Abstract
In hypertensive animals and patients, oxidative stress represents the primary risk factor for progression of left ventricular hypertrophy. Recently, it has been demonstrated that hydrogen, as a novel antioxidant, can selectively reduce hydroxyl radicals and peroxynitrite anion to exert therapeutic antioxidant activity. In the current study, we explored the effect of chronic treatment with hydrogen-rich saline (HRS) on left ventricular hypertrophy in spontaneously hypertensive rats (SHR). The 8-week-old male SHR and age-matched Wistar-Kyoto rats (WKY) were randomized into HRS-treated (6 ml/kg/day for 3 months, i.p.) and vehicle-treated groups. HRS treatment had no significant effect on blood pressure, but it effectively attenuated left ventricular hypertrophy in SHR. HRS treatment abated oxidative stress, restored the activity of antioxidant enzymes including GPx, GST, catalase, and SOD, suppressed NADPH oxidase activity and downregulated Nox2 and Nox4 expression in left ventricles of SHR. HRS treatment suppressed pro-inflammatory cytokines including IL-1β, IL-6, TNF-α, and MCP-1, and inhibited NF-κB activation through preventing IκBα degradation in left ventricles of SHR. HRS treatment preserved mitochondrial function through restoring electron transport chain enzyme activity, repressing ROS formation, and enhancing ATP production in left ventricles of SHR. Moreover, HRS treatment suppressed ACE expression and locally reduced angiotensin II generation in left ventricles of SHR. In conclusion, HRS treatment attenuates left ventricular hypertrophy through abating oxidative stress, suppressing inflammatory process, preserving mitochondrial function, in which suppression of HRS on angiotensin II in left ventricles locally might be involved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypertensive patients and animals exhibit left ventricular hypertrophy [1, 2], which is a compensatory response and often results in poor clinical outcomes, including the development of cardiac systolic and diastolic dysfunction and ultimately heart failure [3–5]. Growing evidence implicates that oxidative stress produced by overproduction of reactive oxygen species/reactive nitrogen species or inefficient antioxidant defenses appears to be involved in the development of hypertension-induced left ventricular hypertrophy [6, 7]. However, clinical trials with antioxidant vitamin C and E failed to show an improved cardiovascular outcome and larger prospective randomized trials in humans looking at hard cardiovascular end-points have been disappointing [8, 9]. Does this mean that antioxidant approaches have no role in prevention or treatment in cardiovascular diseases?. In fact, it is clear that the term oxidative stress covers a diverse array of complex biological actions ranging from highly specific redox signaling involving specialized enzymes to interactions with nitric oxide to more straightforward effects such as ‘‘damage’’ to cells, membranes and macromolecules. Therefore, it is hardly surprising that the use of non-specific antioxidants did not produce the desired results. Moreover, these agents are relatively inefficient and have generally been used without any assessment of the target population for levels of oxidative stress. What may be required are much more specific and more efficient agents that can target defined ROS sources and deleterious redox-dependent signal pathways.
Recently, it has been proved that hydrogen gas, a highly flammable gas, has potent antioxidant property. Compared with normal antioxidants such as vitamin C and vitamin E, hydrogen has two unique characteristics. First, it selectively reduces the hydroxyl radical (−OH) and peroxynitrite anion (ONOO−), the most cytotoxic chemicals of ROS, and effectively protects cells; in addition, hydrogen dose not react with other ROS, which possess physiological roles [10]. Second, since the hydrogen molecule is electrically neutral and much smaller than the other antioxidants, it is able to easily penetrate membranes and enter cells and organelles, such as the nucleus and mitochondria, where most commonly used antioxidants cannot arrive [11].
It has been demonstrated that treatment with hydrogen gas or hydrogen-rich saline (HRS) protects organs damage such as transient cerebral ischemia [12], neonatal cerebral hypoxia–ischemia [13], renal injury [14] and myocardial injury induced by ischemia and reperfusion [15]. However, most of these researches focus on the effect of short-term treatment with hydrogen on acute injury. In the present study, we explored whether chronic treatment with HRS attenuated left ventricular hypertrophy in spontaneously hypertensive rats.
