Abstract
Pleiotropic effects of serotonin (5-HT) in the cardiovascular system are well documented. However, it remains to be elucidated, whether 5-HT is present in adult mammalian cardiomyocytes. To address this issue, we investigated the levels of 5-HT in blood, plasma, platelets, cardiac tissue, and cardiomyocytes from adult mice and for comparison in human right atrial tissue. Immunohistochemically, 5-HT was hardly found in mouse cardiac tissue, but small amounts could be detected in renal preparations, whereas adrenal preparations revealed a strong positive immunoreaction for 5-HT. Using a sensitive HPLC detection system, 5-HT was also detectable in the mouse heart and human atrium. Furthermore, we could identify 5-HT in isolated cardiomyocytes from adult mice. These findings were supported by detection of the activity of 5-HT-forming enzymes—tryptophan hydroxylase and aromatic l-amino acid decarboxylase—in isolated cardiomyocytes from adult mice and by inhibition of these enzymes with p-chlorophenylalanine and 3-hydroxybenzyl hydrazine. Addition of the first intermediate of 5-HT generation, that is 5-hydroxytryptophan, enhanced the 5-HT level and inhibition of monoamine oxidase by tranylcypromine further increased the level of 5-HT. Our findings reveal the presence and synthesis of 5-HT in cardiomyocytes of the mammalian heart implying that 5-HT may play an autocrine and/or paracrine role in the heart.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Serotonin (5-hydroxytryptamine, 5-HT) in blood mainly originates in the enterochromaffine cells of the gastrointestinal tract [1, 2]; 5-HT is released by these cells and is avidly taken up by platelets. Platelets are, therefore, currently thought to be the main source of 5-HT. The cardiovascular effects of 5-HT include vasoconstriction, increase in platelets aggregation, apoptosis of cardiac cells, increase in cardiac beating rate, generation of arrhythmias [3], valvular heart disease [4], a positive inotropic effect and relaxant effects (reviewed in [5]). Moreover, 5-HT can regulate proliferation in the embryonic mouse heart [6] and consequently inactivation of the 5-HT2B receptor gene leads to embryonic and neonatal death caused by heart defects [7, 8].
At present, one can distinguish seven groups of 5-HT receptors (5-HT1–7 receptors [5, 9, 10]). 5-HT uses divergent 5-HT receptors to transduce its cardiac effects in various species. For example 5-HT2A receptors mediate the positive inotropic effect of 5-HT in the rat [11] and 5-HT3 receptors in the guinea pig [12]. In contrast, the 5-HT4 receptor mediates the positive inotropic effect in humans and interestingly in rats after myocardial infarction [13–16].
This study was initiated in order to get further insight into the cardiac origin of 5-HT. 5-HT is generated starting with the amino acid tryptophan (Fig. 1a). In a first rate limiting step, the isoenzyme tryptophan hydroxylase-1 (TPH-1, mainly expressed in the gut) and the isoenzyme tryptophan hydroxylase-2 (TPH-2 present exclusively in the brain) catalyze the formation of 5-hydroxytryptophan (cf. Fig. 1 and Ref. [17]), a biochemical pathway inhibited by p-chlorophenylalanine (Fig. 1). Subsequently, an unspecific decarboxylase of aromatic amino acids leads to the production of 5-HT (Fig. 1a), a step inhibited by 3-hydroxybenzylhydrazine (NSD-1015) (Fig. 1a). 5-HT can be degraded by monoamino oxidases (MAOs) (Fig. 1a). MAO enzymes are mainly active in intestine, liver, and serotoninergic neurons. Knock-out experiments revealed that only MAO A (and not MAO B) is active in degrading 5-HT, at least in the mouse [18]. MAO activity leads to the genesis of first 5-hydroxyindolacetaldehyde and secondly, indirectly via an unspecific dehydrogenase, to 5-hydroxyindolacetic acid (Fig. 1a). MAO A and B activity can be blocked by the unspecific MAO inhibitor tranylcypromine (Fig. 1a), whereas MAO A is preferentially blocked by clorgyline and MAO B by deprenyl. In addition, 5-HT can be metabolized via arylalkylamine N-acetyltransferase (AANAT) to N-acetylserotonin (Fig. 1a). Re-uptake of 5-HT from the extracellular space into cells occurs mainly via the protein 5-HT-transporter (5-HTT), which is specifically blocked by 5-HT reuptake inhibitors like fluoxetine (Fig. 1a). 5-HTT is expressed in thrombocytes and in the lung where 5-HTT is mainly present in endothelial cells and smooth muscle cells [19]. Fittingly, genetic deletion of 5-HTT in mice led to a decrease of 5-HT levels from 29 to 0.4 μM in whole blood due to the loss of 5-HT uptake in platelets, underscoring the functional relevance of this pathway [20].
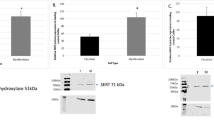
a Scheme for synthesis and degradation of serotonin (5-HT). TPH l-tryptophan-5-monooxygenase, AANAT arylalkylamine N-acetyltransferase, MAO monoamine oxidase, for details see text. b and c Determination of 5-HT and tryptophan by high performance liquid chromatography (HPLC). b Representative chromatogram for 5-HT and tryptophan in isolated adult mouse cardiomyocytes. c Chromatogram of the same sample as in (b), but spiked with authentic 5-HT and tryptophan
Synthesis of 5-HT is generally thought to occur mainly in enterochromaffin cells of the gastrointestinal tract and within the central nervous system [21]. In the past, it was mostly assumed that 5-HT is not present and not synthesized in cardiomyocytes. Any effect of 5-HT in the heart was thought to be due to 5-HT in the plasma, mainly through release from thrombocytes, for instance, during thrombi formation resulting from the development of supraventricular arrhythmias (which are very common). However, more recently the presence of 5-HT and the enzymes relevant for its production have been described in a rat atrium derived tumor line (HL-1) and in neonatal rat cardiomyocytes [22]. However, one can argue that only the mRNAs of the producing enzymes have been described in these cells and not their enzymatic activity [22]. Moreover, it can be questioned whether a tumor cell line (HL-1) has the same 5-HT anabolism/metabolism as adult cardiomyocytes. Similar questions are raised by the use of neonatal cardiac cells. These neonatal cardiac cells have different protein expression patterns than adult cardiomyocytes and therefore may utilize other anabolic and metabolic enzymes (in the present context for 5-HT) than adult cardiac cells. Therefore, we wanted to know whether 5-HT is present and whether it is produced (and degraded) in adult mammalian hearts and cardiomyocytes. We think this issue is relevant in order to know whether 5-HT, produced locally, can affect cardiac function. Further insight into the presence and formation of 5-HT in myocytes may further our understanding of diseases involving 5-HT in the heart (e.g., heart failure, hypertrophy, apoptosis, and arrhythmias) and possibly identify new targets for drug therapy. A progress report of this study has appeared in abstract form [23].
