Abstract
Acidic mammalian chitinase (AMCase) and chitotriosidase (CHIT-1) are two active chitinases expressed in humans. The chitinase activity of AMCase was found to be causative in allergic inflammation and its expression was found to be induced by interleukin-13. CHIT1-1 is expressed by phagocytic cells and extremely high levels are seen in lysosomal storage diseases. Despite that AMCase expression in the inflammation is under investigation, little is known regarding its regulation during macrophages' full maturation and polarization. In this study, we compared AMCase and CHIT-1 modulation during monocyte to macrophage transition and polarization. Gene expression analysis was investigated by real-time PCR from mRNA of human monocytes obtained from buffy coat of healthy volunteers, from mRNA of polarized to classically activated macrophages (or M1), obtained by interferon (IFN)-γ and lipopolysaccharide (LPS) treatment, and from mRNA of alternatively activated macrophages (or M2) obtained by interleukin (IL)-4 exposure. Our results showed that the expression of AMCase and CHIT-1 were differently modulated in HMMs at different stage of maturation. The behavior of these two active chitinase suggests that in the immune response their role is complementary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chitin is the most abundant polysaccharide in nature after cellulose. It is found as a basic component of numerous species such as exoskeleton of crustaceans, parasitic nematodes, and walls of fungi [1]. Chitinases are enzymes that hydrolyze chitin and are a part of the anti-parasitic response against chitin-containing organisms in lower life forms. Although there is no chitin in humans, it has been found that mammals have two genes encoding active chitinases, chitotriosidase (CHIT1) and acidic mammalian chitinase (AMCase or CHIA), that represent an ancient gene duplication event and show sequence homology to bacterial chitinase [2, 3]. AMCase is one of the enzymes with true chitinase activity [4]. The gene is located on chromosome 1q13.1e21.3 and contains 12 exons which transcribes into a 50 kD protein [4]. The gene also encodes various splice forms. Similarly, CHIT-1 gene is localized in chromosome 1q31–q32 [4] and consists of 12 exons. The location of the AMCase gene (CHIA) on human chromosome 1 and the sequence homology and conservation of intron exon boundaries with CHIT-1 confirms that these genes arose from a duplication event in an ancestor gene [4]. The relatively abundant presence of AMCase in the gastrointestinal tract and lung supports a possible role as a food processor in stomach and its involvement in lung inflammation [4, 5]. Furthermore, the expression of AMCase in the lung suggests that the enzyme may have a dual function in digestion of chitinous substrates and host defense. In their pioneering study, Zhu et al. [6] showed that AMCase is expressed in the epithelial cells and alveolar macrophages in patients with asthma with its production driven by Th2-cytokines IL-4 and IL-13, suggesting that it may play a role in the development of this disease. In addition, inhibition with the transition-state analog allosamidin improved the Th2 driven, IL-13-dependent inflammation, suggesting that its chitinase activity play a role in the disease, even in the absence of chitin. Several genetic variants were later proposed to be partially responsible for predisposition to the disease [7]. The precise role of AMCase in immune-mediated diseases is still far from clear since a report suggested that chitinase activity exerts a beneficial effect by negatively regulating chitin-induced tissue infiltration of innate immune cells associated with allergy [8]. In contrast, CHIT-1 is not an effector molecule in allergic inflammation, rather is regarded as a host-defense mechanism against chitin-containing pathogens [9, 10]. CHIT-1 is selectively expressed and highly regulated in activated macrophages and it is also present in human neutrophil-specific granules which release it upon stimulation [11, 12]. The enzyme was first described in Gaucher disease patients. The sources of secreted CHIT-1 are abnormal lipid-laden macrophages formed in tissues Gaucher disease patients [13]. CHIT-1 activity is currently used as a biochemical marker of macrophage activation in some lysosomal diseases. Subsequently, the levels of this enzyme were found elevated not only in Niemann-Pick diseases [14] but also in other pathological conditions including β-thalassemia [15], sarcoidosis [16], multiple sclerosis [17], atherosclerosis [18], and in parasitic infections such as Plasmodium falciparum malaria [19]. Interestingly, other investigations have suggested that CHIT-1 could have a crucial role even in pathological conditions, such as coronary artery disease [18], acute ischemic stroke [20] cerebrovascular dementia (CVD), and Alzheimer’s disease (AD) [21]. Furthermore, it has been shown that CHIT-1 may be involved in the progression of nonalcoholic steatohepatitis [22, 23].
