Abstract
Bone marrow-derived mesenchymal stem cells (MSCs), the most widely used cell source for cartilage tissue engineering, are multipotent cells which have been shown to differentiate into various mesenchyme-lineage cell types including chondrocytes. However, the molecular mechanisms controlling the chondrogenic differentiation of MSCs remain to be fully elucidated. It has been demonstrated that Wnt signaling involves regulating chondrogenesis and MSC differentiation. The aim of the present study was to investigate the role of Wnt11, a member of noncanonical Wnts, in MSCs during chondrogenic differentiation. We observed that overexpression of Wnt11 inhibited proliferation of MSCs and caused a G0/G1 cell cycle arrest. The expression level of chondrogenic markers, aggrecan and Collagen II, was significantly increased in MSCs transduced with Wnt11 as compared with non-transduced cells or MSCs transduced with the empty lentiviral vector. Furthermore, ectopic expression of Wnt11 stimulated gene expression of chondrogenic regulators, SRY-related gene 9, Runt-related transcription factor 2, and Indian hedgehog. Finally, Wnt11 overexpression promoted chondrogenic differentiation of MSCs in synergism with TGF-β. Collectively, these results indicate that Wnt11 plays a crucial role in regulating MSC chondrogenic differentiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Articular cartilage destruction, a central feature in joint diseases such as osteoarthritis and rheumatoid arthritis, is a formidable clinical challenge [1]. It has been demonstrated that damaged articular cartilage exhibits a very limited capacity of self-repair and regeneration [2, 3]. Thus, it is of particular interest to develop novel and biological approaches for cartilage repair and regeneration. Recently, cartilage tissue engineering which is based on the use of the combination of cultured cells, scaffolds, and bioactive factors to generate functional new tissue to replace the damaged tissue has attracted wide attention [4, 5].
Bone marrow-derived mesenchymal stem cells (MSCs), the most extensively used cell source for cartilage tissue engineering, are a population of pluripotent cells with the potential to differentiate into multiple mesenchyme-lineage cell types, including chondrocytes, osteoblasts and adipocytes [6–9]. Chondrogenic differentiation of MSCs has been well established, which makes these cells a highly promising candidate for cartilage tissue repair [10]. Aggrecan and Collagen II (Col-II), two cartilage extracellular matrix proteins, are commonly accepted as chondrogenic-specific markers [11]. It has been demonstrated that the treatment of cytokines such as transforming growth factor-β (TGF-β) successfully induces chondrogenesis of MSCs [12, 13]. Previous study showed that TGF-β induces the expression of chondrogenic marker Col-II in MSC pellets [14]. Recently, Li et al. [10] found that TGF-β promotes chondrogenic differentiation of rat MSCs through stimulating the gene expression of chondrogenic regulators—SRY-related gene 9 (Sox9), Runt-related transcription factor 2 (Runx2), and Indian hedgehog (Ihh). However, the molecular mechanisms controlling the chondrogenic differentiation of MSCs are still poorly understood.
Wnt family members are secreted and highly conserved glycoproteins which have been implicated in regulating various biological processes, such as growth, development, proliferation, and apoptosis [15–17]. Wnt proteins induce signal transduction through multiple pathways, including the noncanonical Wnt signaling pathway [18, 19]. Previous studies suggested the possible involvement of Wnt signaling in regulating chondrogenesis and MSC differentiation. Witte and colleagues [20] reported that all 19 Wnt genes are expressed during mouse limb development and cartilage differentiation. It has been shown that Wnt3a promotes proliferation whereas suppresses chondrogenic differentiation of MSCs [21, 22]. In addition, expression of either Wnt-1 or Wnt7a causes a severe block in chondrogenesis [23]. Recently, Ryu and Chun [24] demonstrated that Wnt11 stimulates the accumulation of Col-II in articular chondrocytes. More importantly, Wnt11 gene expression peaks at the late stage of chondrogenic differentiation of human MSCs in three-dimensional alginate gels, indicating a possible role of Wnt11 in regulating chondrogenic differentiation of MSCs [25].
The aim of the present study was to investigate the function of Wnt11 in chondrogenic differentiation of MSCs. The effects of overexpression of Wnt11 on the proliferation and cell cycle of MSCs were determined. Furthermore, we examined the expression of chondrogenic markers in MSCs transduced with Wnt11. Finally, in vitro chondrogenic induction was used to investigate whether Wnt11 stimulated the chondrogenic differentiation of MSCs.
