Abstract
One etiology related directly to obstructive urinary bladder dysfunction is ischemia/reperfusion resulting in significant oxidative stress to the bladder. Grapes, a natural source of antioxidants, have been proven effective in preventing obstructive and ischemic bladder dysfunction. Many investigators believe that resveratrol is the primary active antioxidant ingredient in grapes. We compared the ability of a whole-grape suspension with pure resveratrol in their ability to protect the bladder from in vitro oxidative stress mediated by hydrogen peroxide (H2O2). Four male rabbit bladders were used. Two strips from each bladder were incubated in the presence of 1 mg/mL grape suspension for 30 min, another two strips were incubated in the presence of 1 mg/mL resveratrol solution, and the last two strips were incubated in the presence of 1 mg/mL sucrose/and fructose as controls. The rest of the bladder was separated into muscle and mucosa, frozen and stored for biochemical evaluation. (1) Chemically, resveratrol has about 20 times the antioxidant capacity of the grape suspension. (2) The grape suspension had significant protective effects when the rate of tension was quantitated at all concentrations of H2O2, while the resveratrol had no effect. (3) Citrate synthase activities of the muscle and mucosa were significantly protected by the grape suspension but not by resveratrol. These data demonstrate that the grape suspension protects the mitochondria to a significantly greater degree than resveratrol, which suggests that the antioxidant activities are due to the combination of active components found in the grape suspension and not just resveratrol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive bladder dysfunction (OBD) is a condition secondary to benign prostatic hyperplasia (BPH) which relates to the enlargement of the prostate and compression of the urethra [1, 2]. BPH involves hyperplasia of prostate cells which ultimately results in an increase in size and mass of the prostate, and when potentially large enough, leads to the progressive obstruction of the urethra. This obstruction interferes with the normal flow of urine which causes lower urinary tract symptoms (LUTS) such as urine hesitancy, frequent and painful urination, and an increased risk of urinary infections [3–5]. This disease is a progressive issue with aging men with more than 80 % over the age of 50 years requiring medical attention due to OBD [3–5].
Based on recent findings, one of the primary etiological factors in obstructed bladder dysfunction is related directly to ischemia followed by reperfusion [6, 7]. Ischemia is the limitation of blood supply to tissues, which results in the decrease of oxygen and glucose needed for cellular metabolism. When blood supply is restored to ischemic tissues, further damage—known as reperfusion injury—can also occur. Reintroduction of blood flow brings oxygen back to the tissues, causing a greater production of free radicals and reactive oxygen species that can result in damage to cells. The restored blood flow also exaggerates the inflammation response of damaged tissues, causing white blood cells to destroy damaged cells that may otherwise still be viable [8]. The combination of ischemia followed by reperfusion is currently referred to as oxidative stress.
Essentially, with OBD, blood flow is decreased to the bladder smooth muscle and mucosa resulting in tissue hypoxia/ischemia, increased free radical generation, decreased contraction, denervation, and mitochondrial dysfunction [9–11].
Hydrogen peroxide (H202), a common oxygen radical and by-product of oxidative metabolism, is known to cause substantial cellular and intracellular damage which potentially leads to oxidative stress even at the low concentrations [12–14]. Two enzymes responsible for antioxidant defense in nearly all cells exposed to oxygen are superoxide dismutase (SOD) and catalase. SOD catalyzes the formation of oxygen and hydrogen peroxide (H202); the enzyme catalase is then responsible for reacting with the hydrogen peroxide species to ultimately form water and oxygen [12–14]. Partial outlet obstruction and in vivo models of ischemia/reperfusion (I/R) have pronounced damaging effects on the ratio of SOD and catalase, which in turn result in a significant increase in the production of H202 and further oxidative damage [12–15].
Many publications support the theory that natural products having strong antioxidant and membrane protective properties can significantly reduce and reverse bladder damage initiated by both partial outlet obstruction and bilateral I/R in rabbits [16–21]. The antioxidant binds to the free radicals ultimately making them harmless. Grapes, a natural source of antioxidants with membrane protective properties, have been proven effective in reducing the effects of ischemia reperfusion, preventing, and reversing obstructed bladder disease when used as a suspension [1, 2]. It is known that grape products contain a variety of antioxidant and membrane protective compounds including resveratrol, quercetin, procyanidins, flavonoids, phenolics, and others [1]. Many investigators believe that resveratrol is the primary active ingredient responsible for grapes’ antioxidant properties [22].
