Abstract
Ginsenoside Rg1 promotes antioxidative protection and intracellular calcium homeostasis in cardiomyocytes hypoxia/reoxygenation (H/R) model. However, the pharmacological effects of G-Rg1 on autophagy in cardiomyocytes have not been reported. In this study, we employed H9c2 cardiomyocytes as a model to investigate the effects of G-Rg1 on autophagy in cardiomyocytes under H/R stress. Our results showed that H/R induced increased level of LC3B-2, an autophagy marker, in a time-dependent manner in association with decreased cell viability and cellular ATP content. H/R-induced autophagy and apoptosis were further confirmed by morphological examination. 100 μmol/l Rg1-inhibited H/R induced autophagy and apoptosis, and this was associated with the increase of cellular ATP content and the relief of oxidative stress in the cells. Mechanistically, we found that Rg1 inhibited the activation of AMPKα, promoted the activation of mTOR, and decreased the levels of LC3B-2 and Beclin-1. In conclusion, our data suggest that H/R induces autophagy in H9c2 cells leading to cell injury. Rg1 inhibits autophagosomal formation and apoptosis in the cells, which may be beneficial to the survival of cardiomyocytes under H/R.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ginseng is a well-known traditional Chinese medicine that has been used widely for thousands of years. It is now one of the most popular alternative medicines consumed in large amounts worldwide. Ginsenosides, which are steroidal saponins, are the cardinal pharmacologically active components of ginseng and appear to be responsible for most of the activities of ginseng [1]. Ginsenosides have potential benefits on the cardiovascular system through various mechanisms, such as antioxidant, modifying vasomotor function, reducing platelet adhesion, and improving lipid profiles [2]. Ginseng root has been used to protect against cardiac ischemia, a major cause of death in the West [3]. As one of the abundant and active ingredients in ginsenosides, ginsenoside Rg1 (G-Rg1) was shown to promote antioxidative protection and intracellular calcium homeostasis in cardiomyocytes hypoxia/reoxygenation (H/R) model [4].
Autophagy emerges as a new pathway responsible for maintaining cellular homeostasis via direct control of cell death and survival [5]. The autophagy-related genes (Atg) have been identified in yeast and mammals [5]. In mammalian cells, the carboxy-terminal region of LC3, the homologues of yeast Atg8, is cleaved to generate a soluble form LC3B-1, which in turn is modified to a membrane-bound form LC3B-2 to prompt its localization to autophagosomes. Subsequently, autophagosomes fuse with lysosomes to form autolysosomes and promote autophagy. Thus, the amount of LC3B-2 in mammalian cells is accepted as an early marker for the formation of autophagosomes [6]. During ischemia and reperfusion in cardiomyocytes, autophagy plays a protective role during ischemia but a detrimental role during reperfusion [7]. In response to ischemia, the extent of autophagy depends on the severity and duration of ischemic insults. For example, modest levels of autophagy appear to be protective by degrading and removing damaged mitochondria, therefore preventing the activation of apoptosis [8, 9]. On the other hand, high levels of autophagy triggered by severe hypoxia or ischemia/reperfusion may cause self-digestion and eventual cell death [10]. Therefore, the level of autophagy may determine whether it is protective or detrimental to the heart in response to ischemia/reperfusion [11]. However, the pharmacological effects of G-Rg1 on autophagy in cardiomyocytes have not been reported.
