Abstract
Survivin, an important inhibitor of apoptosis, has been found to play an important role in the initiation, progression, and chemoradioresistance of human malignancies. Previously, we have reported that upregulation of survivin in oral squamous cell carcinoma correlates with poor prognosis and chemoresistance. The aim of this study was to assess prognostic significance of survivin protein expression in RCC and analyze its correlation with radiosensitivity of RCC cells. RT-PCR and Western blot assays were performed to detect survivin mRNA and protein expression in normal human kidney epithelial cell line (HKEC) or RCC cell lines. The expression of survivin mRNA in RCC and corresponding nontumor kidney tissues was also detected by RT-PCR. Immunohistochemistry was performed to determine survivin protein expression in 75 cases of RCC tissue samples. Moreover, the association of survivin protein expression with clinicopathogical factors and prognosis of RCC patients was statistically analyzed. Small interfering RNA was used to knockdown the endogenous survivin expression in RCC cell line (ACHN) and evaluate the effects of survivin knockdown on proliferation, apoptosis, and radiosensitivity of RCC cell line. RCC cells showed sufficient expression of survivin mRNA and protein, but the expression of survivin gene was not detected in normal HKEC. Moreover, the expression level of survivin mRNA in RCC tissues was significantly higher than that in corresponding nontumor kidney tissues. The immunostaining of survivin protein was mainly located in cytoplasm of RCC tumor cells. Tumor pathological stage (P = 0.028), grade (P = 0.004), and lymph node metastasis (P = 0.017) of RCC patients were significantly correlated with survivin protein expression. In addition, patients with high survivin levels had a significantly shorter overall survival than those with low levels (P < 0.001), and the expression of survivin protein was an independent prognostic factor for RCC patients (P = 0.008). The expression of survivin gene could be reduced in RCC cell line and survivin knockdown could inhibit growth and enhance in vivo radiosensitivity of RCC cell line by inducing apoptosis enhancement. Taken together, the status of survivin protein expression may be an independent factor for predicting the prognosis of RCC patients and tumor-specific survivin knockdown combined with radiotherapy will be a potential strategy for RCC therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal cell carcinoma (RCC) is a type of kidney cancer in which the cancerous cells are found in the lining of very small tubes in the kidney [1]. RCC has become the most common type of kidney cancer in adults, responsible for approximately 80% of cases. In spite of the advances made in clinical treatment including radical or partial nephrectomy, targeted cancer therapies, immunotherapy and chemo- or radiotherapy, the prognosis of RCC patients especially with metastasis is still very poor [2]. Renal tumorigenesis is a complex and a multistep process determined by environmental and genetic factors. Thus, it is essential to identify novel molecular markers underlying the development of RCC and predicting its prognosis, which will help us to explore additional prognostic factors to identify RCC patients at high risk of tumor progression and develop more effective therapeutic strategies.
Survivin, a member of the family of inhibitors apoptosis proteins (IAPs), is a bifunctional protein that suppresses apoptosis and regulates cell division [3]. IAPs are a family of proteins containing one or more characteristic BIR domains. Among the several IAPs, survivin is the smallest member, a 16.5-kDa protein that contains only a single BIR and no RING domain. The overexpression of survivin has been found in a variety of human malignancies, such as hepatocelluar carcinoma, colorectal cancer, lung cancer, pancreatic cancer, and osteosarcoma [4–8]. Moreover, the overexpression of survivin expression has been found to be correlated with radioresistance of human cancers [9, 10]. Thus, survivin might play important roles in malignant transformation and development.
In previous reports, we showed the status of survivin mRNA expression was a potential prognostic factor for oral squamous cell carcinoma (OSCC) patients and siRNA-mediated survivin downregulation could become a novel strategy for chemosensitization of human OSCCs, but the clinical significance of survivin protein expression in RCC has not been systematically investigated. The aim of this study was to investigate the expression of survivin protein in RCC cells or tissues and determine its prognostic significance and in clinical CRC. Meanwhile, we also evaluate the association of survivin protein expression with radiosensitivity of RCC cell lines.