A number of our studies have shown that the mitochondria are one of the most sensitive subcellular organelles to develop oxidative stress and free radical damage from partial outlet obstruction and bilateral I/R [6, 7, 23, 24]. Citrate synthase, an enzyme that exists in nearly all living cells, is commonly used as a quantitative marker for the activity of intact mitochondria [25, 26]. It is involved in the first step of the citric acid cycle (or Krebs Cycle) which is a series of chemical reactions used by all aerobic organisms to generate energy through the oxidization of acetate derived from carbohydrates, fats, and proteins into carbon dioxide. In addition, the cycle provides precursors including certain amino acids as well as the reducing agent (NADH) that are used in numerous biochemical reactions. Citrate synthase’s key role in the citric acid cycle is the catalysis of the condensation reaction with acetyl coenzyme A (Acetyl-CoA) and oxaloacetate. The reaction produces CoA along with citrate, both of which are used substantially throughout the cycle [27, 28].
In the current study, our objective is to determine the comparative effect of grape suspension (Standardized freeze-dried powder made from a variety of whole grapes grown in California) versus pure resveratrol on (A) the contractile response of isolated bladder strips to a field stimulation (FS) and (B) citrate synthase activity to the oxidative effects of H2O2. The contractile studies are important because FS requires the release of acetylcholine, and synaptic function has been demonstrated to be a major target for oxidative damage as is mitochondrial function [29–33].
In these contractile studies, we evaluate both the maximal contractile response which depends on both the levels of intracellular free calcium and the intracellular ATP available. The maximal rate of contraction depends on the rate at which intracellular calcium increases in association with the available intracellular ATP. In our experience, the rate of contractile response is more sensitive to oxidative stress than the maximal tension developed.
Materials and methods
All studies were approved by the Institutional Animal Care and Use Committee of the Stratton VA Medical Center.
Four male New Zealand white rabbits were anesthetized with pentobarbital (25 mg/kg) and the bladder exposed through a midline incision. Each bladder was then removed and sectioned between body and base at the level of the ureteral orifices. The bladder was opened longitudinally and 6 full-thickness isolated strips were taken (1 × 0.3 mm) and mounted in individual baths containing oxygenated Tyrode’s solution (15 mL) at 37 °C for contractile studies. Two of the six strips from each bladder were incubated in the presence of 1 mg/mL grape suspension for 30 min, another two strips were incubated in the presence of 1 mg/mL resveratrol solution, and the last two strips were incubated in the presence of 1 mg/mL sugar composed of equal parts of sucrose and fructose and used as the control. The rest of the bladder was separated by blunt dissection into muscle and mucosal compartments, and each compartment was frozen in liquid nitrogen and stored at −80 °C for biochemical evaluation.
Grape suspension [1, 2, 34]
A standardized freeze-dried powder was kindly supplied by the California Table Grape Commission. The grape powder is a composite of whole red, green, and blue–black California grapes, seeded and seedless varieties, in a freeze-dried powder form. It was created using Good Manufacturing Practices and precautions to preserve the integrity of the biologically active compounds found in fresh grapes. As with fresh grapes, the grape powder is known to contain anthocyanins, catechins, resveratrol, flavonols (including quercetin), flavans, and simple phenolics as well as sugars. The composition has been published previously [1]. Resveratrol was purchased from Sigma. The control for the grape suspension is a sugar suspension made of equal parts of sucrose and fructose, which gives the same carbohydrate content as the grapes but has no significant antioxidant activity.
CUPRAC assay for total antioxidants [35]
The CUPRAC assay was utilized to determine the total antioxidant capacity of the grape and resveratrol solutions. This assay relies on the electron donating capabilities of antioxidants to reduce the copper ion. The CUPRAC working solution consisted of 10 mM copper (II) chloride dihydrate, 1 M ammonium acetate, and 7.5 mM neocuproine. 0.15 mL of the above three solutions was added to 0.15 mL of each sample and allowed to react for 30 min at room temperature, after which the absorbance was read at 450 nm in a Hitachi U-2001 spectrophotometer. Concentrations of resveratrol above 200 mg/ml showed optical densities that were not linear and thus could not be used.
Hydrogen peroxide concentrations
Preliminary studies were performed both on contraction and citrate synthase, and the concentrations that showed progressive inhibition in the two systems were used in the published studies.