Materials and methods
Isolation and characterization of rat bone marrow-derived MSCs
Two-week-old Sprague–Dawley (SD) rats were sacrificed by cervical dislocation, and femurs and tibiae were obtained after removing all the connective tissues. The femurs and tibiae bone marrow were flushed out with Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Manassas, MD, USA). Cell pellets were obtained after centrifugation at 1,800 rpm for 10 min and were resuspended in DMEM supplemented with 10 % fetal bovine serum (FBS; HyClone, Logan, UT, USA). Primary cultures were kept at 37 °C in 5 % humidified CO2. Non-adherent cells were removed and culture medium was changed every 3–4 days. At confluence, primary cultures were digested with 0.25 % trypsin (Beyotime Institute of Biotechnology, Haimen, Jiangsu, China) and were maintained at a density of 1 × 105/mL. Flow cytometry was performed to identify the characteristics of the isolated cells as previously described [26]. Anti-CD34 (Abcam, Cambridge, MA, USA) and CD45 antibodies (eBioscience, San Diego, CA, USA) specific for hematopoietic stem cell as well as anti-CD29 (Abcam) and CD44 antibodies (eBioscience) specific for stem cells were employed. Flow cytometry was carried out with a FACSCalibur cytometer (BD Biosciences, San Diego, CA, USA).
Lentiviral vector construction and transduction in MSCs
The cDNA of rat Wnt11 was amplified and cloned into the lentiviral vector pGCL-GFP (Genechem, Shanghai, China). The CDS of rat Wnt11 was used to prepare the lentiviral vector. The NCBI accession number for the sequence is NM_080401.1. To prepare lentiviral particles, the obtained lentiviral vector (Lv-Wnt11), and the pHelper 1.0 and pHelper 2.0 helper plasmids (Genechem) were co-transfected into HEK293T cells according to the manufacturer’s protocol. The empty lentiviral vector (Lv-GFP) was used as a control for transfection. At about 70 % confluence, MSCs were transduced with the Lv-Wnt11 lentivirus at a multiplicity of infection of ten in the presence of 8 μg/mL Polybrene (Sigma, St. Louis, MO, USA). Transduction with Lv-GFP lentivirus was served as control.
Quantitative real-time PCR
Total RNA was purified using Trizol reagent (Life Technologies, Rockville, MD, USA) according to the manufacturer’s protocol. Equal amount of RNA (1 μg) was used to synthesize cDNA. Reverse transcription was performed using Super M-MLV reverse transcriptase (BioTeke, Beijing, China) and oligo dT. A 20 μL reaction mixture containing 10 μL SYBR green PCR master mix (Solarbio, Beijing, China) and 0.25 μM of forward and reverse primers was applied in a 96-well plate running on an Exicycler™ 96 Real-Time Quantitative Thermal Block (Bioneer, Daejeon, Korea). β-Actin served as an internal control. The primer sequences were indicated as below: Wnt11, forward: 5′-CCTCAGGGACATTCTTACAGC-3′ and reverse: 5′-CAGCCTCATAGATATGGTATGG-3′; Aggrecan, forward: 5′-CGTCCAAACCAACCCGACAA-3′ and reverse: 5′-CAGGGAGCTGATCTCATAGCGA-3′; Col-II, forward: 5′-ACGCCATGAAAGTCTTCTGC-3′ and reverse: 5′-ATCTGGACGTTAGCGGTGTT-3′; Sox9 forward: 5′-AGAACGCACATCAAGACGGA-3′ and reverse: 5′-AGGTGAAGGTGGAGTAGAGC-3′; Runx2 forward: 5′-TACTCTGCCGAGCTACGAAAT-3′ and reverse: 5′-GAGGATTTGTGAAGACCGTTAT-3′; Ihh forward: 5′-CCAACTACAATCCCGACATCA-3′ and reverse: 5′-TCTTCATCCCAGCCTTCCGT-3′; β-actin, forward 5′-GGAGATTACTGCCCTGGCTCCTAGC-3′ and reverse 5′- GGCCGGACTCATCGTACTCCTGCTT-3′.
Western blot
NP-40 lysis buffer (Beyotime Institute of Biotechnology) was used to collect proteins from cells following the manufacturer’s instruction. The extracted proteins were separated on 10 % sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to polyvinylidenefluoride membranes. After blocking with 5 % (w/v) non-fat milk, the membranes were incubated with anti-Wnt11, anti-aggrecan, and anti-Col-II A1 antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA; dilution 1:100), respectively. Subsequently, the membranes were washed with TTBS and probed with horseradish peroxidase (HRP)-labeled secondary antibody (Beyotime Institute of Biotechnology; dilution 1:5,000). The HRP-labeled mouse anti-β-actin antibody (Kangchen, Shanghai, China; dilution 1:10,000) was added to detect the protein levels of internal control in different samples. Blots were developed using an ECL system (Qihaifutai Biological Technology Co. Ltd., Shanghai, China).
