Abstract
Phospholipase C-β (PLCβ) isozymes (PLCβ1 and PLCβ3) have been extensively characterized in cardiac tissue, but no data are available for the PLCβ4 isozyme. In this study, PLCβ(1–4) isozymes mRNA relative expression was studied by real-time PCR (RT-PCR) in human, rat, and murine left ventricle and the presence of PLCβ4 isozyme at the protein level was confirmed by Western blotting in all species studied. Confocal microscopy experiments carried out in HL-1 cardiomyocytes revealed a sarcoplasmic subcellular distribution of PLCβ4. Although there were unexpected significant interspecies differences in the PLCβ(1–4) mRNA expression, PLCβ4 mRNA was the main transcript expressed in all left ventricles studied. Thus, whereas in human and rat left ventricles PLCβ4 > PLCβ3 > PLCβ2 > PLCβ1 mRNA pattern of expression was found, in murine left ventricle the pattern of expression was different, i.e., PLCβ4 > PLCβ1 > PLCβ3 > PLCβ2. However, results obtained in mouse HL-1 cardiomyocytes showed PLCβ3 ≈ PLCβ4 > PLCβ1 > PLCβ2 pattern of mRNA expression indicating a probable cell type specific expression of the different PLCβ isozymes in cardiomyocytes. Finally, RT-PCR experiments showed a trend, even though not significant (P = 0.067), to increase PLCβ4 mRNA levels in HL-1 cardiomyocytes after angiotensin II treatment. These results demonstrate the presence of PLCβ4 in the heart and in HL-1 cardiomyocytes showing a different species-dependent pattern of expression of the PLCβ(1–4) transcripts. We discuss the relevance of these findings in relation to the development of cardiac hypertrophy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myocardial hypertrophy can be initiated by different stressors such as physiological stress of physical exercise or pathological stress, like volume and/or pressure overload [1, 2]. In cellular models, hypertrophy can be induced by activation of certain receptors coupled to the heterotrimeric proteins belonging to the Gq/11 subfamily (angiotensin II AT1 receptor, endothelin-1 ETA receptor, and α1-adrenergic receptor) [3]. Even if other signaling effectors of Gαq/11 have recently been revealed [4], phospholipase Cβ is the best characterized effector of the Gαq/11 mediated signaling [5]. In this sense, a recent study has shown that α1-adrenergic receptor-mediated hypertrophic response is mediated by the PLCβ1b isozyme in neonatal rat cardiomyocytes [6]. Thus, pharmacological interventions that interfere with PLCβ signaling in myocytes have been proposed as a new strategy to influence the hypertrophic process of the heart [6]. PLCβ hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) producing two second messengers, inositol 1,4,5-trisphosphate (IP3), and diacylglicerol (DAG). Both second messengers are involved in signaling pathways that can contribute to the development of cardiac hypertrophy [7, 8]. Four members of the PLCβ(1–4) subfamily and multiple splice variants have been described and two of them (PLCβ1 and PLCβ3) have been extensively studied in cardiac tissue [9]. However, neither PLCβ2 and PLCβ4 isozymes nor the splice variants have been studied to the same extent. Furthermore, only one report has described PLCβ2 expression in rat heart membranes [10], and no data is available to our knowledge on the presence of PLCβ4 in myocardial tissue. However, a recent study using expressed sequence tags (EST) from the human Unigene database has proposed the presence of PLCβ4 (Hs 472101) in human heart [11]. Taking into account the different regulatory properties of the PLCβ isozymes [12] and the different coupling of PLC isozymes to different receptor systems [13], we have considered of interest to study the pattern of expression of the different PLCβ isozymes in human, rat, and murine left ventricles. The aim of this study was to confirm the presence of PLCβ4 in cardiac tissue and to study the pattern of expression of the different isozymes in different species. Furthermore, we have assessed whether key substances in the cardiac hypertrophy development as angiotensin II can influence the mRNA expression levels of this PLCβ isozyme.
Materials and methods
Human left ventricle and blood samples
Human left ventricle biopsy was obtained after informed consent. The patient (50 years old) was diagnosed of eccentric left ventricular hypertrophy secondary to severe aortic valve regurgitation and the myocardial sample was obtained at operation time. Blood sample was obtained from healthy control subject (33 years old) after informed consent. This study has been approved by the institutional review committee and follows the recommendations from the Helsinki Declaration.