Methods
Animals and study design
The 8-week-old male SHR and age-matched normotensive Wistar-Kyoto rats (WKY) were obtained from the Sino-British SIPPR/BK Lab Animal Ltd. All the animals were entrained to controlled temperature (23–25°C), 12-h light and 12-h dark cycles (light 08:00–20:00 h; darkness 20:00–08:00 h), and free access to food and tap water. All the animals used in this study received humane care in compliance with institutional animal care guidelines, and were approved by the Local Institutional Committee. All the surgical and experimental procedures were in accordance with institutional animal care guidelines. Chemicals, reagents, and drugs were purchased from Sigma Chemical (St. Louis, MO, USA) unless otherwise stated.
Animals were divided into four groups and treated for 3 months as follows: (1) WKY rats treated with saline; (2) WKY rats treated with the HRS; (3) SHR treated with saline; (4) SHR treated with the HRS. 6 ml/kg of HRS or saline was intraperitoneally ( i.p.) injected daily in the morning.
Hydrogen-rich saline (HRS)
Two methods including inhalation of hydrogen gas or injection of HRS were usually utilized for treatment. Compared to hydrogen gas, HRS is safe, economical, easily available, and more convenient for long-term treatment. In current study, hydrogen was dissolved in physiological saline for 4 h under the pressure of 0.4 MPa as method described by Ohsawa et al. [10]. HRS was freshly prepared every week to insure a constant concentration more than 0.6 mM.
Blood pressure measurement
24 h after last treatment, systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) were recorded continuously as previously described [16]. In brief, rats were anesthetized with a combination of ketamine (40 mg/kg, i.p.) and diazepam (6 mg/kg, i.p.). A floating polyethylene catheter was inserted into the lower abdominal aorta via the left femoral artery for BP measurement, and another catheter was indwelled in the left femoral vein for intravenous injection. The catheters were exteriorized through the interscapular skin. After a 2-day-recovery period, the animals were placed for BP recording in individual cylindrical cages with food and water. The aortic catheter was connected to a BP transducer via a rotating swivel that allows the animals to move freely in the cage. After about 14-h habituation, the BP signals were digitized by a microcomputer, and beat-to-beat SBP, DBP, and HR values were determined on line. The mean values of these parameters during the 24 h were calculated and served as SBP, DBP, and HR for study.
Sample preparation
The animals were anesthetized and killed by decapitation. Immediately after decapitation, a 2-ml blood sample was collected and heart was immediately excised and rinsed in cold physiological saline.
Assessment of left ventricular hypertrophy
Body and left ventricular weights were recorded. Transversely sectioned left ventricle frozen tissue sections (6 μm) were stained with Alexa Fluor® 594 wheat germ agglutinin and blue-fluorescent Hoechst 33342 dye (Invitrogen, Carlsbad, CA, USA). Four radially oriented microscopic fields from each section were photographed and the cross-sectional area of at least 100 cells, in which the nucleus and a clear staining of the plasma membrane could be visualized, were averaged. The myocyte outlines were traced, and the cell areas were measured using “lasso” tool in Adobe Photoshop.
Measurement of left ventricular and serum malondialdehyde (MDA)
MDA concentration is a presumptive marker of oxidant-mediated lipid peroxidation. Left ventricular homogenates and serum were used for the determination of MDA using a kit (Cayman, Ann Arbor, USA).
