Abstract
Isoorientin (ISO) is a flavonoid compound in the human diet, and has been known to possess various bioactivities. However, the effects of ISO on microglia inflammation have not been investigated. The current study investigates the neuroprotective effect of ISO in LPS-activated mouse microglial (BV-2) cells. ISO significantly increased the BV-2 cells viability, blocked the protein expression of inducible nitric oxide synthase and cyclooxygenase-2, and decreased the production of nitric oxide, pro-inflammatory cytokines including tumor necrosis factor-α and interleukin-1β. The activation of mitogen-activated protein kinases (MAPKs) was blocked by ISO, and NF-κB nuclear translocation was decreased by ISO both alone and together with NF-κB inhibitor (PDTC) and MAPKs inhibitors (U0126, SP 600125, and SB 203580). Furthermore, ISO strongly quenched intracellular reactive oxygen species (ROS) generation. ROS inhibitor (N-acetyl cysteine, NAC) significantly inhibited pro-inflammatory cytokines release and NF-κB and MAPKs activation, indicating that ISO attenuated neuroinflammation by inhibiting the ROS-related MAPK/NF-κB signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microglial are the resident innate immune cells in the central nervous system, and plays a pivotal role in the innate immune response [1]. Microglial activation plays an important role in neurodegenerative diseases through producing several pro-inflammatory enzymes and cytokines. Activated microglial cells are able to scavenge dead cells from the central nervous system and secrete different neurotrophic factors for neuronal survival [2, 3]. While over-activation of microglial cells cause various autoimmune responses and lead to neurodegenerative diseases, such as Alzheimer’s disease, Huntington’s disease, Parkinson’s disease, and multiple sclerosis and stroke [4–6]. Meanwhile, over-activated microglial cells can induce significant and highly detrimental neuronal damage and neurodegenerative processes through excess production of various pro-inflammatory mediators and neurotoxic compounds, such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), reactive oxygen species (ROS), nitric oxide (NO), inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2) [7–9].
Microglial cells can be activated in two ways, neuronal damage and direct stimulation from endogenous proteins or environmental cytotoxic factors (such as lipopolysaccharides, β-amyloid, glutamate, and arachidonate) [1, 10]. Lipopolysaccharides (LPS) is a component of the cell wall of Gram-negative bacteria. It plays critical roles in the pathogenesis of inflammatory responses, and it has been commonly used to pro-inflammatory model and microglial activation [11, 12]. LPS is able to activate the expression of iNOS in the brain, which produces high levels of NO continuously [13]. And later, excess NO induces COX-2 expression which is associated with the management of inflammation and several neuronal diseases [14]. In addition, mitogen-activated protein kinases (MAPKs) also can be activated by LPS. It is classified into three components: extracellular signal-regulated kinases ERK1/2 (p44/p42), c-Jun amino-terminal kinase JNK (p46/p54) and p38 kinase. They have been implicated in the release of immune-related cytotoxic factors, such as NO, COX-2, IL-1β and IL-6 [8–12]. Nuclear factor-κB (NF-κB) is a nuclear transcription factor, and is involved in the regulation of the immune response [15], and is also activated by LPS through MAPK signaling pathway [16, 17]. Moreover, LPS has been shown to induce the production of ROS. Overproduction of ROS is also associated with microglial cells activation and neuroinflammatory processes [1]. An excess production of ROS represents a marker of oxidative stress which usually induces inflammation in microglia and contributes to subsequent neuronal damage and neurodegenerative diseases, such as Alzheimer’s and Parkinson’s diseases [1, 18]. Thus, pharmacological interference with the over activation of microglia presents a reasonable and effective strategy to control the progression of neurodegenerative diseases, and natural compounds have been a fertile source of chemotherapeutic neuroinflammation and chemoprevention agents.
Isoorientin (3′,4′,5,7-tetrahydroxy-6-C-glucopyranosyl flavone; ISO), is a common C-glycosyl flavone in the human diet, and the chemical structure of ISO is shown in Fig. 1a. ISO has been isolated from many plant species, such as Phyllostachys pubescens [19], Crataegus monogyna and C. pentagyna [20], Lythrum salicaria [21], buckwheat [22], Patrinia villosa Juss [23], and Drosophyllum lusitanicum [24]. Previous studies showed various properties of ISO, such as anticancer, antioxidant, anti-bacterial and anti-nociceptive activities [22, 25, 26]. It was also reported that ISO possesses significant anti-inflammatory activity through inhibition of NO production in LPS-stimulated mouse macrophage RAW264.7 cells [27], and inhibition carrageenan-induced hind paw edema model in mice [25]. However, the effects and putative mechanisms of ISO on brain inflammation or microglia have not been investigated.
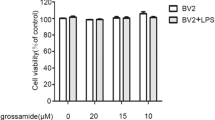
Effect of ISO on cell viability in LPS-activated BV-2 cells. a Chemical structure of ISO. b BV-2 cells were treated with ISO in the presence or absence of LPS (1 μg/mL) for 24 h at the indicated concentrations. The cell viability was determined by MTT assay. Values are presented as the mean ± SD of nine independent experiments. *p < 0.05 and **p < 0.01, compared with LPS in the absence of ISO. ## p < 0.01 and # p < 0.05 compared with control alone
In the present study, we demonstrated the effect and possible mechanisms of ISO on neuroinflammation through evaluating the production of pro-inflammatory mediators (NO, iNOS, COX-2, TNF-α and IL-1β) in LPS-induced BV-2 microglial cells. In addition, NF-κB and MAPK signaling pathway, and ROS level were also examined.
