Abstract
Endothelial dysfunction and increased blood pressure following insulin resistance play an important role in the development of secondary cardiovascular complications. The presence of testosterone is essential for the development of endothelial dysfunction and increased blood pressure. Testosterone regulates the synthesis of vasoconstrictor eicosanoids such as 20-hydroxyeicosatetranoic acid (20-HETE). In a series of studies, we examined: (1) the role of the androgen receptor in elevating blood pressure and (2) the effects of Cyp4A-catalyzed 20-HETE synthesis on vascular reactivity and blood pressure in fructose-fed rats. In the first study, intact and castrated male rats were made insulin resistant by feeding fructose for 9 weeks following which their superior mesenteric arteries (SMA) were isolated and examined for changes in endothelium-dependent relaxation in the presence and absence of 1-aminobenzotriazole (ABT) and N-methylsulfonyl-12,12-dibromododec-11-enamide (DDMS), which are inhibitors of 20-HETE synthesis. In another study, male rats were treated with either ABT or the androgen receptor blocker, flutamide, following which changes in insulin sensitivity, blood pressure, and vascular Cyp4A expression were measured. In the final study, HET0016, which is a more selective inhibitor of 20-HETE synthesis, was used to confirm our earlier findings. Treatment with HET0016 or ABT prevented or ameliorated the increase in blood pressure. Gonadectomy or flutamide prevented the increase in both the Cyp4A and blood pressure. Furthermore, both ABT and DDMS improved relaxation only in the intact fructose-fed rats. Taken together our results suggest that in the presence of testosterone, the Cyp4A/20-HETE system plays a key role in elevating the blood pressure secondary to insulin resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypertension and impaired endothelial relaxation are secondary complications associated with insulin resistance [1]. In the long term, these factors play key roles in the increased incidences of mortality and morbidity associated with the metabolic syndrome. Insulin resistance impairs vascular endothelium-dependent relaxation, which contributes to increasing the blood pressure in both humans and rodents [1]. The attenuated endothelial relaxation in fructose-fed rats is caused by impaired endothelial nitric oxide [1, 2] and endothelium-derived hyperpolarizing factor (EDHF) [3] signaling. Differences in gender and sex hormones play a key role in regulating endothelium-dependent vascular homeostasis and blood pressure. The loss of testosterone by castration [1, 4, 5] or by chemically blocking the androgen receptor [6] prevented endothelial dysfunction and hypertension.
Testosterone has been reported to regulate the synthesis and actions of several vasoconstrictor systems such as the renin–angiotensin system (RAS) in various models of hypertension such as the spontaneously hypertensive and the Dahl salt sensitive rats [7, 8]. Testosterone also influences the synthesis of several arachidonic acid metabolites, which constrict blood vessels and present potential pathways in the induction of endothelial dysfunction and hypertension [9, 10].
The cyclooxygenase-2/thromboxane A2 (COX-2/TXA2) system plays an important role in the development of vascular abnormalities and hypertension secondary to insulin resistance [11–13]. Both insulin resistance and testosterone upregulated the COX-2-dependent responses to phenylephrine along with COX-2 expression, at the protein [12] and mRNA levels, [4] in the thoracic aorta of male fructose-fed rats in addition to increasing the participation of COX-2 in mediating responses to phenylephrine [14].
The second vasoactive cascade involving arachidonic acid as the starting substrate involves metabolism of arachidonic acid by monooxygenases belonging to the cytochrome P450 (Cyp) family [15]. Androgens upregulate Cyp4A expression, which catalyzes the synthesis of the prohypertensive agent, 20-hydroxyeicosatertanoic acid (20-HETE) [16–18]. 20-HETE blocks the calcium-activated potassium (KCa) channels, which mediate hyperpolarization [19]. Recently, Cheng et al. [20] have reported that 20-HETE uncouples eNOS in endothelial cell cultures. Additionally, other papers have also indicated the counteracting effects of 20-HETE and nitric oxide on vascular tone [21, 22]. The attenuation of endothelial relaxation and subsequent increase in intracellular calcium in the vascular smooth muscle may explain the contribution of 20-HETE to impaired relaxation. Several endogenous vasoconstrictors such as norepinephrine and angiotensin-2, whose responses are altered in insulin resistance, recruit 20-HETE as a downstream target [23, 24]. However, the role of 20-HETE in insulin resistance is unclear. The effects of insulin on Cyp4A activity and thereby 20-HETE synthesis vary depending on the organ. Diabetes increases Cyp4A activity in the liver [25, 26], while it is reduced in the kidney [27]. Studies on a high fat fed rat model show decreased 20-HETE levels in the kidney despite obesity and an increase in blood pressure [28, 29]. Although studies in human obese subjects have attempted to ascertain the association between circulating insulin and 20-HETE levels, the findings were inconclusive [30, 31]. In experimental models of hypertension such as the spontaneously hypertensive rat (SHR), stroke-prone SHR [32], and in androgen-infused rats, 20-HETE is elevated [17]. We hypothesized that in the presence of testosterone, insulin resistance increases Cyp4A expression and therefore 20-HETE synthesis in the mesenteric vasculature, which contributes to endothelial dysfunction and hypertension.