Materials and methods
Cell lines
Three human renal cell cancer cell lines (ACHN, 769-P and 786-O) were obtained from Institute of Cell Biology (Shanghai, China). A normal human kidney epithelial cell line (HKEC) was established and preserved in our lab. All cell lines were cultured in RPMI 1640 (Invitrogen, Inc.) supplemented with 10% fetal bovine serum (FBS) in an atmosphere containing 50 ml/l CO2 at 37°C.
Collection of tissue samples
A total of 75 primary RCC and 20 corresponding nontumor tissues were collected from the Department of Urology in Xi Jing Hospital. All patients approved of this study under documented informed consent, and underwent operation at our institute between 2000 and 2002. None of them received preoperative treatments such as chemotherapy and radiotherapy. Clinicopathologic factors were shown in Table 1. Written informed consent was obtained from all patients. Every patient was definitively identified as having RCC based on their clinicopathologic findings. All patients were regularly followed up, and survival data were ascertained through patient records. This study was approved by the Ethics Committee of Shannxi Province Medical Association. All tissue samples were immediately frozen in liquid nitrogen and kept at −80°C.
RT-PCR analysis of survivin mRNA expression
Total RNA was isolated for Trizol reagent according to the instructions and cDNA was reversibly transcribed from the isolated mRNA using an MMLV reagent kit (Clontech, USA) following the manufacturer’s instructions. The primers of survivin gene were designed as follows: sense, 5′-ATGGGTGCCCCGACGTTG-3′; reverse: 5′-AGAGGCCTCAATCCATGG-3′. As an internal control, β-actin primers were designed as: sense, 5′-GTGCGTGACATTAAGGAG-3′; reverse, 5′-CTAAGTCATAGTCCGCC-3′. The amplification conditions: denaturing at 94°C for 3 min, 45 cycles at 94°C for 5 s and at 57°C for 5 s. The amplification products were visualized by electrophoresis on a 1.5% agarose gel stained with ethidium bromide.
Western blot analysis
Cells were lyzed in buffer containing 50 mM Tris–HCl (pH 7.4), 125 mM NaCl, 0.1% Triton-X (Wako Pure Chemical Industries, Osaka, Japan) and 5 mM EDTA containing both 1% (v/v) protease inhibitor and 1% (v/v) phosphatase inhibitor cocktail II (Sigma). Forty micrograms of each extracted protein was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), followed by electrotransfer onto a PVDF membrane (Millipore, Bedford, MA, USA). Rabbit polyclonal antibody to survivin (Upstate, Charlottesville, VA, USA) was used for the primary antibody. As a control, an antibody to β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was also used. Horseradish peroxidase-conjugated sheep anti-rabbit IgG antibody (Boster, Wuhan, China) was used as a secondary antibody for enhanced chemiluminescence using a chemiluminescence kit (NEN™ Life Science Products Inc, Boston, MA).
Immunostaining of survivin protein expression
Tissues were fixed in formalin, embedded in paraffin and cut into 3-μm sections. The sections were deparaffinized in xylene, dehydrated in a graded ethanol series, and then immersed in methanol with 0.3% hydrogen peroxide for 15 min to inhibit endogenous peroxidase activity. Thereafter, the sections were immunostained using two types of rabbit polyclonal antibody against human survivin (1:500 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rinsed five times with PBS, and then incubated with peroxidase-conjugated anti-rabbit IgG (Boster, Wuhan, China). The sections were then developed with 3,3′-diaminobenzidine tetrahydrochloride and counterstained with hematoxylin. Slides were read independently by two observers using light microscopy. Tumors were scored on a four tiered system, with 0–10% of carcinoma cells staining called negative, 10–25% positively scored as 1+, 26–50% scored as 2+, 51–75% scored as 3+, and 76–100% scored as 4+.
Plasmids construction and transfection
DNA oligonucleotides targeting human survivin gene (GenBank NM_001168) were synthesized as the following: shRNA/survivin, sense: 5′-GATCCACTGGACAGAGAAAGAGCCTTCAAGAG-AGGCTCTTTCTCTGTCCAGTTTTTTTGTCGACA-3′; shRNA/control, sense: 5′-GATCCGACT-TCATAAGGCGCATGCACTTCAAGAGAGTGCATGCGCCTTATGAAGTCTTTTTTGTCGACA-3′. Then, these DNA oligonucleotides were inserted into the BamHI and HindIII sites of pSilencer4.1-CMVneo according to the manufacturer’s instructions (Ambion Inc., Austin, Texas, USA). All the inserted sequences were verified by DNA sequencing. Each vector (pSil-shRNA/survivin and pSil-shRNA/control) contains the SV40 early promoter to provide G418 resistance in mammalian cells. Renal cell cancer cell (ACHN) was transfected with different vectors (pSil-shRNA/survivin and pSil-shRNA/control) using LipofectAMINE 2000 (Invitrogen, USA) according to the manufacturer’s instructions. Then, 72 h later after transfection, the cells were collected and used to for next experiments.