Contractile studies [33]
Each isolated bladder strip was allowed to equilibrate for 30 min at 37 °C in an oxygenated Tyrodes bath. Passive tension (2 g) was placed on each strip and they were equilibrated for an additional 30 min. Preliminary studies demonstrated that, at 2 g passive tension, maximal active tension is generated. Each strip was then stimulated by FS at 2, 8, and 32 Hz, 1 ms and 80 V. H2O2 was added to each 15-mL bath to give a final concentration of 0.1 %. The strips were incubated for 10 min, and a second set of stimulations was performed. This procedure was repeated at 0.2, 0.4, and 0.8 % H2O2. We use a Model D Polygraph to get a print out of the contractility curve for each stimulation and then digitize it using the Polyview system, which was designed to work with the polygraph. Using the digital curve, we have the program to calculate the rate of tension generation for the first 10 % of the contractile response, which represents the maximal rate of tension rise for the response.
No pharmacological or chemical contractile agents were utilized because of the need to wash between additions and the re-addition of H2O2. With the FS only, there was no need to wash between increasing concentrations of H2O2.
Citrate synthase studies [25]
Samples of muscle and mucosa were homogenized in 0.05 M Tris buffer (100 mg/mL). Samples were then spun at 2500 rpm for 10 min. 0.9 mL of supernatant plus 0.1 mL of Triton X-100 was combined in a test tube for each sample. Samples (40 μL) were added to ten 0.5-cm cuvettes, along with 1.1 mL of 0.05 M Tris buffer (pH 7.6), 30 μL of 24.6 mM acetyl coenzyme A, and 100 μL of 1 mM 5.5′-dithiobis-2-nitrobenzoic acid (DTNB). 10 mg/mL grape powder was added to all cuvettes to give a final concentration of 1 mg/mL. The final volume in each cuvette was 1,400 mL excluding the 50 μL oxaloacetate (10 mM substrate) that were used to start the reaction. Before oxaloacetate was added, 1.4 μL of the mixtures in cuvettes 1 and 2 was removed and 1.4 μL of Tris buffer added to serve as controls. Similarly, for the remainder of the cuvettes, 1.4 μL of the mixtures was removed, and 1.4 μL of H2O2 was added to give final concentrations of 0.005, 0.01, 0.02, and 0.04 % H2O2. A pair of cuvettes that contained no sample serves as additional controls. A magnetic stir bar was placed in each cuvette to mix the chemicals and, after 1 min, 50 μL of 10 mML oxaloacetate was added to each cuvette. The activity was read every 2 min for 30 min in a Hitachi U-2001 spectrophotometer while the free coenzyme-A generated by citrate synthase activity reacted with DTNB to form a colored compound that was quantified at 412 nm. This experiment was repeated with resveratrol also at a final concentration of 1 mg/mL.
Results
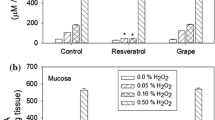
Figure 1 presents a comparison of the total antioxidant capacity of resveratrol and the grape suspension. Chemically, resveratrol has about 20 times the antioxidant capacity of the grape suspension.
In the contractile studies, Fig. 2 presents the maximal tension and rate of tension generation for 2, 8, and 32 Hz FS. Figures 2, 3, 4, and 5 show the effects of 0.1, 0.2, 0.4, and 0.8 % H2O2 on the percentage of no H2O2. The results show that increasing the concentration of H2O2 resulted in a progressive and significant decrease in the contractile responses at all frequencies of stimulation. Interestingly, whereas neither the grape suspension nor resveratrol had any effect on the contractile responses to H2O2 when maximal tension was quantitated, the grape suspension had significant protective effects when the rate of tension was quantitated at all concentrations of H2O2. Resveratrol had a minor positive effect only at 2 Hz and at 0.4 and 0.8 % H2O2.
When comparing the citrate synthase activities of rabbit bladder muscle and mucosa (Fig. 6), it was found that the activity of the citrate synthase is significantly higher in the mucosa than in the muscle. In order to better visualize the comparison of the sensitivities of the muscle and mucosal preparations to H2O2, the citrate synthase activity in the absence of H2O2 has been normalized to 100 % (Fig. 7a, b). Neither the grape suspension nor the resveratrol had any effect on citrate synthase activity in the absence of H2O2. In general, the citrate synthase activities of the muscle and mucosa were protected by the grape suspension and not by the resveratrol. Resveratrol protected the mucosa only at 0.01 % H2O2.