Immunofluorescence staining
Immunofluorescence staining was carried out using the standard indirect immunofluorescence method with slight modification. In brief, MSCs were fixed in 4 % paraformaldehyde for 20 min. After washing with PBS, the fixed cells were incubated with goat serum at room temperature for 15 min. Subsequently, anti-Wnt11 antibody (Santa Cruz Biotechnology; dilution 1:100) was added and incubated with cells at 4 °C overnight. Then, cells were washed with PBS and incubated with cy3-labeled goat anti-rabbit secondary antibody (Beyotime Institute of Biotechnology) at room temperature for 1 h. Samples were observed with a FV1000S-SIM/IX81 laser confocal scanning microscope (Olympus, Tokyo, Japan).
Methyl thiazolyl tetrazolium (MTT) assay
Cell proliferation was measured using MTT assay. MSCs were transferred to 96-well plates at a density of 2 × 103 cells/well with five repeats for each group. Cell proliferation was monitored at 0, 1, 2, 3, and 4 days after transduction. MTT solution (0.2 mg/mL, Sigma) was added to each well after different intervals of incubation. Then, these plates were incubated at 37 °C for an additional 4 h. The generated formazan was dissolved in 20 μL dimethyl sulfoxide (DMSO), and the absorbance was determined at 490 nm with a microplate reader (Bio-Tek, Winooski, VT, USA).
Cell cycle analysis
Cell cycle analysis was performed using a commercially obtained kit (Beyotime Institute of Biotechnology). Briefly, cultured MSCs were collected, washed with PBS, and then fixed with pre-cold 70 % ethanol at 4 °C for 2 h. After centrifugation at 800 rpm for 5 min, cell plates were resuspended with 500 μL staining buffer. Subsequently, 25 μL propidium iodide staining solution and 10 μL RNaseA were added and incubated at 37 °C for 30 min in the dark. Cellular DNA content was determined with a FACSCalibur cytometer (BD Biosciences).
Immunocytochemistry
For immunocytochemistry, cultured MSCs were fixed in 4 % paraformaldehyde for 15 min. To block endogenous peroxidase activity, 0.3 % hydrogen peroxide was added. Then, cells were incubated with normal goat serum at room temperature for 15 min to inhibit nonspecific binding of antibody. Anti-Col-II A1 antibody (Santa Cruz; dilution 1:50) was probed with cells overnight at 4 °C. Subsequently, cells were treated with biotin-labeled secondary antibody at 37 °C for 30 min. The bound antibody was detected with the HRP-conjugated streptavidin detection system (Beyotime Institute of Biotechnology) and 3, 30-diaminobenzidene substrate.
In vitro chondrogenic induction of MSCs
In vitro chondrogenic differentiation of MSCs was induced as previously described with minor modifications [27]. MSCs were transferred into 24-well plates at a density of 1 × 105 cells/well and cultured at 37 °C in 5 % humidified CO2 for 24 h. At around 80 % confluence, MSCs were cultured in chondrogenic inductive medium (high-glucose DMEM, 10 % FBS, 100 mg/mL sodium pyruvate, 40 mg/mL proline, 50 mg/mL vitamin C, 1 mg/mL BSA, and 100 nM dexamethasone) in the presence or absence of TGF-β1 (10 ng/mL). Chondrogenic differentiation of MSCs was induced for 14 days, and then the cells were collected for Alcian blue staining or quantitative real-time PCR analysis. For Alcian blue staining, cells were fixed in 4 % paraformaldehyde for 15 min. Thereafter, cells were exposed to 0.1 % Alcian blue 8GX (Solatbio, Beijing, China) for 40 min. The incorporated Alcian blue dye was determined by measuring absorbance at 590 nm.
Statistical analysis
The statistical analyses were conducted with SPSS16.0 software (SPSS Inc., Chicago, IL, USA). All the results were expressed as mean ± SD. Differences between groups were evaluated by one-way ANOVA. The values of P < 0.05 were considered statistically different.
Results
Characterization of rat bone marrow-derived MSCs
To verify the validity of the isolated MSCs, we carried out flow cytometry analysis. Due to the lack of definitive MSC-specific cellular markers, we used a combination of expression and lack of defined markers to identify the characteristics of the obtained cells [28, 29]. The results showed that the isolated cells were clearly negative for CD34− (Fig. 1a) and CD45− (Fig. 1b), both of which are recognized as hematopoietic stem cell markers. In contrast, these cells exhibited a strong positive signal for the stem cell-specific markers CD29+ (Fig. 1c) and CD44+ (Fig. 1d).