The autophagy process consists of dynamic formation of autophagosomes and their clearance subsequent to lysosomal fusion. Therefore, increase in autophagosomes in the cells can be due to either increased autophagosome formation or decreased autophagosome clearance due to frustrated autophagy. Frustrated autophagy is characterized by the failure of lysosomal fusion, which may cause the catastrophic leakage of lysosomal proteases and cell death [12]. In this study, we employed H9c2 cardiomyocytes as a model to investigate the effects of G-Rg1 on autophagy in cardiomyocytes under H/R stress. Especially, we used the lysosomal inhibitor chloroquine to measure autophagic flux, which reflected the dynamic process of autophagy under H/R condition and elucidated the mechanism of action of G-Rg1. We found that H/R induced pronounced autophagy in H9c2 cells. G-Rg1 caused the decrease of membrane-bound LC3B form of LC3B-2 and LC3B-2-containing organelles in the cells, suggesting the inhibition of autophagosomal formation and apoptosis. G-Rg1 treatment also led to increased ATP content and decreased oxidative stress, LDH and phosphorylated AMPKα, implicating that the inhibition of autophagosomal formation by G-Rg1 is beneficial to the cells to survive H/R.
Materials and methods
Chemicals and reagents
Ginsenoside Rg1 (purity >99%) was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China).
Cell culture and treatment
H9c2 cardiomyocytes derived from rat myocardium were purchased from the Cell Bank of Chinese Academy of Science (Beijing, China). Cells were grown in Dulbecco’s modified Eagle’s medium (DMEM, GIBCO) supplemented with 10% inactivated fetal bovine serum (FBS, GIBCO), 500 μg/ml penicillin, and 500 μg/ml streptomycin (GIBCO) at 37°C in a humidified atmosphere with 5% CO2. Medium was changed every 3 days.
For H/R treatment, H9c2 cardiomyocytes were incubated in nutrient-rich medium for 3 days, then washed with Hank’s solution (5 mM HEPES, 137 mM NaCl, 4 mM KCl, 1 mM MgCl2, and 1.5 mM CaCl2, pH 7.2). The cells were incubated in a glucose-free DMEM base medium and then subjected to hypoxia to mimic the in vivo conditions of myocardial ischemia. The cells were placed in an incubator at 37°C. N2 (95%) and 5% CO2 were flushed into the incubator to bring the oxygen content down to 1%, monitored by an oxygen probe. After different periods of hypoxia, the cells were subjected to reoxygenation by changing the medium into a DMEM base medium with 5.5 mM glucose (pH 7.4) followed by incubation under normoxia for 1 h. Cells were treated for various periods in the presence or the absence of G-Rg1 or other inhibitors. Control cells were cultured in complete medium. Cells were treated with G-Rg1 (100 μmol/l) with or without chloroquine (3 μmol/l, Sigma-Aldrich) under H/R condition for various periods. After the treatment, the cells were harvested and analyzed. No cytotoxic effects on H9c2 cells were observed when they were incubated in complete medium containing 3 μmol/l chloroquine and/or 100 μmol/l G-Rg1 for 24 h.
Cell viability assay
Cell viability was measured by the modified MTT method using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay. H9c2 cells (1 × 105 in 100 μl medium) were seeded in 96-well plates. After the treatment, 20 μl 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide stock solution were added to each well and incubated for 4 h at 37°C. 150 μl Dimethyl sulfoxide was added to each well to dissolve the formazan crystals. The plate was gently shaken for 10 min and was read at 550 nm on a plate reader. The optical density was used as the indicator of cell viability, and was normalized to that from the cells incubated in control medium, which were considered to be 100% viable.
Electron microscopy
H9c2 cells were collected by centrifugation and washed with phosphate-buffered saline (PBS), then fixed with 4% glutaraldehyde, post-fixed with 1% perosmic acid, and dehydrated with acetone. Ultrathin sections were placed on 400-mesh grids and double stained with uranyl acetate and lead citrate. Sections were observed under transmission electron microscopy (TEM; JEM1230; Jeol, Tokyo, Japan).
Measurement of intracellular ATP content
Intracellular ATP content (μmol/mg) was measured using the ATP bioluminescent assay kit (Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions. Experiments were performed in triplicate.