Preparation of crude heart membranes
Animals used in this study were used following the US National Institutes of Health guiding principles in the care and use of animals. Adult male Wistar-Kyoto rats (20–22 weeks old) (CRL: Wi (Han), Charles River, Barcelona, Spain) and adult male BALB/c mice (20–22 weeks old) (BALB/cByJ, Charles River, Barcelona, Spain) fed ad libitum. Animals were killed by decapitation after handling, and heart left ventricles (20–150 mg) were dissected in ice-cold 20 mM Tris–HCl buffer at pH 7.0, containing 1 mM EGTA (Tris-EGTA buffer) prior to homogenization and then homogenized in 20 volumes of the same hypotonic buffer using a polytron homogenizer. A crude membrane preparation was isolated by repeated centrifugations and rehomogenizations in hypotonic buffer as described previously [14, 15]. Briefly, the homogenate was centrifuged for 15 min at 40,000 × g. The pellet obtained was then resuspended in Tris/EGTA buffer, rehomogenized, and centrifuged again. Protein concentration was determined by the Bradford’s method [16]. The samples were aliquoted in microcentrifuge eppendorf tubes and the pellets were kept at −80°C.
Western blot of PLCβ4
Adult human, rat, and murine crude heart membrane proteins were solubilized in 2× sample buffer (final concentration: 10% glycerol, 5% 2-mercaptoethanol, 2% sodium dodecylsulphate (SDS) and 62.5 mM Tris–HCl, pH 6.8), and equal protein amounts (20 μg of membrane proteins) were resolved by electrophoresis in 8% SDS-polyacrylamide gels. Proteins were transferred to nitrocellulose membranes (Amersham Pharmacia, Spain). Blots were blocked in 5% non-fat dry milk/phosphate-buffered saline overnight at 4°C and incubated for 2 h at room temperature with the rabbit polyclonal anti-PLCβ4 antibody (1:400 dilution; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). Blots were washed and incubated with the secondary antibody goat anti-rabbit IgG conjugated to horseradish peroxidase (Sigma Chemical Co. St. Louis, MO, USA) diluted 1:1000 in blocking buffer for 1 h at room temperature. Immunoreactive bands were visualized with the enhanced chemiluminescence reagents (ECL-Plus, Amersham Pharmacia, Spain) and analyzed by imaging analyzer (Chemi-Doc XRS, Bio-Rad, Philadelphia, PA, USA). Blotting with the primary antibody preincubated with blocking peptide specific for the anti-PLCβ4 antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) was used as negative control and mouse cerebellum as positive control. Each gel contained a prestained broad-range protein ladder (Kaleidoscope™, BIO-RAD, CA, USA) to calculate the molecular masses of the individual bands.
Cell culture
HL-1 murine cardiomyocytes (a gift from Dr. W. C. Claycomb, Louisiana State University Medical Center, USA) (passages 61–65) were cultured on fibronectin-covered flasks with Claycomb media™ supplemented with 0.1 mM norepinephrine (Sigma–Aldrich), 2 mM l-glutamine (Life technologies), 100 U/ml/100 μg/ml Penicillin/Streptomycin (Life technologies), and 10% FBS (JRH Biosciences) (maintenance medium). For real-time PCR (RT-PCR) experiments HL-1 cells (106 per treatment) were treated for 24 h with 1 μmol/l angiotensin II without norepinephrine and FBS, and a control group was carried out in parallel. Cell area was quantified from manually outlined cells digitized microscopic images (recorded by a Nikon phase contrast microscope; Nikon corporation, Tokyo, Japan) of randomly chosen cell fields using NIS-Elements AR v3.0 software (Nikon Instruments Europe, Badhoevedorp, Netherlands). 40 cells were measured for each independent experiment, and the experiments were repeated 4 times. For confocal microscopy experiments HL-1 cells were plated on 24-well plates (4 × 104 cells per well) with glass slides previously coated with fibronectin.
Confocal microscopy
HL-1 cardiomyocytes grown on fibronectin-coated slides were stained using DAPI for nuclear visualization and the localization of PLCβ4 was examined by immunofluorescence using a rabbit polyclonal antibody from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA) and an anti-rabbit Alexa Fluor®-594 conjugated secondary antibody from Invitrogen. Confocal images were captured using a Zeiss Meta510 LSM with ×60 oil-immersion objectives (Carl Zeiss, Oberkochen, Germany).
