Abstract
Salidroside, a phenol glycoside of plant origin, has been documented to possess a broad spectrum of pharmacological properties, including protective effects against neuronal death induced by different insults. To provide further insights into the neuroprotective functions peculiar to salidroside, this study used primary cultured cortical neurons of rats as a cell model to examine whether salidroside was able to prevent against cell damage after exposure to cobalt chloride (CoCl2), a hypoxia-inducing agent. The data from 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide test, Hoechst33342 staining, terminal deoxynucleotidyl transferase dUTP-mediated nicked end labeling assay, and Bax/Bcl-2 ratio analysis indicated that salidroside pretreatment attenuated hypoxia-induced apoptotic cell death of primary cultured cortical neurons in a dose-dependent manner. Moreover, preliminary exploration of the possible mechanisms suggested that the protective effects of salidroside, shown in our experimental setting, might probably be mediated by enhancing the expression of hypoxia-inducible factor-1α, alleviating the increase of intracellular reactive oxygen species levels, and inhibiting over-expression of nuclear factor-kappa B protein.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The central nervous system is most susceptible to hypoxia conditions. Cerebral hypoxia refers to a reduced supply of oxygen to the brain. If blood flow is not sufficient to supply the brain’s oxygen needs, mild symptoms such as difficulties with complex learning tasks and reductions in short-term memory will begin to appear. The continuous oxygen deprivation results in cognitive disturbances and decreased motor control, even induces fainting, long term loss of consciousness, coma, seizures, cessation of brain stem reflexes, and brain death. Traumatic brain injury, cerebrovascular disease, high blood pressure, and other brain diseases could cause partial or whole brain hypoxia, which, if not handled properly, will give rise to a variety of complications, even life-threatening. Unfortunately, it remains a difficult task to prevent and treat cerebral hypoxia.
Rhodiola rosea L., a popular medicinal plant, grows in mountains at high altitudes and is long used by Tibetan people to enhance body’s resistance to fatigue. It has been documented that R. rosea has a range of pharmacological properties, such as anti-inflammation, anti-hypoxia, anti-oxidation, anti-aging, anti-cancer, and hepatoprotection activities [1–9]. Salidroside (p-hydroxyphenethyl-β-d-glucoside), separated from R. rosea, is a main active ingredient responsible for most pharmacological effects of the plant. Recently, salidroside has been shown to exert protective effects against cell apoptosis induced by glutamate, by H2O2 or other oxidative stress, or by hypoglycemia and serum limitation in primary culture of rat hippocampal neurons, in rat pheochromocytoma PC12 cells, or in SH-SY5Y human neuroblastoma cells, respectively [10–15]. However, the mechanisms underlying the neuroprotective effect of salidroside have not yet been fully understood.
As is known, cobalt chloride (CoCl2) can serve as a chemical hypoxia-inducing agent to mimic hypoxic/ischemic conditions, including generation of reactive oxygen species (ROS) and transcriptional change of some genes such as hypoxia-inducible transcription factor-1α (HIF-1α), that is associated with various hypoxic responses, p53, p21, and pCNA in promoting the cell death [16, 17]. Therefore, exposure to CoCl2 is commonly employed as an in vitro model simulating hypoxia brain insults [18, 19]. In this study, we investigated the effects of salidroside against CoCl2-induced cell damage in primary cultured cortical neurons and the possible underlying mechanisms.
Materials and methods
Materials
Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS), trypsin, Neurobasal medium, and B27 were provided by Gibco (Grand Island, NY). Poly-l-lysine, Hoechst33342, 2′,7′-Dichlorofluorescin diacetate (DCFH-DA), diacylglycerol kinase inhibitor II(R59949), mouse anti-Bcl-2, anti-Bax, anti-β-actin, anti-Map-2 monoclonal antibodies, and FITC goat anti-mouse IgG were purchased from Sigma (St. Louis, MO). Rabbit anti-HIF-1α polyclonal antibody, TRITC goat anti-rabbit IgG, and FITC goat anti-rabbit IgG were purchased from Santa Cruz Biotechnology Inc. (Santa Crutz, CA). Rabbit anti-NF-κB p65 polyclonal antibody was purchased from ABcam (Cambridge, MA). IRDye 800-conjugated goat anti-rabbit IgG and IRDye 800-conjugated goat anti-mouse IgG were presented by Odyssey corporation Ltd (Pittsburgh, PA). Salidroside was obtained from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Genmed (Westbury, NY). The dUTP-mediated nicked end labeling (TUNEL) assay kit was purchased from Promega (Madison, WI).
