Abstract
Feeding Wistar rats a high calorie “Western” diet (45% fat) for up to 48 weeks induces obesity and cardiac dysfunction, while a high fat diet (60% fat) induces obesity only. Here we investigated the molecular “footprints” of the two forms of diet-induced obesity in the heart. In rats fed Western diet for a long term, cardiac mRNA transcript levels of malic enzyme were decreased (−72%, P < 0.05), suggesting impaired anaplerotic flux of the Krebs cycle (KC) and mitochondrial dysfunction. In addition, there was a marked decrease in the expression of the transcription factor MEF2C (myocyte enhancer factor 2C) and its target gene SERCA2a (sarco-endo-plasmic reticulum Ca2+-ATPase). Oxidative stress was reflected in reduced transcript levels of manganese superoxide dismutase, glutathione peroxidase 1, and increased protein levels of mitochondrial transcription factor A, suggesting compensatory mitochondrial biogenesis in the face of increased mitochondrial damage. Oxidant injury was accompanied by increased protein glycosylation, increased transcript levels of glutamine fructose 6-phosphate amidotransferase 2, and decreased protein levels of acetyl Co-A carboxylase. Lastly, apoptosis was evident by TUNEL positivity and elevated mRNA transcript levels and activity of caspase 3. Consistent with these results, protein levels of Bcl2 were markedly reduced. We conclude that inadequate supplementation of KC intermediates due to reduced levels of malic enzyme, downregulation of MEF2C and its target gene SERCA2a, oxidative stress, and programmed cell death are all potential contributors to contractile dysfunction of the heart.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is an independent risk factor for the development of heart failure [1]. In a prior study we investigated the effects of different obesogenic diets on the heart, and found that a high caloric Western diet (45% of calories from fat) is associated with cardiac contractile dysfunction in a rat model of diet-induced obesity [2]. However, the mechanism underlying this cardiac dysfunction is unclear. This maladaptation of the heart is especially challenging because in parallel-fed obese animals on a high fat diet, contractile function was normal [2].
Diet-induced obesity has been linked to excessive β-oxidation of long-chain fatty acids without a corresponding increase in Krebs cycle (KC) flux [3]. During conditions of overnutrition, the transcriptional activation of the KC enzymes is inadequate to accommodate increased flux. Furthermore, flux is constrained by increased redox pressure and depletion of supporting intermediates. We proposed that this imbalanced environment contributes to impaired mitochondrial performance and to subsequent contractile dysfunction. We were influenced by our previous observations that the depletion of the KC intermediates in the heart results in decreased contractile function, which is reversed by provision of anaplerotic substrates [4–6].
This study was primarily undertaken to identify molecular “footprints” of diet-induced obesity in the heart. This study was based on the following rationale. In diet-induced obesity, excess availability of fat induces a futile cycle in the heart [2], while excess fat and carbohydrate is associated with a loss of synchronization of substrate uptake and oxidation [2]. In the latter we have observed that in animals fed high fat Western diet, dysregulation of hepatic lipogenesis is a major component of heart failure [7]. Furthermore, chronic exposure of the heart to an excess supply of fuels may have deleterious consequences due to the formation of harmful derivatives of glucose and lipid metabolism such as reactive oxygen species (ROS), and protein glycosylation. Excessive generation of ROS affects a number of cellular processes including mitochondrial structure, function, and metabolism. Furthermore, suppression of glucose oxidation by increased fatty acid supply shunts glucose 6-phosphate into nonglycolytic pathways [8]. Lastly, excessive accumulation of fatty acid derivatives is also associated with insulin resistance and type 2 diabetes [9].
The consequences of a loss of synchronization between substrate uptake and oxidation were explored by: (1) investigating the potential link between contractile dysfunction and impaired KC flux by measuring expression levels of carboxylating enzymes; (2) examining the effect of different diets on the induction of cell damage by measuring the markers of oxidative stress; (3) exploring potential glucose sensing components in the context of adaptation of myocardium to excess glucose supply in the earlier feeding phase to subsequent maladaptation in the later phase due to increased inhibition of glucose oxidation by excessive fatty acid availability; (4) evaluating the role of PPARα-regulated genes on glucose and fatty acid metabolism; and (5) measuring the final outcome of imbalance between β-oxidation and KC flux, increased oxidative stress and glucolipotoxicity by markers of apoptosis. The results obtained explain some of our earlier findings on cardiac contractile dysfunction in rats fed Western diet.