Macrophages play a crucial role in regulating the initiation, amplification, and resolution of innate immune responses. Several diseases including atherosclerosis, diabetes, cancer, and rheumatoid arthritis are associated with a deficiency or alteration in macrophage function [24]. These cells derive from granulocyte/macrophage progenitors, which are the precursors of monocytes, in the bone marrow. Monocytes coming out from the bone marrow respond to cytokines and chemokines during their recruitment into tissues where they differentiate into resident macrophages. Macrophages can be classically activated (M1) in the presence of interferon (IFN)-γ and lipopolysaccharide (LPS), while in the presence of T helper 2-type cytokines, such as interleukin (IL)-4 and (IL)-13, macrophages undergo alternative activation or are skewed toward an alternatively activated (M2) phenotype. Therefore, response of these cells is reliant on the extracellular milieu [25]. Macrophage polarization deeply alters the immune properties of these cells as shown by the potent antimicrobial properties of M1 macrophages versus the conspicuous tissue repair properties of M2 macrophages [25]. Since the involvement of AMCase and CHIT-1 in several cases of inflammation not necessarily are activated by chitin-containing organisms, there is a need to dissect the roles played by these chitinases on important effectors of immunity such as monocyte/macrophages.
The aim of our study was to compare the modulation of AMCase and CHIT-1 expression during the differentiation in the population of classical activated macrophages (M1) and alternative activated macrophages (M2).
Materials and methods
Cells
Human monocyte-derived macrophages (HMMs) were isolated from fresh buffy coat of healthy volunteers as described previously [26]. The buffy coat was diluted with phosphate-buffered saline (PBS) supplemented with 2.5 mM EDTA and layered onto Ficoll-Hypaque gradients (Gibco, Invitrogen, Milan, Italy). After 30 min of centrifugation at 400×g at room temperature, the mononuclear cells were collected, washed twice with PBS, and placed in plastic Petri dishes at a concentration of 1 × 106–2 × 106 cells/cm surface areas in Iscove’s medium supplemented with 2 mM glutamine and 50 mg/ml of penicillin/streptomycin. After 2 h incubation, the non-adherent cells were washed out using PBS. The adherent cells (monocytes) were cultured in Iscove’s medium supplemented with rHuman M-CSF 5 ng/ml (Peproteck, BDA, Italy), 10 % fetal bovine serum (FBS), 2 mM glutamine, and 1 % of penicillin/streptomycin (Invitrogen, Milan, Italy).
Macrophage differentiation and polarization
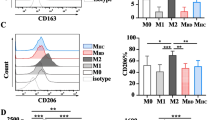
Macrophages were obtained by culturing monocytes for 7 days in Iscove’s medium supplemented with 10 % fetal bovine serum (FBS) 2 mM glutamine, 1 % of penicillin/streptomycin (Invitrogen), and 5 ng/ml M-CSF in Petri dishes at a density 1 × 106–2 × 106 cells/cm2. Macrophage polarization was obtained by removing the culture medium and culturing cells for an additional 18 h in RPMI 1640 supplemented with 5 % FBS and LPS (50 ng/ml) plus IFN-γ (100 U/ml) (for M1 polarization) or IL-4 (20 ng/ml) (for M2 polarization) (Peproteck, Milan, Italy). Five different cell types were generated: freshly isolated monocytes (Mono T0), cells at intermediate differentiation (3 days of culture: Mono T3), resting fully differentiated macrophages (7 days of culture: Macrophages), classical activated macrophages (M1), and alternative activated macrophages (M2) (Fig. 1). Macrophage polarization was confirmed by real-time PCR for two M1 markers (TNF-α and CXCL11) and two M2 markers (CCL18 and CD206) (Fig. 1).