Identity and purity of MSCs. The isolated MSCs were stained with fluorescently labeled antibodies and analyzed by flow cytometry. The results showed that these cells were negative for the hematopoietic stem cell markers CD34− (a) and CD45− (b) and were positive for the stem cell markers CD29+ (c) and CD44+ (d)
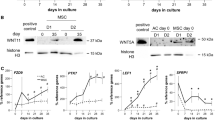
Transduction with lentivirus-bearing Wnt11 cDNA increases the expression of Wnt11 at both mRNA and protein levels in MSCs
To explore the role of Wnt11 in chondrogenic differentiation of MSCs, we used a lentiviral vector carrying GFP reporter (Lv-GFP) or Wnt11 gene (Lv-Wnt11) to transduce the isolated MSCs. As indicated in Fig. 2a, the mRNA level of Wnt11 in MSCs infected with Lv-Wnt11 was significantly increased as compared with the noninfected controls or MSCs infected with Lv-GFP. In line with this, Western blot results revealed that high level of Wnt11 protein was found in MSCs infected with Lv-Wnt11 (Fig. 2b, c).
Overexpression of Wnt11 in MSCs. Quantitative real-time PCR was used to determine the mRNA expression levels of Wnt11 in noninfected control cells and cells infected either with Lv-GFP or with Lv-Wnt11 (a). Western blot analysis of Wnt11 protein levels in noninfected and infected cells (b). Quantification of Wnt11 protein expression levels was calculated by the ratio of the optical density of the band which normalized to β-actin (c). *** P < 0.001 vs control cells
Next, immunofluorescent staining was performed to further confirm the expression of Wnt11 in MSCs. As shown in Fig. 3, weak expression levels of Wnt11 were observed in untreated controls and cells transduced with Lv-GFP. By contrast, strong signal for Wnt11 was detected in cells infected with Lv-Wnt11.
Overexpression of Wnt11 suppresses proliferation of MSCs and arrests the cell cycle at G0/G1 phase
To determine the effects of Wnt11 overexpression on proliferation of MSCs, the MTT assay was conducted. After Lv-Wnt11 transduction, MSCs grew more slowly than the untreated cells or MSCs infected with Lv-GFP (Fig. 4). The inhibition rate for cell proliferation was 21.2 % four days after Lv-Wnt11 transduction.
Furthermore, the effects of Wnt11 overexpression on the cell cycle of MSCs were evaluated by flow cytometry analysis. As revealed in Fig. 5, cell cycle was apparently arrested at G0/G1 phase in MSCs transduced with Lv-Wnt11. Correspondingly, the number of MSCs in the S phase was reduced (Fig. 5). These results demonstrated that overexpression of Wnt11 inhibited proliferation of MSCs by causing a G0/G1 cell cycle arrest.
Overexpression of Wnt11 induces the expression of chondrogenic markers in MSCs
It has been shown that Wnt11 stimulates the accumulation of chondrogenic marker Col-II in articular chondrocytes [24]. Moreover, Wnt11 gene is highly expressed during the late state of MSC chondrogenic differentiation [25]. Thus, we hypothesized that overexpression of Wnt11 induced the expression of chondrogenic markers in MSCs. To test this hypothesis, total RNA and protein were extracted from MSCs, and the expression level of chondrogenic markers, aggrecan and Col-II, was determined by real-time quantitative PCR and Western blot analysis. Both mRNA and protein levels of aggrecan and Col-II were substantially increased in MSCs infected with Lv-Wnt11 as compared with the untreated controls or MSCs infected with Lv-GFP vector (Fig. 6a–c). Furthermore, immunocytochemistry staining was carried out to assess the expression and distribution of Col-II in untreated or treated MSCs. As indicated in Fig. 6d, abundant Col-II proteins were detected in both cytoplasm and extracellular matrix.
Overexpression of Wnt11 induces the expression of chondrocyte markers in MSCs. The mRNA levels of two chondrocyte markers, aggrecan and Col-II, were detected using quantitative real-time PCR (a). Western blot analysis of the protein expression levels of aggrecan and Col-II in control cells and cells infected either with Lv-GFP or with Lv-Wnt11 (b). Densitometric data were normalized by protein levels of β-actin (c). Immunocytochemistry staining of Col-II (d). * P < 0.05, ** P < 0.01 vs control cells. Magnification ×200. Bars = 100 μm
Overexpression of Wnt11 promotes chondrogenic differentiation of MSCs in synergism with TGF-β
Next, we evaluated whether Wnt11 can promote chondrogenic differentiation of MSCs. As TGF-β has been shown to induce MSC chondrogenic differentiation, we investigated the effects of Wnt11 overexpression on chondrogenic differentiation of MSCs in the presence or absence of TGF-β. The accumulation of Alcian blue-positive matrix was not observed when MSCs were treated without TGF-β, indicating that Wnt11 was unable to induce chondrogenic differentiation of MSCs in the absence of TGF-β (Fig. 7a, b). Conversely, MSC chondrogenic differentiation was successfully induced when TGF-β was added to the medium, as evidenced by the incorporation of Alcian blue dye (Fig. 7a, b). The Alcian blue staining signal was stronger in MSCs transduced with Lv-Wnt11 as compared with the noninfected controls or MSCs infected with Lv-GFP vector (Fig. 7a, b).