Western blotting analysis
The protein concentration was determined with bovine serum albumin as a standard by a Bradford assay. Equal amounts of protein preparations (10 μg in 10 μl buffer) were run on SDS-polyacrylamide gels, electrotransferred to polyvinylidine difluoride membranes, and blotted with a primary antibody against Nox2 (1:1000, Abcam, Cambridge, UK), Nox4 (1:500, Abcam), ACE (1:500, Abcam) overnight at 4°C using slow rocking. Then, they were blotted with HRP-conjugated secondary antibody (1:5000) and HRP-conjugated monoclonal antibody against GAPDH (1:10000). Immunoreactive bands were detected by a chemiluminescent reaction (ECL kit, Amersham Pharmacia), and results were expressed as the ratio of the density of specific bands to the corresponding GAPDH. The WKY group was used as the calibrator with a given value of 100%, and the other groups were compared with this calibrator.
NADPH oxidase activity
Lucigenin-enhanced chemiluminescence was used to measure NADPH oxidase activity in left ventricles according to the method described previously [17].
Determination of SOD activity, catalase activity, glutathione-S-epoxide transferase (GST) activity, and glutathione peroxidase (GPx) activity in left ventricles
SOD activity was measured using an SOD-525TM reagent kit (OXIS International, Foster, CA, USA). Catalase activity was measured by the method of Beers and Sizer, as previously described [18]. GST activity was measured using GST Fluorometric Activity Assay Kit (BioVision, Mountain View, CA, USA). GPx activity was determined in left ventricular homogenates using a commercially available kit, according to the manufacturer’s protocol (Cayman Chemicals, Ann Arbor, MI, USA). The final results were corrected for protein content.
Total ROS and OONO− production in left ventricles
Total ROS and OONO− productions were detected as per the method described by Elks et al. [19].
Measurement of mitochondrial ATP production and ROS formation
Mitochondria were isolated by differential centrifugation of left ventricular homogenates. Mitochondrial protein concentration was determined using a DC Protein Assay Kit (Bio-Rad, Hercules, CA, USA). Rates of ATP formation were quantified using a commercially available kit (BioVision, Mountain View, CA, USA). Mitochondrial ROS production was evaluated by lucigenin chemiluminescence. The results were corrected for protein content.
Measurement of mitochondrial electron transport chain enzyme activity
The activity of rotenone-sensitive complex I, malonate-sensitive complex II, antimycin A-sensitive complex III, and sodium cyanide-sensitive complex IV, as well as reduced nicotinamide-adenine dinucleotide cytochrome c reductase (NCCR; marker for electron coupling capacity between complexes I and III) or succinate cytochrome c reductase (SCCR; marker for electron coupling capacity between complexes II and III), were assayed [20] using a thermostatically regulated Thermo-Spectronic spectrophotometer (Fisher Scientific, CA, USA).
ANG II measurement in plasma and left ventricles
Plasma and left ventricular samples were used for the measurement of ANGII using radioimmunoassay kits (China Institute of Atomic Energy, Beijing, China).
Quantitative real-time PCR analysis (RT-PCR)
Total RNA was extracted from left ventricles using TRIzol (Life Technologies Inc., Gaithersburg, USA) according to the manufacturer’s protocol. Real-time PCR analysis was performed with a QuantiTectTM SYBR® Green PCR (Tiangen, Shanghai, China) according to the manufacturer’s instructions. The sequences of primers are listed in Table 1. The highly specific measurement of mRNA was carried out for TNF-α, IL-6, IL-1β, IκBα, NF-κB p65 and GAPDH using the LightCycler system (Bio-Rad, Carlsbad, USA). TNF-α, IL-6, IL-1β, IκBα, and NF-κB p65 mRNA levels were adjusted as the values relative to GAPDH, which was used as the endogenous control to insure equal starting amounts of cDNA. The WKY untreated by HRS was used as the calibrator with a given value of 1, and the other groups were compared with this calibrator.
Measurement of MCP-1 in left ventricles
The contents of MCP-1 in left ventricular homogenates with equal amounts of total protein were determined using a specific sandwich enzyme immunometric assay kit for rat MCP-1 (Assay Design, Ann Arbor, MI). All the samples were assayed in duplicate according to the manufacturer’s instructions. The final result was corrected for protein content.