Materials and methods
Animals
Mice were handled and maintained according to approved protocols of the animal welfare committee of the University of Halle-Wittenberg, Halle, Germany (approval reference number 42502-02-691 MLU). Animals used were of both sex and the age ranged between 7 and 8 months.
Human samples
Samples from the right atrium were obtained from patients undergoing open-heart surgery with coronary artery bypass grafts. Right atrial preparations were removed during surgery in the theatre and placed into a precooled physiological salt solution. Samples were transported quickly into the lab. Adherently blood was dissected and the tissue for biochemical measurements was handled as described below. This study complies with the Declaration of Helsinki and has been approved by the local ethics committee (hm-bü 04.08.2005) and patients gave informed consent.
Tissue harvesting
Isolated hearts were prepared as described previously [24]. Mice were anesthetized intraperitoneally with 2.0 g/kg body weight urethane and treated with 1.5 units of heparin. Hearts, initially filled with blood and therefore red colored, were removed from the opened chest, immediately attached by the aorta to a 20-gauge cannula, and perfused retrogradely with oxygenized Krebs-Henseleit buffer (37.4°C) containing (mM) NaCl 118, NaHCO3 25, Na-EDTA 0.5, KCI 4.7, KH2PO4, 1.2, MgSO4, 1.2, CaCl2 2.5, and glucose 11. After 10 min of perfusion the hearts, free of blood, were quickly frozen in liquid nitrogen and kept at −80°C until analysis. In separate experiments hearts were excised but not perfused but only quickly moved in the buffer given above. Hence these hearts are expected to contain substantial amount of thrombocytes and thus 5-HT. In a similar way, the kidney and the adrenal gland were treated. These tissues were frozen and stored as described above. Fresh frozen human tissue from a serotonin-producing neuroendocrine tumor of the small intestine was provided by the Institute of Pathology, Christian-Albrechts-University, Kiel, Germany.
Immunohistochemical techniques
Immunohistology was performed as described earlier [25, 26]. In brief, human tumor slices and mouse tissue sections were incubated 30 min with the primary antibody against 5-HT (Serotec AHP522HT; AbD Serotec, Düsseldorf, Germany) at 37°C or over night at 4°C, followed by 15 min of incubation with the secondary antibody at room temperature. To visualize bound antibodies, a horse radish peroxidase (HRP) system was used (Zytochem Plus HRP Kit; Zytomed Systems, Berlin, Germany). The controls were (1) omission of incubation with primary antibody, (2) substitution of primary antibody by rabbit IgG (Dianova, Hamburg, Germany) at the same final concentration; and (3) incubation in media containing primary antibody that had been pre-incubated at RT for 2 h with a tenfold molar excess of 5-HT. Control incubations resulted in lack of immunostaining. Images shown are representative of at least five independent experiments, which gave similar results.
Preparation of blood and plasma samples
Blood was withdrawn from the retro orbital sinus or ear vein from anesthetized mice (with pentobarbital) was collected into ice-cold tubes containing ethylene-diamine-tetraacetic acid (EDTA) and supplemented with 0.9 mg/ml glutathione. For determination of 5-HT in whole blood, samples were quickly frozen in liquid nitrogen and stored at −80°C. For preparation of platelet-poor plasma (PPP), samples were centrifuged with 1,800×g for 10 min at 4°C; the PPP was removed and immediately used to analyze by HPLC or quickly frozen in liquid nitrogen and stored at –80°C. Previously frozen or freshly prepared blood or plasma samples were spiked with threefold volume of ice-cold 4% perchloric acid (supplemented with 2 mM EDTA, 1% ascorbic acid, and 0.1% cysteine) and then homogenized two times for 30 s with an Ultra Turrax homogenizer (Janke & Kunkel, Staufen, Germany) at full speed. After centrifugation at 12,000×g for 10 min at 4°C, the supernatant was immediately used for HPLC analysis (see below).
Processing of deep-frozen mammalian tissue for HPLC measurement
Deep-frozen tissue samples were pulverized in a mortar pre-cooled in liquid nitrogen and further then grinded three times for 30 s each with a micro-dismembrator U (Sartorius, Göttingen, Germany) in liquid nitrogen. Five volumes of HClO4, containing 2 mM EDTA, 1% ascorbic acid, and 0.1% cysteine, were added to the frozen pulverized tissue. The mixture was homogenized three times for 30 s each with an Ultra Turrax homogenizer (Janke & Kunkel, Staufen, Germany) at full speed. The homogenate was cleared by centrifugation for 10 min at 12,000×g at 4°C and the supernatant was immediately analyzed by reversed-phase HPLC (see below).
Determination of 5-HT in mouse heart homogenates
Mice were sacrificed by cervical dislocation, the hearts rapidly removed, sliced to facilitate removal from blood within heart chambers, and rinsed with ice-cold 0.9% saline. The tissue was minced with scissors, and homogenized in a Potter–Elvehjem homogenizer with five volumes 0.1 mmol/l phosphate-buffered saline (pH 7.4, Teflon pestle). The homogenates were used to determine activities of tryptophan hydroxylase and aromatic l-amino acid decarboxylase (AADC) by measurement of 5-HT levels in the presence of enzyme inhibitors p-chlorophenylalanine, NSD-1015, tranylcypromine, or clorgyline as well as the intermediate 5-hydroxytryptophan during a 60 min incubation at 37°C, partly in the presence of 0.8 μM pyridoxalphosphate as indicated in the corresponding results. The enzymatic reaction was stopped by the addition of 1.5 volumes HClO4 (containing 1% ascorbic acid 0.1% cysteine). The tubes were vortexed, kept for 60 min at 0°C for complete protein precipitation and centrifuged at 12,000×g. The supernatant was immediately analyzed by HPLC (see below) or quickly frozen in liquid nitrogen and stored at −80°C until further use.