Methods
Animal model and feeding protocol
We used the material from a previously published study [2]. Details are given in the supplement. Acute term (AT) refers to data from animals sacrificed at 1 day or 1 week, short term (ST) to data at 4–8 weeks, intermediate term (IT) at 16–24 weeks, long term (LT) at 32–48 weeks [2]. Using the isolated working heart preparation [10], we have shown that in the same animals contractile function declines by 25% when fed a high fat Western diet [2]. Function was unimpaired in hearts from animals fed only a high fat diet.
Total protein isolation and immunoblotting
Protein isolation, immunoblotting, antibodies, and all other analytical methods are described in the supplement.
Statistical analysis
Results are presented as means ± SEM, and statistically significant differences between groups were calculated by analysis of variance (ANOVA). A value of P < 0.05 was considered significant.
Results
Anaplerosis was decreased with long-term western diet feeding
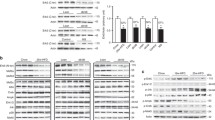
To investigate whether the contractile dysfunction in rats fed Western diet is associated with impaired replenishment of the KC, mRNA transcripts and protein expression of genes involved in anaplerosis were measured. Cardiac mRNA transcript levels of malic enzyme (ME) were reduced with Western diet (−72%, P < 0.05) compared to low fat or high fat diet in the long term (Fig. 1a). The mRNA transcript levels of pyruvate carboxylase (PC) were not changed between three diets (Fig. 1b). Consistent with a decrease in mRNA expression, the protein expression levels of ME were also reduced with Western diet (−40%, P < 0.05) compared to high fat diet in the long term (Fig. 1c). Western blot analysis showed no difference in the content of PC and propionyl-CoA carboxylase (PCC) with three diets (Fig. 1d and e). These findings suggest inadequate supplementation of KC intermediates due to downregulation of ME is responsible for impaired cardiac contractile function.
Anaplerosis: anaplerotic enzymes and the rate limiting enzyme of KC flux were determined by measuring mRNA transcripts of me (a), pc (b), protein expression levels of ME (c), PC (d), and PCC (e) as a function of time on the respective diets. Filled squares represent low fat diet, filled circles represent western diet, and filled triangles represent high fat diet. Values are means ± SEM. * P < 0.05, ** P < 0.01 versus low fat diet at the same age. $ P < 0.05, $$ P < 0.01 compared with Western diet at the same age. See text and abbreviations for details
The majority of research investigating the mechanisms responsible for mitochondrial dysfunction in heart failure has focused on electron transport chain components. Alpha-ketoglutarate dehydrogenase(α-KGDH) catalyzes the conversion of α-ketoglutarate to succinyl-CoA, produces NADH, and directly provides electrons to the respiratory chain. Alterations in the rate of NADH synthesis and delivery to the electron transport chains would, therefore, likely have profound effects on respiratory activity. Recently, it was demonstrated that α-KGDH may be a crucial target of ROS in cells, and inhibition of this enzyme could be critical in the metabolic deficiency induced by oxidative stress and a likely mediator of mitochondrial dysfunction [11]. At the same time, the enzyme itself may generate ROS and, therefore, could contribute to the induction of oxidative stress [12]. The transcript levels of α-kgdh were increased with Western diet in the intermediate term but declined in the long term indicating a dual functional role of this enzyme to oxidative stress with high fat Western diet (Supplement Fig. 1).
