Abstract
Epidemiological studies have demonstrated that diabetes mellitus is a serious health burden for both governments and healthcare providers. This study was hypothesized to evaluate the antihyperglycemic potential of eugenol by determine the activities of key enzymes of glucose metabolism in streptozotocin (STZ)-induced diabetic rats. Diabetes was induced into male albino Wistar rats by intraperitoneal administration of STZ (40 mg/kg body weight (b.w.)). Eugenol was administered to diabetic rats intragastrically at 2.5, 5, and 10 mg/kg b.w. for 30 days. The dose 10 mg/kg b.w. significantly reduced the levels of blood glucose and glycosylated hemoglobin (HbA1c) and increased plasma insulin level. The altered activities of the key enzymes of carbohydrate metabolism such as hexokinase, pyruvate kinase, glucose-6-phosphate dehydrogenase, glucose-6-phosphatase, fructose-1,6-bisphosphatase, and liver marker enzymes (AST, ALT, and ALP), creatine kinase and blood urea nitrogen in serum and blood of diabetic rats were significantly reverted to near normal levels by the administration of eugenol. Further, eugenol administration to diabetic rats improved body weight and hepatic glycogen content demonstrated the antihyperglycemic potential of eugenol in diabetic rats. The present findings suggest that eugenol can potentially ameliorate key enzymes of glucose metabolism in experimental diabetes, and it is sensible to broaden the scale of use of eugenol in a trial to alleviate the adverse effects of diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is a disease due to abnormality of glucose metabolism and it is mainly linked with low plasma insulin level or insensitivity of target organs to insulin and results in chronic hyperglycemia, a clinical hallmark of diabetes [1]. Defects in glucose metabolic machinery and consistent efforts of the physiological system to correct the metabolic imbalance pose an over exertion on the endocrine system leading to the disruption of endocrine control. Deterioration of endocrine control exacerbates the metabolic disturbances by altering glucose metabolic enzymes which leads to hyperglycemia [2]. The sustained hyperglycemia leads to a further impairment of insulin production by β-cells the so-called glucose toxicity [3]. Hyperglycemia occurring in diabetes does not only damage cellular proteins, membrane lipids and nucleic acids, but also increase the rate of onset of disease complications.
Diabetes mellitus affects around 8.3 % of the adult population globally there are 366 million people currently known to have diabetes which is estimated to grow to 552 million by 2030 [4]. India has got 62.4 million people live with diabetes and 77.2 million people are on the threshold leading as diabetic capital of the world [5]. Liver is an insulin-sensitive organ mainly involved in the regulation of glucose metabolism and is responsible for disposal of up to one-third of an oral glucose load and is severely affected during diabetes mellitus. The hallmark of diabetes is unbalanced glucose metabolism due to lack of insulin. It has been shown to decrease the activities of enzymes in the glycolytic and pentose phosphate pathways, while increasing the activities of gluconeogenic and glycogenolytic pathways [6]. Maintenance of normoglycemia involves the integration and coordinated regulation of several metabolic pathways including gluconeogensis and glycolysis.
Renewed attention to alternative medicines and natural therapies has stimulated new wave of research to look for more efficacious agents with lesser side effects. Among the approaches to overcome diabetes, food plays a key role in the maintenance of blood sugar level, in the hyper glycosylation of biomolecules associated with diverse metabolisms, and in the prevention of pathologies. In this connection, a number of bioactive molecules found in fruits, vegetables, food constituents, and other natural sources are being continuously explored for their direct or indirect benefits in preventing and/or management diabetes [7]. Phenolic acids are secondary metabolites, which are commonly found in many food constituents and fruits. Many epidemiological studies have found that the consumption of foods and drinks with high phenolic content is associated with the prevention of diabetes [8].
Eugenol (4-allyl-2-methoxyphenol) (Fig. 1) is a naturally occurring phenolic compound extracted from clove, basil, and nutmeg. It has demonstrated several biological activities such as antioxidant [9], neuroprotective effects [10], and antibacterial activity against both gram positive and gram negative microorganisms [11]. In addition, it has been widely used as a fragrant and favoring agent in a variety of food and cosmetic products [12]. Although eugenol has been subjected to such vast research by many scientists, however, no systematic studies exist in the literature on the effect of eugenol in experimental models of diabetes. Therefore, the objective of this study was to determine the antihyperglycemic property of eugenol by assessing activities of the key glucose metabolic enzymes in streptozotocin (STZ)-induced diabetic rats.
Materials and methods
Chemicals
Eugenol and STZ were purchased from Sigma Chemical Co (St. Louis, Mo. USA). All other chemicals and solvents were of analytical grade and purchased from Himedia Laboratories Pvt. Ltd., Mumbai, India.
