liquid nitrogen and stored in −80 °C until use. No patients received chemotherapy or radiotherapy prior to surgery. Both tumor and normal tissues were histologically confirmed by two independent pathologists. Informed consent was obtained from each patient, and this study was approved by the Ethics Committee of Xi’an No.4 Hospital.
Tca-8113 cells were obtained from the Ninth People’s Hospital, Shanghai Second Medical University. SCC-15 cells were obtained from American Type Culture Collection. Tca-8113, SCC-15, and HEK 293 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % fetal bovine serum (FBS), 100 U/ml streptomycin, and 100 U/ml penicillin and incubated at 37 °C with 5 % CO2. The cells were transfected using Lipofectamine 2000 Reagent (Invitrogen) according to the manufacturer’s instructions.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA (miRNA and mRNA) was isolated using TRIzol (Invitrogen, Carlsbad, CA, USA) from the cells and OSCC samples. All reagents for qRT-PCR were purchased from Takara (Dalian, China). For mRNA analysis, the first-strand cDNA was generated using the PrimeScript® RT reagent kit according to the manufacturer’s instructions. For miRNA analysis, the mature miRNA was reversely transcribed using miRNA-specific primer of miR-338. qRT-PCR analyses for both mRNA and miRNA were performed with the SYBR Premix Ex Taq II (Applied Biosystems). Expression of mRNA and miRNA was normalized to β-actin and U6, respectively. The relative expression was calculated using \(2^{{ - \Delta \Delta C_{t} }}\). Sequences of the qRT-PCR primers are shown in Table 1.
Plasmids
pre-miR-338 coding sequence was chemically synthesized (Beijing AuGCT DNA-SYN Biotechnology Co.Ltd) and subcloned into the pcDNA6.2-GW vector through EcoRI and HindIII restriction sites (Promega). The 3′-UTRs of wide-type (WT) NRP1 and the mutant-type (MT) NRP1 for miR-338 was synthesized (Beijing AuGCT DNA-SYN Biotechnology Co.Ltd) and was inserted into pmirGLO vector by Sac I and Xho I restriction sites (Promega). The ORF sequence of NRP1 was amplified from genomic DNA isolated from the Tca-8813 cells, and the ORF region of the NRP1 cDNA was then subcloned into the GV230 vector (GeneChem, Shanghai, China). The sequences of construced plasmids were confirmed by qRT-PCR in OSCC cells. Their sequences are also shown in Table 1.
Cell proliferation
The cells were seeded in 96-well plates at a density of 3000 cells/well and transfected with pre-miR-338 and miR-control. At 24, 48, and 72 h after transfection, cell proliferation was assessed using the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. Briefly, a 20-μl MTT solution was added into each well and incubated at 37 °C for 4 h, then the supernatant was removed, and 150 μl Dimethyl sulfoxide (DMSO) was added. The OD at 492 nm was measured by a microplate spectrophotometer.
Colony formation assay
The cells were seeded in 12-well plates at 2000 cells/well after 24 h of transfection. Approximately, after 2 weeks, the colonies were then fixed, stained with 0.1 % crystal violet for 15 min, counted, and normalized to the control group.
Migration and invasion assays
Transwell invasion assays were performed with uncoated (for migration) or coated Matrigel (for invasion). 1 × 105cells were seeded into the upper chamber in serum-free medium containing 0.1 % FBS, and 500 μl complete medium was added to the bottom. After 24 h incubation at 37 °C with 5 % CO2, the migrated and invaded cells in the lower membrane were fixed with methanol and stained with 0.1 % crystal violet. Four random fields were captured from each membrane.
Luciferase reporter assay
HEK 293 cells were seeded and co-transfected with wild type (WT) or mutant (Mut) 3′-UTR of NRP1 and pre-miR-338 or miR-control. Cells were harvested after 24 h of transfection, and both firefly and renilla luciferase activities were measured by the Dual-Luciferase Reporter Assay System (Promega, Wisconsin, WI, USA).