Cell proliferation assay
Seventy-two hours later after transfection, a total of 5.0 × 103 mock or transfected ACHN cells/well were seeded into a 24-well plate for 48 h. Then, they were treated with trypsin and stained with trypan blue. Viable cells, which excluded trypsan blue dye, were counted in quadruplicate with a Countess (Invitrogen, USA). The relative number of viable cells was calculated as percentage of mock ACHN cells.
Colonogenic survival assay
Seventy-two hours later after transfection, a total of 5.0 × 105 mock or transfected ACHN cells were plated in six-well plates for 48 h in complete medium, then cells were irradiated using a 6-mV X-ray with a linear accelerator (Elekta, Stockholm, Sweden). The medium was then replaced with fresh medium and incubated for 14 days. Colonies were stained with PBS containing 0.04% crystal violet and 0.5% paraformaldehyde for about 10 min. Staining liquid was aspirated and colonies counted. The PE represents the percentage of cells seeded that grow into colonies under a specific culture condition of a given cell line. The clonogenic survival is PE-normalized percentage of irradiated cells seeded that grow into colonies. The survival fraction, expressed as a function of irradiation, was calculated as: survival fraction = colonies counted/(cells seeded × PE/100).
TUNEL assay
Apoptotic cells were detected by terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuracil triphosphate (dUTP) nick-end labeling (TUNEL), using an in situ cell death detection kit (Boehringer Mannheim, Mannhiem, Germany). The assay was performed as per the manufacturer’s instructions, with minor modifications. Briefly, after routine deparaffinisation and treatment with 3% H2O2, sections were digested with proteinase K (20 mg/ml; 15 min) and incubated with the reaction mixture (1:100; 30 min) at 37°C. Incorporated fluorescein was detected with horse-radish peroxidase (POD) after a 30-min incubation at 37°C and subsequent dyed with DAB.
Statistical analysis
All statistical analyses were performed using the SPSS 13.0 statistical software. Student’s t-and Mann–Whitney U-tests were used to analyze the correlation between survivin expression and clinicopathologic factors. Survival curves were plotted by the Kaplan–Meier method and compared by the log-rank test. Prognostic factors were evaluated by univariate and multivariate analyses. P < 0.05 was considered statistically significance.
Results
Detection of survivin mRNA and protein expression in cell lines
RT-PCR and Western blot assays were performed to detect the expression levels of survivin mRNA and protein in three human RCC cell lines (ACHN, 769-P and 786-O) and a normal HKEC, respectively. As shown in Fig. 1a, we found sufficient expression levels of survivin mRNA in three RCC cell lines, but the expression of survivin mRNA in HKEC cell line was not detected. The results of Western blot analysis were consistent with the results of RT-PCR (Fig. 1b).
Detection of survivin mRNA expression in tissue samples
Then, RT-PCR assay was performed to detect the expression of survivin mRNA in 20 RCC and corresponding nontumor kidney tissues. As shown in Fig. 2, the averaged level of survivin mRNA expression in RCC tissues (0.85 ± 0.11) was significantly higher than that in corresponding nontumor tissues (0.08 ± 0.02; P < 0.05). Therefore, it was concluded that survivin might play an important role in progression of RCC.
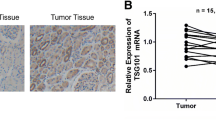
Immunostaining of survivin protein expression in RCC tissue samples
Immunohistochemistry was performed to detect the expression and subcellular localization of survivin protein in RCC tissues. The positive staining of survivin protein was mainly found in the cytoplasm of RCC tumor cells (Fig. 3). An intensity score of ≥2 was used to classify tumor patients with high survivin expression group, and <2 intensity score was used to classify tumor patients with low survivin protein expression group. Thirty-five of 75 cases (46.7%) was considered as survivin-low group, while forty of 75 cases (52.3%) was considered as survivin-high group.