Animals
Adult male rats of Wistar strain weighing 180–220 g were procured from Adhiparasakthi College of Arts and Science, Kalavai, Tamilnadu. Rats were housed in clean, sterile, polypropylene cages under standard vivarium conditions (12 h light/dark cycles) with free access to standard chow (Hindustan Lever Ltd., Bangalore, India) and water. The animals were acclimatized for 2 weeks prior to the start of experiments. The animal experiment was designed and performed in accordance with the ethical norms approved by Ministry of Social Justices and Empowerment, Government of India and Institutional Animal Ethics Committee Guidelines (Reg. No. 1282/ac/09/CPCSEA).
Induction of diabetes
Diabetes was induced in overnight fasted experimental rats by a single intraperitoneal injection of STZ (40 mg/kg body weight (b.w.)) dissolved in freshly prepared citrate buffer (0.1 M, pH 4.5) [13]. STZ injected animals were allowed to drink 20 % glucose solution overnight to overcome the initial drug-induced hypoglycemic mortality. Control rats were injected with same volume of citrate buffer alone. After 96 h, plasma glucose was determined and those rats with fasting blood glucose >250 mg/dl were used in this study.
Experimental design
The animals were randomly divided into six groups of six animals in each group (24 diabetic surviving and 12 normal). Eugenol was dissolved in vehicle solution of olive oil and administered to experimental rats.
- Group I:
-
Normal control (vehicle treated)
- Group II:
-
Normal rats received eugenol (10 mg/kg b.w.) intra gastrically dissolved in 1 ml of olive oil for 30 days
- Group III:
-
Diabetic control
- Group IV:
-
Diabetic rats received eugenol (2.5 mg/kg b.w.) intra gastrically dissolved in 1 ml of olive oil for 30 days
- Group V:
-
Diabetic rats received eugenol (5 mg/kg b.w.) intra gastrically dissolved in 1 ml of olive oil for 30 days
- Group VI:
-
Diabetic rats received eugenol (10 mg/kg b.w.) intra gastrically dissolved in 1 ml of olive oil for 30 days
Body weight, blood glucose level measurements, food and water consumption calculations, and physical examinations were conducted periodically. The dosage was adjusted every week according to any change in body weight to maintain similar dose per kg body weight of rat over the entire period of study for each group.
Oral glucose tolerance test
Oral glucose tolerance test (OGTT) was performed according to the method of Zhang et al. [14]. After overnight fasting, ‘0’ min blood sample (0.2 ml) was taken from control and experimental rats. Without delay, a glucose solution (2 g/kg b.w.) was administered by oral gavage. Blood samples were taken at 30, 60, 90, and 120 min after glucose administration. Blood samples were collected with potassium oxalate and sodium fluoride and glucose levels were determined by the kit method of Trinder [15].
Sample collection
At the end of the experimental period, the animals were fasted overnight, anesthetized using ketamine (24 mg/kg b.w. intramuscular injection), and killed by cervical decapitation. Blood samples were collected in two different tubes, i.e., one tube is containing potassium oxalate and sodium fluoride (3:1) mixture for the estimation of plasma glucose and insulin and another without potassium oxalate and sodium fluoride (3:1) mixture for serum collection. Serum and plasma was separated by centrifugation and used for various biochemical estimations.
Biochemical estimations
Plasma glucose was estimated by using a commercial kit (Sigma Diagnostics Pvt. Ltd., Baroda, India) by the method of Trinder [15]. Hemoglobin (Hb) and glycosylated hemoglobin (HbA1c) were estimated by diagnostic kit (Agappe Diagnostic Pvt. Ltd., India) [16], respectively. The plasma insulin was estimated by using RIA assay kit (for rats) supplied by Linco Research, Inc. (USA). A portion of the liver tissues were dissected out washed with ice-cold saline immediately and were homogenized in 0.1 M Tris–HCl buffer (pH 7.4) for the assay of key enzymes of glucose metabolism. The homogenate was centrifuged at 10,000 rpm to remove the debris and the supernatant was used as enzyme source for the assays of hexokinase [17], pyruvate kinase [18], glucose-6-phosphate dehydrogenase [19], glucose-6-phosphatase [20], and fructose-1,6-bisphosphatase [21]. Another portion of wet liver tissue was used for the estimation of glycogen content [22]. The activities of serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and blood urea nitrogen (BUN) were assayed using commercially available diagnostic kits (Sigma diagnostics (I) Pvt. Ltd., Baroda, India). Serum activity of creatine kinase (CK) was measured according to the method of Szasz et al. [23], respectively. Urine samples was collected by the method of Demirkan and Melli [24] and urine sugar was detected by the method of Benedicts [25].The estimation of protein was determined according to the method of Lowry et al. [26].