Measurements of malondialdehyde level and superoxide dismutase activity
The cultured medium was taken for the measurements of malondialdehyde (MDA) level and superoxide dismutase (SOD) activity using the assay kits (Jiancheng Bioengineering Institute, Nanjing, China). MDA was analyzed by TBA assay and determined at 532 nm. SOD was measured by the degree of inhibition on nitroblue tetrazolium produced by superoxide radicals, which were generated from the xanthine/xanthine oxidase system. SOD activity was measured by taking absorbance at 550 nm.
Western blot analysis
Cells were lysed in RIPA buffer (50 mM Tris–HCl pH7.4, 150 mM NaCl, 1% NP-40, 10.5% sodium deoxycholate, 5% sodium dodecyl sulfate, and 1 mM phenylmethyl sulfonyl fluoride). Protein concentration was quantified using a BCA protein quantity assay kit (Applygen Technologies, Beijing, China). Equal amounts of protein were loaded onto 8, 10, or 15% sodium dodecyl sulfate-polyacrylamide gel, separated by electrophoresis, and transferred onto NC membranes. The membranes were first probed with primary antibodies as follows: rabbit anti-LC3B (Sigma, St. Louis, MO, USA), rabbit anti-AMPKa (cell signaling), rabbit anti-Beclin-1 (cell signaling), rabbit anti-P70S6K (cell signaling), rabbit anti-phospho-P70S6K (cell signaling), or rabbit anti-phospho-AMPKa (cell signaling), then probed with horseradish peroxidase-labeled anti-rabbit IgG secondary antibody (Santa Cruz), and developed with the enhanced chemiluminescent technique.
Immunofluorescence analysis
H9c2 cells were grown on cover slides in a 6-well plate. After the treatment, cells were fixed in 4% paraformaldehyde. The primary antibody used was rabbit anti-LC3B antibody (cell signaling), mouse anti-caspase 9 (Santa Cruz), and the secondary antibody was FITC-labeled anti-rabbit IgG antibody (cell signaling). Images were observed under a confocal microscope (TCS SP/MP, Leica, Bensheim, Germany).
Statistical analysis
Results were presented as mean ± SD. Statistical differences were evaluated by one-way ANOVA. A value of P < 0.05 was considered to be statistically significant.
Results
H/R induces autophagy and cell death in H9c2 cells
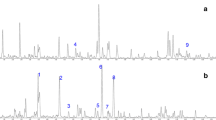
H9c2 cells were subjected to H/R for 0, 1, 2, 3, and 5 h, and then the expression of LC3B-1 and LC3B-2 was examined by immunoblotting. The results showed that the level of LC3B-2, an autophagy marker, increased in a time-dependent manner in H9c2 cells under H/R condition and peaked at 3 h after H/R, although it declined at 5 h after H/R (Fig. 1a), suggesting that H/R induces autophagy in H9c2 cells. To investigate the effects of G-Rg1 on H9c2 cell survival subjected to H/R, we performed MTT assay and found that while 80% cells died after H/R for 5 h, the cells' death was rescued significantly by the treatment with 100 μmol/l G-Rg1, but insignificantly by the treatment with 10 μmol/l G-Rg1 (Fig. 1b). Moreover, cell viability detected at various time points showed that 100 μmol/l G-Rg1 rescued H/R-induced cell death in a time-dependent manner (Fig. 1c).
a H/R induces cell death in a time-dependent manner in H9c2 cells. H9c2 cells were exposed to H/R in the presence or absence of 3 μmol/l chloroquine, the lysosome inhibitor, for 1, 2, 3, and 5 h, and then subjected to immunoblotting for the detection of LC3B-1 (soluble form of LC3B) and LC3B-2 (membrane-bound form of LC3B). H9c2 cells incubated in complete DMEM medium (CM) were used as control. b MTT assay showing the viability of H9c2 cells under H/R for 5 h when treated with 0, 10, 100, and 1000 μmol/l G-Rg1. c MTT assay showing the viability of H9c2 cells under H/R for 0, 0.5, 1, 2, 3, and 5 h in the absence or presence of 100 μmol/l G-Rg1. Experiments were done in triplicate. *P < 0.05 versus 0 h
To confirm that H/R induces autophagy and cell death in H9c2 cells, we compared the ultrastructural morphology between H9c2 cells cultured in complete medium and those subjected to H/R for 3 h. Electron microscopy showed that 3 h after H/R, many small vesicles appeared in the cytoplasm, and these compartments contained membranous structures. Higher magnification showed that most membrane vesicles possessed double or multiple membrane boundaries, with mitochondria or other cellular organelles inside (Fig. 2). In addition to autophagosomes, apoptosis was observed in these cells with altered nuclear morphology, including fragmentation and cell shrinkage (Fig. 2).