Materials and methods
Animals
The fructose hypertensive rat model was used in the studies outlined below. Six-week-old male Wistar rats were obtained from Charles River, Montreal, Canada. In separate studies 1a, 1b, and 1c, half of the rats were castrated surgically at 5 weeks of age, prior to shipment. The rats were cared for as per the guidelines outlined by the Canadian Council on Animal care (CCAC) and the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Subsequent to arriving, the rats were acclimatized in the animal facility at the Faculty of Pharmaceutical Sciences, University of British Columbia, for 1 week prior to treatment. The starch present in normal laboratory rat chow was replaced with 60% fructose, which was obtained commercially as a pre-formulated diet (Teklad Labs, Madison, WI). Feeding this fructose-enriched diet has been shown in previous studies to induce insulin resistance, hyperinsulinemia, hypertriglyceridemia, and an increase in the systolic blood pressure by 15–30 mm Hg, which is independent of obesity [1, 33].
Study design
Study 1
In three separate studies 1a, 1b, and 1c we used the same experimental design to answer different questions. In each study, 6-week-old intact and castrated rats were divided into four groups of six rats each (intact control (C), intact fructose-fed (F), gonadectomized control (G), and gonadectomized fructose-fed (GF)) and were fed with either standard rodent chow or 60% fructose diet for 9 weeks. Rats were matched for age with identical housing, husbandry, and experimental conditions. Following measurement of blood pressure and confirmation of impaired insulin sensitivity, the superior mesenteric arteries were isolated from euthanized rats and evaluated for changes in endothelium-dependent relaxation to increasing concentrations of acetylcholine (ACh; 10−9–10−4 mol/l).
In study 1a, subsequent to determining baseline relaxation to ACh, the SMA was treated with the 20-HETE synthesis inhibitor 1-aminobenzotriazole (ABT; 10−5 mol/l).
In study 1b, the SMA was incubated with the selective 20-HETE synthesis inhibitor N-methylsulfonyl-12, 12-dibromododec-11-enamide (DDMS; 10−5 mol/l, Cayman Chemicals, MI), following which changes in relaxation to ACh were examined. In both the studies, 1a and 1b, following the establishment of functional endothelium, the tissues were equilibrated and incubated with ABT or DDMS for 20 min after which the tissues were contracted with the ED70 dose of PE and changes in relaxation to increasing concentrations of ACh were measured.
In study 1c, the SMA was homogenized and changes in Cyp4A expression were determined by western blotting as described below.
Study 2
To determine the involvement of the androgen receptor and 20-HETE in elevating blood pressure, 6-week-old male Wistar rats (210–240 g) were divided into six groups of 12 rats (control (C), fructose-fed (F), control + flutamide (anti-androgen) (CF), fructose + flutamide (FF), control + 1-aminobenzotriazole (ABT; Cyp4A inhibitor) (CA) and fructose + ABT (FA)). Following determination of baseline systolic blood pressure, the animals were kept on either 60% fructose diet or normal rat chow for 9 weeks after which blood pressure was again measured and treatment regimes were started. Drug treatment was based on data obtained from the literature. Two groups (CF and FF) were injected subcutaneously (s.c.) with a suspension of flutamide in castor oil (8 mg/kg/day) [6], and two groups (CA and FA) were injected intraperitoneally (i.p.) with ABT (25 mg/kg/day) [34]. At the end of 3 weeks of drug treatment, changes in blood pressure and insulin sensitivity index were measured and the animals were sacrificed. Blood was collected at termination by cardiac puncture to obtain plasma for measuring testosterone levels.
Study 3
Since ABT is a non-selective inhibitor of Cyp action, we chose to obtain further evidence with a more potent and Cyp4A selective inhibitor. We used HET0016, which is the most selective inhibitor of Cyp4A and 20-HETE synthesis [35–37] as compared to ABT and DDMS. Thirty-two rats were divided into four groups of eight rats (control + chow-fed (C), control + fructose-fed (F), control + HET0016 (CH) and fructose-fed + HEET0016 (FH). Rats were fed with the normal or fructose 60% chow for 9–11 weeks to induce insulin resistance. Following 8 weeks of fructose feeding, rats in the CH and FH groups were treated with HET0016 (10 mg/kg/day, i.p. injection) [38, 39] for 2 weeks until termination. Induction of insulin resistance was confirmed at the end of 9 weeks (post 1 week of HET0016 treatment). Systolic blood pressure was measured prior to and following 9 weeks of fructose feeding. Rats were sacrificed by an overdose of pentobarbital (60 mg/kg, i.p.; Euthanyl™). Blood was collected by cardiac puncture for measuring 20-HETE.
Blood pressure
Systolic blood pressure was measured in conscious rats using the tail cuff method as previously reported [11, 40].