Histopathological study
The pancreas samples fixed for 48 h in 10 % formal saline were dehydrated by passing successfully in different mixture of ethyl alcohol–water, cleaned in xylene and embedded in paraffin. Sections of pancreas (4–5 μm thick) were prepared and then stained with hematoxylin and eosin (H&E) dye, which mounted in neutral deparaffinated xylene medium for microscopic observations.
Statistical analysis
The statistical significance of the data has been determined using one-way analysis of variance (ANOVA) and significant difference among treatment groups were evaluated by Duncan’s Multiple Range Test (DMRT). The results were considered statistically significant at p < 0.05 [27]. All statistical analyses were made using SPSS 15.0, SPSS Inc, and Cary, NC.
Results
Effect of eugenol on plasma glucose, insulin levels, and glucose tolerance
Figure 2 showed the levels of plasma glucose and insulin in normal control and experimental rats. The levels of plasma glucose as significantly (p < 0.05) increased, whereas plasma insulin level was significantly (p < 0.05) decreased in diabetic control rats. Administration of eugenol (all doses), a significant (p < 0.05) decrease in plasma glucose and increase in insulin were observed at the end of the experimental period. Eugenol at a dose of 10 mg/kg b.w. showed a highly significant (p < 0.05) effect than other dose (2.5 and 5 mg/kg b.w.). Based on these data, the effective dose was fixed as 10 mg/kg b.w. and used for further analysis.
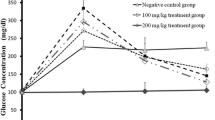
The effect of eugenol on oral glucose tolerance of in control and experimental rats is shown in Fig. 3. The blood glucose concentration elevated to a maximum value at 60 min after glucose load and declined to near basal level at 120 min, whereas, in STZ-induced diabetic rats, the peak increase in blood glucose was noticed even after 60 min and remained high over the next 60 min. Supplementation with eugenol at a dose of 10 mg/kg b.w. to diabetic rats elicited significant (p < 0.05) decrease in blood glucose level than other dose (2.5 and 5 mg/kg b.w.) at 60 min when compared with untreated diabetic rats. However, there is no significant (p < 0.05) alteration in the rats administered with eugenol when compared with control rats.
Changes in the body weight, food, water intake, and urine sugar
The changes in the body weight, food, water intake, and urine sugar in control and experimental rats are represented in Table 1. Food, water intake, and urine sugar were elevated, whereas the body weight significantly (p < 0.05) decreased in diabetic rats compared with normal control rats. In diabetic rats treated with eugenol 10 mg/kg b.w. significantly (p < 0.05) decreased the food, water intake and urine sugar, also increased body weight in diabetic rats when compared with diabetic control rats.
Effect of eugenol on the levels of Hb and HbA1c
The levels of total Hb and HbA1c in normal control and experimental animals are illustrated in Table 2. The diabetic rats showed significant decrease in the level of total Hb and significant (p < 0.05) increase in the level of HbA1c when compared with normal control rats. The levels of total Hb and HbA1c were extensively reversed by the administration of eugenol (10 mg/kg b.w.) in diabetic rats.
Effect of eugenol on the activities of hexokinase, pyruvate kinase, and glucose-6-phosphate dehydrogenase
Table 3 depicts the activities of glucose metabolic enzymes in the liver of control and experimental rats. The activities of hexokinase, pyruvate kinase, and glucose-6-phosphate dehydrogenase were significantly (p < 0.05) diminished in liver tissues of STZ-induced diabetic rats. Oral administration of eugenol to diabetic rats extensively increased the activities of these hepatic enzymes.
Effect of eugenol on the activities of glucose-6-phosphatase and fructose-1,6-bisphosphatase
Table 4 depicts the activities of gluconeogenic enzymes (glucose-6-phosphatase and fructose-1,6-bisphosphatase) in normal control and experimental rats. The actions of glucose-6-phosphatase and fructose-1,6-bisphosphatase were significantly (p < 0.05) increased in liver and kidney of diabetic rats. Treatment with eugenol to diabetic rats inverted the changes in the activities of these enzymes.
Effect of eugenol on the activities of AST, ALT, ALP, CK, and BUN
Table 5 shows the data on the effect of eugenol in the activities of serum AST, ALT, ALP, CK, and BUN of control and experimental rats. The activities of AST, ALT, ALP and the levels of CK and BUN were increased significantly (p < 0.05) in the diabetic group of rats when compared to control group of rats. Oral treatment of eugenol normalized the activities of these enzymes, CK and BUN to near normalcy when compared to control group of rats.
