Abstract
Parkinson’s disease (PD) is a common neurodegenerative condition causing significant disability and thus negatively impacting quality of life. The recent advent of stem cell-based therapy has heralded the prospect of a potential restorative treatment option for PD. In particular, mesenchymal stem cells derived from human umbilical cord (hUC-MSCs) have great potential for developing a therapeutic agent as such. Furthermore, hepatocyte growth factor (HGF), which shows mitogenic and morphogenetic activities in a variety of cells, including MSC, and may be implicated in the pathophysiology of PD. As such, HGF may represent a new therapeutic target for the disease. In this study, we successfully isolated and facilitated the transduction of an adenoviral vector expressing HGF (Ad-HGF) into isolated hUC-MSCs. Following transduction, the hUC-MSCs can differentiate into dopaminergic neuron-like cells secreting dopamine, tyrosine hydroxylase, and dopamine transporter. Our data suggest that hUC-MSCs have the ability to differentiate into dopaminergic neurons after transduction with Ad-HGF, providing encouraging evidence to further explore this approach to the treatment of PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the population ages, the incidence of Parkinson’s disease (PD) increases [1]. Additionally, the age of PD onset continues to decrease. And so this neurodegenerative disease presents an increasingly relevant social problem [2, 3]. Although the underlying cause of PD has not been fully defined, the anatomy of PD has been thoroughly characterized; namely, it involves the loss or dysfunction of dopaminergic neurons in the pars compacta of the substantia nigra [4, 5]. Current medical treatments, such as levodopa, can improve symptoms of early PD patients, but they do not prevent the degeneration of dopaminergic neurons [6]. To combat neuron loss, cell transplantation has emerged as the gold-standard treatment option because of its transdifferentiation and functional replacement capacity [2, 3]. MSCs derived from human umbilical cord (hUC-MSCs) have shown many advantages including ease of access, ethical agreeableness, low immunogenicity, and greater proliferation and differentiation rates. Together, these attributes make hUC-MSCs an ideal candidate for cell-based therapy in the medical field [7].
Hepatocyte growth factor (HGF) is a multifunctional mediator originally identified in hepatocytes. Known as the “scatter factor”, HGF exhibits mitogenic and morphogenetic activities in a variety of cells. In addition, recent data suggest that HGF is an important neurotrophic factor [4, 8–11]. In the nervous system, HGF regulates motor neuron development and survival, axon growth, and guidance. It also plays an important role in neuroprotection after peripheral nerve injury, allowing for axons extension and nerve fiber repair [12–14]. Moreover, HGF is ubiquitous in the nervous system; for example, Schwann cells, microglia, astrocytes, and oligodendrocytes can synthesize and express the HGF/c-met axis. Considering that HGF expression is significantly higher in the CSF of patients with PD relative to controls, these facts together suggest that HGF may be involved in the pathophysiology of PD [10].
The half-life of exogenous HGF protein is relatively short, and even repeated infusions of HGF fail to reach a high level in vivo [8]. Most importantly, as a macromolecular protein, HGF cannot easily pass through the blood–brain barrier. We hypothesized that HGF gene-transferred hUC-MSCs could continuously produce HGF at high levels, which would induce the cells to differentiate into neuron-like cells via autocrine and paracrine signaling pathways. In this article, we isolated and characterized the MSCs from human umbilical cord. After transduction with adenoviral vector expressing HGF (Ad-HGF), hUC-MSCs differentiated into dopaminergic neuron-like cell.
Materials and methods
Preparation of reagents, antibodies, and the expression vectors
Human umbilical cords were obtained from healthy puerpera being treated at The Second Affiliated Hospital of Zhengzhou University, and all biopsies were performed with informed consent and approved by the hospital’s ethics committee. Ham’s F12 nutrient medium (Ham’s F12) and MTT were bought from Gibco (Grand Island, NY, USA), dimethyl sulfoxide from Sigma (Austin, TX, USA), and the DA ELISA kit from IBL (Munich, Germany). All antibodies were obtained from R&D systems (Minneapolis, MN, USA). The recombinant adenovirus carrying green fluorescent protein (GFP) was a generous gift from Beckman Medical Instruments (Pasadena, CA, USA).