Insulin sensitivity
Following an overnight fast, rats were challenged with a 1 g/kg oral gavage dose of a 40% glucose solution. Blood was collected immediately prior to and at 10, 20, 30, 60, and 90 min following the glucose load for measuring plasma glucose and insulin. Using the formula developed by Matsuda and DeFronzo [41], we determined the changes in insulin sensitivity index.
Cyp4A1, 2/3 expression
The mesenteric bed was used in study 3, while SMA was used in study 1c and study 2 for measuring changes in the tissue Cyp4A protein levels (arachidonic acid ω-hydroxylase) by western blotting. A rabbit polyclonal antibody against Cyp4A1, 2/3 was used in our studies (Acris, Herford, Germany) [42]. As the initial western blots were inconclusive due to the high noise and non-specific binding of the antibody, we switched to measuring protein expression in immunoprecipitated samples. Briefly, 500 μl of 1μg/μl protein lysate was prepared with RIPA buffer to which 1.1 μl of antibody was added and rotated overnight at 4°C. The next day 20 μl of protein A Sepharose (Sigma, MO) slurry was added and rotated for 1.5 h at 4°C. The suspension was centrifuged and washed twice with RIPA buffer. After discarding the supernatant, the final pellet was resuspended in 60 μl of resuspension buffer. 20 μl of sample loading buffer was added and boiled at 95°C for 5 min. The immunoprecipitated samples thus obtained were separated on 7.5% SDS-PAGE gels and transferred to PVDF membranes. Subsequent to blocking with 5% non-fat milk in TBS, the membranes were incubated with 1:3000 dilution of antibody in 5% BSA. Following washes in TBS-T, the membranes were incubated with HRP conjugated anti rabbit secondary antibody and then developed with ECL detection reagent (GE Life Sciences).
Plasma
Blood was collected by cardiac puncture into polypropylene tubes containing 20 IU heparin (0.2 ml of 100 units/ml strength). The blood was centrifuged at 4500 rpm, 4°C in a Beckman Tabletop centrifuge for 25 min. The plasma obtained was then aliquoted and stored at −70°C until further use.
Biochemical parameters
Plasma glucose was estimated with an automatic Beckman Glucose Analyzer II. Plasma insulin was measured using commercially available radioimmunoassay kits from Linco Diagnostics Inc, USA.
Drugs
HET0016 was obtained as a gift sample from Taisho Pharma, Japan and prepared as a suspension in lecithin as advised by the company. Briefly, the drug was added to a 20% lecithin suspension, warmed to 70°C and sonicated in an ice bath. The resulting suspension was injected i.p. at a dose of 10 mg/kg daily. Both flutamide and ABT were purchased from Sigma. All other chemicals, unless specified, were obtained from Sigma Chemical Co. (MO).
Statistical analyses
All data are presented as mean ± the standard error of the mean (SEM). In studies involving multiple time points, intergroup comparisons of the dependent variables in each study were performed by general linear model analysis of variance (GLM-ANOVA). The Newman–Keuls multiple comparison test was used for post hoc comparisons upon detection of difference in the means. All statistical analyses were performed using the number cruncher statistical system (NCSS) software package. Mean values were considered significant at P < 0.05.
Results
Study 1a
Insulin sensitivity and blood pressure
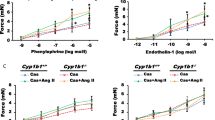
Fructose feeding decreased the insulin sensitivity and elevated the blood pressure in the sham-operated rats. Gonadectomy prevented the increase in blood pressure but not the decrease in insulin sensitivity. These results have been previously reported as part of Vasudevan et al. [5]. Endothelial relaxation was attenuated in the SMA of intact fructose-fed rats [1], which was ameliorated by ABT. Endothelial relaxation was significantly higher in the gonadectomized groups compared to F and was unaffected by inhibiting 20-HETE (Fig. 1).
Inhibition of 20-HETE synthesis by 1-aminobenzotriazole (ABT) improves relaxation to acetylcholine (ACh) in intact fructose-fed rat mesenteric arteries. Male Wistar rats were divided into 4 group: C intact control rats, F fructose-fed intact rats, G gonadectomized, and GF gonadectomized fructose-fed rats (n = 4–6*/group). The response to ACh in the absence (open square) and presence of ABT (filled inverted triangle). Endothelium-dependent relaxation to acetylcholine was unaffected by ABT in superior mesenteric arteries of C, G and GF rats. All groups n = 4–6. *P < 0.05 F (PE + ACh) versus F (PE + ACh + 1-ABT). All values are presented as mean ± SEM
Study 1b
Insulin sensitivity and blood pressure
At the end of 9 weeks, fructose feeding decreased insulin sensitivity in both sham-operated and gonadectomized rats (F: 8.5 ± 1 and GF: 8.5 ± 1) as compared to normal chow-fed controls (C: 11 ± 1 and G: 14.6 ± 1; P < 0.05 F and GF vs. C and G).