Ultrastructural examination of autophagosomal formation in H9c2 cells exposed to H/R. In H9c2 cells cultured in complete medium (CM), few autophagosomes were found. In H9c2 cells exposed to H/R for 3 h, many small vesicles appeared in the cytoplasm. These compartments contained membranous structures, and cytoplasmic proteins or dysfunctional mitochondria were sequestrated in a double membrane-bound vesicle. In addition to autophagosomes, apoptosis was observed in cardiac myocytes with altered nuclear morphology. Autophagosomes are indicated by arrows
G-Rg1 inhibits H/R-induced autophagy in H9c2 cells
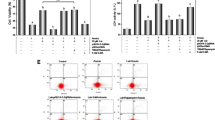
To investigate the effects of G-Rg1 on H/R-induced autophagy in H9c2, we performed western blot analysis and found that H/R-induced LC3B-2 upregulation was significantly inhibited when the cells were treated with 100 μmol/l G-Rg1 (Fig. 3a), suggesting that 100 μmol/l G-Rg1 treatment inhibited the autophagosomal formation induced by H/R in H9c2 cells. Furthermore, the inhibition of autophagosomal vesicles by G-Rg1 in H9c2 cells under H/R was morphologically confirmed by immunofluorescence staining (Fig. 3b, c). Positive fluorescence dots represented autophagosomes on which the membrane-bound LC3B-2 and caspase 9 proteins were located. Few autophagosomes were found in H9c2 cells cultured in complete medium, but the autophagosomes increased in the cells treated with H/R for 3 h. After treatment with 100 μmol/l G-Rg1 for 3 h, the autophagosomes decreased significantly in H9c2 cells exposed to H/R. The inhibitory effects of G-Rg1 on autophagosomal formation and apoptosis were even observed after the blockage of autophagosomal degradation by chloroquine.
G-Rg1 inhibits H/R-induced autophagy in H9c2 cells. H9c2 cells were subjected to H/R for 3 h in the presence or absence of 3 μmol/l chloroquine and 100 μmol/l G-Rg1. a, b Expressions of LC3B-1 and LC3B-2 were examined by immunoblotting. Measurement of the band densities of LC3B-2 and LC3B-1 demonstrated that LC3B-2/LC3B-1 ratio decreased significantly in the cells treated with 100 μmol/l G-Rg1. *P < 0.05, compared to the cells exposed to H/R for 3 h alone. The data were from at least three independent experiments. c LC3B-2 and d caspase-9 were detected by immunofluorescence staining. LC3b was stained as red and caspase-9 as green, and the nuclei were stained by DAPI as blue. Images were observed by a confocal microscope. The number of autophagosomes was less in H9c2 cells cultured in complete medium, and increased in cells exposed to H/R for 3 h. G-Rg1 led to the decrease of autophagosomes and apoptosis
G-Rg1 abrogates the depletion of cellular ATP in H9c2 cells following H/R
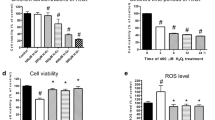
H/R for 3 h significantly depleted cellular ATP content in H9c2 cells even when the cells were treated with chloroquine to block the fusion of autophagosome-lysosome. G-Rg1 treatment largely abrogated the depletion of cellular ATP, apparently favorable to the cell survival (Fig. 4).