Cell culture and treatment
Primary cultures of cortical neurons were prepared from the brain of E18–E19 Sprague-Dawley (SD) rat embryos (obtained from the Experimental Animal Center of Nantong University, China). After treatment with 0.25% trypsin for 15 min at 37°C in Ca2+ and Mg2+-free Hank’s balanced salt solution, the cortex were washed in DMEM supplemented with 10% FBS to stop trypsin activity. Then the cells were re-suspended in DMEM supplemented with 10% FBS and plated onto poly-l-lysine-coated plates for 4 h at 37°C in a humidified atmosphere of 95% air and 5% CO2. After cells were attached to the substrate, the medium was replaced with neuronal culture medium consisting of serum-free Neurobasal medium supplemented with 2% B27, 0.5 mM glutamine, 100 U/ml penicillin, and 100 U/ml streptomycin, followed by re-incubation for 7–8 days, the time required for maturation of cortical neurons, with half of the medium being changed every 2 days. Then, the cells were characterized by immunohistochemistry for neurofilament (NF) protein and fibrillary acidic protein, revealing that the cell cultures contained about 90% neurons.
The primary cultured cortical neurons were pretreated with 120, 240, or 480 μM salidroside, respectively, for 24 h, followed by exposure to 600 μM CoCl2 and 10 μM of glycine in neuronal culture medium for 4 h at 37°C in a humidified atmosphere of 95% air and 5% CO2. The cortical neurons cultured in plain medium served as control. At the end of cell treatments, different tests were carried out as described below.
Cell viability test
The cell viability was determined by conventional MTT assay as previously described [11], which was based on the cleavage of MTT, a yellow tetrazolium salt, into the purple formazan product under the catalysis of mitochondrial enzymes in viable cells. Briefly, the primary cultured cortical neurons were cultured in 96-well plates at a density of 5 × 105 cells per well. After hypoxia stimulation with CoCl2, the cells were further incubated with 10 μl MTT solution in each well at 37°C for 4 h. Afterwards, 100 μl of 20% sodium dodecyl sulfide (SDS) solution were added to each well to dissolve the precipitate for 20 h, and the absorbance was measured by spectrophotometry at 570 nm with an ElX-800 Microelisa reader (Bio-Tek Inc., Winooski, VT). The data were expressed as a percent of control value.
Map-2 immunostaining and Hoechst33342 staining
The primary cultured cortical neurons were cultured on poly-l-lysine-coated glass coverslips at a density of 5 × 104 cells/cm2, and fixed in 4% paraformaldehyde for 20 min at room temperature. The cell sample was allowed to incubate with mouse monoclonal anti-Map-2 (1:200) at 4°C overnight followed by further reaction with FITC-labeled goat anti-mouse IgG (1:200). Afterwards, the cells were stained by 10 μg/ml Hoechst33342 for 10 min. Fluorescent images were processed using a fluorescence microscope.
TUNEL assay
The primary cultured cortical neurons were cultured on poly-l-lysine-coated glass coverslips, at a density of 5 × 104 cells/cm2. Then the cells were fixed in 4% paraformaldehyde for 20 min at room temperature. Then fixed cells were washed, and fragmented DNA was detected in apoptotic cells by adding fluorescein 12-dUTP to nicked ends of DNA, followed by incubation for 1 h at 37°C. After the reaction was terminated with 2 × SSC (300 mM sodium chloride and 30 mM sodium citrate, pH 7.4), the cells were stained with Hoechst33342. Apoptotic cells were detected as localized bright green cells (positive cells) in a blue background by scanning laser confocal microscopy (Leica, Heidelberg, Germany). Data were expressed as the ratio of apoptotic neurons to total neurons.
Western blot analysis
The primary cultured cortical neurons, plated at 1 × 106 cells/ml each plate, were collected and subjected to Western blot analysis for the protein expression of Bax, Bcl-2, HIF-1α, and NF-κB. Cell proteins were extracted and quantified by a BCA-100 Kit, followed by electrophoretic separation on a 12 or 10% SDS-PAGE. After transferred to PVDF membrane (Millipore, Bedford, MA), the protein samples were allowed to react with rabbit anti-HIF-1α polyclonal antibody (1:100), rabbit anti-NF-κB antibody (1:500), mouse anti-Bax monoclonal antibody (1:200), mouse anti-Bcl-2 monoclonal antibody (1:400), and mouse anti-β-actin monoclonal antibody (1:4,000) in blocking buffer at 4°C overnight, and then allowed to incubate with IRDye 800-conjugated goat anti-mouse IgG at room temperature for additional 2 h. The images were scanned with GS800 Densitometer Scanner (Bio-Rad, Hercules, CA), and the absorbance data were analyzed using PDQuest 7.2.0 software (Bio-Rad). The protein β-actin served as an internal reference.