Blood pressure was elevated in intact male rats after 9 weeks of fructose feeding (F: 134 ± 4 mmHg) compared to controls (C: 116 ± 3 mmHg; P < 0.05 F vs. C), which was prevented by gonadectomy (G: 109 ± 2 and GF: 115 ± 2 mmHg; P < 0.05 G and GF vs. F). These data confirm data previously published for study 1a [5] and study 1c [13].
Plasma testosterone
Testosterone was undetectable in gonadectomized rats. Fructose feeding did not affect testosterone levels (C: 1.3 ± 0.3 vs. F: 1.6 ± 0.5 ng/ml).
Vascular reactivity studies
Since ABT was a non-selective inhibitor of eiconsanoid synthesis, DDMS, a more selective inhibitor of 20-HETE synthesis, was used in this study. In the isolated superior mesenteric arteries, relaxation to acetylcholine was impaired in F but not GF as previously reported [1]. Inhibition of 20-HETE synthesis with DDMS (10−5 M) improved the relaxation to ACh in the SMA of F but not GF (Fig. 2). This demonstrates the involvement of androgen-dependent 20-HETE synthesis in impairing endothelium-dependent vasodilation.
Inhibition of 20-HETE synthesis by DDMS improves relaxation to acetylcholine (ACh) in intact fructose-fed rat mesenteric arteries. Male Wistar rats were divided into four groups: C intact control rats, F fructose-fed intact rats, G gonadectomized, and GF gonadectomized fructose-fed rats (n = 4/group). The response to ACh in absence (filled square) and presence of DDMS (open square). Endothelium-dependent relaxation to acetylcholine was unaffected by DDMS in superior mesenteric arteries of C, G and GF rats. Relaxation is shown as AUC values. All groups n = 4. *P < 0.05 F versus F′. All values are presented as mean ± SEM
Study 1c
The changes in insulin sensitivity and blood pressure in intact and gonadectomized rats were similar to that observed in Study 1a. These results were previously published in another manuscript [13].
Cyp4A1, 2/3 expression in the SMA
In the SMA, Cyp4A expression was increased in F compared to C in the SMA, which was prevented by gonadectomy (Fig. 3).
Changes in Cyp4A1, 2/3 expression in the superior mesenteric artery of intact and gonadectomized rats. Male Wistar rats were divided into four groups: C intact control rats, F fructose-fed intact rats, G gonadectomized rats, and GF gonadectomized fructose-fed rats (n = 4/group). Western blotting was performed on immunoprecipitated protein lysates. Controls are bands from the IgG fraction. All groups n = 4. *P < 0.05 F versus C, and GF. All values are presented as mean ± SEM
Study 2
Body weights
Treatment with both flutamide and ABT did not affect body weight in control or fructose-fed rats (data not shown).
Insulin sensitivity
Fructose feeding induced insulin resistance, which was observed by hyperinsulinemia and delayed glucose clearance. Interestingly, treatment with ABT for 3 weeks improved the insulin sensitivity in fructose-fed rats (FA) whereas flutamide had no effect on insulin sensitivity (FF) (Fig. 4c). This was demonstrated by insulin levels similar to C in the FA group (Fig. 4a, inset). Fructose-fed rats were hyperglycemic compared to C or the CA rats (Fig. 4b, inset).
a–c Plasma insulin and glucose profiles and insulin sensitivity index (ISI) following an oral glucose challenge. Male Wistar rats were divided into six groups: C intact control rats, F fructose-fed intact rats, CF control flutamide-treated rats, FF fructose-fed flutamide-treated rats, CA control 1-aminobenzotriazole (ABT)-treated rats and FA-fructose-fed ABT-treated rats (n = 6/group). Flutamide (FF) did not alter ISI values in fructose-fed rats. Treatment with ABT improved the insulin sensitivity as demonstrated by high ISI value (4C). a Plasma insulin levels following a 1 g/kg glucose challenge. Inset shows area under the curve values (AUC) for each group. b Plasma glucose concentration levels following a 1 g/kg glucose challenge. Inset shows area under the curve values (AUC) for each group. c Insulin sensitivity index values for all groups as calculated by the Matsuda and DeFronzo formula [41]. All groups n = 6. *P < 0.05 F and FF versus C, CF, CA, and FA (a, b). **P < 0.05 F and FF versus CA and FA. All values are presented as mean ± SEM
Blood pressure
Treatment with either flutamide or ABT restored the blood pressure to control values suggesting the involvement of both testosterone and Cyp4A (Fig. 5).