Materials and methods
Reagents and antibodies
Isoorientin (purity ≥98 %) was purchased from Forever Biotechnology, Ltd. (Shanghai, China). RPMI 1640 cell cultures, fetal bovine serum (FBS) and the BCA protein kit were purchased from Thermo Fisher (Shanghai, China). MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliunbromide) (purity ≥93 %) was obtained from Wolsen Biotechnology, Ltd. (Xian, China). ELISA kits for TNF-α and IL-1β were obtained from Shanghai Xinle Biotechnology, Ltd. (Shanghai, China). Lipopolysaccharide (LPS), 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA), 1,4-Diamino-2,3-dicyano-1,4-bis(o-aminophenyl-mercapto) butadiene (U0126) (purity ≥98 %), pyrrolidine dithiocarbamate (PDTC, purity ≥99 %), 4-4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)-1H-imidazole(SB203580) (purity ≥98 %), N-acetyl-l-cysteine (NAC) (purity ≥99 %) and 1,9-pyrazoloanthrone (SP600125) (purity ≥98 %) were purchased from Sigma.
Antibodies against iNOS (SC-8310), COX-2 (SC-65239), α-tubulin (SC-5286), lamin B (SC-374015) and horseradish peroxidase-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, USA). NF-κB p65 (8242), IκB (9242), p-p44/42 MAPK (ERK1/2) (9101), p44/42 MAPK (ERK1/2) (9102), p-SAPK/JNK (Thr183/Tyr185) (9251), SAPK/JNK (9252), p-p38 MAPK (9211) and p38 MAPK (9212) polyclonal antibodies were purchased from Cell Signaling Technology Company (Shanghai, China). ELISA kits for TNF-α and IL-1β were obtained from the Xinle Biology Technology (Shanghai, China). All other chemicals made in China were of analytical grade.
Cell culture and treatment
Mouse microglial (BV-2) cells were obtained from Kunming Institute of Zoology, Chinese Academy of Science (Kunming, China), and cultured in RPMI 1640 medium with 10 % FBS and 1 % penicillin-streptomycin at 37 °C in a humidified incubator (5 % CO2, 95 % air). Primary microglial cultures were pretreated with different concentrations of isoorientin (ISO) for 30 min at 37 °C, and then co-treated with LPS (1 μg/mL) for 24 h.
Measurements of cell viability
Cell viability was determined by the MTT assay. Cells were seeded at a density of 1×106 cells/mL in 96-well polystyrene culture plates at 37 °C with 5 % (v/v) CO2. After various treatments for 24 h, the medium was removed and the cells were incubated with 0.5 % (w/v) of MTT for 4 h at 37 °C. The formazan crystals formed by live cells were solubilized with 100 μL DMSO and absorbance at 490 nm was measured with a microplate reader (Bio-Rad Laboratories Ltd., China). Cell viability was expressed as a percentage of the control (untreated cells).
NO assay
The production of NO was expressed by the concentration of nitrite in culture supernatant fluids which was determined by the Griess reagent. Cells (1×106 cells/mL) were plated into 96-well microtiter plates and treated with ISO in the presence or absence of LPS for 24 h. The culture supernatant was incubated with the same volume of the Griess reagent [0.1 % (w/v) N-(1-naphathyl)-ethylenediamine and 1 % (w/v) sulfanilamide in 5 % (v/v) phosphoric acid] at room temperature. Ten minutes later, the absorbance of the chromophoric azo-derivative molecule was measured at 540 nm with an ultraviolet spectrometer (UV-2550, Shimadzu Corporation, Kyoto, Japan). The amount of nitrite was calculated by using a sodium nitrite standard curve.
Measurement of pro-inflammatory cytokines production
Cells of 1×106 cells/mL were plated into 96-well microtiter plates, and treated with ISO in the presence or absence of LPS for 24 h. The levels of TNF-α and IL-1β in the culture medium were measured by using an ELISA kit according to the manufacturer’s instructions.
Detection of intracellular ROS production
Cellular ROS was measured with H2DCFDA. This dye is a stable nonpolar compound, which diffuses readily into the cells and yields DCFH. Intracellular ROS in the presence of peroxidase changes DCFH to the highly fluorescent compound DCF. Thus, the fluorescent intensity is proportional to the amount of ROS, which is produced by the cells. For the assay, the cells were plated in 6-well polystyrene culture plates at a density of 1×105 cells. After incubation, 10 μM H2DCFDA was added to the wells for 30 min at 37 °C. Then, cells were washed twice with PBS, and immediately determined by using a fluorescence microscope.
SDS-PAGE and western blot analysis
After subjected to the indicated treatments, cells were harvested and lysed with cell lysis buffer (Beyotime Institute of Biotechnology, Jiangsu, China) and nuclear extraction reagent (Xianfeng Biotechnology, Xian, China) as cytosolic extract (cytosol) and nuclear extract (nucleus), respectively. The total protein concentration was determined using the BCA Protein Kit (Thermo Fisher, Shanghai, China). The homogenates were treated with SDS sample buffer and then immediately heated at 95 °C for 10 min. The proteins were separated by SDS-PAGE and electrotransferred onto a polyvinylidene fluoride (PVDF) membrane (0.45 μm, Millipore) using a semidry transfer apparatus (Bio-Rad, Shanghai, China). Blocking was carried out for 2 h in 5 % nonfat dry milk in TBST (20 mM Tris, 166 mM NaCl, and 0.05 % Tween 20, pH 7.5). The primary antibodies were added at the manufacturer-recommended dilutions in TBST buffer overnight at 4 °C. Secondary antibodies were added and incubated at 25 °C for 2 h. The blots were subjected to another three washes with TBST, and developed with chemiluminescent substrate (Thermo Fisher, China) and exposed using a Molecular Imager Chemidoc XRS System (Bio-Rad, Shanghai, China).