Immunocytochemistry
The primary cultured cortical neurons were cultured on glass coverslips, fixed in 4% paraformaldehyde for 20 min, and then permeabilized with 0.2% Triton X-100 in phosphate buffer saline (PBS) for 5 min. After blocking, the cells were incubated with rabbit polyclonal anti-HIF-1α (1:50) overnight at 4°C. Afterwards, TRITC-conjugated secondary antibody (1:100) was applied for incubation at room temperature for 30 min. Fluorescent images were processed under fluorescence microscopy.
Measurement of intracellular ROS
The intracellular ROS level was determined as described previously [20] with minor modifications. In brief, at 4 h after cell insult, the primary cultured cortical neurons were incubated with 20 μM DCFH-DA at 37°C for 30 min in the dark, and then gently rinsed with D-Hanks’ solution. The fluorescence of 2′,7′-Dichlorofluorescin (DCF), the oxidation product of DCFH-DA, was excited at 480 nm and detected at 530 nm by flow cytometry (FACScalibur, BD Bioscience, San Jose, CA).
Statistical analysis
Data were expressed as means ± S.D. Statistical significance was determined by one-way analysis of variance (ANOVA) and subsequent Bartlett’s tests. Differences were considered significant at P < 0.05.
Results
Salidroside inhibited CoCl2-induced cell viability loss in cultured cortical neurons
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay indicated that exposure of primary cultured cortical neurons to CoCl2 at different concentrations (150, 300, 600, 1200 μM) for 4 h caused significant decreases in the cell viability, and the cell viability loss exhibited a dose-dependent pattern (Fig. 1a). Exposure to 600 μM CoCl2 for 4 h resulted in 79.3 ± 12.9% viable cells as compared to control (100%), and 600 μM CoCl2 stimulation for 4 h was used in subsequent experiments to induce cell insults in primary cultured cortical neurons.
Protective effects of salidroside against CoCl2-induced cell damage in cultured cortical neurons. a Cultured cortical neurons were exposed to CoCl2 (150, 300, 600, 1200 μM) for 4 h, and the cell viability was measured by MTT assay. b The cell viability was measured by MTT assay after different cell treatments, which included exposure to 600 μM CoCl2 for 4 h without and with pretreatment of salidroside at 120, 240, 480 μM for 24 h, respectively. Cultured cortical neurons undergoing neither salidroside pretreatment nor CoCl2 stimulation serve as control (same as below)
Pretreatment with salidroside at concentrations of 120, 240, 480 μM was found to inhibit CoCl2-induced cell viability loss in primary cultured cortical neurons in a dose-dependent manner, making cell viability back to 84.5 ± 7.8, 91.3 ± 4.3, and 94.4 ± 8.1%, respectively (Fig. 1b). It should be mentioned that treatment with salidroside alone did not induce apparent cytotoxicity in primary cultured cortical neurons (data not shown).
Salidroside protected cultured cortical neurons against CoCl2-induced cell apoptosis
Hoechst33242 staining was carried out to determine the mode of cell death (apoptosis versus necrosis). The results showed that after exposure to 600 μM CoCl2 for 4 h, about 13.4% of primary cultured cortical neurons displayed apoptotic morphology, characterized by the condensation of chromatin, nuclear shrinkage, and formation of a few apoptotic bodies; however, pretreatment with 480 μM salidroside significantly reduced the percentage of the apoptotic cortical neurons (Fig. 2). Primary cultured cortical neurons were also subjected to TUNEL assay, and it was found that exposure to 600 μM CoCl2 yielded about 15.5% TUNEL-positive cells in whole cell population, but pretreatment with 480 μM salidroside significantly decreased the percentage of TUNEL-positive cells in whole cell population (Fig. 3). Both measurements revealed that salidroside attenuated CoCl2-induced cell apoptosis in primary cultured cortical neurons.
Protective effects of salidroside against CoCl2-induced cell apoptosis in cultured cortical neurons. Micrographs (a–c, Scale bar 0.75 μm) were taken following Map-2 immunostaining and Hoechst33342 staining which were performed to confirm that the cells were neurons and to show the cell nuclei, respectively. a Control. b Exposure to 600 μM CoCl2 alone for 4 h. c Salidroside (480 μM) pretreatment for 24 h and then exposure to CoCl2 600 μM for 4 h. d The percentage of nuclear condensation in cultured cortical neurons was determined after above cell treatments.**P < 0.01 versus control, ## P < 0.01 versus exposure to CoCl2 alone. Arrows indicated the apoptotic cells
Effects of salidroside against CoCl2-induced cell apoptosis in cultured cortical neurons. Micrographs (a–c, Scale bar 50 μm) following TUNEL/Hoechst staining displayed TUNEL-positive cells (green) and all the nuclei stained with Hoechst33342 (blue). a Control. b Exposure to CoCl2 600 μM. c Pretreatment with 480 μM salidroside and then exposure to CoCl2 600 μM. d The ratio of TUNEL-positive cells in whole cell population. **P < 0.01 versus control. # P < 0.05 versus exposure to CoCl2 alone. (Color figure online)
Western blot analysis showed that CoCl2 stimulation led to an increased expression of apoptosis-related protein Bax in primary cultured cortical neurons as compared to control, but induced no change in Bcl-2 expression. Salidroside pretreatment suppressed the CoCl2-induced elevation in Bax expression, while Bcl-2 expression remained unchanged by salidroside. In other words, salidroside pretreatment antagonized the up-regulation of Bax/Bcl-2 protein ratio in a dose-dependent manner (Fig. 4).