Gene expression analysis by real-time RT-PCR
Total RNA was extracted from cells using TRIzol reagent (Invitrogen, Milan, Italy). For reverse transcription–polymerase chain reaction (RT-PCR), 2 μg of total RNA was reverse transcribed using High-Capacity cDNA Reverse Trascription Kit (Applied Biosystems, Monza, Italy) in a 20 ml reaction solution. Real-time fluorescence PCR, based on SYBR Green, was carried out in a 30-ml final volume containing 1 SYBR Green PCR Master Mix (Applied Biosystems, Monza, Italy), 200 nM forward and 200 nM reverse primers (Supplementary Table 1) and 20 ng of cDNA. Thermal cycling was performed for each gene in triplicate on cDNA samples in MicroAmp Optical 96-well reaction plate (Applied Biosystems, Monza, Italy) with MicroAmp optical caps (Applied Biosystems) using the ABI PRISM 7700 sequence detection system (Applied Biosystems, Monza, Italy). Amplification was carried out with the following conditions: 50 °C for 2 min, 95 °C for 10 min, and 40 cycles each of 95 °C for 15 s and 60 °C for 1 min. Data are presented as mean % ±SD of at least three independent experiments. Differences were analyzed by Student t test, with p < 0.05 being considered statistically significant.
Flow cytometry
Flow cytometric measurements were performed using a four color FacsCalibur (BD Biosciences, San Jose, CA). Cells were fixed and permeabilized using Cytofix/Cytoperm kit (BD Biosciences, San Jose, CA), as per the manufacturer's instruction. Intracellular staining for CD68 was performed using PE-conjugated mouse anti-human CD68 (BD Biosciences, San Jose, CA). Data were analyzed by Cyflogic software, version 1.2.1
Statistical analysis
Data are expressed as mean ± standard error (SE). Significance was assessed by one-way analysis of variance (ANOVA). p < 0.05 was considered to be statistically significant.
Results
Levels TNF-α and superoxide dismutase (SOD2) during in vitro differentiation and polarization of peripheral blood monocytes
In order to verify that monocytes macrophages were not activated during differentiation, we detected the transcriptional levels of TNF-α and superoxide dismutase SOD2. During the first 7 days of differentiation, the levels of both TNF-α and SOD2 were significantly downregulated, confirming a non-activation of the monocyte. In particular, quantitative real time RT-PCR evaluation of TNF-α expression indicated a twofold decrease during the first five days, and a fivefold (p = 0.015) decrease on the seventh day whereas SOD 2 levels ranged from 3.99- to 3.31-fold decrease (p = 0.005) on the fifth and seventh day, respectively (Fig. 2a, b). In contrast, the polarization of macrophages in M1 induced by LPS (50 ng/ml) and IFN-γ (100 U/ml) resulted in an increase of both TNF-α and SOD2 expression (16.5-fold, p = 0.009; 2.19-fold, p = 0.006; respectively) (Fig. 2a, b).
Real-time PCR confirming the non-activation of the monocyte/macrophages. a variation of TNF-α expression and b SOD2 expression on monocyte/macrophages at day 0, 3, 5, 7 (M Φ), classical activated macrophages (M1), alternative activated macrophages (M2). All RT- PCR values are given as ΔCt values, which correspond to the difference of the maximum number of RT-PCR cycles (40) and the value obtained for each individual sample. Statistical analysis was performed by Student’s t test. *p < 0.05 of differentiated or polarized cells versus day 0 cells
Differential expression of AMCase and CHIT-1 during in vitro differentiation and polarization of peripheral blood monocytes
The modulation of expression was investigated by quantitative real time RT-PCR on primary human monocyte-to-macrophage maturation and subsequent polarization into M1 or M2 cells. The levels of AMCase mRNA expression during the differentiation of monocytes into macrophages and subsequent macrophages polarization into M1 and M2 were modestly changed compared to CHIT-1 mRNA expression. Over the first 3 days of culture, a fold decrease of 1.11 compared to the day 0 was observed (Fig. 3). Instead, the levels of CHIT-1 expression were increased by 1.56-fold after 3 days of culture. Therefore, comparing the level of CHIT-1 with AMCase, we found that CHIT expression increased by 1.75-fold. On the fifth day, AMCase expression increased by 1.09-fold compared to the day 0, whereas the levels of CHIT-1 expression were increased by 15.12-fold compared to the day 0. Comparing the level of CHIT-1 with AMCase, we observed a difference of increase by 13.83-fold (Fig. 3).