Statistical analysis
All the experiments were performed three times, and the data were presented as the means ± standard errors (SE). Significant differences between measurements for the control and treated samples were analyzed using one-way factorial analysis of variance (ANOVA), followed by Duncan’s post-hoc tests (SPSS 16.0).
Results
Cytotoxic effect of ISO on BV-2 cells
In order to investigate the cytotoxic effect of ISO, BV-2 microglia cells were cultured with three different concentrations of ISO. As presented in Fig. 1b, LPS (1 μg/mL) notably (p < 0.01) decreased the cell viability to 88.7 %, while ISO had no significant (p < 0.01) toxic effect on BV-2 microglia cells after 24 h treatment. Compared with LPS-treated control, pretreatment with ISO significantly (p < 0.01) increased the cell viability to 98.8, 98.7 and 96.2 %, respectively. Therefore, 10 μM was selected as the best treatment concentration for further analysis in this study.
ISO inhibits LPS-stimulated production of pro-inflammatory cytokines and mediators
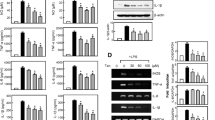
As illustrated in Fig. 2a, b, LPS-induced COX-2 and iNOS productions were decreased by ISO in a dose- and time-dependent manner. ISO also significantly inhibited the protein expression of NF-κB in the nucleus, suggesting that ISO inhibited the translocation of NF-κB from cytosol to nucleus. In addition, stimulation of BV-2 cells with LPS led to increased levels of NO (Fig. 2c), TNF-α (Fig. 2d) and IL-1β (Fig. 2e) in the cell culture medium, while pretreatment with ISO resulted in a significant decrease in the production of these pro-inflammatory cytokines. Additionally, BV-2 cells were stimulated with LPS resulted in the increase of the protein expression of iNOS and COX-2 (Fig. 2f). Pretreatment with ISO notably inhibited the expression iNOS and COX-2 protein compared with LPS-treated control. These results indicated that ISO was able to inhibit the production and protein expressions of NO, TNF-α, IL-1β, iNOS and COX-2 in LPS-stimulated BV-2 microglia cells.
Inhibitory effects of ISO on pro-inflammatory mediators production and expression. Cells were treated with 0–20 μM ISO for 24 h (a) or 10 μM ISO for 0–24 h (b). Aliquots of cell lysates were separated by SDS-PAGE and analyzed for Cox-2, iNOS, NF-κB (in the cytosol and nucleus) protein expression by Western blot as, and the intensity of NF-κB band was quantified by densitometry analysis. α-tubulin and lamin B were employed as loading control, respectively. The results shown here are representative of three independent experiments. *p < 0.05 and **p < 0.01, compared with control groups. # p < 0.05 and ## p < 0.01, versus the LPS-treated groups. The BV-2 cells were pretreated with ISO (10 μM) for 30 min, and then co-treated with 1 μg/mL of LPS at 37 °C for 24 h. The levels of NO (c), TNF-α (d) and IL-1β (e) in the supernatant was measured using the Griess reagent and ELISA assay kit. Values are presented as the mean ± SD of nine independent experiments. **p < 0.01, compared with the control groups. ## p < 0.01 versus the LPS-treated groups. f The protein expression of iNOS and COX-2 was detected by Western Blotting assay using specific antibodies. α-tubulin was used as an internal control
ISO suppresses IκB degradation and NF-κB activation
To explore the mechanisms underlying the anti-inflammatory property of ISO, we examined the degradation of IκB and the translocation of NF-κB from cytosol to nucleus. As shown in Fig. 3a, LPS significantly decreased the protein expressions of IκB and NF-κB in the cytosol, and increased the protein expressions of NF-κB in the nucleus. However, ISO significantly reversed the effects of LPS on IκB and NF-κB levels. To further verify the role of NF-κB in LPS-activated microglia, cells were pretreated with a NF-κB inhibitor (PDTC). PDTC markedly inhibited the decrease of IκB and the nuclear translocation of NF-κB both alone and together with ISO (Fig. 3b). These results showed that ISO was able to inhibit the degradation of IκB and blocked the nuclear translocation of NF-κB.
Inhibitory effects of ISO on IκB degradation and NF-κB activation. BV-2 microglial cells were treated with ISO in the presence or absence of LPS (1 μg/mL) for 24 h. Cytosolic extract (cytosol) and nuclear extract (nucleus) were isolated and analyzed for IκB, NF-κB (in the cytosol) and NF-κB (in the nucleus) were assessed by western Blot analysis (a). Cells were pre-incubated for 30 min with or without ISO and NF-κB inhibitor (PDTC, 50 μM), and then stimulated with LPS (1 μg/ml) for 24 h. The protein expressions of IκB, NF-κB (in the cytosol) and NF-κB (in the nucleus) were assessed by western blot analysis (b). The intensity of NF-κB band was quantified by densitometry analysis. **p < 0.01, compared with the control groups. ## p < 0.01 versus the LPS-treated groups
Effect of NF-κB inhibitor (PDTC) on production of pro-inflammatory cytokines
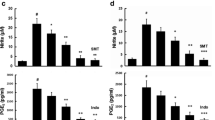
To determine whether the inhibition of NF-κB is correlated with the anti-inflammatory mechanism of ISO, cells were pretreated with PDTC for 30 min, and then co-treated with LPS in the presence or absence with ISO. As shown in Fig. 4, PDTC significantly inhibited the production of NO, TNF-α and IL-1β, as well as the protein expression of iNOS and COX-2 in LPS-activated BV-2 cells. Additionally, the inhibitory effect of ISO on these pro-inflammatory cytokines was also enhanced by PDTC, showing that the inhibitory effect of ISO on inflammatory response is at least partly related to the inhibition of NF-κB activation.