Effects of eugenol on the level of glycogen
Figure 4 represents the effect of eugenol on the level of glycogen in the liver of control and experimental rats. A significant (p < 0.05) decline in the glycogen level was noted in the liver of diabetic group of rats. Treatment with eugenol to diabetic groups of rats reinstated the level of glycogen to near normalcy when compared to control rats.
Effects of eugenol on histology of pancreas
Figure 5a–d represents the histology of pancreas in normal rat. There were no notable changes in pancreas histology in normal rats (Fig. 5a). In contrast, STZ administration showed marked fatty infiltration and shrinkage of islet cells in the diabetic rats (Fig. 5c). Diabetic rats showed cellularity of pancreatic β-cell isets restored to near normal upon treatment with eugenol (Fig. 5d).
a–d Represents the microphotographs of hematoxylin and eosin (40×) staining of pancreas tissues of normal and STZ-induced diabetic rats. a Shows normal pancreatic β-cell islets with normal cellularity and normal histology of pancreas. b Shows normal rat pancreas treated with eugenol (10 mg/kg) showing normal architecture of pancreatic cells. c STZ-induced diabetic rats showing fatty infiltration and shrinkage of islets cells. d Diabetic rats treated with eugenol (10 mg/kg) shows near to normal cellularity of pancreatic β-cell islets when compared to normal pancreas
Discussion
STZ is a 2-deoxy-d-glucose with an N-nitrosomethylurea moiety at the second carbon atom and the existence of 2-deoxy-d-glucose facilitates preferential uptake of STZ into the pancreatic β-cells through GLUT2 and the N-nitrosomethylurea moiety triggers DNA fragmentation in pancreatic β-cells through the formation of alkylating free radicals leading to hasty necrosis of the β-cells thereby the rate of insulin synthesis is diminished [28]. This condition contributes a number of features similar to type 2 diabetes mellitus, and is exemplified by stable hyperglycemia, glucose intolerance, and significantly altered glucose-stimulated insulin secretion both in vivo and in vitro. Eugenol is a remarkably versatile molecule incorporated as a functional ingredient in numerous products and has found application in the pharmaceutical, agricultural, fragrance, flavor, cosmetic, and various other industries. The ability of eugenol to significantly reduce fasting plasma glucose levels in diabetic rats is due to its potential to secrete insulin from existing islet β-cells and increases the utilization of glucose by the tissues. This was confirmed by the increased levels of plasma insulin in diabetic rats treated with eugenol and the increase in the amount of immunoreactive insulin-secreting cells in pancreatic islets as shown by our histopathalogical analysis. Previous data shows that, gallic acid, a phenolic compound increases insulin release in experimental diabetic rats [29].
OGTT is acts as a more sensitive measure to assess the early abnormalities in glucose regulation than fasting plasma glucose. An increase in the plasma glucose level after glucose loading during OGTT in diabetic rats. From the data obtained in the OGTT, it is clear that in untreated diabetic rats the blood glucose level remained high even after 120 min. In contrast, in eugenol treated diabetic rats the blood glucose levels reached a peak and returned again to fasting levels after 120 min. The impaired glucose tolerance observed in diabetic rats was improved to near normal to the treatment with eugenol by restore the function of pancreatic tissues by causing an increase in insulin output or a decrease in the intestinal absorption of glucose.
Diabetes mellitus was characterized by severe loss in body weight. In this study, diabetic rats showed marked reduction in their body weights when compared to normal rats, which could be due to the unavailability of carbohydrates for energy metabolism and the loss or degradation of structural proteins [30]. In addition, the excessive catabolism of protein to provide amino acids for gluconeogenesis during insulin deficiency results in muscle wasting and weight loss in diabetic untreated rats. In our study, initial and final body weight, food and fluid intake were evaluated on daily basis in control and experimental rats. We found an increased food and water intake of diabetic rats that result from a chronic reduction in glucose utilization by the cells and considerable loss of glucose in the urine created a persistent stimulus to eat and drink. Administration of eugenol for 30 days significantly improved glycemic control which prevented the loss of body weight and excess of food and fluid intake on diabetic control rats in dose-dependent manner. These results were similar to the report of Pari and Srinivasan [31], who demonstrated the effect of diosmin in controlling the body weight, desire for food and water intake under diabetic condition.
Estimation of HbA1c has been found to be particularly useful in monitoring the effectiveness of therapy in diabetes. The observed increase in the levels of HbA1c in diabetic control group rats is due to the presence of excessive amounts of blood glucose. During diabetes the excess of glucose present in blood reacts with Hb to form HbA1c, therefore the total Hb level is decreased in diabetic rats [32]. In our study, oral administration of eugenol prevents a significant elevation in HbA1c thereby increasing the level of Hb in diabetic rats. We understand that this may be due to the restoration of blood glucose level, thereby reducing the intensity of Hb glycosylation during the experimental period.