MEF2C and target gene SERCA2a were downregulated with Western diet feeding
We have previously shown that in diabetic patients with nonischemic heart failure, there was a decrease in the expression of MEF2C and its regulated genes [13]. As MEF2C binds to the SERCA2a, GLUT4, and MHCα promoter, we focused our analysis on this transcription factor. MEF2C protein levels were significantly decreased in rats fed the Western diet compared to those fed either high fat or low fat diet (Fig. 2a and b). Studies both in animal models as well as in human heart failure have found that significant decrease in SERCA2a expression leads to abnormal Ca2+ handling and a deficient contractile state [14]. Serca2a mRNA transcript levels were mostly unchanged with high fat diet but significantly decreased (−27%, P < 0.05) in the intermediate term with Western diet (Fig. 2c). However, SERCA2a protein levels were markedly reduced with Western diet (−75%, P < 0.001) and were modestly reduced with high fat diet (−16%) in the long term (Fig. 2d and e). This result is in agreement with other studies that had shown deterioration of contractility and overall cardiac efficiency due to the decreased level of SERCA2a protein [15].
Oxidative stress was increased with Western diet
To investigate whether oxidative stress is responsible for western and high fat diet-mediated detrimental effects, we analyzed the biological pathways that are coordinately altered in heart tissue of rats fed such diets. The oxidative stress pathway is composed of genes for ROS production, stress signaling, and antioxidant enzymes. We measured the transcript levels of antioxidant genes such as manganese superoxide dismutase (MnSOD) and glutathione peroxidase 1 (GPX1), which are known to reduce oxidative stress caused by ROS and lipid peroxides. The transcript levels of both mnsod (Fig. 3a) and gpx1 (Fig. 3b) were significantly reduced with long-term Western diet feeding compared with high fat or low fat diet. Western diet also resulted in reduced protein expression of hemeoxygenase 1 (HO-1) in the intermediate and long term (Fig. 3c and d). The mRNA expression of antioxidant enzyme catalase, however, was not affected with either Western or high fat diet (data not shown). The downregulation of antioxidant enzymes with Western diet, thus, may represent an increase of oxidative stress and subsequent cellular damage.
PPARα regulated genes were differentially affected by Western and high fat diet
We measured transcript as well as protein levels of genes involved in putative PPARα-regulatory pathways. Medium-chain fatty acyl-CoA dehydrogenase (mcad), the enzyme that catalyzes the first step in the β- oxidation of fatty acids and is regulated transcriptionally by PPARα [16] is used as a marker to evaluate the in vivo activity of PPARα. Both mRNA transcripts as well as protein levels of mcad were unchanged with Western or high fat diet (data not shown). This finding is consistent with earlier reports [17]. Among the putative causes of myocardial triacylglycerol accumulation are elevations in long-chain fatty acid (LCFA). In the heart, approximately 50% of LCFA uptake is mediated by the fatty acid translocase CD36. Recent studies have shown that CD36 is not confined to fatty transport in the sarcolemma but may be involved in the translocation of fatty acids across the inner mitochondrial membrane in concert with mCPTI [18]. Both cd 36 mRNA transcript (Supplement Fig. 2a) and protein levels (Supplement Fig. 2b and c) were increased with high fat diet in the long term.
The nuclear receptor PPARα is a transcriptional regulator of multiple genes involved in fatty acid utilization in the heart. In “glucolipotoxicity,” glucose appears to downregulate the expression of fatty acid metabolizing genes, through the repression of PPARα. Recently, animal models of obesity have shown abundant OXPAT protein expression in highly oxidative tissues including heart and skeletal muscle that serve as a marker for PPARα activation and fatty acid oxidation [19]. In our animal model, we found that protein expression of OXPAT was unchanged with Western diet, but it was increased only in the intermediate term with high fat diet (Supplement Fig. 2b and c). This long-term exposure to elevated levels of glucose and fatty acid suppresses the expression of several PPARα-regulated genes involved in fatty acid metabolism.