Real-time PCR
Adult rat and murine left ventricles samples (n = 6) were processed to isolate the RNA using RNeasy® Fibrous Tissue Minikit (Qiagen, Germantown, MD, USA). HL-1 cardiomyocytes (n = 4) were processed to isolate the RNA using RNeasy® Mini Kit (Qiagen, Germantown, MD, USA). Total RNA was quantified using Nano Drop® ND-100 UV–Vis spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). Pool of human PolyA RBA, mRNA, human heart, left ventricle from 15 control subjects was purchased from BD Biosciences (Franklin Lakes, NJ, USA). The cDNA was obtained using random primers and the Taqman Reverse Transcription Reagents (Ref N808-0234, applied Biosystems, Foster City, CA, USA) following the manufacturers instructions.
Rat and murine left ventricle and HL-1 cardiomyocyte PLCβ(1–4) genes were amplified using predesigned SYBR probes from Qiagen (Germantown, MD, USA) (rat assay IDs: QT01685537, QT00188062, QT01620563, and QT00175266, respectively; mouse assay and murine HL-1 cardiomyocytes IDs: QT00173817, QT01052324, QT00155274, and QT00117621, respectively). A rat, mouse, and murine HL-1 cardiomyocyte endogenous control of GAPDH gene was used (assay IDs: QT00199633 and QT00309099). Human PLCβ1–4 genes were amplified using predesigned TaqMan probes from Applied Biosystems (Foster City, CA, USA) (assay IDs: Hs01080542_m1, Hs00248563_m1, Hs00184504_m1, and Hs00168656_m1, respectively). An endogenous control of GAPDH gene was used (assay ID: Hs99999905_m1). Reactions were carried out in an ABI 7300, following manufacturer’s instructions working with 50 ng of cDNA in each well. Each sample was analyzed in triplicate in each plate, and each plate was processed 3 times to test intra- and inter-plate variability, respectively. The relative quantity of the gene target was determined by the 2−∆∆Ct method. All RQ values are expressed as mean ± SEM.
Statistical methods
All Western blot experiments were performed processing simultaneously the human, rat, and murine left ventricle samples within one experiment. Significance of mean difference in PLCβ4 immunodetection and RT-PCR experiments were analyzed using Student’s t-test. P value <0.05 was considered as being statistically significant.
Results
Real-time PCR experiments in human, rat, and mouse ventricle
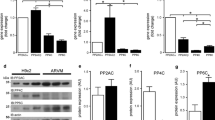
Overall, the mRNA expression pattern analysis of the left ventricle PLCβ isozymes shows PLCβ4 as the main transcript expressed in all the studied species (Fig. 1). However, the relative mRNA expression of the different PLCβ(1–4) isozymes in the human left ventricle differed from those found in rat and murine left ventricles. Thus, in the human left ventricle the PLCβ4 mRNA relative expression was 2.2-fold higher than the PLCβ2 transcript (Fig. 1a). In the rat and murine left ventricles PLCβ4 mRNA relative expression was 115- and 152-fold greater, respectively, than the mouse PLCβ2 transcript (Fig. 1b). It should be noted here that the mouse PLCβ2 transcript was chosen as reference value for the mouse and rat data analysis. Human and murine blood samples were used as a positive control for PLCβ2 mRNA expression and as a control tissue where this PLCβ isozyme expression is well-defined (Fig. 1). PLCβ2 mRNA expression was confirmed in all the studied left ventricles but some differences were shown among the species studied being the rat left ventricle where PLCβ2 mRNA expression was lower. Concerning the PLCβ(1,3) mRNA relative expression there were striking differences among species and the results showed that PLCβ1 is more abundant than PLCβ3 in murine left ventricle whereas the converse (PLCβ3 > PLCβ1) was found in human and rat left ventricles. The relative expression values for PLCβ1 and PLCβ3 transcripts were 48- and 24-fold over the PLCβ2 transcript in murine left ventricle, respectively, whereas 0.036/4.25 and 0.49/1.39-fold values were obtained for rat and human left ventricles, respectively (Fig. 1).