Liver is an insulin-sensitive tissue and plays a major role in glucose metabolism by regulating the interaction between glucose utilization and gluconeogenesis. A partial or total deficiency of insulin causes derangement in glucose metabolism that decreases the activities of hexokinase, pyruvate kinase and glucose-6-phosphate dehydrogenase, causing impaired peripheral glucose utilization and improved hepatic glucose production. The main objective of the study is to determine whether eugenol is implicated as an antihyperglycemic agent, which modulates key enzymes involved in glucose metabolism. In this study, eugenol administration to diabetic rats modulated key glucose metabolizing enzymes in liver, which result in normal blood glucose homeostasis.
Hexokinases serve as the gateway through which glucose enters glycolysis, by catalyzing the phosphorylation of glucose to yield glucose-6-phosphate, the initial and rate-limiting step of the glycolytic pathway. It is an insulin-dependent and insulin-sensitive enzyme and are almost completely inhibited or inactivated in diabetic rat liver in the absence of insulin [33]. Hexokinase is significantly reduced in diabetic rats; this may be the reason for the diminished consumption of glucose in the system and increased blood sugar levels [34]. In our study, the increase in the activity of hexokinase as observed in the diabetic animals administered with eugenol protects the hepatic tissues against STZ-induced diabetes by stimulating insulin from the remnant β-cells. Our results are in harmony with Kanchana et al. [35] who reported that sinapic acid, a phenolic acid improved hexokinase in STZ-induced diabetic rats.
Pyruvate kinase is a ubiquitously expressed key glycolytic enzyme that catalyzes the conversion of phosphoenol pyruvate to pyruvate with the generation of ATP and the altered expression could be expected to impair the glucose metabolism and energy production. Its activity decreases in diabetes mellitus and increases by the administration of insulin to diabetic rats in the liver tissues [36]. Hence, the observed decline in the activity of pyruvate kinase in the liver tissue of STZ-induced diabetic rats is promptly responsible for the reduced glycolysis and amplified gluconeogenesis signifying that these two pathways are distorted in diabetes [37]. Oral administration of eugenol to diabetic rats resulted in a significant enhance in plasma insulin level that augment the Pyruvate kinase activity to near normalcy.
Glucose-6-phosphate dehydrogenase is the first rate limiting enzyme of the pentose phosphate pathway, which results in the production of ribose-5-phosphate and the reducing equivalent, NADPH [38]. A decrease in the activity of glucose-6-phosphate dehydrogenase slows down the pentose phosphate pathway in diabetic conditions, which affects the concentration of NADPH and increases oxidative stress, leading to diabetic complications [39]. In our study, administration of eugenol significantly increased the activity of glucose-6-phosphate dehydrogenase in diabetic state. It provides hydrogen, which binds NADP+ and produce NADPH and enhances the synthesis of fats from carbohydrates, i.e., lipogenesis, finally the plasma glucose levels were decreased.
Gluconeogenesis is a main cause of the elevated hepatic glucose production, contributing 50–60 % of the released glucose. Excessive hepatic glucose production via the gluconeogenesis pathway is partially responsible for the elevated glucose levels observed in diabetes mellitus [40]. The rate of gluconeogenesis is regulated by the activity of two rate limiting gluconeogenic enzyme, glucose-6-phosphatase and fructose-1,6-bisphosphatase. Glucose-6-phosphatase, a key enzyme in the homeostatic regulation of blood glucose and is critical in providing glucose to other organs during diabetes [41]. Fructose-1,6-bisphosphatase is a key regulatory enzyme of the hepatic gluconeogenesis and appeared as a target for efficient and safe glycemic control in diabetes [42]. The activities of the gluconeogenic enzymes such as glucose-6-phosphatase and fructose-1,6-bisphosphatase, increased significantly in the liver and kidney of diabetic rats [43], which may be due to insulin deficiency. In this study, eugenol decreased the activities of glucose 6-phosphatase and fructose-1,6-bisphosphatase in diabetic rats. These results conclusively prove that eugenol normalizes disturbed glucose metabolism by enhancing glucose utilization and by decreasing hepatic glucose production through insulin release and indicates its beneficial effect in the treatment of diabetes mellitus. These results are agreement in with Celik et al. [37] who reported that caffeic acid, a phenolic compound improves glucose metabolism in diabetic rats by the inhibition of gluconeogenesis.