Statistical analysis
All data are presented as mean ± standard deviations. Comparison between groups was analyzed by two-way ANOVA. A probability level of less than 0.05 was considered significant.
Results
Hemodynamic parameters
SHR exhibited higher SBP, DBP, and HR compared with WKY (Table 2). Treatment with HRS had no significant effect on DBP and HR in both strains. Treatment with HRS decreased SBP in SHR, but not significantly.
Left ventricular hypertrophy
LVW-to-BW ratio in SHR was higher than it in WKY, indicating that SHR developed left ventricular hypertrophy (Fig. 1a). Average cardiomyocyte area of left ventricles of SHR was larger than that of WKY, revealing that cardiomyocyte hypertrophied in SHR (Fig. 1b). Treatment with HRS reduced LVW-to-BW ratio and cardiomyocyte area, indicating that HRS treatment attenuated left ventricular hypertrophy in SHR.
Oxidative stress
Compared with WKY, serum MDA content (Fig. 2a), and left ventricular MDA content (Fig. 2b), ROS (Fig. 2c) and OONO− (Fig. 2d) formation were higher in SHR, which were reversed after treatment with HRS.
Effect of HRS on oxidative stress. Column graphs show serum MDA (a), left ventricular MDA (b), ROS formation (c), and OONO− formation (d). n = 11 in each group. LV left ventricular; MDA malondialdehyde; ROS reactive oxygen species; OONO − peroxynitrite; HRS hydrogen-rich saline; Values are means ± SD. *P < 0.05 versus WKY; # P < 0.05 versus SHR
Antioxidant enzymes
Compared with WKY, left ventricular GPx (Fig. 3a), GST (Fig. 3b), catalase (Fig. 3c), and SOD (Fig. 3d) activities were lower in SHR. Treatment with HRS restored them, at least in part.
Effect of HRS on antioxidant enzymes in left ventricles. Column graphs show GPx (a), GST (b), catalase (c), and SOD (d) activities in left ventricles. n = 11 in each group. SOD superoxide dismutase; GST glutathione S epoxide transferase; GPx glutathione peroxidase; HRS hydrogen-rich saline; Values are means ± SD. *P < 0.05 versus WKY; # P < 0.05 versus SHR
NADPH oxidase
Compared with WKY, left ventricular NADPH oxidase activity (Fig. 4a) was increased in SHR. Furthermore, the Nox2 and Nox4 expression levels (Fig. 4b, c) in left ventricles in SHR were higher than in WKY. Treatment with HRS in SHR suppressed NADPH oxidase activity and decreased Nox2 expression, but had no significant effect on Nox4 expression.
Effects of HRS on NADPH oxidase activity and expression in left ventricles. Lucigenin-enhanced chemiluminescence result on enzyme activity of NADPH oxidase (a). n = 11 in each group. Western blot results (b) and responding quantification (c) of Nox2 and Nox4. The WKY group was used as the calibrator with a given value of 100%, and the other groups were compared with this calibrator. HRS hydrogen-rich saline; Values are means ± SD. *P < 0.05 versus WKY; # P < 0.05 versus SHR
Mitochondrial function in left ventricles
Compared to WKY, activities of complex I (Fig. 5a) and III (Fig. 5c), but not complex II (Fig. 5b) or IV (Fig. 5d) were significantly lower in left ventricles of SHR. There was also a significant decline in the electron-coupling capacity between complexes I and III (Fig. 5e) or between complexes II and III (Fig. 5f) in SHR, as demonstrated by the reduced activity of NCCR (Fig. 5g) or SCCR (Fig. 5h). In addition, mitochondria in left ventricles of SHR exhibited higher ROS formation and lower ATP formation. Treatment with HRS restored activities of complex I and III and electron-coupling capacity between complexes I and III and between complexes II and III, suppressed ROS formation, and increased ATP formation.
