Abstract
Pathological levels of homocysteine induce a dramatic degradation of arterial elastic structures. This severe metalloproteinase-dependant elastolysis affects elastic structures all over the media suggesting that smooth muscle cells (SMC) may participate to this process induced by homocysteine. Therefore, we investigated the effect of physiological (10 μM) and pathological (50, 100, and 500 μM) concentrations of homocysteine on the metalloproteinase-dependant proteolytic potential of human arterial SMC in culture. Pathological levels of homocysteine increased concomitantly the secretion of latent MMP-2 and TIMP-2 while the secretion of other elastolytic matrix metalloproteinases (MMPs) and expression of MT1-MMP were not altered. The increased secretion of latent MMP-2 induced by homocysteine was associated with an increased production of reactive oxygen species (ROS). Moreover, the increased secretion of latent MMP-2 induced by homocysteine was inhibited by antioxidant superoxide dismutase alone or in combination with catalase. These results suggest that SMC could participate, through an oxidative stress dependant secretion of elastolytic MMP-2, to the metalloproteinase-dependant degradation of arterial elastic structures induced by homocysteine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High plasma levels of homocysteine, a physiological sulfur-containing amino acid, are well known as an independent risk factor for atherosclerosis on the basis of many epidemiological studies [1, 2]. In vivo studies using genetic [3] and dietary [4] animal models of hyperhomocysteinemia support the concept that homocysteine contributes to and accelerates atherogenesis. According to a wide range of in vitro experiments and, to a lesser extent, in vivo observations, the mechanism by which homocysteine promotes atherogenesis are multiple and likely interrelated, including endothelial dysfunction [5], proliferation of vascular smooth muscle cells (SMC) [6], and extracellular matrix (ECM) remodeling [7].
Among the ECM alterations, it has been clearly ascertained that a dramatic degradation of arterial elastic structures is one of the major features of the arterial lesions associated with hyperhomocysteinemia [8]. The degradation of elastic structures was shown to be due to an elastolytic process supported by an increase in metalloproteinase-dependant elastolytic activity [4, 7, 9]. It was previously shown that endothelial cells could participate to the subendothelial degradation of arterial elastic structures induced by homocysteine through elastolytic matrix metalloproteinases (MMPs) [10]. Interestingly, a well-established feature of this elastolytic process is that it affects elastic structures throughout the media [8, 9]. However, little is known about the participation of SMC to this elastolytic process. Therefore, we investigated the effect of homocysteine on the metalloproteinase-dependant proteolytic potential of human arterial SMC. We cultured SMC with pathological levels of homocysteine matching those encountered in human, focusing our study on elastolytic matrix metalloproteinases MMP-2, MMP-9, MMP-3, and MMP-7 [11, 12]. Furthermore, we investigated whether homocysteine effects involved reactive oxygen species (ROS), which is a key point of MMP synthesis and secretion induced by many stimuli [13] and is responsible for other homocysteine-induced effects on vascular SMC [14]. We showed that homocysteine altered the MMP-2 dependant proteolytic potential of SMC through a mechanism involving ROS. These results suggest that SMC could participate, through the ROS dependant secretion of elastolytic MMP-2, to the metalloproteinase-dependant degradation of arterial elastic structures induced by homocysteine.
Materials and methods
Cell culture
Human arterial SMC were isolated from umbilical arteries as previously described [15]. Briefly, umbilical arteries were isolated from whole cord, opened longitudinally, gently scrap on their luminal face to remove endothelial cells and dissected into 5 mm explants. Explants were laid lumen-side down onto plastic culture plates. Dulbecco Modified Eagle Medium (DMEM, Gibco) supplemented by 20% Fetal Calf Serum (FCS, Gibco), penicillin, and streptomycin (50 U/ml) was added to the culture plates and placed at 37°C in a humidified atmosphere of 5% CO2. Media were replaced with fresh media twice per week. Cell started growing from the explants within 1 week. At confluence, explants were removed and SMC were identified by their typical hill and valley morphology and indirect immunofluorescent positive staining for α-actin. The absence of endothelial cell was confirmed by indirect immunofluorescent negative staining for VE-cadherin.