Liver AST, ALT, and ALP enzymes are used as indices of liver damage. Elevation of biomarker enzymes such as ALT, AST, and ALP in serum was presumed to be due to the decreased blood insulin [44] and mainly due to the leakage of these enzymes from the liver cytosol into the blood stream, which gave an indication on the hepatotoxic effect of STZ. Insulin deficient state leads to breakdown of protein thereby enhancing amino acid catabolism and thus providing substrates for gluconeogenesis. However, eugenol-treated diabetic rats, significantly lower the levels of these hepatic enzymes suggesting that eugenol may protect the hepatic tissue damage caused by STZ-induced diabetes. These results are in agreement with Yogalakshmi et al. [45] who reported that eugenol improved hepatic marker enzymes in thioacetamide-induced hepatotoxicity rats.
Urea is the major nitrogen containing metabolic product of protein metabolism. Accumulation of urea nitrogen in experimental diabetes may due to the enhanced breakdown of both liver and plasma proteins [46]. Alterations in nitrogen homeostasis leads to increased hepatic elimination of urea nitrogen and increased peripheral release of nitrogenous substances. Thus, the observed negative nitrogen balance may partly because of changes occurring within the hepatocytes. Several clinical studies have demonstrated that elevated blood glucose levels are a risk factor for the development of diabetic cardiovascular complications. The cardiotoxicity of xenobiotics can be evaluated using the serum activity of marker enzyme CK. However, Hayden and Tyagi [47] linked the observed increase in the serum CK levels of diabetic rats to cardiac muscular damage caused by the disease. In line with the findings of Hayden and Tyagi [47] the serum CK activities of untreated STZ diabetic rats which were significantly elevated in this study indicates damage to cardiac muscle of the rats. The oral administration of eugenol prevented the increase in the levels of blood urea and improved the CK activities in diabetic rats. These findings suggest that eugenol possesses the potential to attenuate renal and cardiac injury caused by hyperglycemic state.
Glycogen is crucial to glucose homeostasis; muscle glycogen is used as a source of glucose-6-phosphate to generate ATP by rapid anaerobic glycolysis, and liver glycogen is used as a source of glucose that is released into the bloodstream to prevent hypoglycemia during diabetes. Insulin regulates blood glucose homeostasis by stimulating the utilization of glucose by liver [48]. Since STZ causes selective destruction of β-cells of islets of langerhans, resulting in marked decrease in insulin levels, it could be predicted that glycogen levels in hepatic tissues decrease as the influx of glucose in the liver is inhibited in the absence of insulin [49]. Our results showed that upon supplementation of diabetic rats with eugenol significantly increased the glycogen content in liver due to increased secretion of insulin or increased activity of hexokinase in the liver can cause the increased utilization of glucose for energy production and thereby increased the levels of glycogen content in liver. This suggests the possible role of eugenol on the utilization and storage of glucose in the hepatic tissues of the experimental diabetic rats. Our results are also in agreement with the results published in a previous study, the lowering of blood glucose levels was accompanied by increased liver glycogen content after caffeic acid supplementation on glucose metabolism in db/db mice [50].
Conclusion
The administration of eugenol to diabetic rats resulted in a significant restoration of the plasma glucose, insulin, HbA1c, glycogen and the activities of key enzymes involved in the metabolism of glucose. In conclusion, that eugenol possesses significant antihyperglycemic activity without any side effects, it may be useful in the treatment of diabetes even though clinical studies to evaluate this possibility may be warranted.
References
Veerapur VP, Prabhakar KR, Thippeswamy BS, Bansal P, Srinivasan KK, Unnikrishnan MK (2012) Antidiabetic effect of Ficus racemosa Linn. stem bark in high-fat diet and low-dose streptozotocin-induced type 2 diabetic rats: a mechanistic study. Food Chem 132:186–193
Parka H, Junga UJ, Choa S, Jungc HK, Shimd S, Choi M (2013) Citrus unshiu peel extract ameliorates hyperglycemia and hepatic steatosis by altering inflammation and hepatic glucose- and lipid-regulating enzymes in db/db mice. J Nutr Biochem 24:419–427