Isolation of cardiomyocytes
Ventricular myocytes were isolated from adult mouse hearts using a published protocol [24]. In brief, 7- to 8-month-old animals were pretreated with heparin (5 U/g body weight), and later anesthetized. Mouse hearts were excised, and the cannulated aorta was fixed to a Langendorff apparatus. Hearts were perfused for 5 min at 2 ml/min with a Ca2+-free solution (solution A) composed of (in mM) 140 NaCl, 5.8 KCI, 0.5 KH2PO4, 0.4 NaH2PO4, 0.9 MgSO4, 10 HEPES, 11.1 glucose (pH 7.1), followed by a perfusion for 30 min with solution A supplemented with 0.2 mg/ml collagenase (type D, Roche Molecular Biochemicals). Ca2+ concentration was gradually increased during digestion. After enzymatic digestion, the hearts were perfused for 10 min with solution A. The ventricles were cut into several pieces and subjected to gentle agitation through a nylon mesh to separate the myocytes. Cells were allowed to sediment. The supernatant was discarded. Using light microscopy, intact ventricular cardiomyocytes were identified as rod-shaped striated cells, which had no spontaneous contractions. No evidence of non-cardiomyocytes was found in our preparations.
Determination of tryptophan and its metabolites in cardiomyocytes
Freshly isolated cardiomyocytes were gently diluted in serum-free sterile culture medium M199 and used immediately. For each experiment, the isolated cells of one heart were split up to get aliquots for the experiments and the corresponding untreated control. After the experiment has been finished, the protein content of the sample was quantified. 100 μM ascorbate was always present in the medium during the following incubation period as antioxidant. After addition of the enzyme inhibitors p-chlorophenylalanine, NSD-1015, or tranylcypromine as well as 5-hydroxytryptophan, the cell suspensions were incubated for 30 min at 37°C, partly in the presence of 0.8 μM pyridoxalphosphate and 200 μM tetrahydrobiopterin as indicated in the corresponding results. The enzymatic reaction was stopped by the addition of 1.5 volumes HClO4 (containing 1% ascorbic acid and 0.1% cysteine). The tubes were vortexed, kept for 60 min at 0°C and subsequently centrifuged at 12,000×g to remove any proteins. The supernatant was immediately analyzed by HPLC (see below) to assess the indolamines or quickly frozen in liquid nitrogen and stored at −80°C until further use. The M199 medium itself contains no 5-HT.
HPLC detection of 5-HT, 5-hydroxytryptophan, and tryptophan
The levels of 5-HT, 5-hydroxytryptophan, and tryptophan as well as changes caused by enzyme inhibitors were monitored by reversed-phase high performance liquid chromatography (HPLC). For HPLC analysis, the acidic supernatant, prepared as described above, was neutralized with NaOH (10%), directly injected into the HPLC system and analyzed on a reverse-phase column Nucleosil 100-5 C-18 AB (25 cm, Macherey–Nagel, Düren, Germany). The chromatographic apparatus consisted of a degasser (DG1310, Uniflow Co., Ltd., Japan), AS 4000 autosampler, L-7100 pump, F-1050 fluorescence detector, and the HPLC-Manager-Software D-6000 (Merck-Hitachi). The column was isocratically eluted with a flow rate of 0.6 ml/min at 30°C. The mobile phase consisted of methanol (200 ml) and 0.1 M KH2PO4 buffer (600 ml) adjusted to pH 4.5 (containing 170 mg 1-octanesulfonic acid and 60 mg EDTA per liter). Fluorescence detection of indolamines was carried out at 345 nm with excitation at 285 nm. The detection limit of 5-HT amounted to 5 ng/ml.
Data analysis
Data shown are means ± SEM. Statistical significance was estimated by analysis of variance followed by Bonferroni′s modification of Student′s t test or using Wilcoxon signed rank test, as appropriate. A P-value <0.05 was considered to be significant. All statistical calculations were performed with the GraphPad Prism 5 program.
Drugs and materials
5-HT hydrochloride, tryptophan, 5-hydroxytryptophan, NSD-1015 (3-hydroxybenzylhydrazine dihydrochloride), MDMA ((±)-3,4-methylene-dioxy-meth-amphetamine hydrochloride), fluoxetine ((±)-N-methyl-γ-[4-(trifluoro-methyl)-phenoxy]-benzenepropan-amine hydrochloride), d,l-p-chlorophenylalanine, trans-2-phenyl-cyclo-propyl-amine hemisulfate salt, pyridoxal 5′-phosphate monohydrate (3-hydroxy-2-methyl-5-([phosphonooxy]-methyl)-4-pyridinecarboxaldehyde), clorgyline, and (6R)-5,6,7,8-tetra-hydro-biopterin dihydrochloride were obtained from Sigma (Steinheim, Germany). p-Chlorophenylalanine is only poorly soluble in water and therefore it was dissolved directly into the medium at the appropriate concentration. All other chemicals were of analytical grade. Deionized water was used throughout the experiments. Stock solutions were freshly prepared daily.
Results
5-HT in mouse samples
We looked for precursors and metabolites of 5-HT in mouse cardiac tissue. As depicted in Fig. 1a, 5-HT is formed from tryptophan and degraded to, for instance, 5-hydroxyindole acetic acid. In initial studies, we measured the levels of the precursor tryptophan and 5-HT in isolated adult ventricular cardiomyocytes from mice. As seen in a typical HPLC experiment (Fig. 1b), measurable amounts of 5-HT and tryptophan were detected. The identity of the peaks was confirmed by spiking the samples with authentic standards (Fig. 1c).