Real-time PCR of AMCase and CHIT-1 expression during human monocyte/macrophages differentiation and polarization. T0, T3, T5 (monocyte/macrophages at day 0, 3, 5); M Φ 7 (macrophages at day 7), M1:classical activated macrophages, M2: alternative activated macrophages. All RT-PCR values are given as ΔCt values, which correspond to the difference of the maximum number of RT-PCR cycles (40) and the value obtained for each individual sample. Statistical analysis was performed by Student’s t test. *p < 0.05 CHIT-1 expression and # p < 0.05 AMCase expression of differentiated or polarized cells versus day 0 cells
On the seventh day, when monocytes were completely differentiated into macrophages as confirmed by cytoflorometric data (Fig. 1b), AMCase expression was unchanged compared to the day 0, in contrast CHIT-1 levels were increased by 15.75-fold compared to the day 0. Hence, after seven days, comparing the level of CHIT-1, with respect to AMCase, we found that CHIT-1 expression increased by 15-fold. As M1 polarization occurred, AMCase expression increased by 1.39-fold compared to the day 0, instead CHIT-1 levels were increased by 6.62-fold compared to the day 0. Consequently comparing CHIT-1 expression with AMCase, we observed a difference of 4.75 (p = 0.001)-fold. Concerning AMCase expression in M2, no variation was detected compared to the day 0 monocytes, in contrast in M2 CHIT-1 expression increased by 7.52-fold. Hence, comparing CHIT-1 with AMCase expression in M2 macrophages a difference of 7.11-fold was detected (Fig. 3).
Effect of LPS and IFN-γ on the expression of AMCase and CHIT-1 in monocytes
In order to compare the effect exerted by LPS and IFN-γ on AMCase and CHIT-1 expression between undifferentiated monocytes and polarized macrophages, we treated the cells at different time points (2, 4, 8, and 24 h) maintaining the concentration used for the polarization in M1. We found that the treatment with LPS (50 ng/ml) was able to modulate only slightly AMCase expression. As shown in Fig. 4a, LPS increased AMCase expression by 1.32-, 1.33-, 1.30-, and 1.2-fold over that of the control within 2, 4, 8, and 24 h, respectively. In contrast, the expression of CHIT-1 under the effect of LPS (50 ng/ml) was reduced by 1.17-fold since 2 h compared to the control and hold steady within 24 h (Fig. 4a).
Detection of AMCase and CHIT-1 mRNA level by real time RT-PCR in undifferentiated monocytes, untreated and treated for 2, 4, 8, and 24 h with a LPS (50 ng/ml), b IFN-γ (100 U/ml). Statistical analysis was performed by Student’s t test. *p < 0.05, of treated cells versus control; # p < 0.05 AMCase expression versus CHIT-1 expression
Similarly, the treatment with IFN-γ (100 U/ml) powerless modulated AMCase expression. As shown in Fig. 4b, IFN-γ increased AMCase expression by 1.37, 1.48, and 1.50-fold over that of the control within 4, 8, and 24 h, respectively. The expression of CHIT-1 under the effect of IFN-γ (100 U/ml) was increased by 1.60-fold within 2 h and 1.59 at 24 h compared to the control (Fig. 4b).