Cell treatment
SMC from passage second to fifth were plated on 6-well plates (Nunclon), grown to confluence in DMEM containing antibiotics and 10% FBS and changed to basal medium (serum free medium containing 0.1% bovine serum albumin) for 4 h before exposure to experimental treatments. SMC were incubated for 24 h in a basal medium alone or supplemented with physiological (10 μM) or pathological (50, 100, 500 μM) concentrations of homocysteine (d,l-homocysteine, Sigma). At the same time, SMC were incubated for 24 h with medium supplemented with 10 and 100 μM of methionine (d,l-methionine, Sigma) or cysteine (d,l-cysteine, Sigma) to ascertain the specificity of the effect of homocysteine. To assess the involvement of ROS, SMC were incubated for 24 h in basal medium alone or supplemented with 100 μM homocysteine in the absence or presence of superoxide dismutase (SOD, 1,000 U/l, Sigma) and catalase (1,000 U/l, Sigma) alone or in combination.
At the end of the incubation time, conditioned medium were collected and centrifuged, and cells were used to measure ROS production (see below). Thereafter, cells were washed with phosphate-buffered saline and harvested by scrapping into lysis buffer containing 50 mM Tris–HCl, 1% Triton X-100, and anti-protease cocktail inhibitors (Roche Diagnostics). Cell lysates and conditioned medium were centrifuged for 5 min at 1,000×g to remove cell debris. Total cellular protein was quantified using the Bicinchoninic Acid Protein assay reagent (BCA, Sigma).
Zymography
Cell lysates and conditioned medium of HT1080 cells as standard were submitted to gelatin and casein zymography as previously described [16]. The metalloproteinase nature of the detected proteases was confirmed by incubating identical gels in the presence of a 1,10-phenanthroline (1 mmol/l). Proteolytic activities were quantified by densitometry and normalized relatively to HT1080 activities to allow the quantitative comparison between zymograms. Activities were expressed as a percentage of specific activities of SMC cultured in basal medium.
Western blotting
To identify MMPs and TIMP-2, conditioned medium and cell lysates extracts were electrophoresed under non-reducing conditions as previously described [10]. Proteins were transferred onto PVDF membranes and non-specific binding sites were blocked overnight at 4°C in 3% non-fat milk. Membranes were incubated for 1 h with mouse anti-human antibodies directed against MMP-2 (Calbiochem, Ab-3), MMP-9 (Calbiochem, Ab-8), MMP-3 (Calbiochem, Ab-5), MMP-7 (Interchim, Ab-1), TIMP-2 (Calbiochem, Ab-4), or polyclonal goat anti-human MT1-MMP (Tebu-bio, L-15). Then, membranes were incubated for 1 h with anti-goat or anti-mouse IgG horseradish peroxidase conjugated (1/2,000 dilution) and peroxidase activity was revealed by Supersignal® chemiluminescent substrate (Pierce). For negative control experiments, primary antibodies were omitted.
MMP gelatinase activity assay kit
Gelatinolytic activity in conditioned medium was quantified using a MMP gelatinase assay kit (Chemicon) based on a biotinylated gelatinase specific substrate of active MMP-2 and MMP-9. Conditioned medium were incubated with biotinylated gelatinase specific substrate and remaining biotinylated fragments were added to a biotin-binding 96-well plate and detected with streptavidin–enzyme complex. The addition of enzyme substrate resulted in a colored product quantified by its optical density at 450 nm. The color intensity was inversely proportional to gelatinolytic activity. APMA-activated MMP-2 and basal medium were used as positive and negative control, respectively. Gelatinase activities were expressed relatively to negative control.
Measurement of ROS production
The production of ROS was measured by 6-carboxy-2′,7′-dichlorofluorescin diacetate (DCFH-DA) fluorescence as previously described [17]. This assay is based on the oxidation of DCFH-DA to fluorescein by oxygen and nitrogen reactive species, whose fluorescence can be measured at 522 nm. Briefly, after 24 h of culture with the different stimuli conditions and collection of conditioned medium (see above), SMC were washed with medium and incubated with 5 μM of DCFH-DA in PBS for 2 h at 37°C in a humidified atmosphere of 5% CO2. Thereafter, the cells were washed with PBS to remove the excess probe and the fluorescence of oxidized DCFH-DA was measured by a Cytofluor® Series 4000 Fluorescence multi-well plate reader (PerSeptive Biosystems, Framingham, MA, USA). The excitation filter was a 20-nm bandwidth centered at 485 nm and the emission filter was a 25-nm bandwidth centered at 530 nm. Fluorescence intensities reflecting ROS production were expressed as a percentage of fluorescence intensity found in SMC cultured in basal medium alone.
Statistical analysis
Data were analyzed by non-parametric Wilcoxon paired test. Results are expressed as means ± standard deviation. A value of P < 0.05 was considered significant.