Systolic blood pressure values at the end of 9 and 12 weeks of fructose feeding. Male Wistar rats were divided into six groups: C intact control rats, F fructose-fed intact rats, CF control flutamide-treated rats, FF fructose-fed flutamide-treated rats, CA control 1-aminobenzotriazole (ABT)-treated rats and FA fructose-fed ABT-treated rats (n = 8/group). Treatment with flutamide (8 mg/kg/day, subcutaneously) or ABT (25 mg/kg/day, intraperitoneally) was started at the end of 9 weeks. Blood pressure was measured 3 weeks post treatment. All groups n = 8. *P < 0.05 FF and FA (treated) versus FF and FA (untreated) and F (untreated and treated); + P < 0.05 F (treated and untreated) vs. C, CF and CA. All values are expressed as mean ± SEM
Plasma testosterone levels
Blocking the androgen receptor increased plasma testosterone levels in both CF and FF groups (9.33 ± 1 ng/ml and 13.6 ± 1.5 ng/ml) compared to untreated groups (C: 3.19 ± 0.4 ng/ml and F: 3.05 ± 0.6 ng/ml). Fructose diet, by itself, did not affect testosterone levels.
Study 3
Body weights
Body weight at termination was unaffected by either diet or HET0016 treatment (C: 579 ± 14, F: 584 ± 10, CH: 563 ± 21 and FH: 576 ± 8 gm).
Insulin sensitivity
Fructose feeding induced insulin resistance as indicated by the reduced insulin sensitivity index (ISI) (Fig. 6c). Treatment with HET0016 was inconclusive as the ISI values were not different from either C or F groups. HET0016-treated rats did not vary in their total insulin levels as indicated by the area under curve values (Fig. 6a, inset). Fructose feeding and/or HET0016 did not alter plasma glucose levels subsequent to the glucose challenge (Fig. 6b).
a–c Plasma insulin and glucose profiles and insulin sensitivity index (ISI) following an oral glucose challenge. Intact male Wistar rats were divided into four groups: C control, F fructose-fed, CT control HET0016-treated, and FT fructose-fed and HET0016 treated (n = 7/group). HET0016 treated rats did not exhibit statistically different ISI values. a Plasma insulin levels following a 1 g/kg glucose challenge. Inset shows area under the curve values (AUC) for each group. b Plasma glucose levels following a 1 g/kg glucose challenge. Inset shows area under the curve values (AUC) for each group. c Insulin sensitivity index values for all groups as calculated by the Matsuda and DeFronzo formula [41]. All groups n = 7. *P < 0.05 F versus C and CT. All values are presented as mean ± SEM
Blood pressure
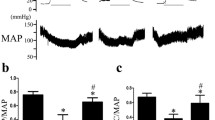
Fructose feeding elevated the blood pressure in F compared to C at the end of 9 weeks. Treatment with HET0016 for 1 week prevented the increase in blood pressure following insulin resistance in FT rats (Fig. 7a).
a Systolic blood pressure values at the end of 9 weeks of fructose feeding. Intact male Wistar rats were divided into four groups: C control, F fructose-fed, CT control HET0016-treated, FT fructose-fed and HET0016 treated (n = 7/group). HET0016 (10 mg/kg/day, intraperitoneally) was started at the end of 8 weeks. Blood pressure was measured 1 week post treatment. All groups n = 7. *P < 0.05 F versus C, CT, and FT. All values are presented as mean ± SEM. b Changes in Cyp4A in the mesenteric bed of control and fructose-fed rats treated with HET0016 (10 mg/kg/day, intraperitoneally). Intact male Wistar rats were divided into four groups: C control, F fructose-fed, CT control HET0016-treated, and FT fructose-fed and HET0016 treated (n = 4/group). Western blotting was performed on samples immunoprecipitated with Cyp4A1, 2/3 Ab. Controls are bands from antibody IgG used in IP. All groups n = 4. *P < 0.05 F versus C, CT and FT. All values are presented as mean ± SEM
Cyp4A protein expression
Insulin resistance elevated Cyp4A1, 2/3 expression in the mesenteric bed of untreated fructose-fed rats (F). Treatment with HET0016 decreased Cyp4A expression and prevented 20-HETE synthesis (Fig. 7b).
Discussion
We have demonstrated for the first time that in the presence of testosterone, Cyp4A expression is elevated in the mesenteric vessels following insulin resistance, which contributes to the development of endothelial dysfunction and increased blood pressure. Both ABT (Fig. 1) and DDMS (Fig. 2) improved the relaxation to acetylcholine only in intact fructose-fed rat SMA. The robust relaxation to ACh in control tissues (C) in spite of Cyp4A expression suggests the presence of functional endothelial nitric oxide synthase (eNOS). The selectivity of drug action towards blood vessels from intact insulin resistant rats supports our previous findings where the loss of functional endothelial nitric oxide system contributes to attenuated relaxation and endothelial dysfunction, which in turn may unmask the effects of vasoconstrictors [1]. The equilibrium between endothelial nitric oxide and endothelial vasoconstrictors such as eicosanoids is further supported by the decrease in vascular Cyp4A expression and its salutary effects on blood pressure in both gonadectomized (G & GF) and flutamide-treated rats (FF) compared to untreated and intact fructose-fed rats (F). Finally treatment with either ABT or HET0016 decreased or prevented, respectively, the increase in blood pressure in fructose-fed rats. In conjunction with previously published data from our laboratory, we suggest that the absence of testosterone prevents endothelial dysfunction and increases in blood pressure by reducing the responses to vasoactive prostanoids and eicosanoids [1, 4, 13]. Our findings are in agreement with the existing evidence in other models to support the role of androgens in regulating Cyp4A/20-HETE synthesis [16, 18].