Having established the method, we compared 5-HT levels of perfused and non-perfused hearts. We reckoned that non-perfused hearts should contain substantial amounts of 5-HT derived from blood. In contrast, in buffer-perfused hearts, blood cells should be removed and all measurable 5-HT should be contained in cardiomyocytes (which should be the major part) and non-muscle cells like smooth muscle cells, endothelial cells, and fibroblasts. Indeed, we measured substantial amounts of 5-HT and very large amounts of its precursor tryptophan even after washout of blood: as predicted levels of both 5-HT and tryptophan were lower in perfused hearts than in non-perfused hearts (Fig. 2a): non-perfused mouse heart tissue contained 2.7 ± 0.3 pmol 5-HT (n = 10) and 25.9 ± 1.7 pmol tryptophan per mg wet weight (n = 15). After a 15 min Langendorff perfusion of the isolated mouse hearts with Krebs-Henseleit solution, the level of 5-HT was reduced to 1.8 ± 0.6 pmol (n = 10) and the level of tryptophan to 15.3 ± 2.1 pmol per mg wet weight (n = 15) (Fig. 2a). For comparison, Lairez [27] found in mouse ventricular tissue (C3H/HeOuJ) about 1.2 ± 0.2 pmol 5-HT per mg, this value is in the range of our own data.
a 5-HT and tryptophan levels of native hearts (=not perfused with saline buffer) and mouse hearts perfused for 15 min with Krebs-Henseleit solution to remove blood. Numbers in bars indicate numbers of experiments. b 5-HT levels in whole blood (n = 6), platelet-poor plasma (PPP, n = 5), and platelets (n = 12) of adult mice. Blood was withdrawn from punctured ear veins. Plasma and blood platelets were handled as described in “Materials and methods”. 5-HT and tryptophan levels were determined by HPLC as described in “Materials and methods”. Values are means ± SEM. *P < 0.05 versus native hearts
To determine whether comparable 5-HT levels are present in the human heart, we analyzed samples from human right atria, taken from patients undergoing open-heart surgery. The level of 5-HT amounted to 0.144 ± 0.028 pmol and the level of tryptophan to 15.83 ± 1.30 pmol per mg wet weight (n = 10).
Next, we measured the concentration of 5-HT in whole blood, PPP as well as platelets of mice (Fig. 2b). As platelets contain much 5-HT and because whole blood contains platelets, the 5-HT concentrations in whole blood were much higher than in plasma (Fig. 2b): The whole blood level of 5-HT amounted to 7.55 ± 1.29 μmol per liter (n = 6) whereas PPP merely contained 11.92 ± 2.25 nmol 5-HT per liter (n = 5). Adult mouse platelets contained 57.5 ± 6.1 pmol 5-HT per mg protein (n = 12).
Experiments for the histochemical detection of 5-HT in cardiac tissue of mouse and man
Next, we tried immunohistochemical detections and compared the results with HPLC findings. Hence, we stained slices from mouse adrenal gland, mouse heart tissue, and a human neuroendocrine tumor. The highest level of 5-HT was detected in the human tumor tissue. We regarded this as a positive control. It proves that immunohistochemistry can detect 5-HT in human tissue. Lower levels of 5-HT were immunologically detectable in slices from adrenal glands of mice and possibly minute amounts in mouse heart samples (Fig. 3a–d). In parallel, we measured 5-HT levels in these mouse tissues and human atrial samples by HPLC (Fig. 3e). Huge amounts of 5-HT (compare different scales of ordinates) were detected in the tumor and high levels in adrenal glands. The tissue of our interest, cardiac tissue, is also plotted. In human neuroendocrine tumor, the 5-HT level was 6.64 ± 0.16 nmol per mg wet weight (performed in triplicates)—i.e., the 5-HT level in the tumor tissue is more than thousand times larger than in mouse heart (2.99 ± 0.74 pmol per mg wet weight, n = 20) as well as in human atrial heart samples (mean value = 0.144 pmol per mg wet weight, see above). In mouse adrenal glands, 5-HT levels amounted to 46.07 ± 19.02 pmol per mg wet weight (n = 5).
a–d Immunohistochemical detection of 5-HT (brown reaction products depict 5-HT). a Mouse heart: hardly any staining was detectable. b Mouse heart: control with blocking of the primary antibody by an excess of 5-HT. No staining was detectable. c Mouse adrenal gland: the brown reaction product depicts 5-HT localization in the adrenal gland. d Human neuroendocrine tumor tissue is known to contain 5-HT and was used as positive control: brown reaction product points out high levels of 5-HT and its localization. e Comparison of 5-HT levels in hearts (n = 20) and adrenal glands (n = 5) of adult mice as well as in human right atria (n = 10) and neuroendocrine tumor tissues (n = 3). Tissue 5-HT levels were measured by HPLC. Bars represent means ± SEM. (Color figure online)
Evidence of 5-HT formation in the mouse heart
Next, we wanted to know whether the enzymes that lead to the formation of 5-HT were functionally detectable in the heart (see Fig. 1a). Indeed, we detected AADC activity in mouse heart homogenates and subsequent in isolated cardiomyocytes.
These activities could be attenuated by enzyme inhibitors. In the presence of 5-hydroxytryptophan (10 μM), the basal 5-HT level in mouse hearts (3.8 ± 0.4 pmol per mg wet weight; “Basal” in Fig. 4a) was enhanced to 109.7 ± 12.2 pmol per mg wet weight during the 60-min incubation period at 37°C (“5-OH-Trp” in Fig. 4a). Using an unselective, irreversible MAO inhibitor (10 μM tranylcypromine), we could increase the level of 5-HT in homogenates of mouse hearts further to 242.2 ± 28.6 pmol per mg wet weight. 5-HT levels were elevated to a similar extent by clorgyline (100 μM), a selective MAO A inhibitor (245.4 ± 23 pmol per mg wet weight, n = 3). In contrast, the l-aromatic AADC inhibitor NSD-1015 (100 μM) abolished any increase of 5-HT (n = 5, Fig. 4a). As expected, the specific tryptophan hydroxylase inhibitor p-chlorophenylalanine did reduce the 5-HT in the presence of the MAO inhibitor and 5-hydroxytryptophan to levels noted with 5-hydroxytryptophan alone (n = 3, Fig. 4b). Tyramine (100 μM) as well as the 5-HT re-uptake inhibitor fluoxetine (50 μM) were without any effect (data not shown).