Effect of IL-4 on the expression of CHIT-1 in monocytes
To examine the effect of IL-4 on AMCase and CHIT-1 expression in undifferentiated monocytes, we treated the cells at different time points (2, 4, 8, 24 h) using the concentration used for the polarization of macrophages in M2. Treatment with IL-4 (20 ng/ml) was able to modulate AMCase expression. We found that the expression of AMCase underwent 2.25- and 2.55-fold increase over that of the control within 2 and 4 h, respectively, showing thereafter at 8 h a 1.31-fold decrease (Fig. 5) compared to treatments at 2 and 4 h, this reduction of AMCase expression was significant at 24 h (2.68-fold, p = 0.04) compared to 2 and 4 h treatments.
Detection of AMCase and CHIT-1 mRNA level by real time RT-PCR in M2-alternative activated macrophages, untreated and treated for 2, 4, 8, and 24 h with IL-4 (20 ng/ml). Statistical analysis was performed by Student’s t test; *p < 0.05, of treated cells versus control; # p < 0.05 AMCase expression versus CHIT-1 expression
The pattern of expression of CHIT-1 was found to be similar to that detected for AMCase. The CHT-1 showed an increase in the early hours of treatment (5.30-fold increase, p = 0.0034 and 5.47-fold increase, p = 0.0079 within 2 and 4 h, respectively, compared to the control cells (Fig. 5). Whereas a significant reduction of CHIT-1 expression was detected within 8 and 24 h (1.04-fold decrease, p = 0.04 and 5.66-fold decrease, p = 0.02 respectively) compared to the untreated cells (Fig. 5).
Discussion
In this study, we compared the modulation of both AMCase and CHIT-1 expression during monocyte/macrophages differentiation and polarization. The results reported here show that the effective maturation of monocytes into macrophages is characterized by the exponential increase of the CHIT-1 levels over time, showing a peak of expression between the fifth and the seventh day of culture. In contrast, we observed that the pattern of AMCase expression does not show significant changes in the diverse stage of HMMs differentiation and/or polarization. Our finding demonstrates different variations of AMCase and CHIT-1 production, during macrophages polarization, and indicates a different rule of these two chitinases in the immune response. The term “macrophage polarization” describes the propensity of fully differentiated macrophages to respond to external stimuli by changing their phenotypic and functional characteristics. The most commonly described subset of macrophages are “classically” polarized macrophages or M1 corresponding to Th1 cells they are considered pro-inflammatory, which is reflected by their production of proinflammatory cytokines, such as IL-1β, IL-6, IL-8, or TNF-α, and of an array of cytotoxic molecules that aid in the clearance of invading pathogens and stimulate the acquired immune response. In our study, verifying the effect that LPS or IFN-γ individually exert on mRNA of AMCase and CHIT-1 expression in undifferentiated monocytes, we found that treatment with LPS barely enhanced AMCase expression, whereas was unable to induce significant changes in CHIT-1 levels, suggesting that AMCase acts precociously, but weakly, against infections, and therefore may be involved in innate immunity. Instead, IFN-γ enhanced both AMCase and CHIT-1 levels. The major sites of expression of AMCase are airway epithelial cells. Our finding indicates that AMCase, unlike CHIT-1, is not selectively expressed and highly regulated in activated macrophages, nevertheless its slight increases in M1 stage and its modulation following treatment with pro-inflammatory stimuli suggests that the antimicrobial pathway in monocyte/macrophages involves also a slight activation of AMCase. Numerous evidences report the central role of CHIT-1 in the expanding spectrum of disorders ranging from granulomatous disease such as sarcoidosis [16] to a large amount of infections including mycobacterial diseases such as tuberculosis [27, 28] and leprosy [29]. In this contest, a great attention must be given to the fact that CHIT-1 is produced by differentiated macrophages themselves and can also be damaging to host tissues and could be implicated in the progression of a number of chronic inflammatory diseases. Consequently, overproduction of CHIT-1 could exert deleterious effect in many degenerative disorders [21]. This idea is also sustained by our recent findings in which we observed that genetic variation within the CHIT-1 gene was strongly associated with human longevity and with several phenotypes of healthy aging [30], and that a functional polymorphism in the CHIT-1 gene protects from NAFLD progression [31]. Alternative or “M2” macrophages, largely reproduce the Th2 response, and since they express an array of phagocytic receptors and release anti-inflammatory cytokines and products that promote tissue regeneration and healing are considered anti-inflammatory [25]. The type of macrophage activation initiated upon phagocytosis of a specific pathogen is essential since it strongly influences the pathogenesis and outcome of the infection. The Th2 cytokine IL-4 promotes immune responses to parasites. Interestingly, in our undifferentiated monocytes, IL-4 treatment induced a significant increase on CHIT-1 expression. Despite in M2 macrophages the level of AMCase were unchanged compared the levels observed in undifferentiated monocytes, IL-4 treatment in undifferentiated monocytes increases significantly AMCase expression in particular at 2 and 4 h, confirming that expression of AMCase is induced by Th2 type. This result confirms the opinion that monocyte/macrophages at the different stages of differentiation have a plastic gene expression and exert diverse activities. Many of these activities appear to be opposing in nature: pro-versus anti-inflammatory effects, immunogenic versus tolerogenic activities, and tissue destruction versus tissue-repair [32]. It has been reported that AMCase is specifically upregulated in response to Th2 inflammation in the lung [6, 33] the effect of IL-4 in undifferentiated monocytes suggests that such upregulation may occur precociously. Allergy and atopic asthma arise as a consequence of poorly controlled Th2 responses [15] our data indicate that inhibition of AMCase may prevent this immune response [6]. In addition, this result clarifies why AMCase is closely associated with pathophysiological conditions dominated by Th2 type cells such as allergy and asthma [6, 33]; as well as our finding explain why increased secretion of CHIT-1 occurs in malaria, fungal infections, fibrosis, allergy, and asthma. In addition, IL-4 is a well-appreciated antagonist of the M1 response and macrophage pro-inflammatory properties [34]. Of interest, IL-4 promotes a M2 phenotype in regressive atherosclerotic lesions [35], providing in such a way a potential explanation of enhanced CHIT-1 activity associated with the presence of atherosclerosis [18]. Although macrophages are paradoxically involved in both generation of fibrosis and its resolution and M2 polarization generates a positive feedback loop during resolution of inflammation, it is unclear what are the events influencing M2 differentiation and interrupting tissue repair/remodeling as well fibrotic outcomes. This finding could support the idea that increased levels of CHIT-1 in this stage could be involved in the modulation of the extracellular matrix affecting cell adhesion and migration during the tissue remodeling processes that take place in fibrogenesis [22, 23]. These same fibrotic changes occur over time in the lung fibrosis as a result of chronic and acute inflammation. Chitin has been shown to induce inflammatory cell recruitment [8], while the immunologic actions of chitin would normally be limited in mammals by chitinase-mediated chitin degradation, interference with chitinase enzymatic activity would likely result in chitin accumulation. Therefore, both CHIT1 and AMCase may be required to insure full degradation and clearance of chitin. The level of chitinase activity in the lung and the predominance of one enzyme over the other may influence the size and quantity of chitin degradation products, which has been shown to determine the inflammatory outcome [36]. In conclusion, the findings presented here emphasize that although our result indicates that AMCase expression is necessary during adaptive and innate immunity and vice versa CHIT-1 may exert an additional role during Th2-driven immune response, and suggest that the confines of action of these two chitinase are not so net, rather their function is interchangeable and/or complementary. This hypothesis opens new view to be explored.