Effects of salidroside on expression of apoptosis-related proteins. The Bax/Bcl-2 protein ratio was changed after different cell treatments (same as in Fig. 1b). Both # P < 0.05 and ## P < 0.01 versus exposure to CoCl2 alone. Also shown is a representative Western blotting image, in which β-actin serves as an internal reference
HIF-1α pathway was involved in neuroprotection of salidroside against CoCl2-induced cell damage in cultured cortical neurons
In order to investigate an involvement of HIF-1α pathway in neuroprotection of salidroside against CoCl2-induced cell apoptosis in cultured cortical neurons, R59949, an inhibitor of HIF-1α pathway, was used to abolish the effect of HIF-1α, and MTT assay was performed to examine the changes in cell viability. We observed that due to R59949 (40 μM) addition, salidroside inhibition of CoCl2-induced cell viability loss was alleviated, implying that HIF-1 played a role in the protective effects of salidroside on CoCl2-induced cell damage in cultured cortical neurons (Fig. 5a).
Involvement of HIF-1α in neuroprotection of salidroside against CoCl2-induced cell apoptosis in cultured cortical neurons. The cell viability as measured by MTT assay (a) or HIF-1α protein expression as analyzed by Western blotting (b) was changed after different cell treatments, which included exposure to CoCl2 (600 μM) alone for 4 h; salidroside (480 μM) pretreatment for 24 h and then exposure to CoCl2 (600 μM) for 4 h; and salidroside (480 μM) pretreatment for 24 h and then addition of R59949 (40 μM) for 30 min followed by exposure to CoCl2 (600 μM) for 4 h. **P < 0.01 versus control. ## P < 0.01 versus exposure to CoCl2 alone. Both † P < 0.05 and †† P < 0.01 versus salidroside pretreatment and then exposure to CoCl2. Also shown in (c) is a representative Western blotting image, in which β-actin serves as an internal reference
Western blot analysis was conducted to further confirm the involvement of HIF-1α pathway. As compared with control, 2.2-fold or 3.8-fold increase in the HIF-1α protein level was observed in primary cultured cortical neurons that exposed to 600 μM CoCl2 or pretreated with 480 μM salidroside and then exposed to 600 μM CoCl2, respectively. However, salidroside (480 μM) pretreatment in couple with R59949 (40 μM) addition and then exposure to 600 μM CoCl2 reduced the HIF-1α protein level to 0.5-fold of control (Fig. 5b).
As shown by immunocytochemistry, HIF-1α proteins were mainly localized in the cytosol of cultured cortical neurons in the presence of oxygen (Fig. 6a); CoCl2 stimulation alone induced the translocation of HIF-1α proteins into the nuclei or perinucleus areas of cultured cortical neurons (Fig. 6b), and pretreatment with 480 μM salidroside led to translocation of HIF-1α proteins (Fig. 6c), which, however, were eliminated by extra addition of 40 μM R59949 (Fig. 6d).
Effects of salidroside on HIF-1α protein location as revealed by micrographs of immunocytochemistry with against HIF-1α antibody. a A little amount of HIF-1α was scattered in cytosol of cultured cortical neurons in normoxia (control). b HIF-1α was concentrated in peri-nucleus regions and within nuclei of cultured cortical neurons on exposure to CoCl2 alone. c Salidroside pretreatment of with 480 μmol/l significantly increased the translocation of HIF-1α in cultured cortical neurons as compared to panel B. d Addition of R59949 abolished the translocation of HIF-1α as compared to panel C. Scale bar 20 μm
Salidroside reduced the expression of ROS production in cultured cortical neurons
Flow cytometry with molecular probe DCFH-DA was used to monitor alterations in the intracellular ROS level. As compared to control, exposure of primary cultured cortical neurons to CoCl2 stimulation significantly increased the number of DCF-positive cells from 57.76 to 73.63% (Fig. 7a, b), indicating an elevation of ROS production. Salidroside pretreatment alone attenuated the CoCl2-induced increase in ROS production, as evidenced by a significant decline in the number of DCF-positive cells from 73.63 to 59.41%, implying inhibition of ROS production within cultured cortical neurons by salidroside (Fig. 7c). Extra addition of R59949, however, attenuated the inhibitory effect of salidroside on ROS production within cells (Fig. 7d).