a Effects of the monoamine inhibitor tranylcypromine (TCYP, 10 μM) and the aromatic l-AADC inhibitor NSD-1015 (100 μM) in the presence of 5-hydroxytryptophan (5-OH-Trp, 10 μM) on 5-HT levels in homogenates from adult mouse hearts. b Inhibition of 5-HT levels in homogenates of adult mouse hearts by addition of the l-tryptophan hydroxylase inhibitor p-chlorophenylalanine (p-Cl-PA, 5 mM) in the presence of 10 μM tranylcypromine (TCYP) and 5-hydroxytryptophan (5-OH-Trp, 10 μM). Bars present means ± SEM from 5 (a) or 3 b experiments. *P < 0.05 versus Basal (=no additions); + P < 0.05 versus 5-OH-Trp; # P < 0.05 versus 5-OH-Trp + TCYP
Presence and formation of serotonin in isolated adult mouse cardiomyocytes
Recent studies revealed that both, tryptophan hydroxylase mRNA and aromatic l-AADC mRNA, are expressed in cardiac tissues and cardiomyocytes of several species [20, 22, 28–30]. These findings give a hunch that 5-HT secreted from cardiomyocytes could be implicated in the cardiac pathophysiology of 5-HT. Therefore, we quantified 5-HT and tryptophan in isolated cardiac myocytes (Fig. 5). Immediately after isolation of cardiomyocytes, the content of 5-HT in the sedimented cell pellets amounted to 2.34 ± 0.58 pmol 5-HT per mg protein (n = 13), in contrast the content of the precursor tryptophan amounted to 52.74 ± 6.97 pmol per mg protein (n = 12). Following resuspension of the pellets in culture medium M199, the cardiomyocytes were incubated 30 min at 37°C. After this, the accumulated 5-HT in the whole culture was enhanced to 3.40 ± 0.80 pmol per mg protein, 1.70 ± 0.38 pmol per mg protein in the cardiomyocyte pellet, and 1.69 ± 0.48 pmol per mg protein in the culture medium. The accumulation of 5-HT in the whole cardiomyocyte cultures varied widely from culture to culture, ranging from 0.21 to 5.89 pmol per mg protein. In initial experiments, the level of the intermediate 5-hydroxytryptophan was frequently near the detection limit of our HPLC method. Therefore, we added the essential cofactor of the tryptophan hydroxylase tetrahydrobiopterin (200 μM) to the culture medium for all further experiments.
5-HT and tryptophan levels in suspension of cardiomyocytes before and after 30 min incubation at 37°C. The cell culture medium contained 0.2 mM tetrahydrobiopterin, a cofactor of the l-tryptophan hydroxylase, as well as 0.8 μM pyridoxal-5-phosphate, a coenzyme of the aromatic l-AAAD. Tryptophan and 5-HT levels were measured in whole cell culture, both cardiomyocytes (CM) and culture medium (M). Numbers in the bars represent the number of experiments. *P < 0.05 versus before incubation
Effect of p-chlorophenylalanine, NSD-1015, and 5-hydroxytryptophan on the 5-HT accumulation in cardiomyocytes
To further characterize the 5-HT synthesizing enzyme system in adult mouse cardiomyocytes, we investigated the effect of the tryptophan hydroxylase inhibitor p-chlorophenylalanine (5 mM) and the aromatic l-AADC inhibitor 3-hydroxybenzylhydrazine (NSD-1015, 100 μM) as well as addition of 5-hydroxytryptophan (10 μM) on the 5-HT formation in isolated cardiomyocytes.
The conversion of tryptophan to 5-hydroxytryptophan catalyzed by the l-tryptophan hydroxylase is the rate limiting step in the biosynthesis of 5-HT. Therefore, at first we studied the effect of tryptophan hydroxylase inhibitor p-chlorophenylalanine. As shown in Fig. 6a, p-chlorophenylalanine (5 mM) decreased the 5-HT accumulation in cultured cardiomyocyte from 2.06 ± 0.38 pmol per mg protein in the absence of the inhibitor to 0.94 ± 0.45 pmol in the presence of the inhibitor (n = 3) indicating the presence of l-tryptophan hydroxylase in mouse cardiomyocytes.
a Inhibition of key enzymes in 5-HT biosynthesis, tryptophan hydroxylase, by p-chlorophenylalanine (p-Cl-PA, 5 mM) in isolated cardiomyocytes of adult mice. Basal 5-HT levels amounted to 5.02 ± 1.28 pmol per mg protein. Bars represent means ± SEM from n = 3 experiments. + P < 0.05 versus Control. CM cardiomyocytes, M cell culture medium. b Enhancement of 5-HT levels in suspensions of cardiomyocytes from adult mice by addition of the key precursor 5-hydroxy-tryptophan (10 μM). Basal 5-HT levels amounted to 3.86 ± 1.38 pmol per mg protein. c Effect of the l-AADC inhibitor NSD-1015 (100 μM) on 5-HT and 5-hydroxytryptophan (5-OH-TRP) levels in mouse cardiomyocytes. Basal 5-HT amounted to 5.45 ± 0.89 pmol per mg protein. Bars represent means ± SEM from n = 6 (a and b [5-HT]) or n = 3 (b [5-OH-TRP]) experiments. *P < 0.05 versus control. CM cardiomyocytes, M cell culture medium)
Next, we studied the effect of 5-hydroxytryptophan (10 μM) on 5-HT accumulation in whole cardiomyocyte culture in the presence of 0.8 μM pyridoxal-5′-phosphate, a cofactor of the l-aromatic AADC. Addition of 5-hydroxytryptophan enhanced the accumulated levels of 5-HT in the cell culture from 2.75 ± 0.79 pmol per mg protein to 12.67 ± 2.40 pmol per mg protein (n = 6, P < 0.05, Fig. 6b). This remarkable augmentation is indicative of a high activity of the 5-hydoxytryptophan decarboxylase in adult mouse cardiomyocytes which might not be active under other conditions. It is obvious that treatment of cardiomyocytes with the aromatic l-AADC inhibitor NSD-1015 (100 μM) caused a significant decrease in 5-HT accumulation from 5.09 ± 1.37 pmol per mg protein to 0.70 ± 1.52 pmol per mg protein (Fig. 6c). The fact that the NSD-1015 caused 5-HT levels lower than basal 5-HT levels (5.45 ± 0.89 pmol per mg protein) is an indication of a functional enzyme system for 5-HT metabolism such as the MAO. Under identical conditions, NSD-1015 treatment caused an increase of the 5-hydroxytryptophan level in the cardiomyocyte culture (control: 1.65 ± 0.49 pmol per mg protein; under NSD-1015 treatment: 1.97 ± 0.56 pmol per mg protein, n = 3, Fig. 6c). Thereby, the basal 5-hydroxytryptophan levels were nearly identical to the corresponding levels under control conditions. We interpret these results as a broad hint to the presence of a tryptophan hydroxylase activity as well as l-aromatic AADC activity in adult mouse cardiomyocytes.