G-Rg1 reverses the depletion of cellular ATP in H9c2 cells exposed to H/R. H9c2 cells were exposed to H/R for 3 h in presence or absence of 3 μmol/l chloroquine and 100 μmol/l G-Rg1, and measured for cellular ATP. G-Rg1 increased ATP content in H9c2 cells exposed to H/R. Data were from 3 independent experiments. *P < 0.05 compared to cells exposed to H/R only
G-Rg1 relieves the oxidative stress induced by H/R in H9c2 cells
T-SOD catalyzes the dismutation of superoxide and is an important antioxidant mechanism. T-SOD activity was reduced in H9c2 cells exposed to H/R for 3 h, indicating the limited clearance of reactive oxygen species in these cells. G-Rg1 treatment significantly increased T-SOD activity in H9c2 cells compared to H9c2 cells untreated by G-Rg1 (Fig. 5a). MDA content in the medium is usually used as a marker to measure the level of oxidative stress in the cells. As expected, cultured media from H9c2 cells exposed to H/R for 3 h contained higher level of MDA, especially from the cells treated with chloroquine to block the fusion of autophagosome-lysosome. However, 100 μmol/l G-Rg1 significantly reduced the MDA content in medium from H9c2 cells exposed to H/R (Fig. 5b). Taken together, these data suggest that G-Rg1 relieves the oxidative stress induced by H/R in H9c2 cells, which may promote cell survival.
G-Rg1 relieves the oxidative stress in H9c2 cells exposed to H/R. H9c2 cells were exposed to H/R for 3 h in the presence or absence of 3 μmol/l chloroquine and 100 μmol/l G-Rg1. The cultured media were collected for the measurement of T-SOD activity (a) and MDA content (b). Experiments were repeated in triplicate. *P < 0.05 compared to cells exposed to H/R only
G-Rg1 inhibits the activation of AMPK in H9c2 cells exposed to H/R
The energy sensor AMP-activated protein kinase (AMPK), which is activated by ATP depletion or glucose starvation, is known to activate autophagy via the inactivation of mTOR complex-1 [12]. AMPK is regulated by the phosphorylation of the catalytic subunit AMPKα, while P70/S6K is one of the main downstream targets of mTOR. Therefore, we examined the activation of AMPK and mTOR in H9c2 cells. We found that 100 μmol/l G-Rg1 decreased p-AMPKα level but increased p-P70S6K level in H9c2 cells exposed to H/R for 2 h (Fig. 6a–d), and this was accompanied by the decreased levels of LC3B-2 and Beclin-1 (Fig. 6e–g). These data suggest that G-Rg1 inhibits H/R-induced autophagy via the regulation of AMPK/mTOR signaling.
G-Rg1 inhibits the activation of AMPK and autophagy in H9c2 cells exposed to H/R. H9c2 cells were exposed to H/R for 2 h and treated as indicated. The phosphorylation of AMPKα (a, b) and P70S6KI (c, d), and the levels of LC3B-2 (e, f) and Beclin-1 (g) were detected by immunoblotting. The specific AMPKα inhibitor compound C and mTOR inhibitor Rapamycin were used as positive control, respectively. Experiments were repeated in triplicate. *P < 0.05 compared to cells exposed to H/R only
Discussion
In this study, we used H9c2 cells exposed to H/R as an experimental model to demonstrate that H/R induced autophagy and impaired cell viability in H9c2 cells in a time-dependent manner. Moreover, we observed that 100 μmol/l G-Rg1 inhibited H/R induced-autophagy and apoptosis, and this was associated with the increase of cellular ATP content and the relief of oxidative stress in the cells. Mechanistically, we found that G-Rg1 inhibited the activation of AMPKα therefore leading to the activation of m/TOR and the inhibition of autophagy.