Salidroside down-regulated nuclear factor-kappa B (NF-κB) expression
To test whether the protective effect of salidroside on CoCl2-induced cell damage in primary cultured cortical neurons was likely to be mediated by other signaling pathways than HIF-1, we performed Western blot analysis to investigate the changes in NF-κB protein expression after different cell treatments. Pretreatment of primary cultured cortical neurons with different concentrations of salidroside (120, 240, 480 μM) was found to suppress the increased expression of NF-κB induced by CoCl2 stimulation, and the suppression exhibited a dose-dependent pattern (Fig. 8).
Effects of salidroside on protein expression transcription factor NF-κB in cultured cortical neurons after different cell treatments (same as in Fig. 4). Both # P < 0.05 and ## P < 0.01 versus exposure to CoCl2 alone. Also shown is a representative Western blotting image, in which β-actin serves as an internal reference
Discussion
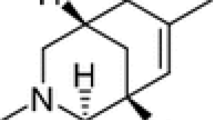
Neurons are the core component of the nervous system, and neuronal damage is the initial reason for hypoxic-ischemic brain injury. Developing new neuroprotective drugs or agents and investigating the molecular mechanism of neuroprotection have become great research concerns. Salidroside, as a small-molecule compound of plant origin with a definite chemical structure of phenol glycoside (Scheme 1), has been documented to possess a broad spectrum of pharmacological properties. Recently, many in vitro studies reveal the protective effects of salidroside against neuronal damage induced by different insults, including glutamate excitotoxicity, hypoglycemia, and oxidative stress, in different neuron or neuron-like models [10–14]. To provide further insights into the neuroprotective functions peculiar to salidroside, this study used primary cultured cortical neurons as a cell model to examine whether salidroside was able to prevent against cell damage induced by chemical hypoxia following CoCl2 stimulation.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay confirmed that exposure to CoCl2 stimulation caused the cell viability loss in primary cultured cortical neurons, and further showed that salidroside pretreatment attenuated the cell viability loss induced by hypoxia in a dose-dependent manner. Since hypoxia-induced neuronal death comprises both apoptosis and necrosis, we conducted Hoechst33342 (a DNA binding dye) staining to display morphological apoptosis in primary cultured cortical neurons in terms of chromatin condensation and nuclear fragmentation, and to determine the percentage of apoptotic cells in whole cell population after cell treatments. The data indicated that salidroside pretreatment antagonized hypoxia-induced cell apoptosis as evidenced by significant reduction in apoptotic cell number and visible changes in apoptotic cell morphology. We also carried out TUNEL assay, a method based on detection of DNA breaks generated during apoptosis, and found that salidroside pretreatment inhibited hypoxia-induced increase in TUNEL-positive cells, providing further evidence for Hoechst staining results.
The Bcl-2 family proteins are involved in major apoptotic signal transduction cascades, and the family members are either anti-apoptotic with cell survival-promoting effects (such as Bcl-2, Bcl-XL) or pro-apoptotic with apoptosis-inducing effects (such as Bax, Bad, Bak, Bik and Bcl-Xs) [21, 22]. The ratio of pro- and anti-apoptotic proteins is a critical factor affecting the ultimate vulnerability of cells to diverse apoptotic stimuli [23, 24]. In this study, we noted that there was a remarkable increase in Bax/Bcl-2 protein ratio on exposure to CoCl2 stimulation, and salidroside pretreatment attenuated the hypoxia-induced increase in Bax/Bcl-2 protein ratio in a dose-dependent manner. The results suggested that neuroprotection of salidroside against CoCl2-induced cell apoptosis in primary cultured cortical neurons was, at least partly, attributable to salidroside-evoked modulation of apoptosis-related protein expression.