Discussion
The results of the present report have shown that we can detect 5-HT with antibodies in human and mouse tissue in principle. We also tried to detect 5-HT immunologically in mouse heart and human heart. However, hardly any signal over background was measurable and these immunohistological data are not easily quantified. It is apparent that HPLC detection of 5-HT is substantially more sensitive than the immunohistological methods employed. Clearly, HPLC also has drawbacks: if homogenates from whole tissues are studied by HPLC no information is given about the cell type where the 5-HT comes from. Therefore, we also studied homogenates from isolated cardiomyocytes, but of course even here we were not able to detect the subcellular localization of the 5-HT. Thus, more sensitive immunohistological methods, e.g., electron microscopy, for detection of subcellular compartments of 5-HT synthesis and storage should be developed and would add useful information about the physiological role of 5-HT in the heart.
The 5-HT which we measured via HPLC in homogenates from perfused hearts or in homogenates from isolated cells could originate theoretically from the blood cells. It could have been taken up into cardiac cells by transporters, like the 5-HTT. The 5-HTT is expressed in the cardiovascular system including the heart and might therefore be involved (reviewed in [31]). Alternatively, however, there is previous preliminary evidence for cardiac production of 5-HT (whole tissue from hamster: [32]). Moreover, in cardiomyocytes (HL-1 cells and neonatal rat cardiomyocytes) 5-HT has already been detected by means of HPLC (65 pmol 5-HT/μg DNA) [22]. As a matter of principle, the detection of tryptophan hydroxylase (isoform 1) and the aromatic l-AADC on mRNA level in the heart [22] are hints to the ability of the heart to synthesize 5-HT. This hypothesis is supported by reports that genetic knock-out of isoform 1 of tryptophan hydroxylase led to low levels of 5-HT in the heart (and dilatative cardiomyopathy) [33]. Furthermore, our data suggest that a release of 5-HT from cells occurs under our experimental conditions. However, it remains to be elucidated how exactly this is brought about, in other words whether, as is well known for the release of noradrenaline, autoreceptors for 5-HT and/or Ca2+-dependent protein kinases may be required or other proteins and receptors play a role. It might be of interest to note that there is evidence for the presence, synthesis, and intracellular action of noradrenaline on β-adrenoceptors in cardiomyocytes [34]. It is tempting to speculate on a similar action of 5-HT which is with regard to evolution more ancient than noradrenaline and therefore might be expected to behave similarly.
We have presented here evidence for the presence of 5-HT synthesizing enzymes in heart tissue. More specifically, we could show that p-chlorophenylalanine, an inhibitor of the tryptophan hydroxylase, as well as 3-hydroxybenzylhydrazine (NSD-1015), an inhibitor of aromatic l-AADC, were effective to reduce the 5-HT level in homogenates of adult mouse hearts. We suggest that cardiomyocytes are able to synthesize 5-HT. The fact that both inhibitors of the 5-HT pathway p-chlorophenylalanine and NSD-1015 reduced the 5-HT level in the cardiomyocyte suspensions on the one hand and addition of the intermediate 5-hydroxytryptophan enhanced the 5-HT level on the other hand demonstrates the existence of both active enzymes, namely, tryptophan hydroxylase as well as aromatic l-AADC in this system. Admittedly, the selected concentration of p-chlorophenylalanine was high but based on cell culture experiments in the literature [35]. Indeed, we cannot rule out the possibility that other hydroxylases were inhibited. Despite the concentration of p-chlorophenylalanine was high, it was less effective than the decarboxylase inhibitor. Here, we can only speculate: other metabolic pathways might be involved which we have not yet been able to dissect or the cellular pool of 5-hydroxytryptophan enabled some 5-HT synthesis in the presence of the hydroxylase inhibitor. Using a pharmacological approach, we also can delineate the degradation of 5-HT in the heart. This pathway is very active, as its inhibition can greatly elevate cellular 5-HT levels. In principle our data, with tranylcypromine (an inhibitor of both MAO A and B), show that this pathway is very effective in getting rid of 5-HT. Using clorgyline (a MAO A inhibitor), we could show that 5-HT degradation under our experimental conditions involves probably only MAO A as this compound was highly effective. Moreover, 10 μM deprenyl (a MAO B inhibitor) was inactive to increase 5-HT levels in mouse cardiac tissue under our experimental conditions (data not shown). Likewise, others [4] who used a knock-out approach of MAO A and B also concluded that MAO B is practically inactive in (mouse) cardiac tissue and that only MAO A is the active isoform of MAO in the (mouse) heart. Whether the same as in mouse heart holds true for the functional relevance of MAO isoforms in human cardiac tissue is of high interest but remains to be elucidated.
In initial experiments, we did not add 5-hydroxytryptophan and could not see any discernible effect of tranylcypromine or tranylcypromine plus p-chlorophenylalanine on 5-HT levels. Therefore, 5-hydroxytryptophan had to be present to have levels of 5-HT that were high enough (Column 5-OH-Trp in Fig. 4a) in order to allow us to detect a substantial effects on 5-HT levels by adding the inhibitors. Under these conditions, p-chlorophenylalanine reduced 5-HT levels to values that nearly where present after addition of 5-hydroxytryptophan alone (compare the second column in Fig. 4a with the third column in Fig. 4b).