Effect of NF-κB inhibitor (PDTC) on production of pro-inflammatory cytokines in LPS-activated BV-2 cells. BV-2 cells were pre-incubated for 30 min with or without ISO and NF-κB inhibitor (PDTC, 50 μM), and then co-treated with 1 μg/mL of LPS at 37 °C for 24 h. The levels of NO (a), TNF-α (b) and IL-1β (c) in the supernatant was measured using the Griess reagent and ELISA assay kit, respectively. Values are presented as the mean ± SD of nine independent experiments. **p < 0.01 versus other treatment groups. ## p < 0.01, compared with LPS in the absence of ISO. (d) The protein expression of iNOS and COX-2 were detected by Western Blotting assay using specific antibodies. α-tubulin was used as an internal control
ISO suppresses MAPKs activation in LPS-activated BV-2 cells
To investigate whether the inhibition of inflammation by ISO is regulated by the MAPK signaling pathway, we examined the effects of ISO on LPS-induced phosphorylation of ERK, JNK and p38 in BV-2 microglia cells by Western Blot analysis. As shown in the Fig. 5, ISO could significantly attenuate LPS-induced phosphorylation of ERK1/2, JNK and p38MAPK, while their non-phosphorylated forms remain unchanged. The phosphorylation of ERK1/2, JNK and p38MAPK induced by LPS were strongly inhibited by their inhibitor U0126 (Fig. 5b), SP 600125 (Fig. 5c) and SB 203580 (Fig. 5d), respectively. These results suggested that the phosphorylation of ERK1/2, JNK and p38MAPK may involve in the inhibitory effect of ISO on the production of pro-inflammatory mediators in LPS-activated BV-2 cells.
Inhibitory effects of ISO on LPS-induced the phosphorylation of MAPKs. BV-2 microglial cells were treated with ISO in the presence or absence of LPS (1 μg/mL) for 24 h. The cellular proteins from the cells were used for the detection of phosphorylated or total forms of ERK1/2, JNK and p38 MAPKs (a). Cells were pre-incubated for 30 min with or without ISO, U0126 (10 μM), SP 600125 (20 μM) and SB 203580 (20 μM), and then stimulated with LPS (1 μg/ml) for 24 h. Total cell lysates were isolated and investigated the phosphorylation of ERK1/2 (b), JNK (c) and p38 (d)
MAPKs inhibit the LPS-stimulated microglial activation
To further evaluate whether MAPK pathways are involved in LPS-induced production of pro-inflammatory mediators, BV-2 cells were pretreated with ERK1/2 inhibitor U0126, JNK inhibitor SP 600125 and p38 MAPK inhibitor SB 203580. As shown in the Fig. 6, the levels of NO (Fig. 6a), TNF-α (Fig. 6b), IL-1β (Fig. 6c), iNOS and COX-2 (Fig. 6d) in response to LPS were strongly decreased by U0126, SP 600125 and SB 203580 in the presence or absence of ISO.
Effect of MAPKs inhibitors on the LPS-stimulated microglial activation. BV-2 cells were pre-incubated for 30 min with or without ISO, U0126 (10 μM), SP 600125 (20 μM) and SB 203580 (20 μM), and then co-treated with 1 μg/mL of LPS at 37 °C for 24 h. The levels of NO (a), TNF-α (b) and IL-1β (c) in the supernatant was measured using the Griess reagent and ELISA assay kit, respectively. Values are presented as the mean ± SD of nine independent experiments. **p < 0.01 versus other treatment groups. # p < 0.05 and ## p < 0.01, compared with LPS in the absence of ISO. The protein expression of iNOS, COX-2 (d), IκB and NF-κB (in the cytosol and nucleus) (e) and were detected by Western Blotting assay using specific antibodies. α-tubulin and lamin B were used as internal controls
Additionally, accumulating evidence indicates that MAPK signaling pathway can activate NF-κB, we next determined whether NF-κB is activated by MAPK signaling pathway in LPS-activated BV-2 cells. To establish a possible linkage between MAPK signaling pathway and NF-κB, the cells were cultivated in the absence or presence of MAPKs inhibitors (U0126, SP 600125 and SB 203580) before LPS stimulation. In response to these three MAPKs inhibitors, the levels of IκB and NF-κB in the cytosol were significantly increased, which was consistent with the levels of NF-κB in the nucleus (Fig. 6d). Taken together, our current data proved that U0126, SP 600125 and SB 203580 could down-regulate pro-inflammatory mediators levels, suppress IκB degradation and NF-κB activation, and promote the inhibitory effect of ISO, suggesting that the anti-inflammatory effect of ISO is mediated through inhibiting MAPK/NF-κB signaling pathways.
Effect of ROS on production of pro-inflammatory cytokines
Since accumulating evidence supports that intracellular ROS is produced by exogenous stimuli and is involved in mediating cell signaling, we nextly determined whether LPS stimulates ROS generation in BV-2 cells. As shown in the Fig. 7a, LPS resulted in increase of the level of intracellular ROS, ISO strongly quenched intracellular ROS generation. Moreover, ROS inhibitor NAC also significantly reduced the generation of ROS both alone and together with ISO.