It has been previously demonstrated by various laboratories that fructose feeding induced insulin resistance and endothelial dysfunction as early as 2 weeks and as long as 9 weeks for observing elevated blood pressure [1, 2, 43]. Currently no studies have investigated the changes in Cyp4A expression/20-HETE synthesis during the development of insulin resistance and high blood pressure in fructose-fed rats. Although no direct evidence exists, our findings along with literature reports, where insulin resistance has been shown to precede and induce hypertension, it may be speculated that elevated Cyp4A expression follows insulin resistance.
We initially used the Cyp4A suicide substrate inhibitor ABT as it was the agent of choice to study the role of 20-HETE. However, the inability of ABT to selectively inhibit Cyp4A [44] and the advent of more potent and Cyp4A selective drugs such as HET0016 [35, 37] led us to revisit our studies. HET0016 inhibited Cyp4A expression (Fig. 7b) and prevented the increase in blood pressure (Fig. 7a). The work published to date has only measured changes in 20-HETE levels and not Cyp4A expression in HET0016 treated rats [18, 45]. The limited availability of HET0016 as a gift sample was a key factor in our starting prophylactic treatment after 8 weeks of fructose feeding. The rationale for the decision was based on data from our laboratory, which showed a significant blood pressure elevation only at the end of 9 weeks of feeding fructose. Compared to untreated fructose-fed rats, HET0016 treatment did not increase the ISI value in FH (Fig. 4c). However, it was also not different from C suggesting there maybe a high variability in the effects of HET0016 on glucose disposal, which warrants future study. Interestingly ABT normalized the glucose disposal profile in fructose-fed rats (Fig. 4a–c). We have no mechanism to support this finding but we believe that ABT reduces blood pressure by improving insulin sensitivity independent of Cyp4A. Studies are required at the molecular level to investigate the effects of ABT on insulin sensitivity and the effects of HET0016 on individual Cyp4A isoforms at the protein and message levels.
Plasma testosterone levels were unaffected by ABT. HET0016-treated samples were not assayed for testosterone, since there are no previous reports that have examined changes in testosterone levels following treatment with HET0016.
Alterations in vascular arachidonic acid metabolism may play an important role in the development of vascular abnormalities and high blood pressure following insulin resistance. Increased vascular Cyp4A expression following insulin resistance increases 20-HETE synthesis, which contributes to the increase in blood pressure. As 20-HETE has been shown to decrease NO synthesis and function by blocking the KCa channels, the above findings could, in part, explain the concomitant decrease in endothelial vasodilator levels and function. Thus, it may be possible that 20-HETE impairs vascular homeostasis by both decreasing endothelial relaxation and increasing vascular smooth muscle (Ca+2) i as a possible common downstream target of angiotensin-2, norepinephrine and endothelin-1, which induces vasoconstriction and increased blood pressure. Furthermore angiotensin-2, norepinephrine and endothelin-1 also induce vascular remodeling by activating various matrix metalloproteinases (MMPs 2/9 and 7), whose activity is elevated in insulin resistant blood vessels following stimulation by phenylephrine [46]. Examining the effects of 20-HETE on MMP activation and therefore remodeling could implicate 20-HETE as a key target in altering hemodynamics following insulin resistance. Further studies need to be conducted to understand the intricate association between insulin resistance and sex hormones in determining the contributions of arachidonic acid metabolites to regulating blood pressure.