Real-time PCR analysis of PLCβ(1–4) gene expression. a Human left ventricle and blood were used and gene expression was normalized to GAPDH mRNA and expressed as fold change relative to PLCβ2 transcript from human left ventricle. b Rat and mouse left ventricle and mouse blood and gene expression was normalized to GAPDH mRNA and expressed as fold change relative to PLCβ2 gene from mouse left ventricle normalized. mRNA was prepared and quantified by RT-PCR as described in the “Material and methods” section. The results represent the mean ± SEM of three independent experiments carried out in triplicate
Effect of angiotensin II treatment in PLCβ(1–4) mRNA expression in HL-1 cardiomyocytes
The mRNA expression pattern of the PLCβ(1–4) isozymes in untreated HL-1 cardiomyocytes differed from results obtained from ventricles, showing similar pattern of mRNA expression for PLCβ3 and PLCβ4 (Fig. 2) and in a lower expression levels for PLCβ1 and PLCβ2. Cell area was significantly increased in HL-1 cardiomyocyte treated with 1 μmol/l of angiotensin II (P < 0.05, data not shown). Treatment with angiotensin II increased, even though not significantly, the mRNA expression levels for all the isozymes studied. The data obtained for PLCβ4 was borderline with the statistical significance (P = 0.067) (Fig. 2).
Real-time PCR analysis of PLCβ(1–4) gene expression in HL-1 cardiomyocytes after angiotensin treatment. HL-1 cardiomyocytes were treated for 24 h with 1 μmol/l angiotensin II and control cells were carried out in parallel. Gene expression was normalized to GAPDH mRNA and expressed as fold change relative to PLCβ2 transcript from control HL-1 cardiomyocyte. mRNA was prepared and quantified by RT-PCR as described in the “Material and methods” section. The results represent the mean ± SEM of four independent experiments carried out in triplicate
Protein analysis for PLCβ4
Western blot experiments performed in human, rat, and murine left ventricles showed the presence of PLCβ4 at the protein level in all the studied samples (Fig. 3). Bands migrating as expected molecular masses corresponding to the two different splice variants [26] (130 and 116 KDa) are shown in all left ventricles studied. Furthermore, the signal obtained with the primary antibody alone disappeared when the sample was preincubated with the specific blocking peptide for the used anti-PLCβ4 antibody. However, the results obtained showed differences at molecular masses of the bands in the different species. Thus, the bands detected for rat and mouse ventricles migrated at lower KDa than the band detected in human left ventricle. Optical density and statistical analysis of the results did not show any significant difference in the PLCβ4 expression among human, rat, and murine left ventricles.
Representative Western blot from three independent experiments of PLCβ4 isozyme from adult heart tissues. Membranes prepared as described in “Material and methods” section were resolved by SDS-PAGE on 8% gels. Each lane represented 20 μg of membrane proteins. Lanes were as follows: human, rat, and mouse left ventricle with and without blocking peptide preincubation and as positive control we used mouse cerebellum
Subcellular distribution of the PLCβ4 isozyme
Confocal microscopy experiments of immunostained HL-1 cardiomyocytes were performed in order to assess the subcellular localization of PLCβ4. Confocal image analysis showed a sarcoplasmic distribution of PLCβ4 in HL-1 cardiomyocytes ruling out a sarcolemmal or nuclear localization (Fig. 4).