Effect of ROS stimulated by LPS on production of pro-inflammatory cytokines. Cells were pre-incubated for 30 min with or without ISO and NAC (10 μM), and then co-treated with 1 μg/mL of LPS at 37 °C for 24 h. a Cells were stained with 10 μM H2DCFDA for 30 min prior to observation under a fluorescent microscope. Scale bar 50 μm. b The protein expression of iNOS and COX-2 were detected by Western Blotting assay using specific antibodies. α-tubulin was used as an internal control. The release of NO (c), TNF-α (d) and IL-1β (e) in the supernatant was measured using the Griess reagent and ELISA assay kit, respectively. Values are presented as the mean ± SD of nine independent experiments. **p < 0.01 compared with other treatment groups. ## p < 0.01 compared with LPS in the absence of ISO
To investigate the effects of ROS on the inhibition of inflammation by ISO, the study examined the effect of antioxidant NAC on inflammatory mediators. Both alone and together with ISO, NAC also effectively inhibited the levels of NO, TNF-α and IL-1β, and the protein expression of iNOS and COX-2 in LPS-stimulated BV-2 cells (Fig. 7b–d), indicating that overproduction of ROS could result in the release of pro-inflammatory cytokines.
Effect of ROS inhibitor (NAC) on MAPK/NF-κB signaling pathway
It has reported that ROS leads to activation of MAPK signaling pathway, next, we investigated whether ROS was also involved in the activation of MAPK/NF-κB pathway in LPS-stimulated BV-2 cells. The phosphorylation of ERK1/2, JNK and p38MAPK were significantly inhibited by NAC in the absence or presence of ISO, while the total ERK1/2, JNK and p38 protein levels remained constant (Fig. 8a). NAC also significantly increased the expression of IκB, attenuated the nuclear translocation of NF-κB, and strongly enhanced the effects of ISO (Fig. 8b). These results showed that ROS is able to activate the MAPK/NF-κB signaling pathway as the upstream signaling, and the inhibitory effect of ISO on inflammatory response is partly related to its antioxidant and free radical scavenging capacity.
Effect of ROS inhibitor (NAC) on MAPK/NF-κB signaling pathway. Cells were treated under the conditions described in Fig. 7. The protein expression of ERK1/2, JNK, p38 (a), IκB and NF-κB (in the cytosol and nucleus) (b) were detected by Western Blotting assay using specific antibodies. α-tubulin and lamin B were used as internal controls. The intensity of NF-κB band was quantified by densitometry analysis. **p < 0.01, compared with the control groups. # p < 0.05 and ## p < 0.01 versus the LPS-treated groups
Discussion
Accumulating evidence suggests that microglia activation is associated with neuroinflammation and neurodegenerative diseases [4, 6]. And neuroinflammation results primarily from the activation of microglia, which can release toxic cytokines and inflammatory mediators [28, 29]. Therefore, inhibition of pro-inflammatory mediators secreted from activated microglia may be an effective therapeutic approach to regulate the progression of neuroinflammation and neurodegenerative diseases. Some natural compounds have been reported to display the effective inhibitory effect on neuroinflammatory response, including fruit pulp fractions [17], honey flavonoid [30], gastrodin [31], icariin [18], obovatol [32], and so on. In this study, we have demonstrated that ISO which is a flavonoid compound attenuates LPS-induced enhancement of expression of proinflammatory enzymes (iNOS and COX-2) and proinflammatory cytokines (TNF-α and IL-1β) through down-regulation of ROS-related MAPK/NF-κB signaling pathway in BV-2 microglia cell.
Sustained up-regulations of pro-inflammatory mediators such as iNOS and COX-2 in microglia by pro-inflammatory stimuli contribute to the progressive damage characteristic of neurodegenerative diseases [29]. Suppression of iNOS and COX-2 productions may be crucial to treat neurodegenerative diseases effectively. Consistent with previous research, our results have shown that ISO significantly reduced the production of NO and the protein expressions of iNOS and COX-2 in LPS-stimulated BV-2 cells (Fig. 2).
NO is a signaling mediator, and is synthesized from l-arginine by a family of nitric-oxide synthases (NOS) which are responsible for the biological synthesis of NO and includes three isoforms, constitutive NOS (nNOS, eNOS) and inducible NOS (iNOS) [33]. NO is able to mediate the interaction between neurons and glial cells within the central nervous system, and it is known to regulate inflammation, neurotransmission and neural cell survival [34]. An excess level of NO can result in neurotoxicity due to inhibition of complex I and II in the respiratory chain [35]. Moreover, it can react with superoxide anion to generate peroxynitrite which is a potentially deleterious reactive molecule and causes striatal neurodegeneration [36, 37]. It is reported that in neuron-glial system, microglial cells are the main source of iNOS/NO after LPS treatment, and NO could contribute to a variety of neurodegenerative pathologies [34, 38]. COX is the key enzyme for pro-inflammatory prostaglandins (PGs) synthesis [39]. COX has two isoforms. COX-1 is constitutively expressed in most tissues, while COX-2 is induced by numerous stimuli including cytokines, LPS, growth factor and astrocytes [8, 34]. COX-2 is localized in postsynaptic sites, and is involved in modulating physiological synaptic transmission, but excessive activation induces neuronal apoptosis, neurodegeneration and cognitive deficits [14, 40, 41].