Fatty acid metabolism was impaired
AMP-activated protein kinase (AMPK) regulates mitochondrial fatty acid oxidation, and is a regulator of insulin sensitivity [20]. When activated, AMPK increases fatty acid oxidation by inhibiting acetyl-CoA carboxylase (ACC) and reducing malonyl-CoA (MCD) levels. As a result it relieves inhibition of CPTI [21]. The activation of AMPK also results in the phosphorylation and inhibition of glycerol-3-phosphate acyltransferase (GPAT), the committed step in de novo synthesis of triglyceride (TG) [22]. We observed no significant change in the expression levels of endogenous and phosphorylated AMPKα (Supplement Fig. 3a). However, there was markedly increased phosphorylation of ACC at Ser-79 with Western diet (and a modest increase with high fat diet) in the long term (Supplement Fig. 3b). The mRNA transcripts of mcd did not change with either Western or high fat diet (data not shown). The mcpt I transcripts were increased in the long term with high fat diet (+29%, P < 0.05) and were not significantly changed with Western diet (Supplement Fig. 3c). This may serve as a compensatory mechanism to preserve fat oxidation in the long term with high fat diet. The increase in the mRNA transcripts of gpat 1 in Western and high fat diet in the long-term feeding was not significant (Supplement Fig. 3d). Thus, sustained levels of GPAT 1 with obesity probably contribute to lipid disorders by reducing fatty acid oxidation and promoting de novo glycerolipid synthesis [23].
Glucotoxic pathways and intermediates were activated with Western diet
Intracellular levels of glucose also activate the hexosamine biosynthetic pathway (HBSP) and further induce glycosylation of serine and threonine residues of cytoplasmic and nuclear proteins. We investigated the efficacy of glucose sensing pathways by measuring mRNA transcripts of two isoforms of glutamine fructose 6-phosphate amidotransferase (GFAT) such as gfat1 and gfat2, which catalyze the flux-generating step in HBP [24]. Of the two isoforms, gfat2 is commonly expressed in the heart. Transcript levels of gfat1 were unchanged with three diet groups at all time points studied (Supplement Fig. 4a). In contrast, gfat2 mRNA transcripts were increased (+33%, P < 0.05) only with Western diet in the long term (Supplement Fig. 4b). Anti-O-GlcNAc immunoblots from hearts of Western, high fat, and low fat diet fed rats are shown in Supplement Fig. 4c. Overall protein glycosylation levels were slightly increased with Western diet in the intermediate (+22%) and in the long term (+34%).
Markers of apoptosis were increased with Western diet feeding
TUNEL positive cells were increased with Western diet
TUNEL staining showed low levels of apoptosis in the hearts of rats fed the high fat and low fat diet. In contrast, apoptosis markers were increased in hearts of rats fed the Western diet (Fig. 4a and b). The results suggest that Western diet is associated with activation of programmed cell death.
Markers of apoptosis: the markers of apoptosis were evaluated by TUNEL staining (a and b), protein expression of Bcl2 (c), protein expression ratio of Bcl2 to BAD (d), and the protein expression ratio of Bcl2 to BAX (e). Markers of apoptosis were increased in hearts from rats fed Western diet. See text for details
Western diet changed the Bcl2 family proteins
Bcl2 and its family members are important modulators of cardiac apoptosis in humans as well as in animal models. Bcl2 mRNA is expressed in both developing and adult hearts [25], and the protein is upregulated after coronary occlusion in rat hearts [26]. Overexpression of Bcl2 protects cardiac myocytes from apoptosis [27]. We show that antiapoptotic Bcl2 protein expression was markedly reduced with Western diet in the long term (−85%, P < 0.001) but not with either a low fat or a high fat diet (Fig. 4c). The ratio of protein expression levels of Bcl2 to pro-apoptotic protein BAD (Fig. 4d) and Bcl2 to pro-apoptotic Bax protein (Fig. 4e) was significantly lower with Western diet, which suggests that the balance between pro survival and pro death signals was tipped to favor the latter. Thus, apoptosis is induced in the hearts of rats fed a Western diet for a prolonged time period.
Caspase-3 mRNA transcript levels and activity were increased
Caspase-3 is the key executioner of apoptosis, as it is either partially or totally responsible for the proteolytic cleavage of many key proteins such as the nuclear enzyme poly (ADP-ribose) polymerase (PARP) [28]. The mRNA transcript levels of caspase-3 were markedly increased in rats fed Western diet, compared with those fed on either low or high fat diet (Supplement Fig. 5a). To determine whether the reduced mRNA transcript levels of caspase-3 were consistent with apoptotic activity, heart homogenates from each experimental group were examined for apoptotic activity by monitoring the rate of cleavage of a fluorogenic caspase-3-specific substrate and, thus, apoptotic activity. Animals fed Western diet exhibited higher caspase-3 activity when compared with those fed on either low fat or high fat diet (Supplement Fig. 5b).