Relations between survivin expression and clinicopathological factors
Relationships between survivin protein expression and clinical factors were analyzed. Table 1 summarized relationships between survivin protein expression and pathological factors. Tumor pathological stage (P = 0.028), grade (P = 0.004), and lymph node metastasis (P = 0.017) were significantly correlated with the expression of survivin protein. However, there was no association between the expression of survivin protein and other factors including age, sex, tumor size, histology and distant metastasis (P = 0.687, 0.578, 0.852, 0.892, and 0.323, respectively).
Correlation between survivin expression and patients’ survival
The correlation between the survivin expression and patients’ survival was explored by the Kaplan–Meier analysis. Patients with high survivin levels had a significantly shorter overall survival than those with low levels (log-rank test, P < 0.001) (Fig. 4). Then, univariate and multivariate regression analyses were performed with the Cox proportional hazards regression model to analyze the independent factors related to prognosis (Table 2). By univariate Cox regression analysis, high pathological stage and grade, lymph node metastasis, distant metastasis, and higher survivin expression were significantly correlated with poor overall survival (P = 0.032, 0.009, 0.016, 0.028, and 0.032, respectively). By multivariate Cox regression analysis, high survivin protein expression, lymph node metastasis, and distant metastasis were independent prognostic factors for overall survival (P = 0.008, 0.005, and 0.013, respectively).
Knockdown of survivin expression by RNAi in human RCC cell line (ACHN)
To investigate the biological role of survivin in RCC progression, small hairpin RNA was employed to knockdown endogenous survivin gene expression in the human RCC cell line ACHN, which has been shown to have robust expression of survivin. Compared with those in mock or pSil-shRNA/control-transfected ACHN cells, the levels of survivin mRNA and protein expression in pSil-shRNA/survivin-transfected ACHN cells were significantly downregulated by approximately 44.5% and 50.6%, respectively (Fig. 5; P < 0.05). It was shown that most survivin mRNA or protein could be degraded by anti-survivin siRNA in RCC cell line.
RT-PCR and Western blot analysis of survivin mRNA and protein expression in ACHN cells with different treatments. The levels of survivin mRNA and protein expression in pSil-shRNA/survivin-transfected ACHN cells were significantly lower than those in mock or pSil-shRNA/control-transfected ACHN cells. β-actin was used as an internal control
Knockdown of survivin expression by RNAi could inhibit growth of RCC cells
MTT assay was performed to investigate the effect of survivin knockdown on cellular proliferation of ACHN cells. ACHN cells were transfected with pSil-shRNA/survivin, pSil-shRNA/control or medium alone, and the proliferation of cells was evaluated. At day 5, ACHN cells transfected with pSil-shRNA/survivin displayed a significant reduction in cell proliferation by 57.3 ± 3.6% (P < 0.01). A slight decrease in cell proliferation was also observed in ACHN cells transfected with pSil-shRNA/control, but these differences were not statistically significant compared with the mock-treated ACHN cells (P > 0.05) (Fig. 6a).
Effects of RNAi-mediated survivin knockdown on proliferation and apoptosis of ACHN cells. a MTT analysis of cell viability. The viability of ACHN cells was significantly reduced by pSil-shRNA/survivin. * P < 0.05 and ** P < 0.01 versus mock or pSil-shRNA/control-transfected ACHN cells. b TUNEL analysis of cell apoptosis. The apoptotic rate of pSil-shRNA/survivin-transfected ACHN cells was significantly higher than that of mock or pSil-shRNA/control-transfected ACHN cells (P < 0.05)
Knockdown of survivin expression by RNAi could induce apoptosis of RCC cells
To investigate whether RNAi-mediated survivin knockdown could induce apoptosis of RCC cells, TUNEL assay was performed to analyze the apoptosis of mock or transfected ACHN cells. As shown in Fig. 6b, the apoptotic rate of pSil-shRNA/survivin-transfected ACHN cells (18.4 ± 3.2%) was significantly enhanced in comparison with those of mock or pSil-shRNA/control-transfected ACHN cells, which were 6.8 ± 1.2% and 8.5 ± 2.3% (P < 0.05). Therefore, the growth inhibition of ACHN cells induced by survivin knockdown might be correlated with increased apoptosis.