In addition to NO, activated microglia cells also secrete other pro-inflammatory cytokines, such as TNF-α, IL-1β. Inhibition of pro-inflammatory cytokines has been evaluated as a key mechanism to suppress neuroinflammation. It has reported that ISO effectively inhibits LPS-induced NO production in the murine monocytic macrophage cell line RAW264.7 [27]. To further investigate the effect of ISO on the secretion of pro-inflammatory cytokines, we measured the levels of TNF-α and IL-1β in the culture medium using ELISA. The results demonstrated for the first time that ISO significantly decreased the productions of TNF-α and IL-1β in LPS-stimulated BV-2 cells (Fig. 2b, c). TNF-α and IL-1β are two main proinflammatory cytokines that are produced by activated microglia. A number of stimuli, such as LPS, β-amyloid and traumatic brain injury have been shown to abundantly produce TNF-α and IL-1β [42, 43]. TNF-α-positive astrocytes and microglial cells were found in brain lesions of multiple sclerosis patients [35]. IL-1β is a prototypic proinflammatory cytokine considered the gatekeeper of inflammation. Pro-IL-1β is biologically inactive until it is enzymatically cleaved by the caspase-1 complex to generate the bioactive IL-1β, which is then secreted and is mediated through the type I IL-1 receptor [44, 45]. It has been suggested that IL-1β blocking strategies show clinical benefit in inflammation treatment [46].
It is well known that NF-κB is a nuclear transcription factor, and is an important factor in the microglia-mediated inflammatory response. In resting cells, NF-κB is retained in the cytosol as heterodimer in complex with its inhibitory protein, IκB which is able to mask the nuclear localization signal. When cells are stimulated, IκB is phosphorylated and is subjected to rapid degradation by IκB kinase. This effect disassociates NF-κB from IκB and allows NF-κB to translocate to the nucleus, causing activation of NF-κB-mediated pro-inflammatory genes [31, 47]. Consistent with previous reports, in this study, ISO strongly inhibited the activation of NF-κB in LPS-stimulated BV-2 cells (Fig. 3), and showing that the anti-neuroinflammatory effect of ISO may be attributed to its inhibition of the NF-κB signaling pathway. This is also supported by the observation that NF-κB inhibitor (PDTC) significantly inhibits the productions of NO, TNF-α and IL-1β, and the protein expression of iNOS and COX-2 in LPS-activated BV-2 cells (Fig. 4). Taken together, these findings indicate that the inhibitory effect of ISO on neuroinflammatory response is partly related to the inhibition of NF-κB signaling pathway.
Additionally, MAPKs (p38, ERK, and JNK) play an important role in the processes of inflammation, and they can control the synthesis and release of pro-inflammatory substances and activate NF-κB signaling pathway [17, 48]. Numerous studies have shown that MAPKs are all activated by LPS and are important upstream modulators for the production of pro-inflammatory mediators in BV-2 cells, such as NO, TNF-α and IL-1β [17, 31, 49]. The present study showed that a marked activation of MAPKs by LPS, and an suppression of LPS-activated MAPKs by ISO (Fig. 5a). We also found that the phosphorylation of MAPKs (Fig. 5b–d), the levels of pro-inflammatory mediators (NO, TNF-α, IL-1β, iNOS and COX-2) and the activation of NF-κB (Fig. 6) in LPS-activated BV-2 cells were strongly inhibited by U0126 (an ERK1/2 inhibitor), SP 600125 (a JNK inhibitor) and SB 203580 (a p38 inhibitor) both alone and together with ISO, respectively. Therefore, these results suggested that the inhibition of ISO on proinflammatory mediators is associated with down-regulation of the MAPK signaling pathway, and MAPKs is able to activate NF-κB as the upstream signaling.
It is well documented that ROS are commonly produced during inflammatory process, and LPS induces production of ROS via NADPH oxidase activation and leads to activation of MAPK signaling pathway [32, 49]. In present study, we also found a significant increase of the intracellular ROS in LPS-activated BV-2 cells, and ISO strongly quenched the levels of intracellular ROS (Fig. 7a). Moreover, the release of pro-inflammatory mediators (Fig. 7) and the activation of MAPKs and NF-κB (Fig. 8) were also markedly inhibited by ROS inhibitor NAC in the presence or absence of ISO in LPS-activated BV-2 cells. These results demonstrates that LPS-stimulated ROS was able to activate the MAPK/NF-κB signaling pathway as the upstream signaling, and the subsequent release of proinflammatory mediators and cytokines, while ISO was able to inhibit the generation of ROS and the subsequent inflammatory response in BV-2 cells.
Conclusion
In summary, this study demonstrates the novel findings that ISO attenuates LPS-induced pro-inflammatory mediator production in activated BV-2 microglia cells via inhibition of the ROS-related MAPK/NF-κB signaling pathway. A schematic diagram of this model is shown in the Fig. 9. This study clarified a previously unknown mechanism of the inhibition of inflammatory reaction by ISO, suggested that ISO can be an effective approach to prevent microglia cells and neuronal cell damage, and emphasized the neuroprotective effect and therapeutic potential of ISO in neuroinflammatory diseases.