Construction of recombinant adenovirus vectors carrying human HGF cDNA
A complementary DNA (cDNA) clone for human HGF (2,184 bp) was isolated from a human placental cDNA library by PCR, then subcloned into the HindIII/NotI site in pXCJL1/CMV/pA (7,650 bp, kindly provided by the Gene Therapy Unit, Baxter Healthcare Company, USA) using T4 ligase (New England Biolabs) to generate the shuttle recombinant plasmid pXCJL1–CMV/HGF/pA. In the final stage, the recombinant pXCJL1–CMV/HGF/pA vector was packaged into infectious adenovirus by transfecting human embryonic kidney 293 cells. Recombinant adenovirus was harvested by lysing transfected cells. Recombinant adenoviruses titer was determined by Titer Kit (Clontech Laboratories). Purified viruses were stored in PBS and kept at −80 °C until use.
Cell isolation, culture, and characterization
In order to remove the remnant blood under sterile conditions, the newborn umbilical cords of full-term pregnancies were washed by PBS. After separating the umbilical cord artery and vein, the remaining sections were cut to approximately 2 mm in size and directly transferred to a 25 cm2 culture flasks in Ham’s F12 supplemented with 10 % fetal calf serum, penicillin 100 IU/ml, and streptomycin 100 μg/ml, in an atmosphere of 5 % CO2 at 37 °C. In order to determine the “stemness” of the isolated cells, hUC-MSCs were harvested within 3–6 passages via trypsinization. The cells were then fixed in neutralized 2 % paraformaldehyde (PFA) solution for 30 min. The fixed cells were washed twice with PBS and incubated with antibodies against CD44, CD29, CD105, CD31, and CD45 for 30 min. Primary antibodies were directly conjugated with FITC and phycoerythrin. For purposes of isotype control, non-specific FITC-conjugated IgG was substituted for the primary antibodies. Lastly, the samples were subjected to flow cytometry.
Determination of the optimal multiplicity of transduction
The hUC-MSCs were seeded in 6-well plates at 5 × 105 cell/well. When the cells reached about 80 % confluency, they were washed with PBS for three times, and then subjected to Ad-GFP transduction at 50, 100, 200, or 400 MOI for 2 h at 37 °C. The vibrance of the GFP was qualified 12 h later by fluorescent microscopy. After 48 h, flow cytometry was used to detect the transduction efficiency of the hUC-MSCs.
Detection of TH and DAT expression by immunocytochemistry and western blot
After fixation with 4 % PFA/PBS, cells were treated with blocking solution (PBS containing 4 % goat serum and 0.1 % Triton X-100) for 45 min at RT. The cells were incubated in primary antibodies in PBS/0.1 % Triton X-100 and 1 % goat serum overnight at 4 °C. The following antibodies used were anti-TH and anti-DAT (1:300, R&D, USA). After washing with PBS, slides treated with biotin-labeled secondary antibodies (1:500, R&D, USA) were incubated at RT for 1 h. The chromogenic reagent DAB was used to show the antibody conjugation. After immunostaining, cells on coverslips were mounted and analyzed by an image analysis system. The control group was no adenovirus transduction.
Following treatment, cells were washed in ice-cold phosphate-buffered saline, and total cell lysates were prepared by scraping the cells in lysis buffer. Lysates were rotated at 4 °C for 1 h and the insoluble material was removed by centrifugation at 12,000×g for 10 min. Equal amounts of denatured proteins were separated by 12 % SDS-PAGE and transferred to PVDF membranes (Pharmarcia). The membranes were blocked by incubation in Tris–buffered saline non-fat dry milk for 2 h, followed by incubation at room temperature with indicated antibodies (against TH, DAT, and β-actin) at room temperature for 2 h. After extensively washing in Tris–buffered saline containing 0.1 % Tween-20, the membranes were incubated for 1 h with horseradish peroxidase-conjugated secondary antibody. Membranes were then washed and developed using enhanced chemiluminescence substrate (ECL, Amersham Pharmacia Biotech).