Effect of HRS on mitochondrial function in left ventricles. Enzyme activities of complexes I to IV of mitochondrial electron transport chain (a–d) or electron coupling capacity between complexes I and II or complexes II and III, as denoted by the activity of NCCR or SCCR (e, f) and mitochondrial ROS formation (g) and ATP formation (h) in left ventricles. Values are means ± SD. n = 11 in each group. HRS hydrogen-rich saline; SCCR ; NCCR nicotinamide-adenine dinucleotide cytochrome c reductase; *P < 0.05 versus WKY; # P < 0.05 versus SHR
Pro-inflammatory cytokines in left ventricles
Compared with WKY, IL-1β (Fig. 6a), IL-6 (Fig. 6b), TNF-α (Fig. 6c) mRNA levels, and MCP-1 (Fig. 6d) content in left ventricles were higher in SHR, which were reversed after treatment with HRS.
Effect of HRS on pro-inflammatory cytokines in left ventricles. RT-PCR results on IL-1β (a), IL-6 (b), and TNF-α (c) mRNA expression, and MCP-1 concentration (d) in left ventricles. Values are means ± SD. n = 11 in each group. HRS hydrogen-rich saline; LV left ventricular; MCP-1 macrophage chemoattractant protein 1; *P < 0.05 versus WKY; # P < 0.05 versus SHR
NF-κB pathway
Compared with WKY, IκBα (Fig. 7a) mRNA level was decreased, while NF-κB p65 (Fig. 7b) mRNA level was increased in left ventricles of SHR. Furthermore, NF-κB p65 activity (Fig. 7c) in left ventricles of SHR was higher than that of WKY. Treatment with HRS restored them in left ventricles of SHR.
Effect of HRS on NF-κB pathway in left ventricles. RT-PCR results on IκBα (a) and NF-κB p65 (b) mRNA expression in left ventricles and NF-κB activity (c) in nuclear extract of the left ventricles. HRS hydrogen-rich saline; RT-PCR reverse transcription polymerase chain reaction; NF-κB nuclear factor-kappa B; Values are means ± SD. n = 11 in each group. *P < 0.05 versus WKY; # P < 0.05 versus SHR
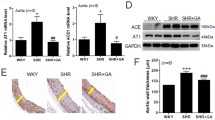
Angiotensin II (Ang II)
Compared with WKY, Ang II levels of plasma (Fig. 8a) and left ventricles (Fig. 8b) were higher in SHR. Furthermore, left ventricular ACE expression (Fig. 8c, d) was higher in SHR than in WKY. Treatment with HRS decreased ACE expression and Ang II level in left ventricles, but had no significant effect on Ang II level of plasma.
Effects of HRS on Ang II formation and ACE expression. Radioimmunoassay results on plasma (a) and left ventricular (b) Ang II concentration. Western blot results (c) and
corresponding quantification (d) of ACE. The WKY group was used as the calibrator with a given value of 100%, and the other groups were compared with this calibrator. HRS hydrogen-rich saline; Ang II angiotensin II; ACE angiotensin converting enzyme; Values are means ± SD. n = 11 in each group. *P < 0.05 versus WKY; # P < 0.05 versus SHR
Discussion
In the current study, we examined the effects of long-term treatment with HRS on left ventricular hypertrophy in SHR. The salient findings of present study were summarized as follows: (1) Treatment with HRS alleviated left ventricular hypertrophy in SHR without a reduction of blood pressure level. (2) Treatment with HRS abated oxidative stress through upregulating activities of anti-oxidant enzymes and suppressing NADPH oxidase activity and mitochondrial ROS formation. (4) Treatment with HRS suppressed inflammation through decreasing pro-inflammatory molecules including IL-6, TNF-α, IL-1β, and MCP-1 in left ventricles of SHR. (5) Treatment with HRS exerted inhibitory effects on NF-κB by impeding IκBα degradation in left ventricles of SHR. (6) Treatment with HRS decreased left ventricular Ang II formation through suppressing ACE expression in SHR, but had no significant effect on circulating Ang II.