What is the possible physiological relevance of our findings? In the cardiovascular system, several 5-HT receptor subtypes are expressed and 5-HT is important for cardiac morphogenesis [reviewed in 36]. Whereas in the human heart, 5-HT4 receptors mediate inotropic and chronotropic effects of 5-HT the situation in the mouse heart seems to be different. In the adult mouse heart, inotropic and chronotropic effects of 5-HT are missing [37]. Nevertheless, local 5-HT synthesis, storage, and release should influence vascular function via 5-HT1 and 5-HT2 receptors in the mouse heart and possibly play a role in physiological and pathophysiological states of the heart [36]. The way 5-HT is stored and released by cardiac myocytes has to be explored by further studies. In murine mast cells, it was demonstrated that serglycin proteoglycan containing secretory vesicles are mainly responsible for 5-HT storage and release. Moreover, also mast cells of serglycin knockout mice, lacking intracellular stores, are able to release 5-HT but to a lesser extent. Because of the high expression of TPH-1 in mast cells, it was supposed that 5-HT released due to de novo synthesis [38]. This could also be a possible mechanism in cardiac myocytes.
The EC50-value for the positive inotropic effect of 5-HT in isolated preparations from human right atrium is between 39 and 230 nM [15]. The concentration of 5-HT in myocytes was estimated to be about 2.9 pmol/mg protein. Concentrations of 5-HT in isolated samples from human hearts range from 0.08 (ca. 0.45 μM: [39], human left ventricle and human apex, post mortem) to 0.4 μg/g (about 2.3 μM, [32], human papillary muscle, during cardiac surgery). However, in this study human right atria contain 5-HT levels of much less namely to 0.22 ng/g (ca. 1.3 nM).
It is conceivable that the apparent differences in human cardiac 5-HT levels between our study and that of others may be due to different levels of 5-HT in different regions of the heart and/or the consequence of unequal platelet contaminations between studies. Moreover, we cannot exclude the possibility that during handling and transport of the human tissue samples in this study, some 5-HT is degraded (though tissue was frozen in the theatre), and hence our data probably give the lower limit of human atrial concentrations for 5-HT.
Assuming a homogenous distribution of 5-HT in myocytes and taking into account their protein content, one can calculate a 5-HT concentration of about 200 nM in adult cardiomyocytes. This concentration is expected to stimulate 5-HT receptors because it is, for instance, well in the range of the EC50 for the positive inotropic effect [14], but also in the range of other physiological effects of 5-HT like vasoconstriction, stimulation of thrombocytes, or action on the sinus node [9].
It is of interest in this context that high concentrations of 5-HT in the heart can also lead to apoptosis or necrosis. This involves dual mechanisms of action in part mediated by 5-HT receptors but also through receptor-independent degradation of 5-HT by MAO A and generation of hydrogen peroxide in the myocytes [1, 40]. Lower concentrations of 5-HT (<1 μM) act via 5-HT receptors in an antiapoptotic way [40].
In summary, we have presented in the present work indications of enzyme activities responsible for the production (and degradation) of 5-HT in mouse cardiac preparations and we suggest that 5-HT may be produced in cardiomyocytes which serves autocrine and/or paracrine roles in the mammalian heart.
References
Verbeuren T (1990) The distribution and biochemistry of 5-HT in the cardiovascular system. Kluver Academic Press, Dordrecht, pp 3–13
Verbeuren T (1992) Distribution, synthesis, metabolism, release, uptake, and passage across body membranes in cardiovascular tissues including blood-brain barrier. Raven Press, New York, pp 29–39
Kaumann AJ (1994) Do human atrial 5-HT4 receptors mediate arrhythmias? Trends Pharmacol Sci 15:451–455
Robiolio PA, Rigolin VH, Wilson JS, Harrison JK, Sanders LL, Bashore TM, Feldman JM (1995) Carcinoid heart disease. Correlation of high serotonin levels with valvular abnormalities detected by cardiac catheterization and echocardiography. Circulation 92:790–795
Kaumann AJ, Levy FO (2006) 5-hydroxytryptamine receptors in the human cardiovascular system. Pharmacol Ther 111:674–706
Yavarone MS, Shuey DL, Tamir H, Sadler TW, Lauder JM (1993) Serotonin and cardiac morphogenesis in the mouse embryo. Teratology 47:573–584
Nebigil CG, Choi DS, Dierich A, Hickel P, Le Meur M, Messaddeq N, Launay JM, Maroteaux L (2000) Serotonin 2B receptor is required for heart development. Proc Natl Acad Sci USA 97:9508–9513
Nebigil CG, Maroteaux L (2001) A novel role for serotonin in heart. Trends Cardiovasc Med 11:329–335
Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP (1994) International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol Rev 46:157–203
Hoyer D, Hannon JP, Martin GR (2002) Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav 71:533–554
Laer S, Remmers FO, Scholz H, Stein B, Muller FU, Neumann J (1998) Receptor mechanisms involved in the 5-HT-induced inotropic action in the rat isolated atrium. Br J Pharmacol 123:1182–1188
Tramontana M, Giuliani S, Del Bianco E, Lecci A, Maggi CA, Evangelista S, Geppetti P (1993) Effects of capsaicin and 5-HT3 antagonists on 5-hydroxytryptamine-evoked release of calcitonin gene-related peptide in the guinea-pig heart. Br J Pharmacol 108:431–435
Du XY, Schoemaker RG, Bos E, Saxena PR (1994) Different pharmacological responses of atrium and ventricle: studies with human cardiac tissue. Eur J Pharmacol 259:173–180
Gergs U, Neumann J, Simm A, Silber RE, Remmers FO, Laer S (2009) Phosphorylation of phospholamban and troponin I through 5-HT(4) receptors in the isolated human atrium. Naunyn Schmiedebergs Arch Pharmacol 379:349–359