Overexpression of Wnt11 stimulates chondrogenic differentiation through promoting the expression of chondrogenic regulators, Sox9, Runx2, and Ihh, in MSCs. Chondrogenic differentiation of control cells, Lv-GFP- and Lv-Wnt11-infected cells in the absence or presence of TGF-β1 (a). Quantification of the incorporated Alcian blue dye was determined by measuring absorbance at 590 nm (b). The mRNA expression levels of Sox9, Runx2, and Ihh were analyzed by quantitative real-time PCR during chondrogenic differentiation of MSCs in the absence or presence of TGF-β1 (c). * P < 0.05, ** P < 0.01, and *** P < 0.001
To uncover the molecular mechanisms of Wnt11 in regulating MSC chondrogenic differentiation, we examined the gene expression of Sox9, Runx2, and Ihh, respectively. Sox9 is a master transcription factor for chondrocyte-specific genes, whereas Runx2 and Ihh are important regulators of chondrocyte differentiation [10, 30]. Quantitative real-time PCR results showed that the mRNA levels of all these three genes were significantly increased in Lv-Wnt11-transduced MSCs as compared with the controls or MSCs transduced with Lv-GFP, irrespective of the presence or absence of TGF-β (Fig. 7c). Moreover, the amount of mRNA of the above genes was more abundant when Lv-Wnt11-transduced MSCs were treated with TGF-β. In accordance with the previous reports, TGF-β stimulated the expression of Sox9, Runx2, and Ihh in MSCs (Fig. 7c). These data demonstrated that Wnt11 upregulated the expression of cartilage-specific genes. Furthermore, these findings suggest that Wnt11 exhibits a synergistic effect with TGF-β in promoting chondrogenic differentiation of MSCs.
Discussion
Bone marrow-derived MSCs are widely used as a cell source for cartilage tissue engineering which aims at generating functional new tissue to replace the damaged one [4, 5]. These MSCs are pluripotent cells and thus capable of differentiating into multiple mesenchyme-lineage cell types, such as chondrocytes, osteoblasts, and adipocytes [6–9]. In the present study, we investigated the function of Wnt11 in MSCs during chondrogenic differentiation. Our results showed that lentivirus-mediated overexpression of Wnt11 inhibited cell proliferation of MSCs and induced cell cycle arrest. Moreover, enhanced expression of Wnt11 resulted in upregulation of aggrecan and Col-II, both of which are recognized as chondrogenic markers. Finally, Wnt11 overexpression promoted chondrogenic differentiation of MSCs in synergism with TGF-β.
Wnts are a family of secreted glycoproteins which involved in regulating cell differentiation and proliferation; and inducing chondrogenesis [20, 31]. Wnt11 belongs to the Wnt5a subclass in which they exert the diverse effects through activation of the noncanonical Wnt signaling pathway [32]. Lentivirus-mediated Wnt11 gene transfer enhances cardiomyogenic differentiation of skeletal muscle-derived stem cells, indicating a key role of Wnt11 in regulating stem cell differentiation [33]. A study by Ryu and Chun [24] showed that Wnt11 stimulates the accumulation of chondrogenic marker Col-II in articular chondrocytes. Moreover, Wnt11 gene is highly expressed at the late stage of chondrogenic differentiation of human MSCs in three-dimensional alginate gels [25]. These findings lead us to hypothesize that Wnt11 may enhance chondrogenic differentiation of MSCs. To test this, we used lentivirus-mediated gene transfer to overexpress Wnt11 in MSCs. The enhanced expression of Wnt11 in MSCs infected with Lv-Wnt11 lentivirus was confirmed by quantitative real-time PCR and Western blot analysis. The expression and distribution of Wnt11 were further revealed by immunofluorescent staining. Overexpression of Wnt11 resulted in the inhibition of MSC proliferation as demonstrated by the results of MTT and flow cytometry assay. This observation was different from the other Wnt members. Qu et al. [22] reported that overexpression of Wnt3a promotes MSC proliferation via the β-catenin-mediated canonical Wnt pathway. In contrast, a previous study showed that overexpression of Wnts (i.e., Wnt4, Wnt5a, and Wnt5b) does not change proliferation of wing mesenchymal cells in vitro [31]. It should be noted that the inhibition rate of Wnt11 overexpression for MSC proliferation was relatively low. Further investigation is needed to elucidate the inhibitory mechanism revealed in the current study.