Accumulating evidence has indicated that autophagy is implicated in many cardiovascular diseases such as myocardial ischemia, heart hypertrophy, and cardiomyopathies [13–16]. To investigate the role of autophagy in myocardial ischemia, we treated H9c2 cardiomyocyte cells with H/R and found that the amount of LC3B-2 was markedly increased in a time-dependent manner. As LC3B-2 is produced in response to H/R and is quickly degraded in lysosomes, LC3B-2 protein levels in cells only provides the dynamic balance between the production and the degradation in lysosomes. Chloroquine is a lysosomal inhibitor that inhibits lysosome-autophagosome fusion. We therefore assayed LC3B-2 in cells incubated in medium containing chloroquine to evaluate the actual autophagy flux. The fusion processes between autophagosomes and lysosomes were blocked by chloroquine, and LC3B-2 accumulated in the cells. In this study, the amount of LC3B-2 increased in a time-dependent manner by H/R treatment, indicating the higher autophagic flux in H9c2 cells under H/R. To determine whether the active autophagy is protective or detrimental, we measured cell viability in H/R H9c2 cells. Cell viability dramatically decreased following H/R for 3 h, demonstrating that autophagy has reached to the extent beyond its capacity to protect cells from death.
Before we examined the effect of G-Rg1 on H9c2 cells, we evaluated the toxicity of G-Rg1 and chloroquine to the cells. H9c2 cells treated with 100 μmol/l G-Rg1 and 3 μmol/l chloroquine showed no changes in cell viability. We therefore used 100 μmol/l G-Rg1 to examine its effect on autophagy in H9c2 cells under H/R for 3 h. G-Rg1 treatment decreased LC3B-2 level in H9c2 cells in the presence or absence of chloroquine, indicating that G-Rg1 blocks the autophagy flux by inhibiting autophagosome formation.
To elucidate the mechanisms responsible for the protective effects of G-Rg1 on H9c2 cells under H/R, we measured the cellular ATP content and oxidative stress and found that H/R depleted cellular ATP content, increased MDA level, and decreased SOD level. Cells have evolved various defense mechanisms to cope with oxidative stress, among which autophagy plays a major role [17]. For example, autophagy induces cell death due to ROS accumulation [18]. It was shown that G-Rg1 scavenges ROS by increasing the activity of endogenous antioxidants, including T-SOD, CAT, and GSH [4]. Consistent with this, our results show that G-Rg1 decreased MDA level and increased SOD level, suggesting that G-Rg1 promotes cell survival by relieving oxidative stress and sustaining ATP production.
ATP depletion is known to activate AMPK, a major regulator of energy homeostasis [19, 20]. AMPK plays a critical role in mediating autophagy during myocardial ischemia, whereas ischemia/reperfusion stimulates autophagy through a Beclin-1-dependent but AMPK-independent mechanism [7]. Valentim et al. [16] showed that the inhibition of autophagy, by both genetic and pharmacological inhibition of Beclin-1, reduced cell death in cardiomyocytes subjected to simulated ischemia–reperfusion. Whether autophagy is protective against stress in cardiac myocytes has been controversial. Both protective and detrimental effects of autophagy have been reported, using either primary cardiac myocytes or cardiac cell lines in vitro [16, 21, 22]. In our report, we found that G-Rg1 inhibited the activation of AMPK because the phosphorylation of AMPKa was reduced under H/R condition. Moreover, we found that G-Rg1 decreased the LC3-II and Beclin-1 levels. These results suggest that autophagy plays detrimental effects in our model and G-Rg1 provides protective effects by inhibiting H/R-induced autophagy.
In summary, our results show that G-Rg1 has protective effects on cardiac myocytes exposed to H/R. We propose that H/R induces ATP depletion, which then activates AMPK and in turn promotes autophagy to impair cell survival. G-Rg1 helps to sustain cellular ATP level and prevent the activation of AMPK and autophagy. Our findings provide new insight into the cardioprotective effects of G-Rg1 and help develop new agents for the prevention and therapy of heart diseases.