Confocal image analysis of immunofluorescent staining of HL-1 cardiomyocytes with PLCβ4 primary antibody and Alexa 594 anti-rabbit secondary antibody (red channel), and nuclear staining (DAPI). PLCβ4 shows a sarcoplasmic distribution and is not present in the nuclei or in sarcolemma. At least 100 cells were examined. Scale bar = 10 μm
Discussion
Although PLCβ1 and PLCβ3 isozymes have been extensively studied in cardiac tissue and several studies have linked the cardiac hypertrophy to PLCβ(1,3) activation [6, 17–19], no studies have been carried out in order to study the presence of PLCβ4 in mammalian heart and the role that PLCβ4 could play in the development of cardiac hypertrophy. The inhibition induced by ribonucleotides on PLCβ4 activity and the fact that PLCβ4 was discovered after the characterization of PLCβ isozymes in cardiac tissue done by Rhee and coworkers in 1993 [20] could be the reason for the unavailable data characterizing PLCβ4 in cardiac tissue [21, 22]. However, in the absence of ribonucleotides the specific PLCβ4 induced PIP2 hydrolysis is 4–5 times the average specific activity of PLCβ1 and PLCβ3 [22] and data obtained in vivo showed a Gq/11-dependent activation of the PLCβ4 [23]. Although the PLCβ4 expression has been circumscribed to retina and cerebellum [12, 24] and visual and motor functions are related to this isozyme by data obtained in studies using knockout mice [25], a recent report using the EST from human Unigene database has suggested the presence of PLCβ4 in human heart [11]. The data obtained in our study demonstrate that PLCβ4 is expressed in human, rat, and murine left ventricles and in HL-1 cardiomyocytes. Furthermore, RT-PCR results obtained showed that PLCβ4 is the main transcript expressed in the left ventricles of all the studied species and one of the most expressed in HL-1 cardiomyocytes. In addition, results obtained by Western blot in human, rat, and murine left ventricles and by confocal microscopy in HL-1 cardiomyocytes confirm the protein expression of PLCβ4. However, the results obtained by Western blot experiments showed differences in the molecular mass of PLCβ4 detected in the different species. The results obtained are in agreement with the presence of two different splice variants, PLCβ4a expression in human ventricle whereas PLCβ4b would be the splice variant expressed in rat and murine ventricles. Differences between rat and mouse PLCβ4 mRNA length could explain the differences observed. However, post-transcriptional modifications or peptide splicing processes can not be excluded. Confocal imaging of murine HL-1 cardiomyocytes showed a sarcoplasmic distribution of PLCβ4 which is in agreement with the well-documented cytoplasmic distribution of PLCβ4b [26]. The eventual differential splice variant expression of PLCβ4 in human ventricle could be functionally relevant since whereas PLCβ4a is activated by Gq/11, PLCβ4b is insensitive to Gαq and Gβγ activation [26].
Treatment of HL-1 cardiomyocytes with angiotensin II increased, even though not significantly, all the PLCβ isozymes mRNA expression but this increase was more pronounced for PLCβ4 mRNA. Further studies will be required in order to dissect whether the PLCβ4 expression is altered by hypertrophic agents in other cardiomyocyte models as neonatal rat cardiomyocytes. In relation to this, recently it has been reported that α1-adrenergic receptor-mediated hypertrophy is mediated by PLCβ1b in neonatal rat cardiomyocytes [6]. However, this study has not analyzed whether PLCβ4 is involved in this effect or whether other hypertrophic agents as angiotensin II mediate their effect through PLCβ1b. In this respect, differences among receptor coupling to the different PLCβ isozymes have been described [13] and therefore, different receptor-PLCβ isozyme coupling could be possible depending on which PLCβ isozymes are present downstream to these receptors. In this sense, the special regulatory properties of the PLCβ4 led us to speculate that the interconversion from ATP or GTP, which inhibit PLCβ4, into cyclic guanine nucleotides (cGMP and cAMP), which exert no inhibitory effect on PLCβ4, induced by increased norepinephrine levels and NOS1 activity found in cardiac hypertrophy and heart failure, could relieve the inhibition of PLCβ4 activity and therefore increase the activity of downstream signaling pathways related to cardiac hypertrophy and heart failure development in human ventricle [27–29]. Therefore, further studies will be necessary, in order to dissect the role of the PLCβ4 and its splice variants in the etiopathology of cardiac hypertrophy and heart failure.
The data obtained in the present study showed PLCβ4 as the main transcript expressed in all the studied left ventricles and one of the most expressed in HL-1 cardiomyoctes. Moreover, we found remarkable interspecies differences when considering the relative expression of the better studied PLCβ1 and PLCβ3 transcripts. Expression of PLCβ1 is greater than PLCβ3 in murine left ventricle, whereas the opposite was found in human and rat left ventricles. No studies are available in the literature where the PLCβ1 and PLCβ3 mRNA expression pattern have been compared across human, rat and murine left ventricles to contrast our findings. However, there are several reports where PLCβ activity was determined in mouse and rat cardiomyocytes showing interspecies differences in response to selective Gq/11-coupled receptor agonists that are reminiscent of our results [30, 31]. The differences found in those studies and the data obtained in the present study highlight the use of caution when comparing the results obtained in different animal models. In this regard, the present results suggest a more similar pattern of expression between rat and human left ventricles. However, the differences found at the PLCβ1 and PLCβ2 mRNA level of expression and the PLCβ4a and PLCβ4b splice variants between human and rat left ventricles raise the necessity to study in greater depth the differences existing among the different species in the PLCβ isozyme expression profile and more importantly the functional significance of these differences in the role of PLCβ in the development of cardiac hypertrophy.