Results
Homocysteine modulates the MMP-2 dependant proteolytic potential of human arterial SMC
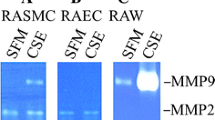
MMP-2 and MMP-9 were examined by gelatin zymography in both conditioned medium and cell lysates. In conditioned medium from SMC cultured in basal medium, one major gelatinolytic band was detected at molecular weight corresponding to latent MMP-2 (72 kDa) while no band was detected at molecular weight corresponding to active MMP-2 as well as latent or active MMP-9 (Fig. 1b). Gelatinolytic activities were confirmed as metalloproteinase by inhibition with PHE (results not shown). In conditioned medium, densitometric quantification showed a significantly increased secretion of the 72 kDa gelatinase with 50 and 100 μM homocysteine, while its secretion was not significantly increased with 500 μM homocysteine when compared to 10 μM homocysteine (Fig. 1a). This result was confirmed by western blotting (Fig. 1c). At the same time, active MMP-2 (68 kDa) and both latent (92 kDa) and active (88 kDa) MMP-9 remained undetectable in conditioned medium whatever the concentration of homocysteine used in culture (Fig. 1b). In cell lysates from SMC cultured in basal medium, significant amount of latent MMP-2, no active MMP-2 and very low amount of latent and active MMP-9 were detected (Fig. 1d). However, none of these gelatinolytic bands was altered by homocysteine whatever the concentration used in culture (Fig. 1d).
Homocysteine increases the secretion of latent MMP-2. Representative gelatin zymography of conditioned medium (b) and cell lysates (d) and western blot analysis of conditioned medium (c) from SMC cultured in basal medium alone or supplemented with 10, 50, 100, and 500 μM of homocysteine. Conditioned medium of HT1080 cells was used as internal standard. Each zymogram and western blot is the representative of four independent experiments. The 92, 72, and 68 kDa gelatinolytic band are, respectively, the latent form of MMP-9, the latent form of MMP-2 and the active form of MMP-2. In a, gelatinolytic bands corresponding to latent MMP-2 were quantified by densitometry analysis and specific activities were expressed as a percentage of specific activities of SMC cultured in basal medium. Values are means ± standard deviation of six independent experiments. * P < 0.05 vs. 10 μM homocysteine
Culture of SMC with methionine or cysteine did not altered the secretion of latent MMP-2 and confirmed the specificity of the inducing effect of homocysteine on the secretion of latent MMP-2 by SMC (Fig. 2).
Methionine and cysteine have no effect on the secretion of latent MMP-2. b Representative gelatin zymography of conditioned medium from SMC cultured in basal medium alone or supplemented with 10 and 100 μM of methionine (MET) or cysteine (CYS). Conditioned medium of HT1080 cells was used as internal standard. The zymogram is representative of four independent experiments. In a, gelatinolytic bands corresponding to latent MMP-2 were quantified by densitometry analysis and specific activities were expressed as a percentage of specific activities of SMC cultured in basal medium. Values are means ± standard deviation of six independent experiments
Gelatinase activities in conditioned medium were measured against a specific substrate of active MMP-2 and MMP-9. In conditioned medium from SMC cultured in basal medium, no gelatinase activity was detected when compared to a negative control free of gelatinase activity (Fig. 3). Gelatinase activities were not altered in conditioned medium from SMC cultured with 50, 100, and 500 μM of homocysteine compared to 10 μM homocysteine.
Homocysteine does not alter gelatinase activity in conditioned medium from cultured SMC. Gelatinase activity in conditioned medium was measured against a specific substrate cleaved by active MMP-2 and MMP-9. APMA-activated MMP-2 and basal medium were, respectively, supplied as positive and negative control. Gelatinase activities were expressed as a percentage of negative control. Values are means ± standard deviation of four independent experiments
Weak caseinolytic bands corresponding to latent MMP-3 (57 kDa) and MMP-7 (28 kDa) were detected in conditioned medium by casein zymography. Both caseinolytic activities were not altered in conditioned medium from SMC cultured with 50, 100, and 500 μM homocysteine compared to 10 μM homocysteine (data not shown).
Homocysteine altered the secretion of TIMP-2 but not the expression of MT1-MMP
We determined the effect of homocysteine on the expression of MT1-MMP in cell lysates by western blotting. A major and a weak band were, respectively, detected at molecular weight corresponding to the latent (62 kDa) and active (56 kDa) forms of MT1-MMP in cell lysates from SMC cultured in basal medium (Fig. 4a). The expression of both forms was not altered in cell lysates from SMC cultured with 50, 100, and 500 μM of homocysteine compared to 10 μM homocysteine. The secretion of TIMP-2 in conditioned medium was determined by western blotting. As show in Fig. 4b, a weak band at molecular weight of 22 kDa equivalent to standard of TIMP-2 was detected in conditioned medium from control SMC. The secretion of TIMP-2 was increased in conditioned medium from SMC cultured with, 50, 100, and 500 μM of homocysteine compared to 10 μM homocysteine.