Analysis of DA by ELISA
The hUC-MSCs were transfected with Ad-GFP and Ad-HGF at a MOI of 200 in serum-free F12 for 2 h. The conditioned media (CMs) were harvested at different time-points post-transduction. Concentrations of immunoreactive DA in the supernatant were measured by enzyme-linked immunosorbent assay (ELISA). ELISA plates (R&D system) were coated overnight at room temperature with 100 μl of a 12 μg/ml solution of affinity purified anti-DA diluted in 1× antibody coating buffer. Following two washes with 1× wash buffer, the plate was blocked with 300 μl of 1× general blocker buffer for 3–6 h at room temperature. Blocking solution was removed, the plate firmly tapped on absorbent paper to remove excess liquid and used immediately. Ninety-five microliter of general assay diluent was added to each well, followed by 5 μl of standard blank (purified DA) or serum samples. The plate was then sealed and incubated overnight at 4 °C. Following five washes with 1× washing buffer, 100 μl of HRP-conjugated secondary antibody was added to each well and the plate incubated again for 1 h at room temperature. The plate was washed five additional times. After complete removal of excess solution, 100 μl of TMB substrate was added to each well. Following a 15-min incubation period at room temperature, 100 μl of stop solution was added and the absorbance at 450 nm was read using a plate reader (Bio-Rad). Concentrations of DA in the samples were calculated relative to the exponential standard curve obtained from the standard included in each assay.
Statistical analysis
All data were expressed as mean ± standard deviation (X ± SD). Comparisons between groups were made using one-way analysis of variance. A P value of less than 0.05 was considered to be statistically significant.
Results
Isolation of hUC-MSCs from tissue explant
In our study, a tissue explant technique was used to separate hUC-MSCs. After cleaning the dissected umbilical cord, the tissue slices were cultured. Using inverted microscopy, we observed that cells emigrated from the tissue 3–4 days. The cells were spindle and polygonal in shape with visible nuclei. Following binary fission, the cells gradually developed into long spindles—the typical fibroblast morphology. When 90 % confluent, cells assumed a parallel arrangement or swirling pattern. Cells were then passaged at the ratio of 1–2. The molecular surface markers at the third subpassage were detected by flow cytometry. Our results suggest that hUC-MSCs can be readily isolated using the tissue explant technique. They express the MSCs-related antigens CD29, CD44, and CD105, but lowly expressed endothelial cells phenotype and hematopoietic cells CD31 and CD45 [CD29 (94.20 ± 1.56 %), CD44 (95.63 ± 1.23 %), and CD105 (90.03 ± 0.64 %), CD31 (6.89 ± 0.09 %) and CD45 (5.07 ± 0.068 %) (Fig. 1)]. CD29 is the most important member of the integrin family, mediating the cell and extracellular matrix adhesion. As a receptor, CD44 is a cell surface transmembrane glycoprotein that can identify hyaluronan (HA) and collagen I and IV, and is also involved in cell–cell and cell–matrix-specific adhesion processes. Similarly, CD105 is a specific mesenchymal stem cell surface marker [15, 16]. CD31, a member of the immunoglobulin superfamily, is a surface marker of endothelial cells, and CD45 is specifically expressed in hematopoietic stem cells [17, 18].