Palanisamy U, Manaharan T, Teng LL, Radhakrishnan AKC, Subramaniam T, Masilamani T (2011) Rambutan rind in the management of hyperglycemia. Food Res Int 44:2278–2282
Hwang CK, Han PV, Zabetian A, Ali MK, Narayan KM (2012) Rural diabetes prevalence quintuples over twenty-five years in low- and middle-income countries: a systematic review and meta-analysis. Diabetes Res Clin Pract 96:271–285
Misra P, Upadhyay RP, Misra P, Anand K (2011) A review of the epidemiology of diabetes in rural India. Diabetes Res Clin Pract 92:303–311
Sundaram R, Naresh R, Shanthi P, Sachdanandam P (2012) Efficacy of 20-OH-ecdysone on hepatic key enzymes of carbohydrate metabolismin streptozotocin induced diabetic rats. Phytomedicine 19:725–729
Srinivasan S, Pari L (2013) Antihyperlipidemic effect of diosmin: a citrus flavonoid on lipid metabolism in experimental diabetic rats. J Funct Foods 5:484–492
Karthikesan K, Pari L, Menon VP (2010) Protective effect of tetrahydrocurcumin and chlorogenic acid against streptozotocin–nicotinamide generated oxidative stress induced diabetes. J Funct Foods 2:134–142
Jirovetz L, Buchbauer G, Stoilova I, Stoyanova A, Krastanov A, Schmidt E (2006) Chemical composition and antioxidant properties of clove leaf essential oil. J Agric Food Chem 54:6303–6307
Kabuto H, Tada M, Kohno M (2007) Eugenol [2-methoxy-4-(2-propenyl)] phenol prevents 6-hydroxydopamine-induced dopamine depression and lipid peroxidation inductivity in mouse striatum. Biol Pharm Bull 30:423–427
Kalemba D, Kunicka A (2003) Antibacterial and antifungal properties of essential oils. Curr Med Chem 10:813–829
Nuchuchua O, Saesoo S, Sramala I, Puttipipatkhachorn S, Soottitantawat A, Ruktanonchai U (2009) Physicochemical investigation and molecular modeling of cyclodextrin complexation mechanism with eugenol. Food Res Int 42:1178–1185
Prabakaran D, Ashokkumar N (2012) Antihyperglycemic effect of esculetin modulated carbohydrate metabolic enzymes activities in streptozotocin induced diabetic rats. J Funct Foods 4:776–783
Zhang M, Lv XY, Li J, Xu ZG, Chen L (2008) The characterization of high fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Exp Diabetes Res 70:40–45
Trinder P (1969) Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem 6:24–27
Bisse E, Abragam EC (1999) New less temperature sensitive, microchromato graphic method for the separation and quantitation of glycosylated haemoglobin using a non cyanide buffer system. J Chromatogr 344:81–91
Brandstrup N, Kirk JE, Bruni C (1957) The hexokinase and phosphoglucoisomerase activities of aortic and pulmonary artery tissue in individuals of various ages. J Gerontol 12:166–171
Pogson CI, Denton RM (1967) Effect of alloxan diabetes, starvation and refeeding on glycolytic kinase activities in rat epididymal adipose tissue. Nature 216:156–157
Ells HA, Kirkman HN (1961) A colorimetric method for assay of erythrocytic glucose-6-phosphate dehydrogenase. Proc Soc Exp Biol Med 106:607–609
Koide H, Oda T (1959) Pathological occurrence of glucose-6-phosphatase in serum in liver diseases. Clin Chim Acta 4:554–561
Gancedo JM, Gancedo C (1971) Fructose-1,6-diphosphatase, phosphofructokinase and glucose-6-phosphate dehydrogenase from fermenting and non fermenting yeasts. Arch Microbiol 76:132–138
Ong KC, Khoo HE (2000) Effects of myricetin on glycemia and glycogen metabolism in diabetic rats. Life Sci 67:1695–1705
Szasz G, Gerhardt W, Gruber W (1977) Creatine kinase in serum: further study of adenylate kinase inhibitors. Clin Chem 23:1888–1892
Demirkan A, Melli M (2007) A simple and inexpensive device for collecting urine samples from rats. Lab Anim 36:39–41
Benedicts SR (1911) The detection and estimation of glucose in urine. Ann Med Assoc 57:1193–1196
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Duncan BD (1957) Multiple ranges tests for correlated and heteroscedastic means. Biometrics 13:359–364
Lenzen S (2008) The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 51:216–226
Latha RC, Daisy P (2011) Insulin-secretagogue, antihyperlipidemic and other protective effects of gallic acid isolated from Terminalia bellerica Roxb. in streptozotocin-induced diabetic rats. Chem Biol Interact 189:112–118
Saravanan G, Ponmurugan P, Senthilkumar GP, Rajarajan T (2009) Modulatory effect of S-allylcysteine on glucose metabolism in streptozotocin induced diabetic rats. J Funct Foods 1:336–340