Oxidative stress plays an important role in the pathogenesis of left ventricular hypertrophy [21, 22]. In the present study, it was found that hydrogen not only neutralized with the toxic ROS marked by decreased OONO−, but also influenced the antioxidant systems and pro-oxidant enzymes. It restored the activities of GST, GPx, SOD, and catalase, and suppressed the ROS formation derived from NADPH oxidase in left ventricles of SHR. In addition, HRS treatment exerted beneficial effect on mitochondrial function. Recently, increasing evidence linked mitochondrial dysfunction and mitochondria-derived ROS to the pathogenesis of cardiovascular diseases [23–25]. Here, we found that HRS treatment restored mitochondrial electron transport chain enzyme activity, suppressed mitochondrial ROS formation, and enhanced ATP formation in left ventricles of SHR. The protective effect of HRS on mitochondria might be due to its effect on NADPH oxidase. Several studies have revealed that NADPH oxidase-derived ROS lie upstream of mitochondria-produced ROS, and after mitochondrial dysfunction [26, 27]. However, since the hydrogen molecule is electrically neutral and much smaller than the other antioxidants, it is able to easily penetrate membranes and enter mitochondria to neutralize the excessive ROS. The unique property of hydrogen might also contribute to its protective effect on mitochondria.
Apart from antioxidant property, hydrogen exerted anti-inflammatory property in this study, which was also observed in several previous studies [28, 29]. Inflammation also plays a pivotal role in the progression of hypertensive left ventricular hypertrophy [30]. Here, we found that treatment with HRS suppressed pro-inflammatory cytokines including IL-6, TNF-α, IL-1β, and MCP-1 in left ventricles of SHR. The anti-inflammatory effect of hydrogen might be secondary to its anti-oxidative effect. Excess ROS activate the redox-sensitive transcription factor NF-κB, resulting in enhancement of its expression and activity [31, 32]. Increased expression and activity of NF-κB induces gene transcription of pro-inflammatory cytokines, such as IL-6 and IL-1β, to increase their production [31, 33]. In this study, left ventricular NF-κB activation in SHR was inhibited by treatment with HRS, which might interpret the anti-inflammatory property of HRS, at least in part.
In the current study, it was first observed that HRS treatment in SHR locally suppressed Ang II generation in left ventricles of SHR. Many reports have demonstrated important roles of Ang II in the cardiovascular system under pathological conditions, such as hypertension and heart failure [34, 35]. Ang II was implicated in the development of cardiomyocyte hypertrophy in humans as well as in animal models [36, 37]. Moreover, Ang II could induce ROS formation derived from NADPH oxdase, which in turn induced mitochondrial ROS formation and mitochondrial dysfunction [26]. Ang II is also capable of inducing an inflammatory response in the cardiac tissue through the activation of NF-κB [38, 39]. In the current study, HRS treatment suppressed Ang II generation through down-regulating ACE expression in left ventricles of SHR, which interpreted why HRS treatment could suppress NADPH oxidase, preserve mitochondrial function, and inhibit inflammation in left ventricles of SHR, at least in part.
Conclusion
Chronic treatment with HRS attenuates left ventricular hypertrophy in SHR, at least in part, because of its antioxidant, mitochondria-protective, and anti-inflammatory properties.