Western blotting analysis
Total proteins extracted from SCC-15 cells were separated by 10 % SDS-PAGE and transferred to PVDF membrane (Millipore, MA, USA). The membranes were blocked with 5 % non-fat milk in Tris-buffered saline with 0.1 % Tween-20 (TBST) for 2 h. The membranes were then incubated with anti-NRP1 antibody (Proteintech Group Inc., China. 1:1000) at 4 °C overnight, and subsequently probed with secondary antibodies conjugated with HRP and detected by ECL. Protein levels were normalized to β-actin.
Statistical analysis
All data sets were presented as mean ± SEM. The differences between groups were analyzed using one-way ANOVA or non-paired t test with SPSS 13.3, and P < 0.05 was considered to be statistically significant.
Results
miR-338 was decreased in both OSCC tissues and cell lines
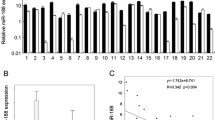
Expression of miR-338 in 24-paired OSCC tissue samples and adjacent nontumor tissues was examined by qRT-PCR. Data showed that the expression of miR-338 was significantly reduced in OSCC compared with the nontumor tissues (Fig. 1a, P < 0.001). Similarly, miR-338 was dramatically decreased in Tca-8113 and SCC-15 cells compared with the normal oral epithelial cells (Fig. 1b, P < 0.001, P = 0.006, respectively).
miR-338 was decreased in both OSCC tissues and cell lines. a Expression level of miR-338 between OSCC and normal tissues was detected by qRT-PCR. b Expression level of miR-338 between in two OSCC cell lines (Tca-8113 and SCC-15 cells) and normal oral epithelial cells was detected by qRT-PCR. U6 was used as control. *P < 0.05, **P < 0.001 compared with the control group
miR-338 inhibited growth in OSCC cells
To explore the effect of miR-338 on cell growth, Tca-8113 and SCC-15 cells were transiently transfected with pre-miR-338. The expression of miR-338 was identified by qRT-PCR (Fig. 2a, P < 0.001). As shown in Fig. 2b, the results of MTT assay displayed that overexpression of miR-338 significantly inhibited the cell proliferation of Tca-8113 and SCC-15 cells compared to the miR-control (P < 0.05). Similarly, miR-338 overexpression suppressed colony formation of Tca-8113 and SCC-15 cells (Fig. 2c, P = 0.006, P = 0.002, respectively).
miR-338 suppressed OSCC cell proliferation and colony formation. a Expression of miR-338 in two OSCC cell lines (Tca-8113 and SCC-15 cells) transfected with pre-miR-338 or the miR-control was detected by qRT-PCR. b MTT assay was use to examine the proliferation of two OSCC cell lines (Tca-8113 and SCC-15 cells). c Colony formation of two OSCC cell lines (Tca-8113 and SCC-15 cells) transfected with pre-miR-338 or the miR-control. Experiments were performed in triplicate. *P < 0.05, **P < 0.001 compared with the control group
miR-338 suppressed OSCC cell migration and invasion
We further evaluated whether miR-338 could also inhibit the migration and invasion of OSCC cells. As shown in Fig. 3a, overexpression of miR-338 inhibited tumor cell migration in SCC-15 cells compared with the miR-control (P = 0.001). In consistent with this result, transwell assays with Matrigel demonstrated that miR-338 significantly suppressed the invasive capacity of SCC-15 cells (Fig. 3b, P = 0.004).
miR-338 inhibited OSCC cell migration and invasion. a Migration assay of SCC-15 cells transfected with pre-miR-338 or the miR-control. b Invasion assay of SCC-15 cells transfected with pre-miR-338 or the miR-control. Experiments were performed in triplicate. *P < 0.05 compared with the control group
NRP1 was a target of miR-338 in OSCC cells
To identify the target of miR-338 in OSCC, TargetScan 6.2 (http://www.targetscan.org/) was used to select the potential target of miR-338. NRP1 was predicted as a target of miR-338 (Fig. 4a). Luciferase activity assay showed that miR-338 significantly inhibited the luciferase activity of WT NRP1 (NRP1-WT) but not the mutant (NRP1-MT) in HEK 293 cells (Fig. 4b, P = 0.005). Furthermore, SCC-15 cells were transfected with pre-miR-338 or miR-control, then qRT-PCR and Western blot were performed. The results showed that overexpression of miR-338 significantly increased both the mRNA (Fig. 4c, P = 0.001) and protein levels of NRP1 (Fig. 4d).