Cartilage matrix proteins, aggrecan and Col-II, are generally recognized as chondrogenic-specific markers [11]. The expression level of aggrecan and Col-II in MSCs was determined by quantitative real-time PCR and Western blot analysis. In accordance with a previously published paper, we observed that overexpression of Wnt11 significantly increased the expression level of aggrecan and Col-II in MSCs [24]. Previously, it has been shown that Wnt11 enhances cardiomyogenic differentiation of bone marrow-derived MSCs. He et al. [34] found that transduction of Wnt11 promotes MSCs transdifferentiation into cardiac phenotypes. However, these reports typically used MSCs that were cocultured with cardiomyocytes or with other factors such as bone morphogenetic protein-2. To clarify, we determined the mRNA expression levels of cardiomyocyte markers, including GATA-4, brain natriuretic peptide (BNP), and α-actinin in MSCs transduced with Lv-Wnt11. However, the mRNA levels of these markers in Lv-Wnt11-transduced cells were not significantly different from those in the non-transduced control cells (Supplemental Fig. 1). These data imply that Wnt11 may specifically enhance MSCs chondrogenic differentiation.
Alcian blue staining, which is specific for the highly sulfated proteoglycans of the cartilage matrix, is frequently used to assess chondrogenic differentiation [35, 36]. Our results showed that overexpression of Wnt11 failed to stimulate the accumulation of Alcian blue-positive matrix in the absence of TGF-β. The incorporation of Alcian blue dye was successfully observed when MSCs were treated with TGF-β. Additionally, Wnt11 overexpression cells induced stronger Alcian blue staining signal compared to the nontreated controls or MSCs infected with empty lentiviral vector. To clarify the role of Wnt11 and TGF-β in the regulation of MSC chondrogenic differentiation, we measured the gene expression levels of chondrogenic regulators, Sox9, Runx2, and Ihh, in cells treated with various conditions. These factors have been shown to regulate the synthesis of aggrecan and to transcriptionally activate Col-II gene [37, 38]. The transcription factor Runx2 is required for chondrocyte hypertrophy and maturation [39, 40], whereas Ihh is essential for regulating chondrocyte proliferation and differentiation [10]. Regardless of TGF-β, the expression levels of these genes were significantly higher in Lv-Wnt11-transduced MSCs as compared with the controls. Interestingly, the amount of mRNA of the above genes was more abundant when Lv-Wnt11-transduced MSCs were treated with TGF-β. These results suggest that Wnt11 may act in synergism with TGF-β to promote the chondrogenic differentiation of MSCs. Our findings are in consistent with a prior report in which the authors demonstrated that TGF-β stimulates chondrocyte differentiation and inhibits adipocyte differentiation in human MSCs in synergism with Wnt signaling pathways [41]. In addition to this, Wnt11 may enhance the chondrogenic differentiation of MSCs via repressing canonical Wnt signaling, such as Wnt1 and Wnt3a, which has been shown to inhibit chondrogenic differentiation of MSCs [22, 23, 42]. Further studies are needed to test this hypothesis in the future.
Taken together, our observations showed that overexpression of Wnt11 inhibited proliferation of MSCs and induced cell cycle arrest at G0/G1 phase. Furthermore, the expression level of aggrecan and Col-II, two chondrogenic-specific markers, was higher in Wnt11-transduced MSCs compared to controls or MSCs infected with the empty lentiviral vector. Finally, Wnt11 overexpression induced MSC chondrogenic differentiation in synergism with TGF-β. These results suggest that Wnt11 functions in regulating chondrogenic differentiation of MSCs.