References
Lü JM, Yao Q, Chen C (2009) Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol 7:293–302
Zhou W, Chai H, Lin PH (2004) Molecular mechanisms and clinical applications of ginseng root for cardiovascular disease. Med Sci Monit 10:187–192
Gillis CN (1997) Panax ginseng pharmacology: a nitric oxide link? Biochem Pharmacol 54:1–8
Zhu D, Wu L, Li CR (2009) Ginsenoside Rg1 protects rat cardiomyocyte from hypoxia/reoxygenation oxidative injury via antioxidant and intracellular calcium homeostasis. J Cell Biochem 108:117–124
Cuervo AM (2004) Autophagy: many paths to the same end. Mol Cell Biochem 263(1–2):55–72
Kabeya Y, Mizushima N, Ueno T (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19:5720–5728
Matsui Y, Takagi H, Qu XP (2007) Distinct roles of autophagy in the heart during ischemia and reperfusion : roles of AMP-activated protein kinase and Beclin-1 in mediating autophagy. Circ Res 100:914–922
Decker RS, Wildenthal K (1980) Lysosomal alterations in hypoxic and reoxygenated hearts. I. Ultrastructural and cytochemical changes. Am J Pathol 98:425–444
Hamacher-Brady A, Brady NR, Gottlieb RA (2006) The interplay between pro-death and pro-survival signaling pathways in myocardial ischemia/reperfusion injury: apoptosis meets autophagy. Cardiovasc Drugs Ther 20:445–462
Gustafsson AB, Gottlieb RA (2008) Eat your heart out: role of autophagy in myocardial ischemia/reperfusion. Autophagy 4:416–421
Wagner C, Tillack D, Simonis G (2010) Ischemic post-conditioning reduces infarct size of the in vivo rat heart: role of PI3-K, mTOR, GSK-3beta, and apoptosis. Mol Cell Biochem 339(1–2):135–147
Gwinn DM, Shackelford DB, Egan DF (2008) AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30:214–226
Gottlieb RA, Mentzer RM (2010) Autophagy during cardiac stress: joys and frustrations of autophagy. Annu Rev Physiol 72:45–59
Whelan RS, Kaplinskiy V, Kitsis RN (2010) Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol 72:19–44
Dorn GW 2nd (2009) Apoptotic and non-apoptotic programmed cardiomyocyte death in ventricular remodeling. Cardiovasc Res 81:465–473
Valentim L, Laurence KM, Townsend PA (2006) Urocortin inhibits Beclin1-mediated autophagic cell death in cardiac myocytes exposed to ischaemia/reperfusion injury. J Mol Cell Cardiol 40:846–852
Kiffin R, Christian C, Knecht E (2004) Activation of chaperone-mediated autophagy during oxidative stress. Mol Biol Cell 15:4829–4840
Yu L, Wan F, Dutta S (2006) Autophagic programmed cell death by selective catalase degradation. Proc Natl Acad Sci USA 103:4952–4957
Hardie DG (2007) AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 8:774–785
Laderoute KR, Amin K, Calaoagan JM (2006) 50-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol Cell Biol 26:5336–5347
Hamacher-Brady A, Brady NR, Gottlieb RA (2006) Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem 281:29776–29787
Aki T, Yamaguchi K, Fujimiya T (2003) Phosphoinositide 3-kinase accelerates autophagic cell death during glucose deprivation in the rat cardiomyocyte-derived cell line H9c2. Oncogene 22:8529–8535
Acknowledgment
This work was supported by National Science and Technology Major Projects for “Major New Drugs Innovation and Development” (No. 2009ZX09103-441).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, ZL., Fan, Y. & Liu, ML. Ginsenoside Rg1 inhibits autophagy in H9c2 cardiomyocytes exposed to hypoxia/reoxygenation. Mol Cell Biochem 365, 243–250 (2012). https://doi.org/10.1007/s11010-012-1265-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-012-1265-3