To further explore the possible mechanisms underlying the protective effects of salidroside on cultured cortical neurons, we investigate the involvement of the HIF-1 pathway. Hypoxia inducible factor-1 belongs to the family of HIF, an important transcription factor when cells or tissues are under hypoxia conditions, and regulates a variety of hypoxia-associated cell adaptation. The protein consists of an oxygen-regulated subunit HIF-1α and a constitutively expressed subunit HIF-1β [25, 26]. Under lower oxygen tension, hydroxylation is inhibited because of O2 substrate deprivation, whereas HIF-1α accumulates, dimerizes with HIF-1β, and mediates profound changes in hypoxia-inducible gene expression [27, 28]. It has been shown that hypoxia-induced accumulation of HIF-1α protein is strongly impaired by the inhibitor of diacylglycerol kinase, R59949 [29]. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay showed that due to extra addition of R59949, the neuroprotective effect of salidroside against hypoxia insult was reduced but not eradicated. The reasons for this were assumed to be that: (1) the protective effect of salidroside on cultured cortical neurons damaged by CoCl2 was not entirely through HIF-1α pathway; (2) R59949 failed to completely degrade HIF-1α, making the protective effect of HIF-1 partly retained. To test our two assumptions, Western blot analysis was carried out which showed that salidroside pretreatment alone caused the increase in HIF-1α protein expression, but salidroside pretreatment in couple with R59949 addition greatly reduced the increased level of HIF-1α expression, even to come close to the level under normoxic conditions. This suggested that R59949 was able to completely degrade HIF-1α through the same pathway as that in normoxia (namely through the hydroxylation of two proline residues and acetylation of a lysine residue), and the downstream target genes in relation to hypoxia adaptation were not activated following the complete degradation of HIF-1α, thereby reducing but not eliminating the protective effects by salidrocide. In other words, the above second assumption seems to be not valid, and the protective effect of salidroside on cultured cortical neurons exposed to CoCl2 might be through multiple pathways, of which HIF-1 was only one pathway.
Since some cell apoptosis is related to mitochondrial dysfunction, which could be caused by intracellular ROS accumulation, we detected the intracellular ROS level in an attempt to figure out the protection of salidroside against cell apoptosis. Flow cytometry showed that on exposure to CoCl2 stimulation, the intracellular ROS level in primary cultured cortical neurons was significantly increased as compared to control, while salidroside pretreatment alleviated the intracellular ROS level elevation. Extra addition of R59949 impaired HIF-1α accumulation, and concurrently increased the intracellular ROS production even in the presence of salidroside, thus suggesting that the reduced HIF-1α accumulation might be related to the increased production of the intracellular ROS. It should be mentioned that the correlation between HIF-1α accumulation and ROS production under hypoxia conditions is very complex and far beyond conclusive, depending on the cytotoxic model used. For example, controversial observations have been reported in different hypoxic cells, thus raising an issue of whether ROS induces HIF-1α accumulation or promotes HIF-1α degradation [30–32]. As a result, our finding on the relationship of ROS and HIF-1α was only valid in the specific experimental setting used in this study.
Nuclear factor-kappa B is widely expressed in the nervous system, and related to many physiological and pathological activities. Nuclear factor-kappa B can be activated by a number of external stimuli, and in vivo studies have demonstrated that cerebral ischemia/hypoxia could also trigger NF-κB activation, playing an anti-apoptotic role [33–39]. However, there are some seemingly contrary results that NF-κB over-expression causes cell apoptosis [40–42]. In this study, Western blot analysis showed that NF-κB protein expression was significantly increased in primary cultured cortical neurons which had undergone CoCl2 stimulation, while pretreatment with different concentrations of salidroside alleviated the increase in NF-κB expression. Our results suggested that NF-κB overexpression might contribute to the CoCl2-induced cell viability loss in primary cultured cortical neurons, and the neuroprotective effect of salidroside might be associated with stabilization of NF-κB protein expression. Despite several studies demonstrating cross-talk between the NF-κB and HIF signaling pathways, a direct link of them has yet to be elucidated [43]. Very intriguingly, NF-κB regulation of HIF-1α could be stimulus-specific or even cell-type-specific [44]. In this sense, our data perhaps contribute to the accumulation of experimental evidence on the relationship between NF-κB and HIF-1α.
Our previous studies confirm that the protective effects of salidroside on cultured hippocampal neurons or PC12 cells against glutamate-, hypoglycemia-, or oxidative stress-induced cell damage are mostly mediated by modulation of apoptosis-related proteins expression, mitochondrial membrane potentials, intracellular calcium flux, and/or ROS production [10–13]. By contrast, this study showed that salidroside could also inhibit the CoCl2-induced cell apoptosis in cultured cortical neurons. In addition to the aforementioned common apoptosis-related mechanisms, however, some hypoxia-dependent molecular mechanisms, including the regulation of HIF-1α and NF-кB, were particularly involved in its neuroprotection under hypoxia conditions. The presence of different mechanisms suggest that salidroside performs various neuroprotective actions in a challenge-specific manner.
In summary, we showed that salidroside pretreatment could protect primary cultured cortical neurons against CoCl2-induced cell apoptosis. The protective effects might be mediated by enhancing the expression of HIF-1α, alleviating the increase of intracellular ROS levels, and inhibiting the increased expression of NF-кB.