Discussion
As mentioned in the introduction, the maximal tension developed is based primarily on the maximal concentration of intracellular calcium and the available intracellular ATP, whereas the maximal rate of tension development is based primarily on the rate at which calcium enters the cell during the stimulation and the concentration of intracellular ATP available. If oxidative stress slows down calcium entry into the muscle cell but not the concentration of intracellular calcium achieved, then antioxidants may very well protect the rate of tension generation but not the maximal tension achieved.
From the contractile results, the above may be true since only the rate of tension generation was protected by the grape suspension but not by the resveratrol. Previous supportive studies have demonstrated that the rate of tension generation to FS is significantly more sensitive to oxidative stress, thus correlating with the current study [36, 37].
In regard to the citrate synthase study, it was observed that the activity of citrate synthase was significantly higher in the mucosa than in muscle. A higher SOD and catalase activity in the mucosa compared to that of the muscle would explain the difference in sensitivity to H2O2 between the two preparations [2, 38]. Also, previous studies have demonstrated that oxidative phosphorylation, glucose metabolism, and the rate of ATP generation are also significantly higher in the mucosal tissue than in the muscle [39–42].
The citrate synthase activity of the muscle was protected by the grape suspension at 0.005, 0.01, and 0.02 % concentrations of H2O2 but surprisingly not sufficiently by the resveratrol suspension. For the mucosa, the citrate synthase activity was protected at all concentrations of H2O2 by the grape suspension and protected by the resveratrol suspension at 0.01 and 0.02 % concentrations of H2O2. This indicates that the grape suspension was more protective of the citrate synthase activity of both muscle and mucosa against damage by H2O2 when compared to resveratrol. This comes as a surprise since the data obtained using the CUPRAC method of total antioxidant capacity revealed that resveratrol at the same concentration as the grape suspension had a significantly greater antioxidant activity (chemically) than grapes. One can speculate that the resveratrol is not being absorbed sufficiently by the tissue in comparison with the absorption of the antioxidants of the grape suspension.
In accord with previous studies [1, 2], we believe that it is the combination of antioxidants found in the grape suspension rather than an individual component that work in synergy to produce the protective effect.
Conclusion
These data clearly demonstrate that the standardized grape suspension protects the mitochondria to a significantly greater degree than pure resveratrol. The grape suspension showed greater protective effect, suggesting that the antioxidant activities are due to the combination of active components found in the grape suspension and not just one as seen with resveratrol.
References
Agartan CA, Whitbeck C, Sokol R, Chichester P, Levin RM (2004) Protection of urinary bladder function by grape suspension. Phytother Res 18:1013–1018. doi:10.1002/ptr.1620
Lin AD, Mannikarottu A, Kogan BA, Whitbeck C, Leggett RE, Levin RM (2007) Effect of bilateral in vivo ischemia/reperfusion on the activities of superoxide dismutase and catalase: response to a standardized grape suspension. Mol Cell Biochem 296:11–16. doi:10.1007/s11010-005-9068-4
Barry MJ (1990) Epidemiology and natural history of benign prostatic hyperplasia. Urol Clin North Am 17:495–507
Barry MJ, Fowler FJ Jr, Bin L, Pitts JC 3rd, Harris CJ, Mulley AG Jr (1997) The natural history of patients with benign prostatic hyperplasia as diagnosed by North American urologists. J Urol 157:10 (discussion 14–5)
Girman CJ (1998) Natural history and epidemiology of benign prostatic hyperplasia: relationship among urologic measures. Urology 51:8–12