The hUC-MSC membrane proteins were evaluated by flow cytometry with CellQuest software. Acquisitions are shown in histograms; the number of acquired events was 20,000 in each acquisition. The hUC-MSCs were positive for CD29 (94.20 ± 1.56 %), CD44 (95.63 ± 1.23 %), and CD105 (90.03 ± 0.64 %) and lowly expressed CD31 (6.89 ± 0.09 %) and CD45 (5.07 ± 0.068 %). IgG1-FITC and IgG1-PE antibodies were utilized as isotype controls. Our results suggest that hUC-MSCs can be readily isolated using the tissue explant technique
Adenoviral vector-mediated transduction of hUC-MSCs
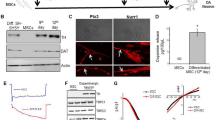
In order to test the transduction efficiency of adenoviral vectors, we transduced the hUC-MSCs with Ad-GFP allowing for identification of GFP. As the MOI increased, the transduction efficiency of hUC-MSCs rose in a dose-dependent manner. When MOI reached 200, 99.55 % of the cells became fluorescent (Fig. 2A, B). Under this MOI, little cell damage was observed (data not shown). Previous reports have documented that Ad-HGF expresses HGF increased up to 128 ng/ml in tissue culture media [19].
The detection of transduction efficiency of hUC-MSCs. A The cells were transduced with Ad-GFP at different MOI (0, 50, 100, 150, 200, and 400) for 2 h and cultured for 48 h at 37 °C. The transduction efficiencies were determined by flow cytometry (a 0.02 ± 0.0096 %, b 71.54 ± 1.98 %, c 82.45 ± 1.64 %, d 98.38 ± 2.24 %, e 99.55 ± 1.99 %, f 99.99 ± 2.38 %). With changes in viral titer, the transduction efficiency increased up to 99.99 % at the highest MOI. However, the difference between 200 and 400 MOI groups was not significant, however. This thus indicates that the transduction efficiency did not become larger as the titer further increased. B Green fluorescence in cells was observed under fluorescence microscopy after transduction with Ad-GFP at 200 MOI. The results show that the recombinant adenovirus vector can be efficiently transferred into the hUC-MSCs
The hUC-MSCs express TH and DAT neuronal markers after transduction with Ad-HGF
The hUC-MSCs were transduced with Ad-HGF at 200 MOI (Ad-HGF group). The control groups were divided into two. One was transduced with Ad-GFP at 200 MOI (Ad-GFP group), and the other was a blank control (the control group). After continuous observation for 72 h, we found that cell morphology gradually changed in the Ad-HGF group. Cells became polygonal and irregular with long protrusions. These phenomena were not observed in Ad-GFP and control groups. At the same time, the expression of TH and DAT in hUC-MSCs was detected by immunocytochemistry. This expression became stronger at 7 and 10 days in the Ad-HGF group (Fig. 3a). The analysis of western blot corresponded to the results of immunocytochemistry (Fig. 3b). The results of western blot were analyzed by an image analysis system. The IOD values were used for statistical analysis (Fig. 3c). Significant differences in IOD values were found between the control, Ad-GFP, and Ad-HGF groups.
Immunocytochemistry assay performed on the hUC-MSCs revealed that the cells could express TH and DAT after transduction with Ad-HGF. a The expression of TH and DAT in hUC-MSCs in the Ad-HGF group gradually increased over a 10-day time period, with intervals at 3, 7, and 10 days. At the same time, the morphology of hUC-MSCs changed, becoming polygonal and irregular with a number of long protrusions (denoted by the arrow). These phenomena were not observed in Ad-GFP and control groups. b TH and DAT expression in cultured hUC-MSCs was analyzed by western blot. An increase of TH and DAT expression was observed in hUC-MSCs treated with Ad-HGF, but not in Ad-GFP and control groups. That is to say, after hUC-MSCs were transfected with Ad-HGF, the expression of TH and DAT up-regulated. c Graph represents densitometric analysis (**P < 0.01). The results of the western blot were analyzed by an image analysis system. The IOD values obtained showed that there were significant differences between the control (Ad-GFP and the blank control) and Ad-HGF groups (# P < 0.01). Thus, HGF can up-regulate the expression of TH and DAT in the hUC-MSCs after transduction with Ad-HGF
The concentration of DA increased in hUC-MSCs supernatant after transduction with Ad-HGF
After transduction was completed with all groups, the concentration of immunoreactive DA in the hUC-MSCs supernatant was measured at different time-points by ELISA (0, 3, 7, and 10 days). As shown in Fig. 4, DA accumulated to about 10 ng/ml in the supernatant at 3 days in Ad-HGF groups. It gradually increased, peaking at 15 ng/ml at 7 days. At 10 days, concentration of DA declined slightly to approximately 13 ng/ml. In blank control and Ad-GFP groups, DA concentration remained stable at 5 ng/ml (Fig. 4).