References
Darbinyan V, Kteyan A, Panossian A, Gabrielian E, Wikman G, Wagner H (2000) Rhodiola rosea in stress induced fatigue: a double blind cross-over study of a standardized extract SHR-5 with a repeated low-dose regimen on the mental performance of healthy physicians during night duty. Phytomedicine 7:365–371
Lanza A, Martinez M, Matellano L, Carretero C, Castillo L, Sen A, Benito P (2001) Lignan and phenylpropanoid glycosides from Phillyrea latifolia and their in vitro anti-inflammatory activity. Planta Med 67:219–223
Iaremii I, Grigor’eva N (2002) Hepatoprotective properties of liquid extract of Rhodiola rosea. Eksp Klin Farmakol 65:57–59
De Sanctis R, De Bellis R, Scesa C, Mancini U, Cucchiarini L, Dachà M (2004) In vitro protective effect of Rhodiola rosea extract against hypochlorous acid-induced oxidative damage in human erythrocytes. Biofactors 20:147–159
Kucinskaite A, Briedis V, Savickas A (2004) Experimental analysis of therapeutic properties of Rhodiola rosea L. and its possible application in medicine. Medicina (Kaunas, Lithunia) 40:614
Mattioli L, Perfumi M (2007) Rhodiola rosea L. extract reduces stress-and CRF-induced anorexia in rats. J Psychopharmacol 21:742–750
Ming D, Hillhouse B, Guns E, Eberding A, Xie S, Vimalanathan S, Towers G (2005) Bioactive compounds from Rhodiola rosea (Crassulaceae). Phytother Res 19:740–743
Perfumi M, Mattioli L (2007) Adaptogenic and central nervous system effects of single doses of 3% rosavin and 1% salidroside Rhodiola rosea L. extract in mice. Phytother Res 21:37–43
Prasad D, Ram M, Kumar R, Sawhney R, Sharma S, Ilavazhagan G, Kumar D, Banerjee P (2005) Cytoprotective and antioxidant activity of Rhodiola imbricata against tert-butyl hydroperoxide induced oxidative injury in U-937 human macrophages. Mol Cell Biochem 275:1–6
Chen X, Liu J, Gu X, Ding F (2008) Salidroside attenuates glutamate-induced apoptotic cell death in primary cultured hippocampal neurons of rats. Brain Res 1238:189–198
Chen X, Zhang Q, Cheng Q, Ding F (2009) Protective effect of salidroside against H2O2-induced cell apoptosis in primary culture of rat hippocampal neurons. Mol Cell Biochem 332:85–93
Yu S, Liu M, Gu X, Ding F (2008) Neuroprotective effects of salidroside in the PC12 cell model exposed to hypoglycemia and serum limitation. Cell Mol Neurobiol 28:1067–1078
Yu S, Shen Y, Liu J, Ding F (2010) Involvement of ERK1/2 pathway in neuroprotection by salidroside against hydrogen peroxide-induced apoptotic cell death. J Mol Neurosci 40:321–331
Zhang L, Yu H, Sun Y, Lin X, Chen B, Tan C, Cao G, Wang Z (2007) Protective effects of salidroside on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. Eur J Pharmacol 564:18–25
Zhang L, Yu H, Zhao X, Lin X, Tan C, Cao G, Wang Z (2010) Neuroprotective effects of salidroside against beta-amyloid-induced oxidative stress in SH-SY5Y human neuroblastoma cells. Neurochem Int 57:547–555
Wang G, Hazra TK, Mitra S, Lee HM, Englander EW (2000) Mitochondrial DNA damage and a hypoxic response are induced by CoCl2 in rat neuronal PC12 cells. Nucleic Acids Res 28:2135
Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT (1998) Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci USA 95:11715–11720
Hou RCW, Huang HM, Tzen JTC, Jeng KCG (2003) Protective effects of sesamin and sesamolin on hypoxic neuronal and PC12 cells. J Neurosci Res 74:123–133
Jung JY, Roh KH, Jeong YJ, Kim SH, Lee EJ, Kim MS, Oh WM, Oh HK, Kim WJ (2008) Estradiol protects PC12 cells against CoCl2-induced apoptosis. Brain Res Bull 76:579–585
Wang H, Joseph J (1999) Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med 27:612–616
Crompton M (2000) Bax, bid and the permeabilization of the mitochondrial outer membrane in apoptosis. Curr Opin Cell Biol 12:414–419
Gross A, McDonnell JM, Korsmeyer SJ (1999) BCL-2 family members and the mitochondria in apoptosis. Genes Dev 13:1899–1911
Bar-Am O, Weinreb O, Amit T, Youdim MBH (2005) Regulation of Bcl-2 family proteins, neurotrophic factors, and APP processing in the neurorescue activity of propargylamine. FASEB J 19:1899–1901
Yang E, Korsmeyer SJ (1996) Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood 88:386–401
Pugh C, Ratcliffe P (2003) Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med 9:677–684
Wenger R (2002) Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J 16:1151