Gosling JA, Kung LS, Dixon JS, Horan P, Whitbeck C, Levin RM (2000) Correlation between the structure and function of the rabbit urinary bladder following partial outlet obstruction. J Urol 163:1349–1356
Levin RM, Haugaard N, O’Connor L, Buttyan R, Das A, Dixon JS, Gosling JA (2000) Obstructive response of human bladder to BPH vs. rabbit bladder response to partial outlet obstruction: a direct comparison. Neurourol Urodyn 19:609–629. doi:10.1002/1520-6777(2000)19:5<609:AID-NAU7>3.0.CO;2-H
Sims NR, Muyderman H (2010) Mitochondria, oxidative metabolism and cell death in stroke. Biochim Biophys Acta 1802:80–91. doi:10.1016/j.bbadis.2009.09.003
Schroder A, Chichester P, Kogan BA, Longhurst PA, Lieb J, Das AK, Levin RM (2001) Effect of chronic bladder outlet obstruction on blood flow of the rabbit bladder. J Urol 165:640–646. doi:10.1097/00005392-200102000-00087
Greenland JE, Brading AF (2001) The effect of bladder outflow obstruction on detrusor blood flow changes during the voiding cycle in conscious pigs. J Urol 165:245–248. doi:10.1097/00005392-200101000-00072
Greenland JE, Hvistendahl JJ, Andersen H, Jorgensen TM, McMurray G, Cortina-Borja M, Brading AF, Frokiaer J (2000) The effect of bladder outlet obstruction on tissue oxygen tension and blood flow in the pig bladder. BJU Int 85:1109–1114
Aikawa K, Leggett RE, Levin RM (2003) Effect of age on hydrogen peroxide mediated contraction damage in the male rat bladder. J Urol 170:2082–2085. doi:10.1097/01.ju.0000081461.73156.48
Matsumoto S, Leggett RE, Levin RM (2003) The effect of vitamin E on the response of rabbit bladder smooth muscle to hydrogen peroxide. Mol Cell Biochem 254:347–351
Kalorin CM, Mannikarottu A, Neumann P, Leggett R, Weisbrot J, Johnson A, Kogan BA, Levin RM (2008) Protein oxidation as a novel biomarker of bladder decompensation. BJU Int 102:495–499. doi:10.1111/j.1464-410X.2008.07567.x
Malone L, Schuler C, Leggett RE, Levin RM (2013) The effect of in vitro oxidative stress on the female rabbit bladder contractile response and antioxidant levels. ISRN Urol 2013:639685. doi:10.1155/2013/639685
Levin RM, Das AK (2000) A scientific basis for the therapeutic effects of Pygeum africanum and Serenoa repens. Urol Res 28:201–209
Ishani A, MacDonald R, Nelson D, Rutks I, Wilt TJ (2000) Pygeum africanum for the treatment of patients with benign prostatic hyperplasia: a systematic review and quantitative meta-analysis. Am J Med 109:654–664
Levin RM, Riffaud JP, Bellamy F, Rohrmann D, Habib M, Krasnopolsky L, Zhao Y, Wein AJ (1996) Protective effect of tadenan on bladder function secondary to partial outlet obstruction. J Urol 155:1466–1470
Levin RM, Riffaud JP, Bellamy F, Rohrmann D, Krasnopolsky L, Haugaard N, Zhao Y, Wein AJ (1996) Effects of tadenan pretreatment on bladder physiology and biochemistry following partial outlet obstruction. J Urol 156:2084–2088
Levin RM, Kawashima Y, Leggett RE, Whitbeck C, Horan P, Mizutani K (2002) Effect of oral Kohki tea on bladder dysfunction induced by severe partial outlet obstruction. J Urol 167:2260–2266
Juan YS, Hydery T, Mannikarottu A, Kogan B, Schuler C, Leggett RE, Lin WY, Huang CH, Levin RM (2008) Coenzyme Q10 protect against ischemia/reperfusion induced biochemical and functional changes in rabbit urinary bladder. Mol Cell Biochem 311:73–80. doi:10.1007/s11010-007-9696-y
Hung LM, Chen JK, Huang SS, Lee RS, Su MJ (2000) Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovasc Res 47:549–555
Levin RM, Hudson AP (2004) The molecular genetic basis of mitochondrial malfunction in bladder tissue following outlet obstruction. J Urol 172:438–447. doi:10.1097/01.ju.0000129560.25005.0e
Nevel-McGarvey CA, Levin RM, Haugaard N, Wu X, Hudson AP (1999) Mitochondrial involvement in bladder function and dysfunction. Mol Cell Biochem 194:1–15
Haugaard N, Potter L, Wein AJ, Levin RM (1992) Effect of partial obstruction of the rabbit urinary bladder on malate dehydrogenase and citrate synthase activity. J Urol 147:1391–1393