References
Vasudevan H, Nagareddy PR, McNeill JH (2006) Gonadectomy prevents endothelial dysfunction in fructose-fed male rats, a factor contributing to the development of hypertension. Am J Physiol Heart Circ Physiol 291:H3058–H3064
Verma S, Bhanot S, Yao L, McNeill JH (1996) Defective endothelium-dependent relaxation in fructose-hypertensive rats. Am J Hypertens 9:370–376
Katakam PV, Ujhelyi MR, Miller AW (1999) EDHF-mediated relaxation is impaired in fructose-fed rats. J Cardiovasc Pharmacol 34:461–467
Song D, Arikawa E, Galipeau D, Battell M, McNeill JH (2004) Androgens are necessary for the development of fructose-induced hypertension. Hypertension 43:667–672
Vasudevan H, Xiang H, McNeill JH (2005) Differential regulation of insulin resistance and hypertension by sex hormones in fructose-fed male rats. Am J Physiol Heart Circ Physiol 289:H1335–H1342
Reckelhoff JF, Zhang H, Srivastava K, Granger JP (1999) Gender differences in hypertension in spontaneously hypertensive rats: role of androgens and androgen receptor. Hypertension 34:920–923
Reckelhoff JF, Zhang H, Srivastava K (2000) Gender differences in development of hypertension in spontaneously hypertensive rats: role of the renin-angiotensin system. Hypertension 35:480–483
Yanes LL, Sartori-Valinotti JC, Iliescu R, Romero DG, Racusen LC, Zhang H, Reckelhoff JF (2009) Testosterone-dependent hypertension and upregulation of intrarenal angiotensinogen in Dahl salt-sensitive rats. Am J Physiol Renal Physiol 296:F771–F779
Bogatcheva NV, Sergeeva MG, Dudek SM, Verin AD (2005) Arachidonic acid cascade in endothelial pathobiology. Microvasc Res 69:107–127
Vanhoutte PM, Feletou M, Taddei S (2005) Endothelium-dependent contractions in hypertension. Br J Pharmacol 144:449–458
Galipeau D, Arikawa E, Sekirov I, McNeill JH (2001) Chronic thromboxane synthase inhibition prevents fructose-induced hypertension. Hypertension 38:872–876
Jiang J, Tran L, Vasudevan H, Xia Z, Yuen VG, McNeill JH (2007) Endothelin-1 blockade prevents COX2 induction and TXA2 production in the fructose hypertensive rat. Can J Physiol Pharmacol 85:422–429
Vasudevan H, Lau SM, Jiang J, Galipeau D, McNeill JH (2010) Effects of insulin resistance and testosterone on the participation of cyclooxygenase isoforms in vascular reactivity. J Exp Pharmacol 2:169–179
Vasudevan H (2009) Testosterone-dependent vascular arachidonic acid metabolism in the regulation of insulin resistance and blood pressure. Faculty of Pharmaceutical Sciences, University of British Columbia, Vancouver, pp 1–151
Roman RJ (2002) P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82:131–185
Holla VR, Adas F, Imig JD, Zhao X, Price E Jr, Olsen N, Kovacs WJ, Magnuson MA, Keeney DS, Breyer MD, Falck JR, Waterman MR, Capdevila JH (2001) Alterations in the regulation of androgen-sensitive Cyp 4a monooxygenases cause hypertension. Proc Natl Acad Sci USA 98:5211–5216
Nakagawa K, Marji JS, Schwartzman ML, Waterman MR, Capdevila JH (2003) Androgen-mediated induction of the kidney arachidonate hydroxylases is associated with the development of hypertension. Am J Physiol Regul Integr Comp Physiol 284:R1055–R1062
Singh H, Cheng J, Deng H, Kemp R, Ishizuka T, Nasjletti A, Schwartzman ML (2007) Vascular cytochrome P450 4A expression and 20-hydroxyeicosatetraenoic acid synthesis contribute to endothelial dysfunction in androgen-induced hypertension. Hypertension 50:123–129
Miyata N, Roman RJ (2005) Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. J Smooth Muscle Res 41:175–193
Cheng J, Ou JS, Singh H, Falck JR, Narsimhaswamy D, Pritchard KA Jr, Schwartzman ML (2008) 20-Hydroxyeicosatetraenoic acid causes endothelial dysfunction via eNOS uncoupling. Am J Physiol Heart Circ Physiol 294:H1018–H1026
Cheng J, Ou JS, Singh H, Falck JR, Narsimhaswamy D, Pritchard KA Jr, Schwartzman ML (2008) 20-Hydroxyeicosatetraenoic acid causes endothelial dysfunction via eNOS uncoupling. American journal of physiology. Heart and circulatory physiology 294:H1018–H1026
Cheng J, Wu CC, Gotlinger KH, Zhang F, Falck JR, Narsimhaswamy D, Schwartzman ML (2010) 20-hydroxy-5,8,11,14-eicosatetraenoic acid mediates endothelial dysfunction via IkappaB kinase-dependent endothelial nitric-oxide synthase uncoupling. J Pharmacol Exp Ther 332:57–65
Berezan DJ, Dunn KM, Falck JR, Davidge ST (2008) Aging increases cytochrome P450 4A modulation of alpha1-adrenergic vasoconstriction in mesenteric arteries. J Cardiovasc Pharmacol 51:327–330
Croft KD, McGiff JC, Sanchez-Mendoza A, Carroll MA (2000) Angiotensin II releases 20-HETE from rat renal microvessels. Am J Physiol Renal Physiol 279:F544–F551
Barnett CR, Rudd S, Flatt PR, Ioannides C (1993) Sex differences in the diabetes-induced modulation of rat hepatic cytochrome P450 proteins. Biochem Pharmacol 45:313–319