Mitochondrial biogenesis was increased with Western diet
Mitochondrial damage is accompanied by markers of mitochondrial biogenesis including elevation in the level of mitochondrial regulatory protein such as mitochondrial transcription factor A (mtTFA) also known as mtTF1, or TFAM [29]. mtTFA is required for many aspects of mitochondrial biogenesis including replication and transcription of mitochondrial DNA (mtDNA). Protein expression of mtTFA was significantly increased in the hearts of rats fed Western diet (Supplement Fig. 5c) in the long term, which suggests a compensatory mitochondrial biogenesis in the face of increased mitochondrial damage due to oxidative stress with Western diet.
Discussion
The five main findings of our study are summarized in Fig. 5, and these suggest that (1) in rats fed Western diet, there is decrease in the expression of ME, suggesting impaired anaplerotic flux of the KC; (2) oxidative injury in the hearts of rats fed a Western diet is suggested by reduced mRNA transcript levels of antioxidant enzymes mnsod and gpx1; (3) exacerbation of oxidant injury due to glucolipotoxicity in rats fed Western diet is indicated by increased protein glycosylation and mRNA transcript levels of gfat2 and inhibition of ACC; (4) induction of apoptosis as a consequence of oxidant injury in hearts of obese animals fed on Western diet is demonstrated by increased TUNEL staining, increased mRNA transcripts and activity of caspase 3, significant decrease in the expression of anti apoptotic protein Bcl2, and increased expression of mtTFA; and (5) increased expression of mtTFA further suggests compensatory mitochondrial biogenesis in the face of increased mitochondrial damage due to oxidative stress with Western diet.
The changes in cardiac metabolism with Western diet over the extended time course of our study largely reflect adaptations and maladaptations in mitochondria. In a parallel study to this study, we have shown that high fat Western diet decreases the unsaturated-to-saturated fatty acid ratio and impairs cardiac mitochondrial membrane fluidity [7]. In addition, the inadequate induction of a cassette of fatty acid responsive genes (especially CTE1, MTE1, and UCP3) and resultant impaired fatty acid oxidation with Western diet suggest that mitochondrial dysfunction is the most likely candidate for the development of cardiac dysfunction that occurs with the Western diet and not with the high fat diet [2]. The majority of investigations into potential sites responsible for degeneration of mitochondrial function during the development of heart failure have focused on alterations in the activities and composition of various electron transport chain components. α-KGDH is the rate-limiting enzyme of the KC [30], and the inhibition of α-KGDH has been reported to have detrimental effects on mitochondrial respiration in various diseases such as cardiac ischemia–reperfusion injury, Alzheimer’s disease, and Parkinson’s disease [11]. In addition, α-KGDH seems to be highly sensitive to free radical-mediated inactivation [12]. The findings presented in this study suggest that α-KGDH is a likely candidate responsible for mitochondrial dysfunction due to its unique ability for activation and inhibition in response to oxidative stress.
We have shown previously that in the heart there is a greater activation of fatty acid oxidation with high fat diet as compared to Western diet [2]. According to Randle’s hypothesis we observed that glucose oxidation rates were suppressed, especially with high fat diet and in the long term with Western diet [2]. We also observed that glucose oxidation decreases not only acutely and chronically with Western and high fat diet in the earlier feeding phase, but also in rats fed low fat diet in the long term [2]. This decrease in glucose oxidation may be associated with the development of insulin resistance with aging [31].