References
Merzendorfer H, Zimoch L (2003) Chitin metabolism in insects: structure, function and regulation of chitin synthases and chitinases. J Exp Biol 206:4393e412
Bussink AP, Speijer D, Aerts JM, Boot RG (2007) Evolution of mammalian chitinase (-like) members of family 18 glycosyl hydrolases. Genetics 177:959–970
Funkhouser JD, Aronson NN Jr (2007) Chitinase family GH18: evolutionary insights from the genomic history of a diverse protein family. BMC Evol Biol 7:96
Boot RG, Blommaart EF, Swart E, Ghauharali-van der Vlugt K, Bijl N, Moe C, Place A, Aerts JM (2001) Identification of a novel acidic mammalian chitinase distinct from chitotriosidase. J Biol Chem 276:6770e8
Chou YT, Yao S, Czerwinski R, Fleming M, Krykbaev R, Xuan D, Zhou H, Brooks J, Fitz L, Strand J, Presman E, Lin L, Aulabaugh A, Huang X (2006) Kinetic characterization of recombinant human acidic mammalian chitinase. Biochemistry 45:4444–4454
Zhu Z, Zheng T, Homer RJ, Kim YK, Chen NY, Cohn L, Hamid Q, Elias JA (2004) Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science 304:1678e82
Bierbaum S, Nickel R, Koch A, Lau S, Deichmann KA, Wahn U, Superti-Furga A, Heinzmann A (2005) Polymorphisms and haplotypes of acid mammalian chitinase are associated with bronchial asthma. Am J Respir Crit Care Med 172:1505–1509
Reese TA, Liang HE, Tager AM, Luster AD, van Rooijen N, Voehringer D, Locksley RM (2007) Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature 447:92–96
Malaguarnera L (2006) Chitotriosidase: the yin and yang. Cell Mol Life Sci 63:3018–3029
Labadaridis I, Dimitriou E, Theodorakis M, Kafalidis G, Velegraki A, Michelakakis H (2005) Chitotriosidase in neonates with fungal and bacterial infections. Arch Dis Child Fetal Neonatal Ed 90:F531–F532
Boot RG, Renkema GH, Strijland A, van Zonneveld AJ, Aerts JM (1995) Cloning of a cDNA encoding chitotriosidase, a human chitinase produced by macrophages. J Biol Chem 270:26252–26256
van Eijk M, Voorn-Brouwer T, Scheij SS, Verhoeven AJ, Boot RG, Aerts JM (2010) Curdlan-mediated regulation of human phagocyte-specific chitotriosidase. FEBS Lett 584:3165–3169
Boven LA, van Meurs M, Boot RG, Mehta A, Boon L, Aerts JM, Laman JD (2004) Gaucher cells demonstrate a distinct macrophage phenotype and resemble alternatively activated macrophages. Am J Clin Pathol 122:359–369
Brinkman J, Wijburg FA, Hollak CE, Groener JE, Verhoek M, Scheij S, Aten J, Boot RG, Aerts JM (2005) Plasma chitotriosidase and CCL18: early biochemical surrogate markers in type B Niemann-Pick disease. J Inherit Metab Dis 28:13–20
Barone R, Bertrand G, Simporè J, Malaguarnera M, Musumeci S (2001) Plasma chitotriosidase activity in beta-thalassemia major: a comparative study between Sicilian and Sardinian patients. Clin Chim Acta 306:91–96
Bargagli E, Maggiorelli C, Rottoli P (2008) Human chitotriosidase: a potential new marker of sarcoidosis severity. Respiration 76:234–238
Comabella M, Domínguez C, Rio J, Martín-Gallán P, Vilche A, Vilarrasa N, Espejo C, Montalban X (2009) Plasma chitotriosidase activity in multiple sclerosis. Clin Immunol 131:216–222
Artieda M, Cenarro A, Gañán A, Lukic A, Moreno E, Puzo J, Pocoví M, Civeira F (2007) Serum chitotriosidase activity, a marker of activated macrophages, predicts new cardiovascular events independently of C-reactive protein. Cardiology 108:297–306
Malaguarnera L, Simporè J, Prodi DA, Angius A, Sassu A, Persico I, Barone R, Musumeci S (2003) 24-bp duplication in exon 10 of human chitotriosidase gene from the sub-Saharan to the Mediterranean area: role of parasitic diseases and environmental conditions. Genes Immun 4:570–574