Kaumann AJ, Sanders L, Brown AM, Murray KJ, Brown MJ (1990) A 5-hydroxytryptamine receptor in human atrium. Br J Pharmacol 100:879–885
Qvigstad E, Brattelid T, Sjaastad I, Andressen KW, Krobert KA, Birkeland JA, Sejersted OM, Kaumann AJ, Skomedal T, Osnes JB, Levy FO (2005) Appearance of a ventricular 5-HT4 receptor-mediated inotropic response to serotonin in heart failure. Cardiovasc Res 65:869–878
Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H, Bader M (2003) Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 299:76
Popova NK (2006) From genes to aggressive behavior: the role of serotonergic system. BioEssays 28:495–503
Lee SL, Fanburg BL (1986) Serotonin uptake by bovine pulmonary artery endothelial cells in culture. I. Characterization. Am J Physiol 250:C761–C765
Mekontso-Dessap A, Brouri F, Pascal O, Lechat P, Hanoun N, Lanfumey L, Seif I, Benhaiem-Sigaux N, Kirsch M, Hamon M, Adnot S, Eddahibi S (2006) Deficiency of the 5-hydroxytryptamine transporter gene leads to cardiac fibrosis and valvulopathy in mice. Circulation 113:81–89
Yusuf S, Al-Saady N, Camm AJ (2003) 5-hydroxytryptamine and atrial fibrillation: how significant is this piece in the puzzle? J Cardiovasc Electrophysiol 14:209–214
Ikeda K, Tojo K, Otsubo C, Udagawa T, Kumazawa K, Ishikawa M, Tokudome G, Hosoya T, Tajima N, Claycomb WC, Nakao K, Kawamura M (2005) 5-hydroxytryptamine synthesis in HL-1 cells and neonatal rat cardiocytes. Biochem Biophys Res Commun 328:522–525
Pönicke K, Gergs U, Hauptmann S, Buchwalow IB, Neumann J (2008) Release of 5-hydroxytryptamine from heart and isolated adult mice cardiomyocytes. Naunyn Schmiedebergs Arch Pharmacol 377(Suppl 1):57
Kirchhefer U, Neumann J, Baba HA, Begrow F, Reinke U, Schmitz W, Kobayashi YM, Jones LR (2001) Cardiac hypertrophy and impaired relaxation in transgenic mice overexpressing triadin 1. J Biol Chem 276:4142–4149
Buchwalow IB, Podzuweit T, Bocker W, Samoilova VE, Thomas S, Wellner M, Baba HA, Robenek H, Schnekenburger J, Lerch MM (2002) Vascular smooth muscle and nitric oxide synthase. FASEB J 16:500–508
Buchwalow IB, Podzuweit T, Samoilova VE, Wellner M, Haller H, Grote S, Aleth S, Boecker W, Schmitz W, Neumann J (2004) An in situ evidence for autocrine function of NO in the vasculature. Nitric Oxide 10:203–212
Lairez O, Calise D, Bianchi P, Ordener C, Spreux-Varoquaux O, Guilbeau-Frugier C, Escourrou G, Seif I, Roncalli J, Pizzinat N, Galinier M, Parini A, Mialet-Perez J (2009) Genetic deletion of MAO-A promotes serotonin-dependent ventricular hypertrophy by pressure overload. J Mol Cell Cardiol 46:587–595
Izikki M, Hanoun N, Marcos E, Savale L, Barlier-Mur AM, Saurini F, Eddahibi S, Hamon M, Adnot S (2007) Tryptophan hydroxylase 1 knockout and tryptophan hydroxylase 2 polymorphism: effects on hypoxic pulmonary hypertension in mice. Am J Physiol Lung Cell Mol Physiol 293:L1045–L1052
Kubovcakova L, Krizanova O, Kvetnansky R (2004) Identification of the aromatic l-amino acid decarboxylase gene expression in various mice tissues and its modulation by immobilization stress in stellate ganglia. Neuroscience 126:375–380
Lopez-Contreras AJ, Galindo JD, Lopez-Garcia C, Castells MT, Cremades A, Penafiel R (2008) Opposite sexual dimorphism of 3,4-dihydroxyphenylalanine decarboxylase in the kidney and small intestine of mice. J Endocrinol 196:615–624
Ni W, Watts SW (2006) 5-hydroxytryptamine in the cardiovascular system: focus on the serotonin transporter (SERT). Clin Exp Pharmacol Physiol 33:575–583
Sole MJ, Shum A, Van Loon GR (1979) Serotonin metabolism in the normal and failing hamster heart. Circ Res 45:629–634
Cote F, Thevenot E, Fligny C, Fromes Y, Darmon M, Ripoche MA, Bayard E, Hanoun N, Saurini F, Lechat P, Dandolo L, Hamon M, Mallet J, Vodjdani G (2003) Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc Natl Acad Sci USA 100:13525–13530
Vaniotis G, Del Duca D, Trieu P, Rohlicek CV, Hébert TE, Allen BG (2011) Nuclear β-adrenergic receptors modulate gene expression in adult rat heart. Cell Signal 23:89–98
Kelly CJ, Johnson TC (1978) Effects of p-chlorophenylalanine and alpha-methylphenylalanine on amino acid uptake and protein synthesis in mouse neuroblastoma cells. Biochem J 174:931–938
Monassier L, Laplante MA, Ayadi T, Doly S, Maroteaux L (2010) Contribution of gene-modified mice and rats to our understanding of the cardiovascular pharmacology of serotonin. Pharmacol Ther 128:559–567
Gergs U, Baumann M, Böckler A, Buchwalow IB, Ebelt H, Fabritz L, Hauptmann S, Keller N, Kirchhof P, Klöckner U, Pönicke K, Rueckschloss U, Schmitz W, Werner F, Neumann J (2010) Cardiac overexpression of the human 5-HT4 receptor in mice. Am J Physiol Heart Circ Physiol 299:H788–H798
Ringvall M, Rönnberg E, Wernersson S, Duelli A, Henningsson F, Abrink M, García-Faroldi G, Fajardo I, Pejler G (2008) Serotonin and histamine storage in mast cell secretory granules is dependent on serglycin proteoglycan. J Allergy Clin Immunol 121:1020–1026
Correa-Araujo R, Oliveira JS, Ricciardi Cruz A (1991) Cardiac levels of norepinephrine, dopamine, serotonin and histamine in Chagas’ disease. Int J Cardiol 31:329–336
Mialet-Perez J, Bianchi P, Kunduzova O, Parini A (2007) New insights on receptor-dependent and monoamine oxidase-dependent effects of serotonin in the heart. J Neural Transm 114:823–827
Acknowledgments
The technical assistance of A. Spiess-Dunemann and I. Adler is greatly appreciated. This study was supported by the Deutsche Forschungsgemeinschaft (DFG).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pönicke, K., Gergs, U., Buchwalow, I.B. et al. On the presence of serotonin in mammalian cardiomyocytes. Mol Cell Biochem 365, 301–312 (2012). https://doi.org/10.1007/s11010-012-1270-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-012-1270-6