While fatty acids are able to modulate gene expression in the heart most likely through activation of nuclear receptor PPARα [32], information concerning glucose-regulated gene expression in the context of diet-induced obesity in the heart is relatively limited. Further studies are necessary to define the effects of excess glucose availability on cardiac function and metabolism in the setting of a rat model of diet-induced obesity. Increased flux through the protein glycosylation pathway has been implicated in the pathogenesis of diabetes [33]. The targets of glycosylation that mediate cadiotoxicity are not clearly defined. There is some evidence that SERCA2a is regulated by glycosylation and inactivation of Sp1 [34]. As Sp1 serves as a master transcriptional regulator of metabolic processes in all cells, exploring Sp1-mediated signaling may provide important insights into the effects of aberrant glycosylation on Sp1. Previous studies in our laboratory have shown downregulation of MEF2C and its regulated genes (SERCA2a and GLUT4) in the failing hearts of patients with diabetes, which further suggests a transcriptional mechanism that might contribute to the pathogenesis and contractile dysfunction of heart failure [13]. Decrease in SERCA2a expression with a concomitant decrease in the expression of MEF2C in response to Western diet feeding is consistent with previous studies showing a decrease in MEF2C and SERCA2a expression with diabetes [35].
A study of this magnitude has also many limitations. For example, we have not assessed anaplerotic substrate fluxes or the possible reversibility of the remodeling of genes involved in glucose and fatty acid metabolism; especially whether the cardiac dysfunction in Western diet is reversible. Our own clinical observations suggest that weight loss in patients after bariatric surgery is accompanied by favorable changes in cardiac function as well as gene expression and triglyceride levels in muscle [36].
In conclusion, hearts from rats fed Western diet demonstrate a decline in the expression of ME, suggesting impaired anaplerotic flux of the KC. Oxidant injury is exacerbated by decreased antioxidant gene expression. Downregulation of Bcl2, increased mitochondrial biogenesis, and caspase-3 activation suggest oxidative stress-induced apoptosis underlying impaired contractile function of the heart. The results provide a new aspect for the physiological importance of anaplerosis in maintaining metabolic homeostasis and contractile function in the heart.
Abbreviations
- ACC:
-
Acetyl-CoA carboxylase
- αKGDH:
-
a-Ketoglutarate dehydrogenase
- AMPK:
-
5′ AMP-activated protein kinase
- ANOVA:
-
Analyses of variance
- AT:
-
Acute term (1–7 days)
- CPT:
-
Carnitine palmitoyl transferase
- CTE:
-
Cytosolic thioesterase
- GFAT:
-
Glutamine fructose 6-phosphate amidotransferase
- GLUT:
-
Glucose transporter
- GPAT:
-
Glycerol 3-phosphate acyltransferase
- GPX1:
-
Glutathione peroxidase 1
- HBSP:
-
Hexosamine biosynthetic pathway
- HO-1:
-
Heme oxygenase 1
- IT:
-
Intermediate term (16–24 weeks)
- KC:
-
Krebs cycle
- LT:
-
Long term (32–48 weeks)
- ME:
-
Malic enzyme
- MERF2c:
-
Myocyte enhancer factor 2c
- MnSOD:
-
Manganese superoxide dismutase
- MTE:
-
Mitochondrial thioesterase
- mtTFA/TFAM:
-
Mitochondrial transcription factor A
- NAD (H):
-
Nicotinamide adenine dinucleotide (reduced)
- OXPAT:
-
Lipid droplet proteins of the PAT (perilipin, adipophilin, and TIP47) family in highly oxidative tissues
- PC:
-
Pyruvate carboxylase
- PCC:
-
Propionyl-CoA carboxylase
- PARP:
-
Poly (ADP-ribose) polymerase
- PPAR:
-
Peroxisome proliferator-activated receptor
- ROS:
-
Reactive oxygen species
- SERCA:
-
Sarco-endoplasmatic reticulum calcium ATPase
- ST:
-
Short term (4–8 weeks)
- TG:
-
Triglycerides
- UCP:
-