The concentration of DA in cells supernatant was detected by ELISA after the hUC-MSCs were transduced with Ad-HGF and Ad-GFP. After transduction with Ad-HGF, DA gradually accumulated in hUC-MSCs supernatant, peaking at about 15 ng/ml at 7 days. Levels remained stable at 5 ng/ml in the Ad-GFP and blank control group. Indeed, statistically significant differences emerged between the control and Ad-HGF groups upon analysis (# P < 0.01)
Discussion
PD is detrimental to the quality of life. Age, genetic, and environmental factors have generally been thought to be involved in the etiology of PD but the pathogenesis is still not entirely clear. The reduction of nigrostriatal dopaminergic neurons in the brain, however, is a cellular phenotype that has proven true for all PD patients [8, 20]. The repair of the damaged dopaminergic neurons thus appears to be critical to cure of the disease. That said, the current treatment for PD is the administration of exogenous dopamine. This, however, only alleviates the symptoms and does not repair the fundamentally damaged neurons. And exogenous dopamine only goes so far in alleviating the symptoms of PD because, as PD progresses, the patients gradually become less sensitive to such therapies. As a result, the debilitating symptoms reappeared [21, 22].
Cell replacement therapies in PD may provide long-lasting relief of patients’ symptoms. Stem cells (SCs) represent a class of pluripotent cells with self-renewing ability which can, under certain conditions, differentiate into a variety of functional cells. More and more evidences show that SCs, when transplanted into recipients, exhibit plasticity [23, 24]. Among SCs, hUC-MSCs are particularly advantageous in particular for the following reasons: (a) they are easily obtained without risk of injury to the mother or newborn; (b) they can be repeatedly passaged because of their potent proliferation and differentiation abilities, (c) they do not cause host immune rejection as their immune activity is minimal, (d) they rarely transfect with tumor cells, viruses, and pathogenic microorganisms; and (e) the isolation of hUC-MSCs is not controversial in the ethical and legal realms [4, 25].
Kim et al. [26] propose that abnormalities of the immune system may be related to the pathogenesis of PD. An increasing amount of data confirms that cytokines—products of the immune response—lead to the chronic inflammation that occurs as PD progresses [27]. HGF, a multifunctional cytokine, shows mitogenic and morphogenetic activities in a variety of cells, including MSCs. As an example, literature suggests that HGF regulates the proliferation and migration of dopamine neural progenitor cells. Lan et al. [28] isolated dopaminergic neural progenitor cells from the placenta and showed that said cell’s proliferation was mediated by exogenous HGF and could be inhibited by U0126, a MAPK inhibitor. Cell migration mediated by HGF is completely halted by LY294002, an inhibitor of the protein kinase signaling pathway.
In the study of Koike et al. [29], a rat model is available for the study of PD, which is injected unilaterally into substantia nigra with 6-hydroxydopamine. They directly injected human HGF plasmid DNA into substantia nigra, instead of striatum. At 7 days, after transfection of naked human HGF plasmid, human HGF protein could be detected in the striatum as assessed by immunohistochemical staining. Koike et al. found over 90 % of dopaminergic neurons were lost in PD rats transfected with lacZ, whereas over 70 % survived in rats transfected with HGF. Lan et al. found that the HGF plasmid group, in a dose-dependent manner, showed significantly reduced symptoms after 24 weeks. Immunohistochemical results showed that about 90 % of the control group of dopaminergic neurons disappeared compared to a 70 % decrease observed in the HGF group, suggesting that HGF overexpression can prevent dopaminergic neuronal death in PD [28]. Similarly, Salehi and Rajaei [10] found increased HGF concentrations in the cerebrospinal fluid of PD patients compared to the normal population, further demonstrating that HGF may be involved in the pathophysiology of PD.