Possible cellular mechanisms involved in the inhibitory effects of ISO on LPS-stimulated microglial activation. LPS triggers ROS production in BV-2 cells, which activates the MAPKs signaling pathway, and the subsequent activation of NF-κB and release of proinflammatory mediators and cytokines, while ISO is able to inhibit the generation of ROS and neuroinflammatory response in BV-2 cells induced by LPS
Abbreviations
- BV-2 cell:
-
Mouse microglial cell line
- ISO:
-
Isoorientin
- LPS:
-
Lipopolysaccharide
- ROS:
-
Reactive oxygen species
- NO:
-
Nitric oxide
- MAPKs:
-
Mitogen-activated protein kinases
- NF-κB:
-
Nuclear factor κB
- TNF-α:
-
Tumor necrosis factor
- IL:
-
Interleukin
- iNOS:
-
Inducible nitric oxide synthase
- COX:
-
Cyclooxygenase
References
Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8:57–69
Nakajima K, Kohsaka S (1998) Functional roles of microglia in the central nervous system. Hum Cell 11:141–155
Neumann H, Kotter MR, Franklin RJ (2009) Debris clearance by microglia: an essential link between degeneration and regeneration. Brain 132:288–295
Amor S, Puentes F, Baker D, Valk P (2010) Inflammation in neurodegenerative diseases. Immunology 129:154–169
Long-Smith CM, Sullivan AM, Nolan YM (2009) The influence of microglia on the pathogenesis of Parkinson’s disease. Prog Neurobiol 89:277–287
Lue LF, Kuo YM, Beach T, Walker DG (2010) Microglia activation and anti-inflammatory regulation in Alzheimer’s disease. Mol Neurobiol 41:115–128
Cao Q, Li P, Lu J, Dheen ST, Kaur C et al (2010) Nuclear factor-κB/p65 responds to changes in the Notch signaling pathway in murine BV-2 cells and in amoeboid microglia in postnatal rats treated with the gamma-secretase complex blocker DAPT. J Neurosci Res 88:2701–2714
Choi Y, Lee MK, Lim SY, Sung SH, Kim YC (2009) Inhibition of inducible NO synthase, cyclooxygenase-2 and interleukin-1β by torilin is mediated by mitogen-activated protein kinases in microglial BV2 cells. Br J Phamrmacol 156:933–940
Wang MJ, Huang HY, Chen WF, Chang HF, Kuo JS (2010) Glycogen synthase kinase-3β inactivation inhibits tumor necrosis factor-a production in microglia by modulating nuclear factor κB and MLK3/JNK signaling cascades. J Neuroinflammation 7:99–116
Brown GC, Neher JJ (2010) Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol 41:242–247
Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT (2007) Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55:453–462
Wilms H, Sievers J, Rickert U, Rostami-Yazdi M, Mrowietz U, Lucius R (2010) Dimethylfumarate inhibits microglial and astrocytic inflammation by suppressing the synthesis of nitric oxide, IL-1beta, TNF-alpha and IL-6 in an in-vitro model of brain inflammation. J Neuroinflammation 7:30
Murphy S (2000) Production of nitric oxide by glial cells: regulation and potential roles in the CNS. Glia 29:1–13
Liang X, Wu L, Wang Q, Hand T, Bilak M et al (2007) Function of COX-2 and prostaglandins in neurological disease. J Mol Neurosci 33:94–99
Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ (2011) Natural innate and adaptive immunity to cancer. Annu Rev Immunol 29:235–271
Jang SI, Kim HJ, Kim YJ, Jeong SI, You YO (2006) Tanshinone IIA inhibits LPS-induced NF-κB activation in RAW 264.7 cells: possible involvement of the NIK-IKK, ERK1/2, p38 and JNK pathways. Eur J Pharmacol 542:1–7
Poulose SM, Fisher DR, Larson J, Bielinski DF, Rimando AM, Carey AN et al (2012) Anthocyanin-rich Acai (Euterpe oleracea Mart.) Fruit pulp fractions attenuate inflammatory stress signaling in mouse brain BV-2 microglial cells. J Agric Food Chem 60:1084–1093
Zeng KW, Fu H, Liu GX, Wang XM (2010) Icariin attenuates lipopolysaccharide-induced microglial activation and resultant death of neurons by inhibiting TAK1/IKK/NF-κB and JNK/p38 MAPK pathways. Int Immunopharmacol 10:668–678
Yong-Chun J, Lin Y, Yuan K (2012) A novel high-performance liquid chromatography fingerprint approach to discriminate Phyllostachys pubescens from China. Pharmacogn Mag 8:42–48
Prinz S, Ring A, Huefner A, Pemp E, Kopp B (2007) 4’’’- Acetylvitexin-2’’ -O-rhamnoside, isoorientin, orientin, and 8-methoxykaempferol-3-O-glucoside as markers for the differentiation of Crataegus monogyna and Crataegus pentagyna from Crataegus laevigata. Chem Biodivers 4:2920–2931
Tunalier Z, Koşar M, Küpeli E, Çaliş Ì, Başer KHC (2007) Antioxidant, anti-inflammatory, anti-nociceptive activities and composition of Lythrum salicaria L. extracts. J Ethnopharmacol 110:539–547
Watanabe W (2007) An anthocyanin compound in buckwheat sprouts and its contribution to antioxidant capacity. Biosci Biotechnol Biochem 71:579–582
Peng J, Fan G, Hong Z, Chai Y, Wu Y (2005) Preparative separation of isovitexin and isoorientin from Patrinia villosa Juss by high-speed counter-current chromatography. J Chromatogr 1074:111–115
Budzianowski J, Budzianowska A, Kromerb K (2002) Naphthalene glucoside and other phenolics from the shoot and callus cultures of Drosophyllum lusitanicum. Phytochemistry 61:421–425
Küpeli E, Aslan M, Gürbüz I, Yesilada E (2004) Evaluation of in vivo biological activity profile of isoorientin. Z Naturforsch 59:787–790