In conclusion, our findings obtained by RT-PCR show PLCβ4 as the main PLCβ transcript in human, rat, and murine left ventricles and one of the most expressed in HL-1 cardiomyocytes. On the other hand, PLCβ4 protein expression was confirmed by Western blotting in human, rat, and murine left ventricles and by confocal microscopy in HL-1 cardiomyocytes. Even though angiotensin II increase in PLCβ expression did not reach statistical significance, the results obtained for PLCβ4 mRNA expression levels raise the necessity to study in greater depth the role of PLCβ4 in the cardiac hypertrophic development. Further studies will be required to understand the pathophysiological relevance of PLCβ4 in the origin of cardiac hypertrophy and heart failure.
References
Hill JA, Olson EN (2008) Cardiac plasticity. N Engl J Med 358:1370–1380
Lorell BH, Carabello BA (2000) Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation 102:470–479
Clerk A, Sugden PH (1999) Activation of protein kinase cascades in the heart by hypertrophic G protein-coupled receptor agonists. Am J Cardiol 83:64H–69H
Golebiewska U, Scarlata S (2008) Gαq binds two effectors separately in cells: evidence for predetermined signalling pathways. Biophys J 95:2575–2582. doi:10.1529/biophysj.108.129353
Taylor SJ, Chae HZ, Rhee SG, Exton JH (1991) Activation of β1 isozyme of phospholipase C by α subunits of the Gq class of G proteins. Nature 350:516–518. doi:10.1038/350516a0
Filtz TM, Grubb DR, McLeod-Dryden TJ, Luo J, Woodcock EA (2009) Gq-inititated cardiomyocyte hypertrophy is mediated by phospholipase Cβ1b. FASEB J. doi:10.1096/fj.09-133983
Sabri A, Steinberg SF (2003) Protein kinase C isoform-selective signals that lead to cardiac hypertrophy and the progression of heart failure. Mol Cell Biochem 251:97–101. doi:10.1023/A:1025490017780
Luo DL, Gao J, Lan XM, Wang G, Wei S, Xiao RP, Han QD (2006) Role of inositol 1,4,5-trisphosphate receptors in alpha1-adrenergic receptor-induced cardiomyocyte hypertrophy. Acta Pharmacol Sin 27:895–900
Tappia PS (2007) Phospholipid-mediated signaling systems as novel targets for treatment of heart disease. Can J Physiol Pharmacol 85:25–41. doi:10.1139/Y06-098
González-Yanes C, Santos-Alvarez J, Sánchez-Margalet V (2001) Pancreastatin, a chromogranin A-derived peptide, activates Gα16 and phospholipase C-β2 by interacting with specific receptors in rat heart membranes. Cell Signal 13:43–49. doi:10.1016/S0898-6568(00)00127-3
Suh P-G, Park JI, Manzoli L, Cocco L, Peak JC, Katan M, Fukami K, Kataoka T, Yun S, Ryu SH (2008) Multiple roles of phosphoinositide-specific phospholipase C isozymes. BMB Rep 41:415–434
Rebecchi MJ, Pentyala SN (2000) Structure, function and control of phosphoinositide-specific phospholipase C. Physiol Rev 80:1291–1335
Strassheim D, Williams CL (2000) P2Y2 purinergic and M3 muscarinic acetylcholine receptors activate different phospholipase C-beta isoforms that are uniquely susceptible to protein kinase C-dependent phosphorylation and inactivation. J Biol Chem 275:39767–39772. doi:10.1074/jbc.M007775200
Wallace MA, Claro E (1993) Transmembrane signaling through phospholipase C in human cortical membranes. Neurochem Res 18:139–145
Garro MA, López de Jesús M, Ruíz de Azúa I, Callado LF, Meana JJ, Sallés J (2001) Regulation of phospholipase Cβ activity by muscarinic acetylcholine and 5-HT2 receptors in crude and synaptosomal membranes from human cerebral cortex. Neuropharmacology 40:686–695. doi:10.1016/S0028-3908(00)00206-9
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Kawaguchi H, Sano H, Iizuka K, Okada H, Kudo T, Kageyama K, Muramoto S, Murakami T, Okamoto H, Mochizuki N, Kitabatake A (1993) Phosphatidylinositol metabolism in hypertrophic rat heart. Circ Res 72:966–972