In this article, we sought to combine the use of hUC-MSCs and HGF to develop and study new approaches for the treatment of PD. To do so, we constructed Ad-HGF and facilitated its transduction into the hUC-MSCs. As an effect, transdifferentiation among the hUC-MSCs followed. Previous literature reported that an adenovirus carrying the HGF vector could enter the MSCs and efficiently expressed HGF protein. However, the role of HGF on cell proliferation was not made clear [19]. Although, it was shown that HGF can promote migration of the hUC-MSCs. In this study, we focused on the ability of HGF to promote hUC-MSCs differentiation into dopaminergic neuron-like cells. Our results suggest that, after transduction with Ad-HGF, hUC-MSCs express TH, DAT, and DA. The shape of hUC-MSCs also changes from a long and spindle-like to polygonal in shape with some long protrusions. Their appearance is similar to the morphology of neural SCs. It needs to be further in vivo studies that the survival, differentiation, and function of hUC-MSCs gene-modified by HGF gene in substantia nigra.
TH is often used as a target gene of PD in gene therapy, as TH is the rate-limiting enzyme in the catecholamine neurotransmitter synthesis process, playing a catalytic role in the process of tyrosine conversion to l-Dopa [30, 31]. DAT is mainly synthesized and expressed by the nigrostriatal DA nerve cell bodies, dendrites, and axons, eventually distributing in the plasma membrane of the dendrites and axons. The two receptor binding sites on DAT are equivalent to the DA binding sites although their transmembrane structures are not the same [32, 33]. In biological fluids, DAT exists in a cationic form, and its main function is to reuptake the DA in the synaptic cleft following DA transmission [34, 35]. In recent years, studies have shown that DAT does not only serve to reuptake DA but that it is also an important regulator of the DA nervous system function and the dopamine transporter molecular vesicle regulates movement, emotion, learning, memory, and endocrine functions related to the DA system. DAT is thus recognized as the most specific marker of DA neurons.
Our findings reveal the conversion of cellular phenotype in hUC-MSCs following transduction with Ad-HGF, thereafter expressing markers specific markers to dopaminergic neuron (TH, DAT, and DA). This indicates that HGF promotes hUC-MSCs differentiation into dopaminergic-like neurons. Future studies are warranted to investigate the effect of transplanting HGF gene-modified hUC-MSCs to PD animal models in vivo.
Abbreviations
- PD:
-
Parkinson’s disease
- hUC-MSCs:
-
Mesenchymal stem cells derived from human umbilical cord
- HGF:
-
Hepatocyte growth factor
- TH:
-
Tyrosine hydroxylase
- DAT:
-
Dopamine transporter
- DA:
-
Dopamine
References
Pluck GC, Brown RG (2002) Apathy in Parkinson’s disease. J Neurol Neurosurg Psychiatry 73(6):636–642
Bjorklund A, Kordower JH (2013) Cell therapy for Parkinson’s disease: what next? Mov Disord 28(1):110–115. doi:10.1002/mds.25343
Sharma R, McMillan CR, Niles LP (2007) Neural stem cell transplantation and melatonin treatment in a 6-hydroxydopamine model of Parkinson’s disease. J Pineal Res 43(3):245–254
Ali SF, Binienda ZK, Imam SZ (2011) Molecular aspects of dopaminergic neurodegeneration: gene–environment interaction in parkin dysfunction. Int J Environ Res Public Health 8(12):4702–4713. doi:10.3390/ijerph8124702