Uncoupling protein
References
Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan R (2002) Obesity and the risk of heart failure. N Engl J Med 347:305–313
Wilson CR, Tran MK, Salazar KL, Young ME, Taegtmeyer H (2007) Western diet, but not high fat diet, causes derangements of fatty acid metabolism and contractile dysfunction in the heart of Wistar rats. Biochem J 406:457–467
Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM (2008) Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7:45–56
Russell RR, Taegtmeyer H (1991) Pyruvate carboxylation prevents the decline in contractile function of rat hearts oxidizing acetoacetate. Am J Physiol 261:H1756–H1762
Gibala MJ, Young ME, Taegtmeyer H (2000) Anaplerosis of the citric acid cycle: role in energy metabolism of heart and skeletal muscle. Acta Physiol Scand 168:657–665
Sorokina N, O’Donnell JM, McKinney RD, Pound KM, Woldegiorgis G, LaNoue KF, Ballal K, Taegtmeyer H, Buttrick PM, Lewandowski ED (2007) Recruitment of compensatory pathways to sustain oxidative flux with reduced carnitine palmitoyltransferase I activity characterizes inefficiency in energy metabolism in hypertrophied hearts. Circulation 115:2033–2041
Harmancey R, Wilson CR, Wright NR, Taegtmeyer H (2010) Western diet changes cardiac acyl-CoA composition in obese rats: a potential role for hepatic lipogenesis. J Lipid Res 51:1380–1393
Young ME, Yan Z, Razeghi P, Cooksey RC, Guthrie PH, Stepkowski SM, McClain DA, Tian R, Taegtmeyer H (2007) Proposed regulation of gene expression by glucose in rodent heart. Gene Reg Syst Biol 1:251–262
Zhang D, Liu ZX, Choi CS, Tian L, Kibbey R, Dong J, Cline GW, Wood PA, Shulman GI (2007) Mitochondrial dysfunction due to long-chain Acyl-CoA dehydrogenase deficiency causes hepatic steatosis and hepatic insulin resistance. Proc Natl Acad Sci USA 104:17075–17080
Taegtmeyer H, Hems R, Krebs HA (1980) Utilization of energy-providing substrates in the isolated working rat heart. Biochem J 186:701–711
Huang HM, Zhang H, Xu H, Gibson GE (2003) Inhibition of the alpha-ketoglutarate dehydrogenase complex alters mitochondrial function and cellular calcium regulation. Biochim Biophys Acta 1637:119–126
Tretter L, Adam-Vizi V (2005) Alpha-ketoglutarate dehydrogenase: a target and generator of oxidative stress. Philos Trans R Soc Lond B Biol Sci 360:2335–2345
Razeghi P, Young ME, Cockrill TC, Frazier OH, Taegtmeyer H (2002) Downregulation of myocardial myocyte enhancer factor 2C and myocyte enhancer factor 2C-regulated gene expression in diabetic patients with nonischemic heart failure. Circulation 106:407–411
Byrne MJ, Power JM, Preovolos A, Mariani JA, Hajjar RJ, Kaye DM (2008) Recirculating cardiac delivery of AAV2/1SERCA2a improves myocardial function in an experimental model of heart failure in large animals. Gene Ther 15:1550–1557
Trost SU, Belke DD, Bluhm WF, Meyer M, Swanson E, Dillmann WH (2002) Overexpression of the sarcoplasmic reticulum Ca(2+)-ATPase improves myocardial contractility in diabetic cardiomyopathy. Diabetes 51:1166–1171
Gulick T, Cresci S, Caira T, Moore D, Kelly D (1994) The peroxisome proliferator-activated receptor regulates mitochondrial fatty acid oxidative enzyme gene expression. Proc Natl Acad Sci USA 91:11012–11016
Nagao M, Parimoo B, Tanaka K (1993) Developmental, nutritional, and hormonal regulation of tissue-specific expression of the genes encoding various acyl-CoA dehydrogenases and alpha-subunit of electron transfer flavoprotein in rat. J Biol Chem 268:24114–24124
Bonen A, Campbell SE, Benton CR, Chabowski A, Coort SL, Han XX, Koonen DP, Glatz JF, Luiken JJ (2004) Regulation of fatty acid transport by fatty acid translocase/CD36. Proc Nutr Soc 63:245–249