Benter IF, Yousif MH, Canatan H, Akhtar S (2005) Inhibition of Ca2+/calmodulin-dependent protein kinase II, RAS-GTPase and 20-hydroxyeicosatetraenoic acid attenuates the development of diabetes-induced vascular dysfunction in the rat carotid artery. Pharmacol Res 52:252–257
Chen YJ, Li J, Quilley J (2008) Deficient renal 20-HETE release in the diabetic rat is not the result of oxidative stress. Am J Physiol Heart Circ Physiol 294:H2305–H2312
Laffer CL, Laniado-Schwartzman M, Nasjletti A, Elijovich F (2004) 20-HETE and circulating insulin in essential hypertension with obesity. Hypertension 43:388–392
Wang MH, Smith A, Zhou Y, Chang HH, Lin S, Zhao X, Imig JD, Dorrance AM (2003) Downregulation of renal CYP-derived eicosanoid synthesis in rats with diet-induced hypertension. Hypertension 42:594–599
Tsai IJ, Croft KD, Mori TA, Falck JR, Beilin LJ, Puddey IB, Barden AE (2009) 20-HETE and F2-isoprostanes in the metabolic syndrome: the effect of weight reduction. Free Radic Biol Med 46:263–270
Ward NC, Hodgson JM, Puddey IB, Beilin LJ, Croft KD (2006) 20-Hydroxyeicosatetraenoic acid is not associated with circulating insulin in lean to overweight humans. Diabetes Res Clin Pract 74:197–200
Dunn KM, Renic M, Flasch AK, Harder DR, Falck J, Roman RJ (2008) Elevated production of 20-HETE in the cerebral vasculature contributes to severity of ischemic stroke and oxidative stress in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 295:H2455–H2465
Hwang IS, Ho H, Hoffman BB, Reaven GM (1987) Fructose-induced insulin resistance and hypertension in rats. Hypertension 10:512–516
Llinas MT, Alexander BT, Capparelli MF, Carroll MA, Granger JP (2004) Cytochrome P-450 inhibition attenuates hypertension induced by reductions in uterine perfusion pressure in pregnant rats. Hypertension 43:623–628
Miyata N, Taniguchi K, Seki T, Ishimoto T, Sato-Watanabe M, Yasuda Y, Doi M, Kametani S, Tomishima Y, Ueki T, Sato M, Kameo K (2001) HET0016, a potent and selective inhibitor of 20-HETE synthesizing enzyme. Br J Pharmacol 133:325–329
Sato M, Ishii T, Kobayashi-Matsunaga Y, Amada H, Taniguchi K, Miyata N, Kameo K (2001) Discovery of a N′-hydroxyphenylformamidine derivative HET0016 as a potent and selective 20-HETE synthase inhibitor. Bioorg Med Chem Lett 11:2993–2995
Seki T, Wang MH, Miyata N, Laniado-Schwartzman M (2005) Cytochrome P450 4A isoform inhibitory profile of N-hydroxy-N′-(4-butyl-2-methylphenyl)-formamidine (HET0016), a selective inhibitor of 20-HETE synthesis. Biol Pharm Bull 28:1651–1654
Hoagland KM, Flasch AK, Roman RJ (2003) Inhibitors of 20-HETE formation promote salt-sensitive hypertension in rats. Hypertension 42:669–673
Blanton A, Nsaif R, Hercule H, Oyekan A (2006) Nitric oxide/cytochrome P450 interactions in cyclosporin A-induced effects in the rat. J Hypertens 24:1865–1872
Vasudevan H (2005) Potential role of sex hormones in altered vascular relaxation following insulin resistance. Faculty of Pharmaceutical Sciences, University of British Columbia, Vancouver, pp 1–99
Matsuda M, DeFronzo RA (1999) Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22:1462–1470
Ito O, Alonso-Galicia M, Hopp KA, Roman RJ (1998) Localization of cytochrome P-450 4A isoforms along the rat nephron. Am J Physiol 274:F395–F404
Katakam PV, Ujhelyi MR, Hoenig ME, Miller AW (1998) Endothelial dysfunction precedes hypertension in diet-induced insulin resistance. Am J Physiol 275:R788–R792
Linder CD, Renaud NA, Hutzler JM (2009) Is 1-aminobenzotriazole an appropriate in vitro tool as a nonspecific cytochrome P450 inactivator? Drug Metab Dispos 37:10–13
Hong HJ, Liu JC, Chan P, Juan SH, Loh SH, Lin JG, Cheng TH (2004) 17b-estradiol downregulates angiotensin-II-induced endothelin-1 gene expression in rat aortic smooth muscle cells. J Biomed Sci 11:27–36
Nagareddy PR (2009) Mechanisms of vascular dysfunction in diabetes and hypertension. Faculty of Pharmaceutical Sciences, University of British Columbia, Vancouver, p 267
Acknowledgments
This project was funded by grants-in aid from The Heart and Stroke Foundation of BC and Yukon (HSFBCY) and Canadian Institutes of Health Research (CIHR-Priority announcement grant from the Institute of Gender and Health) to Dr. McNeill. Harish Vasudevan was funded by a Doctoral Research Award from the Heart and Stroke Foundation of Canada and a Senior Graduate Studentship from the Michael Smith Foundation for Health Research British Columbia, Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vasudevan, H., Yuen, V.G. & McNeill, J.H. Testosterone-dependent increase in blood pressure is mediated by elevated Cyp4A expression in fructose-fed rats. Mol Cell Biochem 359, 409–418 (2012). https://doi.org/10.1007/s11010-011-1035-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-1035-7