Wickremaratchi MM, Ben-Shlomo Y, Morris HR (2009) The effect of onset age on the clinical features of Parkinson’s disease. Eur J Neurol 16(4):450–456. doi:10.1111/j.1468-1331.2008.02514.x
Pedrosa DJ, Timmermann L (2013) Review: management of Parkinson’s disease. Neuropsychiatr Dis Treat 9:321–340. doi:10.2147/NDT.S32302
Can A, Balci D (2011) Isolation, culture, and characterization of human umbilical cord stroma-derived mesenchymal stem cells. Methods Mol Biol 698:51–62. doi:10.1007/978-1-60761-999-4_5
Michalopoulos GK, Zarnegav R (1992) Hepatocyte growth factor. Hepatology 15(1):149–155
Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, Vande Woude GF, Aaronson SA (1991) Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science 251(4995):802–804
Salehi Z, Rajaei F (2010) Expression of hepatocyte growth factor in the serum and cerebrospinal fluid of patients with Parkinson’s disease. J Clin Neurosci 17(12):1553–1556. doi:10.1016/j.jocn.2010.04.034
Funakoshi H, Nakamura T (2001) Identification of HGF-like protein as a novel neurotrophic factor for avian dorsal root ganglion sensory neurons. Biochem Biophys Res Commun 283(3):606–612
Hu ZX, Geng JM, Liang DM, Luo M, Li ML (2010) Hepatocyte growth factor protects human embryonic stem cell derived-neural progenitors from hydrogen peroxide-induced apoptosis. Eur J Pharmacol 645(1–3):23–31. doi:10.1016/j.ejphar.2010.07.011
Powell EM, Mars WM, Levitt P (2001) Hepatocyte growth factor/scatter factor is a motogen for interneurons migrating from the ventral to dorsal telencephalon. Neuron 30(1):79–89
Hashimoto N, Yamanaka H, Fukuoka T, Dai Y, Obata K, Mashimo T, Noguchi K (2001) Expression of HGF and c-Met in the peripheral nervous system of adult rats following sciatic nerve injury. NeuroReport 12(7):1403–1407
Halfon S, Abramov N, Grinblat B, Ginis I (2011) Markers distinguishing mesenchymal stem cells from fibroblasts are downregulated with passaging. Stem Cells Dev 20(1):53–66. doi:10.1089/scd.2010.0040
Bae S, Shim SH, Park CW, Son HK, Lee HJ, Son JY, Jeon C, Kim H (2011) Combined omics analysis identifies transmembrane 4 L6 family member 1 as a surface protein marker specific to human mesenchymal stem cells. Stem Cells Dev 20(2):197–203. doi:10.1089/scd.2010.0127
Philippova M, Suter Y, Toggweiler S, Schoenenberger AW, Joshi MB, Kyriakakis E, Erne P, Resink TJ (2011) T-cadherin is present on endothelial microparticles and is elevated in plasma in early atherosclerosis. Eur Heart J 32(6):760–771. doi:10.1093/eurheartj/ehq206
Gaiba S, França LP, França JP, Ferreira LM (2012) Characterization of human adipose-derived stem cells. Acta Cir Bras 27(7):471–476
Duan HF, Wu CT, Wu DL, Lu Y, Liu HJ, Ha XQ, Zhang QW, Wang H, Jia XX, Wang LS (2003) Treatment of myocardial ischemia with bone marrow-derived mesenchymal stem cells overexpressing hepatocyte growth factor. Mol Ther 8(3):467–474
Toovey S, Jick SS, Meier CR (2011) Parkinson’s disease or Parkinson symptoms following seasonal influenza. Influenza Other Respir Viruses 5(5):328–333. doi:10.1111/j.1750-2659.2011.00232.x
Fox SH, Chuang R, Brotchie JM (2008) Parkinson’s disease-opportunities for novel therapeutics to reduce the problems of levodopa therapy. Prog Brain Res 172:479–494. doi:10.1016/S0079-6123(08)00923-0
Rascol O, Lozano A, Stern M, Poewe W (2011) Milestones in Parkinson’s disease therapeutics. Mov Disord 26(6):1072–1082. doi:10.1002/mds.23714