Chi J, Wang Z, Nuyten D, Rodriguez E, Schaner M, Salim A, Wang Y, Kristensen G, Helland A, Borresen-Dale A (2006) Gene expression programs in response to hypoxia: cell type specificity and prognostic significance in human cancers. PLoS Med 3:395
Lee J, Bae S, Jeong J, Kim S, Kim K (2004) Hypoxia-inducible factor (HIF-1) alpha: its protein stability and biological functions. Exp Mol Med 36:1
Aragones J, Jones DR, Mart NS, Juan MAS, Alfranca A, Vidal F, Vara A, Merida I, Landazuri MO (2001) Evidence for the involvement of diacylglycerol kinase in the activation of hypoxia-inducible transcription factor 1 by low oxygen tension. J Biol Chem 276:10548–10555
Shi H (2009) Hypoxia inducible factor 1 as a therapeutic target in ischemic stroke. Curr Med Chem 16:4593–4600
Liu J, Narasimhan P, Yu F, Chan PH (2005) Neuroprotection by hypoxic preconditioning involves oxidative stress-mediated expression of hypoxia-inducible factor and erythropoietin. Stroke 36:1264–1269
Callapina M, Zhou J, Schmid T, Köhl R, Brüne B (2005) NO restores HIF-1 [alpha] hydroxylation during hypoxia: Role of reactive oxygen species. Free Radic Biol Med 39:925–936
Domanska-Janik K, Bronisz-Kowalczyk A, Zajac H, Zablocka B (2000) Interrelations between nuclear-factor kappa B activation, glial response and neuronal apoptosis in gerbil hippocampus after ischemia. Acta Neurobiol Exp 61:45–52
Mattson M, Culmsee C, Yu Z, Camandola S (2000) Roles of nuclear factor κB in neuronal survival and plasticity. J Neurochem 74:443–456
Ravati A, Ahlemeyer B, Becker A, Klumpp S, Krieglstein J (2001) Preconditioning-induced neuroprotection is mediated by reactive oxygen species and activation of the transcription factor nuclear factor-κB. J Neurochem 78:909–919
Crawford M, Krishnamoorthy R, Rudick V, Collier R, Kapin M, Aggarwal B, Al-Ubaidi M, Agarwal N (2001) Bcl-2 overexpression protects photooxidative stress-induced apoptosis of photoreceptor cells via NF-[kappa] B preservation* 1. Biochem Biophys Res Commun 281:1304–1312
Kim G, Xu J, Song S, Yan P, Ku G, Xu X, Hsu C (2001) Tumor necrosis factor receptor deletion reduces nuclear factor-{kappa} B activation, cellular inhibitor of apoptosis protein 2 expression, and functional recovery after traumatic spinal cord injury. J Neurosci 21:6617
Ramirez S, Sanchez J, Dimitri C, Gelbard H, Dewhurst S, Maggirwar S (2001) Neurotrophins prevent HIV Tat-induced neuronal apoptosis via a nuclear factor-κB (NF-κB)-dependent mechanism. J Neurochem 78:874–889
Yabe T, Wilson D, Schwartz J (2001) NFκB activation is required for the neuroprotective effects of pigment epithelium-derived factor (PEDF) on cerebellar granule neurons. J Biol Chem 276:43313
Clemens J, Stephenson D, Yin T, Smalstig E, Panetta J, Little S, Dietrich W, Bethea J (1998) Drug-induced neuroprotection from global ischemia is associated with prevention of persistent but not transient activation of nuclear factor-{kappa} B in rats* editorial comment. Stroke 29:677
Levites Y, Youdim M, Maor G, Mandel S (2002) Attenuation of 6-hydroxydopamine (6-OHDA)-induced nuclear factor-kappaB (NF-[kappa] B) activation and cell death by tea extracts in neuronal cultures1. Biochem Pharmacol 63:21–29
Ueno T, Sawa Y, Kitagawa-Sakakida S, Nishimura M, Morishita R, Kaneda Y, Kohmura E, Yoshimine T, Matsuda H (2001) Nuclear factor-{kappa} B decoy attenuates neuronal damage after global brain ischemia: A future strategy for brain protection during circulatory arrest. J Thorac Cardiovasc Surg 122:720
Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M (2008) NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 453:807–811
Van Uden P, Kenneth NS, Rocha S (2008) Regulation of hypoxia-inducible factor-1 by NF- B. Biochem J 412:477–484
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant No. 30870881), a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, and the key project of Jiangsu Education Department (Grant No. 08KJA310002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, S., Chen, X., Yang, Y. et al. Neuroprotection against cobalt chloride-induced cell apoptosis of primary cultured cortical neurons by salidroside. Mol Cell Biochem 354, 161–170 (2011). https://doi.org/10.1007/s11010-011-0815-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-0815-4