Knockdown of survivin expression by RNAi could enhance in vitro radiosensitivity of RCC cells
Some studies have shown that the overexpression of survivin is associated with radioresistance of human cancers, but the role of survivin expression in radiosensitivity of RCC cells is still unclear. To ascertain if siRNA-mediated attenuation of survivin expression leads to a subsequent sensitizing effect to irradiation, cellular radiosensitivity was assessed by colonogenic survival assay (Fig. 7a). The ACHN cells transfected with pSil-shRNA/survivin displayed significantly increased sensitivity to irradiation. The surviving fraction of ACHN cells transfected pSil-shRNA/survivin at 2.0 and 6.0 Gy was decreased by 13.4 ± 2.5% (P < 0.05) and 32.6 ± 1.4% (P < 0.01), respectively. Next, we investigated the possible mechanisms of radiosensitivity enhancement by pSil-shRNA/survivin. As shown in Fig. 7b, pSil-shRNA/survivin combined with irradiation could significantly enhance apoptosis of ACHN cells, compared with mock treatment or pSil-shRNA/control combined with the same dose of irradiation. Therefore, RNAi-mediated survivin knockdown enhances radiosensitivity of RCC cells by inducing apoptosis enhancement.
Effects of RNAi-mediated survivin knockdown on in vitro radiosensitivity of ACHN cells. a Colonogenic survival analysis of cell radiosensitivity. The surviving fraction of pSil-shRNA/survivin-transfected ACHN cells was significantly reduced at different doses of irradiation. Colony efficiencies (determined by dividing the number of survival colonies by the number of cells seeded) were plotted as a function of each individual treatment. b TUNEL analysis of apoptosis in ACHN cells with different doses of irradiation. The apoptotic rate of pSil-shRNA/survivin-transfected ACHN cells was significantly increased at different doses of irradiation. * P < 0.05 and ** P < 0.01 versus mock or pSil-shRNA/control-transfected ACHN cells
Discussion
Renal carcinogenesis is a complex and incompletely understood process which is determined by environmental and genetic factors [11]. Up to now, the molecular mechanisms underlying the development of RCC are still poorly understood. Therefore, it is crucial to exploit molecular markers that can accurately represent biological features of tumors and predict the outcome, which will help us to perform tailored therapy for individual cases.
Many studies have documented the overexpression of anti-apoptotic factors such as the inhibitors of apoptosis proteins (IAPs) in a variety of solid tumors [12, 13]. The IAPs, which are widely expressed in all kinds of malignancies, are encoded by the highly conservative anti-apoptosis gene family and play important roles in the regulation of apoptosis [14]. Survivin, a new member of IAP gene family that has been found recently, inhibits apoptosis via its baculovirus inhibitor of apoptosis repeat (BIR) protein domain by either directly or indirectly interfering with the function of caspases [15, 16]. The overexpression of survivin has been detected in many human solid tumors. Furthermore, in several tumors, survivin overexpression has been reported to be associated with poor prognosis of tumor patients. Nouraee et al. reported that overexpression of survivin and survivin-deltaEx3 in bladder tumors correlates with poor prognosis of bladder cancer [17]. Fields et al. also showed that survivin expression correlates with poor prognostic parameters (high nuclear and histologic grade, microvascular invasion), increased proliferation (mitotic count, MIB-1), local recurrence, and shorter disease-free survival of hepacelluar carcinoma patients [18]. Additionally, Cohen and his study groups found that the survivin expression correlates with poor prognostic parameters (high grade, histologic type, and p53 mutation) but not with survival of the majority of ovarian carcinoma patients [19]. In our previous study, we also found that the status of survivin mRNA could be an independent prognostic factor for OSCC patients. Meanwhile, the prognosis of survivin expression has also been evaluated in other human malignancies [20–22].