Flici H, Giangrande A (2012) Stem cell aging and plasticity in the Drosophila nervous system. Fly (Austin) 6(2):108–112. doi:10.4161/fly.19797
Phinney DG, Isakova I (2005) Plasticity and therapeutic potential of mesenchymal stem cells in the nervous system. Curr Pharm Des 11(10):1255–1265
Zhou C, Yang B, Tian Y, Jiao H, Zheng W, Wang J, Guan F (2011) Immunomodulatory effect of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells on lymphocytes. Cell Immunol 272(1):33–38. doi:10.1016/j.cellimm.2011.09.010
Kim S, Jeon BS, Heo C, Im PS, Ahn TB, Seo JH, Kim HS, Park CH, Choi SH, Cho SH, Lee WJ, Suh YH (2004) Alpha-synuclein induces apoptosis by altered expression in human peripheral lymphocyte in Parkinson’s disease. FASEB J 18(13):1615–1617
Fiszer U (2001) Does Parkinson’s disease have an immunological basis? The evidence and its therapeutic implications. BioDrugs 15(6):351–355
Lan F, Xu J, Zhang X, Wong VW, Li X, Lu A, Lu W, Shen L, Li L (2008) Hepatocyte growth factor promotes proliferation and migration in immortalized progenitor cells. NeuroReport 19(7):765–769. doi:10.1097/WNR.0b013e3282fdf69e
Koike H, Ishida A, Shimamura M, Mizuno S, Nakamura T, Ogihara T, Kaneda Y, Morishita R (2006) Prevention of onset of Parkinson’s disease by in vivo gene transfer of human hepatocyte growth factor in rodent model: a model of gene therapy for Parkinson’s disease. Gene Ther 13(23):1639–1644
Kastner A, Hirsch EC, Agid Y, Javoy-Agid F (1993) Tyrosine hydroxylase protein and messenger RNA in the dopaminergic nigral neurons of patients with Parkinson’s disease. Brain Res 606(2):341–345
Corbitt J, Hagerty T, Fernandez E, Morgan WW, Strong R (2002) Transcriptional and post-transcriptional regulation of tyrosine hydroxylase messenger RNA in PC12 cells during persistent stimulation by VIP and PACAP38: differential regulation by protein kinase A and protein kinase C-dependent pathways. Neuropeptides 36(1):34–45
Kish SJ, Shannak K, Hornykiewicz O (1988) Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease: pathophysiologic and clinical implications. N Engl J Med 318(14):876–880
Harrington KA, Augood SJ, Kingsbury AE, Foster OJ, Emson PC (1996) Dopamine transporter (Dat) and synaptic vesicle amine transporter (VMAT2) gene expression in the substantia nigra of control and Parkinson’s disease. Brain Res Mol Brain Res 36(1):157–162
Tolosa E, Coelho M, Gallardo M (2003) DAT imaging in drug-induced and psychogenic parkinsonism. Mov Disord 18(Suppl 7):S28–S33
Berendse HW, Ponsen MM (2009) Diagnosing premotor Parkinson’s disease using a two-step approach combining olfactory testing and DAT SPECT imaging Parkinsonism. Parkinsonism Relat Disord 15(Suppl 3):S26–S30. doi:10.1016/S1353-8020(09)70774-6
Acknowledgments
This project was supported, in part, by the Chinese National Science Foundation (No. 30500208) and IAEA Research Project (No. CPR-13305).
Conflict of interest
There is no conflict of interest between all authors.
Author information
Authors and Affiliations
Corresponding authors
Additional information
All experiments were reviewed by the Ethics Committee of the 148th Hospital.
Rights and permissions
About this article
Cite this article
Li, JF., Yin, HL., Shuboy, A. et al. Differentiation of hUC-MSC into dopaminergic-like cells after transduction with hepatocyte growth factor. Mol Cell Biochem 381, 183–190 (2013). https://doi.org/10.1007/s11010-013-1701-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-013-1701-z