NRP1 was a target of miR-338 in OSCC cells. a The potential miR-338 targeting site in NRP1 3′-UTR and the mutated sequences. b HEK-293 cells were co-transfected with pre-miR-338 or the miR-control with wild-type (WT) or mutant-type (MT) NRP1 3′-UTR, luciferase activity was detected. c Expression of NRP1 mRNA was detected by qRT-PCR in SCC-15 cells transfected with pre-miR-338 or the miR-control. d NRP1 protein level was detected by Western blotting in SCC-15 cells transfected with pre-miR-338 or the miR-control. β-actin was used as an internal control. *P < 0.05 compared with the control group
Restoration of NRP1 attenuated the tumor-suppressive effects of miR-338
We further investigated whether recovery of NRP1 could attenuate the tumor-suppressive effects of miR-338. SCC-15 cells were transfected with NRP1 expression vector, and the effect of NRP1 was confirmed by qRT-PCR and Western blot (Fig. 5a, b). MTT assay, colony formation, migration, and invasion assays showed that restoration of NRP1 attenuated the tumor-suppressive effects of miR-338 (Fig. 5c–f, P < 0.05).
NRP1 overexpression attenuated the tumor-suppressive effects of miR-338. a NRP1 mRNA level was detected by qRT-PCR in SCC-15 cells transfected with NRP1 vector. b NRP1 protein level was detected by Western blotting in SCC-15 cells transfected with NRP1 vector. c MTT assay was use to examine the proliferation in SCC-15 cells which were co-transfected with pre-miR-338 and NRP1 or NRP1 vector. d Colony formation assay was performed in SCC-15 cells which were co-transfected with pre-miR-338 and NRP1 or NRP1 vector. e Migration assay was performed in SCC-15 cells which were co-transfected with pre-miR-338 and NRP1 or NRP1 vector. f Invasion assay was performed in SCC-15 cells which were co-transfected with pre-miR-338 and NRP1 or NRP1 vector. Experiments were performed in triplicate. *P < 0.05, **P < 0.001 compared with the control group, # P < 0.05
NRP1 was inversely correlated with miR-338 in OSCC
Expression of NRP1 in 24-paired OSCC tissues was examined by qRT-PCR. The expression of NRP1 was remarkably increased in OSCC tissue samples compared with adjacent nontumor tissues (Fig. 6a, P < 0.001). Moreover, NRP1 mRNA level was inversely correlated with miR-338 level in OSCC (Fig. 6b, r = −0,984, P < 0.001).
Discussion
Accumulating evidence reveals the vital roles of miRNAs in OSCC progression. In this study, we validated that the expression of miR-338 was significantly decreased in OSCC tissue samples and cell lines compared with normal controls. Forced expression of miR-338 could remarkably suppress proliferation, colony formation, migration, and invasion of OSCC cells, which was consistent with previous studies from other cancers [11, 13, 14] suggesting that miR-338 might play a vital role in the development of OSCC. In addition, we identified that NRP1 was a direct target of miR-338 in OSCC cells. Restoration of NRP1 dramatically attenuated the tumor-suppressive effects of miR-338 in OSCC cells. Moreover, NRP1 was inversely correlated with miR-338 in OSCC tissues.