Yuan L, Wang J, Xiao HF, Xiao CX, Wang YT, Liu XB (2012) Isoorientin induces apoptosis through mitochondrial dysfunction and inhibition of PI3K/Akt signaling pathway in HepG2 cancer cells. Toxicol Appl Pharm 265:83–92
Conforti F, Rigano D, Menichini F, Loizzo MR, Senatore F (2009) Protection against neurodegenerative diseases of Iris pseudopumila extracts and their constituents. Fitoterapia 80:62–67
Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A (2011) The role of microglia in the healthy brain. J Neurosci 31:16064–16069
Ha SK, Moon E, Kim SY (2010) Chrysin suppresses LPS-stimulated proinflammatory responses by blocking NF-κB and JNK activations in microglia cells. Neurosci Lett 485:143–147
Candiracci M, Piatti E, Dominguez-Barragan M, García-Antras D, Morgado B, Ruano D et al (2012) Anti-inflammatory activity of a honey flavonoid extract on lipopolysaccharide-activated N13 microglial cells. J Agric Food Chem 60:12304–12311
Dai JN, Zong Y, Zhong LM, Li YM, Zhang W, Bian LG, Ai QL et al (2011) Gastrodin inhibits expression of inducible NO synthase, cyclooxygenase-2 and proinflammatory cytokines in cultured LPS-stimulated microglia via MAPK pathways. Plos One 6:e21891
Ock J, Han HS, Hong SH, Lee SY, Han YM, Kwon BM et al (2010) Obovatol attenuates microglia-mediated neuroinflammation by modulating redox regulation. Brit J Pharmacol 159:1646–1662
Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzell S et al (2008) The chemical biology of nitric oxide: implications in cellular signaling. Free Radical Biol Med 45:18–31
Kim EJ, Kwon KJ, Park JY, Lee SH, Moon CH, Baik EJ (2002) Effects of peroxisome proliferator-activated receptor agonists on LPS-induced neuronal death in mixed cortical neurons: associated with iNOS and COX-2. Brain Res 941:1–10
Wilms H, Claasen J, Röhl C, Sievers J, Deuschl G, Lucius R (2003) Involvement of benzodiazepine receptors in neuroinflammatory and neurodegenerative diseases: evidence from activated microglial cells in vitro. Neurobiol Dis 14:417–424
Korhonen R, Lahti A, Kankaanranta H, Moilanen E (2005) Nitric oxide production and signaling in inflammation. Curr Drug Targets 4:471–479
Mihm MJ, Schanbacher BL, Wallace BL, Wallace LJ, Uretsky NJ, Bauer JA (2001) Free 3-nitrotyrosine causes striatal neurodegeneration in vivo. J Neurosci 21:1–5
Steinert JR, Chernova T, Forsythe ID (2010) Nitric oxide signaling in brain function, dysfunction, and dementia. Neuroscientist 16:435–452
Ricciotti E, FitzGerald GA (2011) Prostaglandins and inflammation. Arterioscl Throm Vasc 31:986–1000
Wyss-Coray T, Rogers J (2012) Inflammation in Alzheimer disease-A brief review of the basic science and clinical literature. Cold Spring Harbor Perspect Med 2:a006346
Andreasson KI, Savonenko A, Vidensky S, Goellner JJ, Zhang Y, Shaffer A, Kaufmann WE, Worley PF, Isakson P, Markowska AL (2001) Age-dependent cognitive deficits and neuronal apoptosis in cyclooxygenase-2 transgenic mice. J Neurosci 21:8198–8209
Nam KN, Park YM, Jung HJ, Lee JY, Min BD et al (2010) Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur J Pharmacol 648:110–116
Szczepanik AM, Ringheim GE (2003) IL-10 and glucocorticoids inhibit Abeta (1-42)- and lipopolysaccharide-induced pro-inflammatory cytokine and chemokine induction in the central nervous system. J Alzheimers Dis 5:105–117
Lee Y, Schulte DJ, Shimada K, Chen S, Crother TR, Chiba N et al (2012) Interleukin-1β is crucial for the induction of coronary artery inflammation in a mouse model of Kawasaki disease. Circulation 125:1542–1550
Thornberry N, Bull H, Calaycay J, Chapman K, Howard A, Kostura M, Miller D, Molineaux S, Weidner J, Aunins J (1992) A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 356:768–774
Goldbach-Mansky R (2011) Immunology in clinic review series; focus on autoinflammatory diseases: update on monogenic autoinflammatory diseases: the role of interleukin (IL)-1 and an emerging role for cytokines beyond IL-1. Clin Exp Immunol 167:391–404
Fang IM, Yang CH, Yang CM, Chen MS (2007) Linoleic acid-induced expression of inducible nitric oxide synthase and cyclooxygenase II via p42/44 mitogen-activated protein kinase and nuclear factor-kB pathway in retinal pigment epithelial cells. Exp Eye Res 85:667–677
Kaminska B (2005) MAPK signalling pathways as molecular targets for anti-inflammatory therapy-from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta 1754:253–262
Pangestuti R, Bak SS, Kim SK (2011) Attenuation of pro-inflammatory mediators in LPS-stimulated BV2 microglia by chitooligosaccharides via the MAPK signaling pathway. Int J Biol Macromol 49:599–606
Acknowledgments
This work was financially supported by the Young Scientists Fund of the National Natural Science Foundation of China (No. 31000757), and the National “Twelfth Five-Year” Plan for Science and Technology Support (No. 2012BAH30F03).
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yuan, L., Wu, Y., Ren, X. et al. Isoorientin attenuates lipopolysaccharide-induced pro-inflammatory responses through down-regulation of ROS-related MAPK/NF-κB signaling pathway in BV-2 microglia. Mol Cell Biochem 386, 153–165 (2014). https://doi.org/10.1007/s11010-013-1854-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-013-1854-9