Chemo- or radioresistance is a major cause of treatment failure in cancer patients. Many researches indicated that the upregulated levels of survivin expression were significantly associated with chemo- or radioresistance of human cancers [23, 24]. Therefore, it was concluded that survivin would be a potential molecular target for cancer therapy. Several novel experimental therapeutic strategies developed to target survivin include vaccination strategies to generate an antigen-specific immune response, the development of antisense oligonucleotides, ribozymes, or siRNA molecules targeting survivin; and small molecule inhibitors of survivin function [25, 26]. At the same time, survivin inhibition could overcome chemo- or radioresistance of human cancer cells. For example, Kami et al. report that siRNA-mediated downregulation of survivin diminishes radioresistance of pancreatic cancer cells [27]. Shen et al. show that knockdown of survivin expression by siRNAs enhances chemosensitivity of prostate cancer cells and attenuates its tumorigenicity [28]. In other reports, inhibition of survivin could enhance the antitumor activity of several cytotoxics and targeted therapies, such as topoisomerase inhibitors, alkylating agents, tumor necrosis factor α-related apoptosis-inducing ligand, and so on [29–31]. Thus, survivin might be an ideal molecular target for cancer therapy. Although some studies have shown that survivin played important roles in RCC progression and development [32, 33], the prognosis significance of survivin expression and its correlation with radiosensitivity of RCC cells are still unclear.
In this study, we firstly found that the expression of survivin gene was sufficient in different RCC cell lines at both transcriptional and translational levels, but the expression of survivin gene was not detected in normal HKEC. Additionally, the level of survivin mRNA in RCC tissues was also significantly higher than that in corresponding nontumor kidney tissues. Then, we detected the status of survivin protein expression in 75 RCC tissue samples by immunohistochemistry and analyzed the correlation between survivin protein expression and clinicopathologic factors or prognosis of RCC patients. From our experimental results, high level of survivin expression was significantly correlated with tumor pathological stage, grade, and lymph node metastasis, but not with other clinicopathological factors including age, sex, tumor size, histology, and distant metastasis of RCC patients. Furthermore, multivariate analysis showed that the status of survivin protein expression might be an independent prognostic factor for RCC patients. Meanwhile, we also analyzed the correlation of survivin protein expression with radiosensitivity of RCC cells. Results showed that RNAi-mediated survivin knockdown could significantly inhibit proliferation and enhance in vitro radiosensitivity of RCC cells, which might be associated with increased apoptosis. Whether survivin knockdown could enhance in vivo radiosensitivity of RCC cells is under way and we will publish our research in next article.
In conclusion, survivin is usually overexpressed in RCC cells and high survivin protein expression might be an independent prognostic factor for RCC. Moreover, knockdown of survivin reduced growth, induce apoptosis and enhance in vitro radiosensitivity of RCC cells. Therefore, tumor-specific downregulation of survivin gene may become a novel therapeutic strategy to RCC patients.
References
Parkin DM, Bray F, Ferlay J et al (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108
Buzaid AC, Todd MB (1989) Therapeutic options in renal cell carcinoma. Semin Oncol 16(Suppl 6):12–16
Altieri DC (2003) Survivin, versatilemodulation of cell division and apoptosis in cancer. Oncogene 22:8581–8589
Chau GY, Lee AF, Tsay SH et al (2007) Clinicopathological significance of survivin expression in patients with hepatocellular carcinoma. Histopathology 51:204–218
Chen WC, Liu Q, Fu JX et al (2004) Expression of survivin and its significance in colorectal cancer. World J Gastroenterol 10:2886–2889
Falleni M, Pellegrini C, Marchetti A et al (2003) Survivin gene expression in early-stage non-small cell lung cancer. J Pathol 200:620–626
Sarela AI, Verbeke CS, Ramsdale J et al (2002) Expression of survivin, a novel inhibitor of apoptosis and cell cycle regulatory protein, in pancreatic adenocarcinoma. Br J Cancer 86:886–892