References
Loeser RF (2006) Molecular mechanisms of cartilage destruction: mechanics, inflammatory mediators, and aging collide. Arthritis Rheum 54:1357–1360. doi:10.1002/art.21813
O’Driscoll SW (1998) The healing and regeneration of articular cartilage. J Bone Joint Surg Am 80:1795–1812
Hunziker EB (1999) Articular cartilage repair: are the intrinsic biological constraints undermining this process insuperable? Osteoarthr Cartil 7:15–28. doi:10.1053/joca.1998.0159
Moreira-Teixeira LS, Georgi N, Leijten J, Wu L, Karperien M (2011) Cartilage tissue engineering. Endocr Dev 21:102–115. doi:10.1159/000328140
Hwang NS, Varghese S, Elisseeff J (2007) Cartilage tissue engineering: directed differentiation of embryonic stem cells in three-dimensional hydrogel culture. Methods Mol Biol 407:351–373. doi:10.1007/978-1-59745-536-7_24
Kagami H, Agata H, Tojo A (2011) Bone marrow stromal cells (bone marrow-derived multipotent mesenchymal stromal cells) for bone tissue engineering: basic science to clinical translation. Int J Biochem Cell Biol 43:286–289. doi:10.1016/j.biocel.2010.12.006
Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI (2001) The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs 169:12–20
Prockop DJ (1997) Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276:71–74
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Li J, Zhao Z, Liu J, Huang N, Long D, Wang J, Li X, Liu Y (2010) MEK/ERK and p38 MAPK regulate chondrogenesis of rat bone marrow mesenchymal stem cells through delicate interaction with TGF-beta1/Smads pathway. Cell Prolif 43:333–343. doi:10.1111/j.1365-2184.2010.00682.x
Huang CY, Reuben PM, D’Ippolito G, Schiller PC, Cheung HS (2004) Chondrogenesis of human bone marrow-derived mesenchymal stem cells in agarose culture. Anat Rec A Discov Mol Cell Evol Biol 278:428–436. doi:10.1002/ar.a.20010
Huang CY, Hagar KL, Frost LE, Sun Y, Cheung HS (2004) Effects of cyclic compressive loading on chondrogenesis of rabbit bone-marrow derived mesenchymal stem cells. Stem Cells 22:313–323. doi:10.1634/stemcells.22-3-313
Jian H, Shen X, Liu I, Semenov M, He X, Wang XF (2006) Smad3-dependent nuclear translocation of beta-catenin is required for TGF-beta1-induced proliferation of bone marrow-derived adult human mesenchymal stem cells. Genes Dev 20:666–674. doi:10.1101/gad.1388806
Longobardi L, O’Rear L, Aakula S, Johnstone B, Shimer K, Chytil A, Horton WA, Moses HL, Spagnoli A (2006) Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-beta signaling. J Bone Miner Res 21:626–636. doi:10.1359/jbmr.051213
Cohen ED, Tian Y, Morrisey EE (2008) Wnt signaling: an essential regulator of cardiovascular differentiation, morphogenesis and progenitor self-renewal. Development 135:789–798. doi:10.1242/dev.016865
Klaus A, Birchmeier W (2008) Wnt signalling and its impact on development and cancer. Nat Rev Cancer 8:387–398. doi:10.1038/nrc2389
Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20:781–810. doi:10.1146/annurev.cellbio.20.010403.113126
Widelitz R (2005) Wnt signaling through canonical and non-canonical pathways: recent progress. Growth Factors 23:111–116. doi:10.1080/08977190500125746
Pandur P, Lasche M, Eisenberg LM, Kuhl M (2002) Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature 418:636–641. doi:10.1038/nature00921
Witte F, Dokas J, Neuendorf F, Mundlos S, Stricker S (2009) Comprehensive expression analysis of all Wnt genes and their major secreted antagonists during mouse limb development and cartilage differentiation. Gene Expr Patterns 9:215–223. doi:10.1016/j.gep.2008.12.009
Boland GM, Perkins G, Hall DJ, Tuan RS (2004) Wnt3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem 93:1210–1230. doi:10.1002/jcb.20284
Qu F, Wang J, Xu N, Liu C, Li S, Wang N, Qi W, Li H, Li C, Geng Z, Liu Y (2013) Wnt3a modulates chondrogenesis via canonical and non-canonical Wnt pathways in MSCs. Front Biosci 18:493–503
Rudnicki JA, Brown AM (1997) Inhibition of chondrogenesis by Wnt gene expression in vivo and in vitro. Dev Biol 185:104–118. doi:10.1006/dbio.1997.8536
Ryu JH, Chun JS (2006) Opposing roles of Wnt-5a and Wnt-11 in interleukin-1beta regulation of type II collagen expression in articular chondrocytes. J Biol Chem 281:22039–22047. doi:10.1074/jbc.M601804200