Dent M, Dhalla NS, Tappia PS (2004) Phospholipase C gene expression, protein content, and activities in cardiac hypertrophy and heart failure due to volume overload. Am J Physiol Heart Circ Physiol 287:719–727. doi:10.1152/ajpheart.01107.2003
Dent MR, Aroutiounova N, Dhalla NS, Tappia PS (2006) Losartan attenuates phospholipase C isozyme gene expression in hypertrophied hearts due to volume overload. J Cell Mol Med 10:470–479. doi:10.1111/j.1582-4934.2006.tb00412.x
John DY, Lee HH, Park D, Lee CW, Lee KH, Yoo OJ, Rhee SG (1993) Cloning, sequencing, purification and Gq-dependent activation of phospholipase C-β3. J Biol Chem 268:6654–6661
Lee CW, Lee KH, Lee SB, Park D, Rhee SG (1994) Regulation of phospholipase C-β4 by ribonucleotides and the α subunit of Gq. J Biol Chem 269:25335–25338
Lee CW, Park DJ, Lee KH, Kim CG, Rhee SG (1993) Purification, molecular cloning, and sequencing of phospholipase C-β4. J Biol Chem 268:21318–21327
Jiang H, Wu D, Simon MI (1994) Activation of phospholipase C β4 by heterotrimeric GTP-binding proteins. J Biol Chem 269:7593–7596
Rhee SG (2001) Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem 70:281–312. doi:10.1146/annurev.biochem.70.1.281
Kim D, Jun KS, Lee SB, Kang NG, Min DS, Kim YH, Ryu SH, Suh PG, Shin HS (1997) Phospholipase C isozymes selectively couple to specific receptors. Nature 389:290–293. doi:10.1038/38508
Kim MJ, Min DS, Ryu SH, Suh P-G (1998) A cytosolic, Gαq- and βγ-insensitive splice variant of phospholipase C-β4. J Biol Chem 273:3618–3624
Esler M, Kaye D (2000) Measurement of sympathetic nervous system activity in heart failure: the role of norepinephrine kinetics. Heart Fail Rev 5:17–25. doi:10.1023/A:1009889922985
Damy T, Ratajczak P, Shah AM, Camors E, Marty I, Hasenfuss G, Marotte F, Samuel JL, Heymes C (2004) Increased neuronal nitric oxide synthase-derived NO production in the failing human heart. Lancet 363:1365–1367. doi:10.1016/S0140-6736(04)16048-0
Bendall JK, Damy T, Ratajczak P, Loyer X, Monceau V, Marty I, Milliez P, Robidel E, Marotte F, Samuel JL, Heymes C (2004) Role of myocardial neuronal nitric oxide synthase-derived nitric oxide in beta-adrenergic hyporesponsiveness after myocardial infarction-induced heart failure in rat. Circulation 110:2368–2375. doi:10.1161/01.CIR.0000145160.04084.AC
Hilal-Dandan R, Kanter JR, Brunton LL (2000) Characterization of G-protein signaling in ventricular myocytes from the adult mouse heart: differences from the rat. J Mol Cell Cardiol 32:1211–1221. doi:10.1006/jmcc.2000.1156
Sabri A, Pak E, Alcott SA, Wilson B, Steinberg SF (2000) Coupling of endogenous α1- and β-adrenergic receptors in mouse cardiomyocytes. Circ Res 86:1047–1053
Acknowledgments
The authors would like to thank Dr Eneko Urizar for his valuable advice at the time of this manuscript preparation. We would like to thank the cardiac surgeons from Policlinica Guipuzcoa for providing the human left ventricle sample biopsy. We thank to Dr. A. Aiastui for help in immunofluorescence technique; A. Pavón and A. Dorronsoro from Inbiomed for assistance with confocal microscopy This work was supported by the Universidad del País Vasco/Euskal Herriko Unibertsitatea (1/UPV 00079.252-E-15424/2003 to M.A.G.); and the Gobierno Vasco (GV2005111012 to M.A.G.). A.L. holds a fellowship from the Diputacion Foral from Gipuzkoa.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Otaegui, D., Querejeta, R., Arrieta, A. et al. Phospholipase Cβ4 isozyme is expressed in human, rat, and murine heart left ventricles and in HL-1 cardiomyocytes. Mol Cell Biochem 337, 167–173 (2010). https://doi.org/10.1007/s11010-009-0296-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0296-x