Pari L, Srinivasan S (2010) Antihyperglycemic effect of diosmin on hepatic key enzymes of carbohydrate metabolism in streptozotocin-nicotinamide-induced diabetic rats. Biomed Pharmacother 64:477–481
Saudek CD, Derr RL, Kalyani RR (2006) Assessing glycemia in diabetes using self-monitoring blood glucose and hemoglobin A1c. JAMA 295:1688–1697
Silva DD, Zancan P, Coelho WS, Gomez LS, Sola-Penna M (2010) Metformin reverses hexokinase and 6-phosphofructo-1-kinase inhibition in skeletal muscle, liver and adipose tissues from streptozotocin-induced diabetic mouse. Arch Biochem Biophys 496:53–60
Gardiner NJ, Wang Z, Luke C, Gott A, Price SA, Fernyhoug P (2007) Expression of hexokinase isoforms in the dorsal root ganglion of the adult rat and effect of experimental diabetes. Brain Res 1175:143–154
Kanchana G, Jerine Shyni W, Rajadurai M, Periasamy R (2011) Evaluation of antihyperglycemic effect of sinapic Acid in normal and streptozotocin-induced diabetes in albino rats. Global J Pharmacol 5:33–39
Anand P, Murali KY, Tandon V, Murthy PS, Chandra R (2010) Insulinotropic effect of cinnamaldehyde on transcriptional regulation of pyruvate kinase, phosphoenolpyruvate carboxykinase, and GLUT4 translocation in experimental diabetic rats. Chem Biol Interact 186:72–81
Celik S, Erdogan S, Tuzcu M (2009) Caffeic acid phenethyl ester (CAPE) exhibits significant potential as an antidiabetic and liver-protective agent in streptozotocin-induced diabetic rats. Pharmacol Res 60:270–276
Xu Y, Osborne BW, Stanton RC (2005) Diabetes causes inhibition of glucose-6-phosphate dehydrogenase via activation of PKA, which contributes to oxidative stress in rat kidney cortex. Am J Physiol Renal Physiol 289:1040–1047
Ugochukwu NH, Babady NE (2003) Antihyperglycemic effect of aqueous and ethanolic extracts of Gongronema latifolium leaves on glucose and glycogen metabolism in livers of normal and streptozotocin-induced diabetic rats. Life Sci 73:1925–1938
He L, Sabet A, Djedjos S, Miller R, Sun X, Hussain MA, Radovick S, Wondisford FE (2009) Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell 137:635–646
Nordlie RC, Foster JD (2010) A retrospective review of the roles of multifunctional glucose-6-phosphatase in blood glucose homeostasis: genesis of the tuning/retuning hypothesis. Life Sci 87:339–349
Zhang Y, Xie Z, Zhou G, Zhang H, Lu J, Zhang WJ (2010) Fructose-1,6-bisphosphatase regulates glucose-stimulated insulin secretion of mouse pancreatic β-cells. Endocrinology 151:4688–4695
Palsamy P, Subramanian S (2009) Modulatory effects of resveratrol on attenuating the key enzymes activities of carbohydrate metabolism in streptozotocin–nicotinamide- induced diabetic rats. Chem Biol Interact 179:356–362
Barbora V, Norbert S, Robert LS, Aramesh S, Richard PE, Clifton B (2002) High alanine amino transferase associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes 51:1889–1895
Yogalakshmi B, Viswanathan P, Anuradha CV (2010) Investigation of antioxidant, anti-inflammatory and DNA-protective properties of eugenol in thioacetamide-induced liver injury in rats. Toxicology 268:204–212
Alderson NL, Chachich ME, Frizzell N, Canning P, Metz TO, Januszewski AS (2004) Effect of antioxidants and ACE inhibition on chemical modification of proteins and progression of nephropathy in streptozotocin diabetic rat. Diabetologia 47:1385–1395
Hayden MR, Tyagi SC (2002) Intimal redox stress: accelerated atherosclerosis in metabolic syndrome and type 2 diabetes mellitus. Atheroscleropathy. Cardiovasc Diabetol 1:3–8
Canada SE, Weaver SA, Sharpe SN, Pederson BA (2011) Brain glycogen super compensation in the mouse after recovery from insulin-induced hypoglycemia. J Neurosci Res 89:585–591
Thamizhiniyan V, Subramanian S (2012) Antidiabetic activity of gossypin, a pentahydroxyflavone glucoside, in streptozotocin-induced experimental diabetes in rats. J Diabetes 4:41–46
Jung UJ, Lee MK, Park YB, Jeon SM, Choi MS (2006) Antihyperglycemic and Antioxidant Properties of Caffeic Acid in db/db Mice. J Pharmacol Exp Ther 318:476–483
Conflict of interest
The authors of this article do not have any conflict of interest to disclose. No part of the manuscript has been submitted or is under consideration in any other publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Srinivasan, S., Sathish, G., Jayanthi, M. et al. Ameliorating effect of eugenol on hyperglycemia by attenuating the key enzymes of glucose metabolism in streptozotocin-induced diabetic rats. Mol Cell Biochem 385, 159–168 (2014). https://doi.org/10.1007/s11010-013-1824-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-013-1824-2