Wolins NE, Quaynor BK, Skinner JR, Tzekov A, Croce MA, Gropler MC, Varma V, Yao-Borengasser A, Rasouli N, Kern PA, Finck BN, Bickel PE (2006) OXPAT/PAT-1 is a PPAR-induced lipid droplet protein that promotes fatty acid utilization. Diabetes 55:3418–3428
Ruderman N, Prentki M (2004) AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat Rev Drug Discov 3:340–351
McGarry JD (2002) Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 51:7–18
Cao J, Li JL, Li D, Tobin JF, Gimeno RE (2006) Molecular identification of microsomal acyl-CoA:glycerol-3-phosphate acyltransferase, a key enzyme in de novo triacylglycerol synthesis. Proc Natl Acad Sci USA 103:19695–19700
Nagle CA, An J, Shiota M, Torres TP, Cline GW, Liu ZX, Wang S, Catlin RL, Shulman GI, Newgard CB, Coleman RA (2007) Hepatic overexpression of glycerol-sn-3-phosphate acyltransferase 1 in rats causes insulin resistance. J Biol Chem 282:14807–14815
Fulop N, Marchase RB, Chatham JC (2007) Role of protein O-linked N-acetyl-glucosamine in mediating cell function and survival in the cardiovascular system. Cardiovasc Res 73:288–297
Kajstura J, Mansukhani M, Cheng W, Reiss K, Krajewski S, Reed JC, Quaini F, Sonnenblick EH, Anversa P (1995) Programmed cell death and expression of the protooncogene bcl-2 in myocytes during postnatal maturation of the heart. Exp Cell Res 219:110–121
Misao J, Hayakawa Y, Ohno M, Kato S, Fujiwara T, Fujiwara H (1996) Expression of bcl-2 protein, an inhibitor of apoptosis, and Bax, an accelerator of apoptosis, in ventricular myocytes of human hearts with myocardial infarction. Circulation 94:1506–1512
Kirshenbaum L, de Moissac D (1997) The bcl-2 gene product prevents programmed cell death of ventricular myocytes. Circulation 96:1580–1585
Thornberry NA, Lazebnik Y (1998) Caspases: enemies within. Science 281:1312–1316
Shen X, Zheng S, Thongboonkerd V, Xu M, Pierce WM Jr, Klein JB, Epstein PN (2004) Cardiac mitochondrial damage and biogenesis in a chronic model of type 1 diabetes. Am J Physiol Endocrinol Metab 287:E896–E905
Cooney GJ, Taegtmeyer H, Newsholme EA (1981) Tricarboxylic acid cycle flux and enzyme activities in the isolated working rat heart. Biochem J 200:701–703
Slawik M, Vidal-Puig AJ (2006) Lipotoxicity, overnutrition and energy metabolism in aging. Ageing Res Rev 5:144–164
Barger PM, Kelly DP (2000) PPAR signaling in the control of cardiac energy metabolism. Trends Cardiovasc Med 10:238–245
Cooksey RC, McClain DA (2002) Transgenic mice overexpressing the rate-limiting enzyme for hexosamine synthesis in skeletal muscle or adipose tissue exhibit total body insulin resistance. Ann NY Acad Sci 967:102–111
Clark RJ, McDonough PM, Swanson E, Trost SU, Suzuki M, Fukuda M, Dillmann WH (2003) Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J Biol Chem 278:44230–44237
Netticadan T, Temsah RM, Kent A, Elimban V, Dhalla NS (2001) Depressed levels of Ca2+-cycling proteins may underlie sarcoplasmic reticulum dysfunction in the diabetic heart. Diabetes 50:2133–2138
Leichman JG, Wilson EB, Scarborough T, Aguilar D, Miller CC III, Yu S, Algahim MF, Reyes M, Moody FG, Taegtmeyer H (2008) Dramatic reversal of derangements in muscle metabolism and diastolic left ventricular function after bariatric surgery. Am J Med 121:966–973
Acknowledgments
This study was supported in part by a National Heart, Lung and Blood Institute grant (RO1HL73162). We thank Mei Gong for technical assistance, Tommy Reese for TUNEL staining, and Roxy A. Tate and Rebecca Salazar for help with the preparation of the manuscript.
Disclosures
There are no financial conflicts.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ballal, K., Wilson, C.R., Harmancey, R. et al. Obesogenic high fat western diet induces oxidative stress and apoptosis in rat heart. Mol Cell Biochem 344, 221–230 (2010). https://doi.org/10.1007/s11010-010-0546-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-010-0546-y