References
Paolisso G, Galzerano D, Gambardella A, Varricchio G, Saccomanno F, D’Amore A, Varricchio M, D’Onofrio F (1995) Left ventricular hypertrophy is associated with a stronger impairment of non-oxidative glucose metabolism in hypertensive patients. Eur J Clin Invest 25:529–533
Li L, Yi-Ming W, Li ZZ, Zhao L, Yu YS, Li DJ, Xia CY, Liu JG, Su DF (2008) Local RAS and inflammatory factors are involved in cardiovascular hypertrophy in spontaneously hypertensive rats. Pharmacol Res 58:196–201
Kass DA, Bronzwaer JG, Paulus WJ (2004) What mechanisms underlie diastolic dysfunction in heart failure? Circ Res 94:1533–1542
Satoh M, Ogita H, Takeshita K, Mukai Y, Kwiatkowski DJ, Liao JK (2006) Requirement of Rac1 in the development of cardiac hypertrophy. Proc Natl Acad Sci USA 103:7432–7437
Nadal-Ginard B, Kajstura J, Leri A, Anversa P (2003) Myocyte death, growth, and regeneration in cardiac hypertrophy and failure. Circ Res 92:139–150
Giordano FJ (2005) Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest 115:500–508
Alvarez MC, Caldiz C, Fantinelli JC, Garciarena CD, Console GM, Chiappe de Cingolani GE, Mosca SM (2008) Is cardiac hypertrophy in spontaneously hypertensive rats the cause or the consequence of oxidative stress? Hypertens Res 31:1465–7146
The Heart Outcomes Prevention Evaluation Study Investigators (2000) Vitamin E supplementation and cardiovascular events in high-risk patients. N Engl J Med 342:154–160
Heart Protection Study Collaborative G (2002) MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20536 high-risk individuals: a randomised placebo-controlled trial. Lancet 360:23–33
Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S (2007) Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med 13:688–694
Maher P, Salgado KF, Zivin JA, Lapchak PA (2007) A novel approach to screening for new neuroprotective compounds for the treatment of stroke. Brain Res 1173:117–125
Ji Q, Hui K, Zhang L, Sun X, Li W, Duan M (2011) The effect of hydrogen-rich saline on the brain of rats with transient ischemia. J Surg Res 168:e95–e101
Ji X, Liu W, Xie K, Liu W, Qu Y, Chao X, Chen T, Zhou J, Fei Z (2010) Beneficial effects of hydrogen gas in a rat model of traumatic brain injury via reducing oxidative stress. Brain Res 1354:196–205
Wang F, Yu G, Liu SY, Li JB, Wang JF, Bo LL, Qian LR, Sun XJ, Deng XM (2011) Hydrogen-rich saline protects against renal ischemia/reperfusion injury in rats. J Surg Res 167:e339–e344
Hayashida K, Sano M, Ohsawa I, Shinmura K, Tamaki K, Kimura K, Endo J, Katayama T, Kawamura A, Kohsaka S, Makino S, Ohta S, Ogawa S, Fukuda K (2008) Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem Biophys Res Commun 373:30–35
Yu YS, Xie HH, Li L, Song SW, Han P, Cai GJ, Su DF (2010) Effects of low-dose ketanserin on atherosclerosis in rats and rabbits. Can J Physiol Pharmacol 88:1054–1060
Li YL, Gao L, Zucker IH, Schultz HD (2007) NADPH oxidase-derived superoxide anion mediates angiotensin II-enhanced carotid body chemoreceptor sensitivity in heart failure rabbits. Cardiovasc Res 75:546–554
Harrison DG, Gongora MC, Guzik TJ, Widder J (2007) Oxidative stress and hypertension. J Am Soc Hypertens 1:30–44
Elks CM, Mariappan N, Haque M, Guggilam A, Majid DS, Francis J (2009) Chronic NF-κB blockade reduces cytosolic and mitochondrial oxidative stress and attenuates renal injury and hypertension in SHR. Am J Physiol Renal Physiol 296:F298–F305
Maher P, Salgado KF, Zivin JA, Lapchak PA (2007) A novel approach to screening for new neuroprotective compounds for the treatment of stroke. Brain Res 1173:117–125
Seddon M, Looi YH, Shah AM (2007) Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart 93:903–907