Osaka E, Suzuki T, Osaka S et al (2006) Survivin as a prognostic factor for osteosarcoma patients. Acta Histochem Cytochem 39:95–100
Asanuma K, Kobayashi D, Furuya D et al (2002) A role for survivin in radioresistance of pancreatic cancer cells. Jpn J Cancer Res 93:1057–1062
Rödel F, Hoffmann J, Distel L et al (2005) Survivin as a radioresistance factor, and prognostic and therapeutic target for radiotherapy in rectal cancer. Cancer Res 65:4881–4887
Motzer RJ, Bander NH, Nanus DM (1996) Renal-cell carcinoma. N Engl J Med 335:865–875
La Casse EC, Baird S, Korneluk RG et al (1998) The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene 17:3247–3259
Deveraux QL, Takahashi R, Salvesen GS et al (1997) X-linked IAP is a direct inhibitor of cell death proteases. Nature 388:300–304
Deveraux QL, Reed JC (1999) IAP family proteins: suppressors of apoptosis. Genes Dev 13:239–252
Ambrosini G, Adida C, Altieri DC (1997) A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med 3:917–921
Johnson ME, Howerth EW (2004) Survivin: a bifunctional inhibitor of apoptosis protein. Vet Pathol 41:599–607
Nouraee N, Mowla SJ, Ozhand A et al (2009) Expression of survivin and its spliced variants in bladder tumors as a potential prognostic marker. Urol J 6:101–108
Fields AC, Cotsonis G, Sexton D et al (2004) Survivin expression in hepatocellular carcinoma: correlation with proliferation, prognostic parameters, and outcome. Mod Pathol 17:1378–1385
Cohen C, Lohmann CM, Cotsonis G et al (2003) Survivin expression in ovarian carcinoma: correlation with apoptotic markers and prognosis. Mod Pathol 16:574–583
Song KY, Jung CK, Park WS et al (2009) Expression of the antiapoptosis gene Survivin predicts poor prognosis of stage III gastric adenocarcinoma. Jpn J Clin Oncol 39:290–296
Troeger A, Siepermann M, Escherich G et al (2007) Survivin and its prognostic significance in pediatric acute B-cell precursor lymphoblastic leukemia. Haematologica 92:1043–1050
Adida C, Recher C, Raffoux E et al (2000) Expression and prognostic significance of survivin in de novo acute myeloid leukaemia. Br J Haematol 111:196–203
Virrey JJ, Guan S, Li W et al (2008) Increased survivin expression confers chemoresistance to tumor-associated endothelial cells. Am J Pathol 173:575–585
Yuan QZ, Wang CT, Mao YQ et al (2010) Enhanced tumor radiosensitivity by a survivin dominant-negative mutant. Oncol Rep 23:97–103
Zaffaroni N, Daidone MG (2002) Survivin expression and resistance to anticancer treatments: perspectives for new therapeutic interventions. Drug Resist Updat 5:65–72
Ryan BM, O’Donovan N, Duffy MJ (2009) Survivin: a new target for anti-cancer therapy. Cancer Treat Rev 35:553–562
Kami K, Doi R, Koizumi M et al (2005) Downregulation of survivin by siRNA diminishes radioresistance of pancreatic cancer cells. Surgery 138:299–305
Shen J, Liu J, Long Y et al (2009) Knockdown of survivin expression by siRNAs enhances chemosensitivity of prostate cancer cells and attenuates its tumorigenicity. Acta Biochim Biophys Sin 41:223–230
Sato A, Ito K, Asano T et al (2007) Synergistic effect of survivin-specific small interfering RNA and topotecan in renal cancer cells: topotecan enhances liposome-mediated transfection by increasing cellular uptake. Int J Oncol 30:670–695
Song X, Wang JB, Yin DL et al (2009) Down-regulation of lung resistance related protein by RNA interference targeting survivin induces the reversal of chemoresistances in hepatocellular carcinoma. Chin Med J 122:2636–2642
Fulda S, Debatin KM (2004) Sensitization for tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by the chemopreventive agent resveratrol. Cancer Res 64:337–346
Zamparese R, Pannone G, Santoro A et al (2008) Survivin expression in renal cell carcinoma. Cancer Invest 26:929–935
Byun SS, Yeo WG, Lee SE et al (2007) Expression of survivin in renal cell carcinomas: association with pathologic features and clinical outcome. Urology 69(1):34–37
Acknowledgment
The authors thank the Members of Department of Pathology in Xijing Hospital for their sincere help and technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yu Lei and Zhang Geng are contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lei, Y., Geng, Z., Guo-Jun, W. et al. Prognostic significance of survivin expression in renal cell cancer and its correlation with radioresistance. Mol Cell Biochem 344, 23–31 (2010). https://doi.org/10.1007/s11010-010-0525-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-010-0525-3