NRP-1 is a co-receptor for vascular endothelial growth factor (VEGF) [15, 16] which includes five extracellular domains, a transmembrane domain, and an intracellular domain [17, 18]. Accumulating evidence suggests that NRP1 was increased in a variety of tumors, including gastric cancer [19], pancreatic cancer [20], oral cancer [21], and esophageal carcinoma [22]. The roles of NRP1, as an oncogene, in cancer cells are still controversial, as NRP1 overexpression is involved in proliferation, angiogenesis, metastasis, and immunity [20, 23]. However, the potential molecular mechanisms have not been elucidated. Recently, several studies revealed that NRP1 could be regulated by a number of miRNAs in human cancers [19, 20, 24]. NRP1 has been found to be targeted by miR-1247 inhibits cell proliferation using in vitro and in vivo models in pancreatic cancer [20]. Cui et al. [25] demonstrated that miR-9 and miR-181b inhibited the arsenic-induced angiogenesis by targeting NRP1, which was consistent with studies of Wu et al. that miR-320 regulated tumor angiogenesis driven by vascular endothelial cells in oral cancer by silencing NRP1 [21]. Here, we found that NRP1 was a target of miR-338 in OSCC cells, and overexpression of NRP1 could dramatically attenuate the tumor-suppressive effects of miR-338 on OSCC cells. Furthermore, NRP1 was inversely correlated with miR-338 in OSCC tissues, suggesting that NRP1 overexpression may be involved in progression of OSCC.
In conclusion, in the present study, we demonstrated that miR-338 was significantly decreased in OSCC. Overexpression of miR-338 inhibited tumor growth and metastasis of OSCC through targeting NRP1, suggesting that miR-338 might serve as a potential diagnostic marker and therapeutic target for OSCC.
References
Petersen PE (2009) Oral cancer prevention and control–the approach of the World Health Organization. Oral Oncol 45(4–5):454–460. doi:10.1016/j.oraloncology.2008.05.023
Funk GF, Karnell LH, Robinson RA, Zhen WK, Trask DK, Hoffman HT (2002) Presentation, treatment, and outcome of oral cavity cancer: a National Cancer Data Base report. Head Neck 24(2):165–180. doi:10.1002/hed.10004
Croce CM (2008) Oncogenes and cancer. N Engl J Med 358(5):502–511
Winter J, Jung S, Keller S, Gregory RI, Diederichs S (2009) Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol 11(3):228–234. doi:10.1038/ncb0309-228
Zhou X, Li L, Su J, Zhang G (2014) Decreased miR-204 in H. pylori-associated gastric cancer promotes cancer cell proliferation and invasion by targeting SOX4. PLoS ONE 9(7):e101457. doi:10.1371/journal.pone.0101457
Karatas OF, Guzel E, Suer I, Ekici ID, Caskurlu T, Creighton CJ, Ittmann M, Ozen M (2014) miR-1 and miR-133b are differentially expressed in patients with recurrent prostate cancer. PLoS ONE 9(6):e98675. doi:10.1371/journal.pone.0098675
Cao Q, Lu K, Dai S, Hu Y, Fan W (2014) Clinicopathological and prognostic implications of the miR-200 family in patients with epithelial ovarian cancer. Int J Clin Exp Pathol 7(5):2392–2401
Wang RT, Xu M, Song ZG, Xu CX, Jin H (2014) Decreased expression of miR-216a contributes to non-small cell lung cancer progression. Clin Cancer Res. doi:10.1158/1078-0432.ccr-14-0517
Gao SL, Wang LZ, Liu HY, Liu DL, Xie LM, Zhang ZW (2014) miR-200a inhibits tumor proliferation by targeting ap-2gamma in neuroblastoma cells. Asian Pac J Cancer Prev 15(11):4671–4676
Samaraweera L, Grandinetti KB, Huang R, Spengler BA, Ross RA (2014) MicroRNAs define distinct human neuroblastoma cell phenotypes and regulate their differentiation and tumorigenicity. BMC Cancer 14(1):309. doi:10.1186/1471-2407-14-309