Palasik W, Fiszer U, Lechowicz W, Czartoryska B, Krzesiewicz M, Lugowska A (2005) Assessment of relations between clinical outcome of ischemic stroke and activity of inflammatory processes in the acute phase based on examination of selected parameters. Eur Neurol 5:188–193
Di Rosa M, Dell’Ombra N, Zambito AM, Malaguarnera M, Nicoletti F, Malaguarnera L (2006) Chitotriosidase and inflammatory mediator levels in Alzheimer’s disease and cerebrovascular dementia. Eur J Neurosci 23:2648–2656
Malaguarnera L, Di Rosa M, Zambito AM, Dell’Ombra N, Di Marco R, Malaguarnera M (2006) Potential role of chitotriosidase gene in nonalcoholic fatty liver disease evolution. Am J Gastroenterol 101:2060–2069
Malaguarnera L, Di Rosa M, Zambito AM, Dell’Ombra N, Nicoletti F, Malaguarnera M (2006) Chitotriosidase gene expression in Kupffer cells from patients with non-alcoholic fatty liver disease. Gut 55:1313–1320
Ross R, Ross XL, Ghadially H, Lahr T, Schwing J, Knop J, Reske-Kunz AB (1999) Mouse langerhans cells differentially express an activated T cell attracting CC chemokine. J Invest Dermatol 113:991–998
Martinez FO, Sica A, Mantovani A, Locati M (2008) Macrophage activation and polarization. Front Biosci 13:453–461
Di Rosa M, Zambito AM, Marsullo AR, Li Volti G, Malaguarnera L (2009) Prolactin induces chitotriosidase expression in human macrophages through PTK, PI3-K, and MAPK pathways. J Cell Biochem 107:881–889
Cakır G, Gumus S, Ucar E, Kaya H, Tozkoparan E, Akgul EO, Karaman B, Deniz O, Kurt I, Ozkan M, Bilgic H (2012) Serum chitotriosidase activity in pulmonary tuberculosis: response to treatment and correlations with clinical parameters. Ann Lab Med 32:184–189
Bargagli E, Margollicci M, Nikiforakis N, Luddi A, Perrone A, Grosso S, Rottoli P (2007) Chitotriosidase activity in the serum of patients with sarcoidosis and pulmonary tuberculosis. Respiration 74:548–552
Iyer A, van Eijk M, Silva E, Hatta M, Faber W, Aerts JM, Das PK (2009) Increased chitotriosidase activity in serum of leprosy patients: association with bacillary leprosy. Clin Immunol 131:501–509
Malaguarnera L, Ohazuruike LN, Tsianaka C, Antic T, Di Rosa M, Malaguarnera M (2010) Human chitotriosidase polymorphism is associated with human longevity in Mediterranean nonagenarians and centenarians. J Hum Genet 55:8–12
Di Rosa M, Mangano K, De Gregorio C, Nicoletti F, Malaguarnera L (2012) Association of Chitotriosidase Genotype with the development of nonalcoholic fatty liver disease. Hepat Res. doi:10.1111/j.1872-034X.2012.01063.x
Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Imm 8:958–969
Zimmermann N, Mishra A, King NE, Fulkerson PC, Doepker MP, Nikolaidis NM, Kindinger LE, Moulton EA, Aronow BJ (2004) Rothenberg Transcript signatures in experimental asthma: identification of STAT6-dependent and -independent pathways. J Immunol 172:1815–1824
Martinez FO, Helming L, Gordon S (2009) Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol 27:451–483
Pourcet B, Feig JE, Vengrenyuk Y, Hobbs A, Kepka-Lenhart D, Garabedian M, Morris SM Jr, Fisher EA, Pineda-Torra I (2011) LXR{alpha} regulates macrophage arginase 1 through PU.1 and interferon regulatory factor 8. Circ Res 109:492–501
Da Silva CA, Hartl D, Liu W, Lee CG, Elias JA (2008) TLR-2 and IL-17A in chitin-induced macrophage activation and acute inflammation. J Immunol 181:4279–4286
Acknowledgments
Giulia Malaguarnera is supported by the International Ph.D. Program in Neuropharmacology (Coordinator Prof. Filippo Drago), University of Catania Medical School, Catania, Italy.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Di Rosa, M., De Gregorio, C., Malaguarnera, G. et al. Evaluation of AMCase and CHIT-1 expression in monocyte macrophages lineage. Mol Cell Biochem 374, 73–80 (2013). https://doi.org/10.1007/s11010-012-1506-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-012-1506-5