Xu J, Wang W, Ludeman M, Cheng K, Hayami T, Lotz JC, Kapila S (2008) Chondrogenic differentiation of human mesenchymal stem cells in three-dimensional alginate gels. Tissue Eng Part A 14:667–680. doi:10.1089/tea.2007.0272
Wu B, Ma X, Zhu D, Liu Y, Sun Z, Liu S, Xue B, Du M, Yin X (2013) Lentiviral delivery of biglycan promotes proliferation and increases osteogenic potential of bone marrow-derived mesenchymal stem cells in vitro. J Mol Histol 44:423–431. doi:10.1007/s10735-013-9497-4
Tominaga H, Maeda S, Miyoshi H, Miyazono K, Komiya S, Imamura T (2009) Expression of osterix inhibits bone morphogenetic protein-induced chondrogenic differentiation of mesenchymal progenitor cells. J Bone Miner Metab 27:36–45. doi:10.1007/s00774-008-0003-0
Buhrmann C, Mobasheri A, Matis U, Shakibaei M (2010) Curcumin mediated suppression of nuclear factor-kappaB promotes chondrogenic differentiation of mesenchymal stem cells in a high-density co-culture microenvironment. Arthritis Res Ther 12:R127. doi:10.1186/ar3065
Wei H, Shen G, Deng X, Lou D, Sun B, Wu H, Long L, Ding T, Zhao J (2013) The role of IL-6 in bone marrow (BM)-derived mesenchymal stem cells (MSCs) proliferation and chondrogenesis. Cell Tissue Bank 14(4):699–706. doi:10.1007/s10561-012-9354-9
Kawakami Y, Rodriguez-Leon J, Izpisua Belmonte JC (2006) The role of TGFbetas and Sox9 during limb chondrogenesis. Curr Opin Cell Biol 18:723–729. doi:10.1016/j.ceb.2006.10.007
Church V, Nohno T, Linker C, Marcelle C, Francis-West P (2002) Wnt regulation of chondrocyte differentiation. J Cell Sci 115:4809–4818
Du SJ, Purcell SM, Christian JL, McGrew LL, Moon RT (1995) Identification of distinct classes and functional domains of Wnts through expression of wild-type and chimeric proteins in Xenopus embryos. Mol Cell Biol 15:2625–2634
Xiang G, Yang Q, Wang B, Sekiya N, Mu X, Tang Y, Chen CW, Okada M, Cummins J, Gharaibeh B, Huard J (2011) Lentivirus-mediated Wnt11 gene transfer enhances Cardiomyogenic differentiation of skeletal muscle-derived stem cells. Mol Ther 19:790–796. doi:10.1038/mt.2011.5
He Z, Li H, Zuo S, Pasha Z, Wang Y, Yang Y, Jiang W, Ashraf M, Xu M (2011) Transduction of Wnt11 promotes mesenchymal stem cell transdifferentiation into cardiac phenotypes. Stem Cells Dev 20:1771–1778. doi:10.1089/scd.2010.0380
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:4279–4295. doi:10.1091/mbc.E02-02-0105
Ogawa R, Mizuno H, Watanabe A, Migita M, Shimada T, Hyakusoku H (2004) Osteogenic and chondrogenic differentiation by adipose-derived stem cells harvested from GFP transgenic mice. Biochem Biophys Res Commun 313:871–877
Bell DM, Leung KK, Wheatley SC, Ng LJ, Zhou S, Ling KW, Sham MH, Koopman P, Tam PP, Cheah KS (1997) SOX9 directly regulates the type-II collagen gene. Nat Genet 16:174–178. doi:10.1038/ng0697-174
Xie WF, Zhang X, Sakano S, Lefebvre V, Sandell LJ (1999) Trans-activation of the mouse cartilage-derived retinoic acid-sensitive protein gene by Sox9. J Bone Miner Res 14:757–763. doi:10.1359/jbmr.1999.14.5.757
Kim IS, Otto F, Zabel B, Mundlos S (1999) Regulation of chondrocyte differentiation by Cbfa1. Mech Dev 80:159–170
Wang Y, Belflower RM, Dong YF, Schwarz EM, O’Keefe RJ, Drissi H (2005) Runx1/AML1/Cbfa2 mediates onset of mesenchymal cell differentiation toward chondrogenesis. J Bone Miner Res 20:1624–1636. doi:10.1359/JBMR.050516
Zhou S, Eid K, Glowacki J (2004) Cooperation between TGF-beta and Wnt pathways during chondrocyte and adipocyte differentiation of human marrow stromal cells. J Bone Miner Res 19:463–470. doi:10.1359/JBMR.0301239
Maye P, Zheng J, Li L, Wu D (2004) Multiple mechanisms for Wnt11-mediated repression of the canonical Wnt signaling pathway. J Biol Chem 279:24659–24665. doi:10.1074/jbc.M311724200
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11010_2014_1963_MOESM1_ESM.tif
Supplemental Fig. 1 Quantitative real-time PCR analysis of cardiomyocyte markers, GATA-4, BNP, and α-actinin. (TIFF 218 kb)
Rights and permissions
About this article
Cite this article
Liu, S., Zhang, E., Yang, M. et al. Overexpression of Wnt11 promotes chondrogenic differentiation of bone marrow-derived mesenchymal stem cells in synergism with TGF-β. Mol Cell Biochem 390, 123–131 (2014). https://doi.org/10.1007/s11010-014-1963-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-014-1963-0