Takimoto E, Kass DA (2007) Role of Oxidative stress in cardiac hypertrophy and remodeling. Hypertension 49:241–248
Zimmerman MC, Zucker IH (2009) Mitochondrial dysfunction and mitochondrial-produced reactive oxygen species. Hypertension 53:112–114
Chan SH, Wu KL, Chang AY, Tai MH, Chan JY (2009) Oxidative impairment of mitochondrial electron transport chain complexes in rostral ventrolateral medulla contributes to neurogenic hypertension. Hypertension 53:217–227
Thomas SR, Witting PK, Drummond GR (2008) Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal 10:1713–1765
Doughan AK, Harrison DG, Dikalov SI (2008) Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 102:488–496
de Cavanagh EM, Piotrkowski B, Basso N, Stella I, Inserra F, Ferder L, Fraga CG (2003) Enalapril and losartan attenuate mitochondrial dysfunction in aged rats. FASEB J 17:1096–1098
Zhang Y, Sun Q, He B, Xiao J, Wang Z, Sun X (2011) Anti-inflammatory effect of hydrogen-rich saline in a rat model of regional myocardial ischemia and reperfusion. Int J Cardiol 148:91–95
Wang C, Li J, Liu Q, Yang R, Zhang JH, Cao YP, Sun XJ (2011) Hydrogen-rich saline reduces oxidative stress and inflammation by inhibit of JNK and NF-κB activation in a rat model of amyloid-beta-induced Alzheimer’s disease. Neurosci Lett 491:127–132
Kai H, Kuwahara F, Tokuda K, Imaizumi T (2006) Perivascular inflammation and hypertensive cardioavscular remodeling. Curr Hypertens Rev 2:263–269
Janssen-Heininger YMW, Poynter ME, Baeuerle PA (2000) Recent advances towards understanding redox mechanisms in the activation of nuclear factor κB. Free Radic Biol Med 28:1317–1327
Schreck R, Rieber P, Baeuerle PA (1991) Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-κB transcription factor and HIV-1. EMBO J 10:2247–2258
Sanlioglu S, Williams CM, Samavati L, Butler NS, Wang G, McCray PB, Ritchie TC, Hunninghake GW, Zandi E, Engelhardt JF (2001) Lipopolysaccharide induces Rac1-dependent reactive oxygen species formation and coordinates tumor necrosis factor-alpha secretion through IKK regulation of NF-kappa B. J Biol Chem 276:30188–30198
Daemen MJ, Lombardi DM, Bosman FT, Schwartz SM (1991) Angiotensin II induces smooth muscle proliferation in the normal and injured rat arterial wall. Circ Res 68:450–456
Baker KM, Booz GW, Dostal DE (1992) Cardiac action of angiotensin II: role of an intracardiac renin-angiotensin system. Annu Rev Physiol 54:227–241
Sadoshima J, Xu Y, Slayter HS, Izumo S (1993) Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell 75:977–984
Weber KT (1997) Extracellular matrix remodeling in heart failure: a role for de novo angiotensin II generation. Circulation 96:4065–4082
Brasier AR, Jamaluddin M, Han Y, Patterson C, Runge MS (2000) Angiotensin II induces gene transcription through cell-type-dependent effects on the nuclear factor-kappaB (NF-kappaB) transcription factor. Mol Cell Biochem 212:155–169
Takemoto M, Egashira K, Tomita H, Usui M, Okamoto H, Kitabatake A, Shimokawa H, Sueishi K, Takeshita A (1997) Chronic angiotensin-converting enzyme inhibition and angiotensin II type 1 receptor blockade: effects on cardiovascular remodeling in rats induced by the long-term blockade of nitric oxide synthesis. Hypertension 30:1621–1627
Author information
Authors and Affiliations
Corresponding author
Additional information
Yong-Sheng Yu and Hao Zheng contributed equally to the work.
Rights and permissions
About this article
Cite this article
Yu, YS., Zheng, H. Chronic hydrogen-rich saline treatment reduces oxidative stress and attenuates left ventricular hypertrophy in spontaneous hypertensive rats. Mol Cell Biochem 365, 233–242 (2012). https://doi.org/10.1007/s11010-012-1264-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-012-1264-4