Guo B, Liu L, Yao J, Ma R, Chang D, Li Z, Song T, Huang C (2014) miR-338-3p suppresses gastric cancer progression through a PTEN-AKT axis by targeting P-REX2a. Mol Cancer Res 12(3):313–321. doi:10.1158/1541-7786.mcr-13-0507
Scapoli L, Palmieri A, Lo Muzio L, Pezzetti F, Rubini C, Girardi A, Farinella F, Mazzotta M, Carinci F (2010) MicroRNA expression profiling of oral carcinoma identifies new markers of tumor progression. Int J Immunopathol Pharmacol 23(4):1229–1234
Xue Q, Sun K, Deng HJ, Lei ST, Dong JQ, Li GX (2014) MicroRNA-338-3p inhibits colorectal carcinoma cell invasion and migration by targeting smoothened. Jpn J Clin Oncol 44(1):13–21. doi:10.1093/jjco/hyt181
Chen X, Pan M, Han L, Lu H, Hao X, Dong Q (2013) miR-338-3p suppresses neuroblastoma proliferation, invasion and migration through targeting PREX2a. FEBS Lett 587(22):3729–3737. doi:10.1016/j.febslet.2013.09.044
Pan Q, Chathery Y, Wu Y, Rathore N, Tong RK, Peale F, Bagri A, Tessier-Lavigne M, Koch AW, Watts RJ (2007) Neuropilin-1 binds to VEGF121 and regulates endothelial cell migration and sprouting. J Biol Chem 282(33):24049–24056. doi:10.1074/jbc.M703554200
Fuh G, Garcia KC, de Vos AM (2000) The interaction of neuropilin-1 with vascular endothelial growth factor and its receptor flt-1. J Biol Chem 275(35):26690–26695. doi:10.1074/jbc.M003955200
Appleton BA, Wu P, Maloney J, Yin J, Liang WC, Stawicki S, Mortara K, Bowman KK, Elliott JM, Desmarais W, Bazan JF, Bagri A, Tessier-Lavigne M, Koch AW, Wu Y, Watts RJ, Wiesmann C (2007) Structural studies of neuropilin/antibody complexes provide insights into semaphorin and VEGF binding. EMBO J 26(23):4902–4912. doi:10.1038/sj.emboj.7601906
Staton CA, Kumar I, Reed MW, Brown NJ (2007) Neuropilins in physiological and pathological angiogenesis. J Pathol 212(3):237–248. doi:10.1002/path.2182
Peng Y, Liu YM, Li LC, Wang LL, Wu XL (2014) MicroRNA-338 inhibits growth, invasion and metastasis of gastric cancer by targeting NRP1 expression. PLoS ONE 9(4):e94422. doi:10.1371/journal.pone.0094422
Shi S, Lu Y, Qin Y, Li W, Cheng H, Xu Y, Xu J, Long J, Liu L, Liu C, Yu X (2014) miR-1247 is correlated with prognosis of pancreatic cancer and inhibits cell proliferation by targeting neuropilins. Curr Mol Med 14(3):316–327
Wu YY, Chen YL, Jao YC, Hsieh IS, Chang KC, Hong TM (2014) miR-320 regulates tumor angiogenesis driven by vascular endothelial cells in oral cancer by silencing neuropilin 1. Angiogenesis 17(1):247–260. doi:10.1007/s10456-013-9394-1
Alattar M, Omo A, Elsharawy M, Li J (2014) Neuropilin-1 expression in squamous cell carcinoma of the oesophagus. Eur J Cardiothorac Surg 45(3):514–520. doi:10.1093/ejcts/ezt380
Prud’homme GJ, Glinka Y (2012) Neuropilins are multifunctional coreceptors involved in tumor initiation, growth, metastasis and immunity. Oncotarget 3(9):921–939
Wu YY, Chen YL, Jao YC, Hsieh IS, Chang KC, Hong T-M (2014) miR-320 regulates tumor angiogenesis driven by vascular endothelial cells in oral cancer by silencing neuropilin 1. Angiogenesis 17(1):247–260. doi:10.1007/s10456-013-9394-1
Cui Y, Han Z, Hu Y, Song G, Hao C, Xia H, Ma X (2012) MicroRNA-181b and microRNA-9 mediate arsenic-induced angiogenesis via NRP1. J Cell Physiol 227(2):772–783. doi:10.1002/jcp.22789
Conflict of interest
none
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, C., Wang, Z., Wang, Y. et al. miR-338 suppresses the growth and metastasis of OSCC cells by targeting NRP1. Mol Cell Biochem 398, 115–122 (2015). https://doi.org/10.1007/s11010-014-2211-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-014-2211-3