Makeeva AV, Popova TN, Matasova LV, Yama IN (2008) Effects of lipoic acid on citrate content, aconitate hydratase activity, and oxidative status during myocardial ischemia in rats. Biochemistry (Mosc) 73:76–79
Remington SJ (1992) Structure and mechanism of citrate synthase. Curr Top Cell Regul 33:209–229
Wiegand G, Remington SJ (1986) Citrate synthase: structure, control, and mechanism. Annu Rev Biophys Biophys Chem 15:97–117. doi:10.1146/annurev.bb.15.060186.000525
Levin RM, Longhurst PA, Monson FC, Kato K, Wein AJ (1990) Effect of bladder outlet obstruction on the morphology, physiology, and pharmacology of the bladder. Prostate Suppl 3:9–26
Kato K, Monson FC, Longhurst PA, Wein AJ, Haugaard N, Levin RM (1990) The functional effects of long-term outlet obstruction on the rabbit urinary bladder. J Urol 143:600–606
Liu F, Yao L, Yuan J, Liu H, Yang X, Qin W, Wu G, Yang L, Wang H, Takahashi N, Yamaguchi O (2009) Protective effects of inosine on urinary bladder function in rats with partial bladder outlet obstruction. Urology 73:1417–1422. doi:10.1016/j.urology.2008.10.032
Lu SH, Chang LS, Yang AH, Lin AT, Chen KK, Wei YH (2000) Mitochondrial DNA deletion of the human detrusor after partial bladder outlet obstruction-correlation with urodynamic analysis. Urology 55:603–607
Lu SH, Wei YH, Chang LS, Lin AT, Chen KK, Yang AH (2000) Morphological and morphometric analysis of human detrusor mitochondria with urodynamic correlation after partial bladder outlet obstruction. J Urol 163:225–229
Lin AD, Mannikarottu A, Chaudhry A, Whitbeck C, Kogan BA, Chichester P, Levin RM (2005) Protective effects of grape suspension on in vivo ischaemia/reperfusion of the rabbit bladder. BJU Int 96:1397–1402. doi:10.1111/j.1464-410X.2005.05832.x
Bean H, Radu F, De E, Schuler C, Leggett RE, Levin RM (2009) Comparative evaluation of antioxidant reactivity within obstructed and control rabbit urinary bladder tissue using FRAP and CUPRAC assays. Mol Cell Biochem 323:139–142. doi:10.1007/s11010-008-9972-5
Agartan CA, Leggett RE, Kogan BA, Levin RM (2007) Effect of age on the response to in vitro ischemia and reperfusion of the rabbit bladder. Urol Int 78:155–159. doi:10.1159/000098075
Aikawa K, Sugino T, Matsumoto S, Chichester P, Whitbeck C, Levin RM (2003) The effect of ovariectomy and estradiol on rabbit bladder smooth muscle contraction and morphology. J Urol 170:634–637. doi:10.1097/01.ju.0000068723.05004.ca
Onal B, Levin RM, Kogan BA, Guven A, Leggett RE, Mannikarottu AS (2007) Novel alterations in superoxide dismutase and catalase activities in the female rabbit bladder subjected to hormonal manipulations. Int Urol Nephrol 39:1049–1054. doi:10.1007/s11255-007-9176-z
Hypolite JA, Longhurst PA, Gong C, Briscoe J, Wein AJ, Levin RM (1993) Metabolic studies on rabbit bladder smooth muscle and mucosa. Mol Cell Biochem 125:35–42
Kato K, Lin AT, Haugaard N, Longhurst PA, Wein AJ, Levin RM (1990) Effects of outlet obstruction on glucose metabolism of the rabbit urinary bladder. J Urol 143:844–847
Lin AT, Monson FC, Kato K, Haugaard N, Wein AJ, Levin RM (1989) Effect of chronic ischemia on glucose metabolism of rabbit urinary bladder. J Urol 142:1127–1133
Longhurst PA, Briscoe JA, Leggett RE, Samadzadeh S, Levin RM (1993) The influence of diabetes mellitus on glucose utilization by the rat urinary bladder. Metabolism 42:749–755
Acknowledgments
This material is based upon work supported in part by the Office of Research and Development Department of the Veterans Affairs and in part by the Capital Region Medical Research Foundation. The grape powder was the kind gift from the California Table Grape Commission.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Francis, JA., Leggett, R.E., Schuler, C. et al. Effect of hydrogen peroxide on contractility and citrate synthase activity of the rabbit urinary bladder in the presence and absence of resveratrol and a whole-grape suspension. Mol Cell Biochem 391, 233–239 (2014). https://doi.org/10.1007/s11010-014-2007-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-014-2007-5