Western blot analysis of a MT1-MMP in cell lysates and b TIMP-2 in conditioned medium from SMC cultured in basal medium alone or supplemented with 10, 50, 100, and 500 μM of homocysteine. Purified TIMP-2 and cell lysates from SMC stimulated with phorbol myristyl acetate (PMA) were used as standard. Each western blot is representative of four independent experiments
ROS are involved in the increased secretion of MMP-2 induced by homocysteine
To determine whether ROS are involved in the increased secretion of MMP-2 induced by homocysteine, we first examined whether the increased secretion of latent MMP-2 induced by homocysteine was associated with an increased production of ROS in SMC using DCFH-DA assay. The production of ROS was significantly increased in SMC cultured with 50, 100, and 500 μM homocysteine compared to 10 μM homocysteine (Fig. 5). Moreover, we studied the effects of free oxygen radical scavengers SOD and catalase alone or in combination on the production of ROS and on the secretion of latent MMP-2 in presence or absence of homocysteine. In SMC cultured in absence of homocysteine, catalase alone or in combination with SOD decreased concomitantly the production of ROS (Fig. 6a) and the secretion of latent MMP-2 (Fig. 6b), while SOD alone increased both production of ROS (Fig. 6a) and secretion of latent MMP-2 (Fig. 6b). These effects of SOD and catalase on ROS production in cultured SMC are in agreement with previous in vitro study [18, 19]. Catalase alone had no effect on increased secretion of latent MMP-2 and increased production of ROS induced by 100 μM homocysteine, while SOD alone or in combination with catalase both completely inhibited the increased secretion of latent MMP-2 (Fig. 6b) and the increased production of ROS (Fig. 6a) induced by 100 μM homocysteine.
Homocysteine increases the production of ROS in SMC. Oxidation of DCFH-DA to fluorescein was measured in SMC cultured in absence or with 10, 50, 100, and 500 μM of homocysteine. Fluorescence intensities reflecting ROS production were expressed as a percentage of fluorescence intensity found in SMC cultured in basal medium. Values are means ± standard deviation of six independent experiments. * P < 0.05 vs. 10 μM homocysteine
Effect of SOD and catalase on the production of ROS and the secretion of latent MMP-2 induced by homocysteine (HCY) in SMC. SMC were cultured in basal medium alone or supplemented with 100 μM homocysteine in the absence or presence of SOD (1,000 U/l) and catalase (1,000 U/l) alone or in combination. a ROS production was determined by the oxidation of DCFH-DA to fluorescein in SMC. Fluorescence intensities reflecting ROS production were expressed as a percentage of fluorescence intensity found in SMC cultured in basal medium. b Secretion of latent MMP-2 was determined by gelatin zymography analysis of conditioned medium. Gelatinolytic bands corresponding to latent MMP-2 were quantified by densitometry analysis and specific activities were expressed as a percentage of specific activities of SMC cultured in basal medium. Values are means ± standard deviation of five independent experiments. Effect of homocysteine was considered significant (*) when P < 0.05
Discussion
In this study, we showed that pathological levels of homocysteine increased the MMP-2 dependant proteolytic potential of arterial SMC through a ROS dependant process. SMC were cultured with homocysteine at concentrations matching plasma levels encountered in human hyperhomocysteinemia (50–500 μM). In these conditions, high levels of homocysteine increased the secretion of latent MMP-2. Extracellular free-radical inhibitors markedly reduced SMC secretion of latent MMP-2 induced by homocysteine.
A dramatic degradation of arterial elastic structures throughout the media is one of the major features of the arterial lesions associated with hyperhomocysteinemia [8, 9]. It was shown to be due to an elastolytic process supported by an increase in metalloproteinase-dependant elastolytic activity [9]. Our results suggest that SMC could participate, through the secretion of latent elastolytic MMP-2, to the metalloproteinase-dependant degradation of arterial elastic structures induced by homocysteine.
Latent MMP-2 was constitutively secreted by SMC, while neither latent nor active forms of MMP-9 were detectable. Concomitantly, latent MMP-3 and MMP-7 were weakly secreted while their active forms were undetectable (data not shown). This elastolytic MMP pattern closely matched the basal pattern previously described in non-atherosclerotic arteries [20] as well as in cultured SMC derived from macrovasculature [21]. Pathological levels of homocysteine (50 and 100 μM) increased SMC secretion of latent MMP-2 alone whereas secretion of its active form and secretion of latent and active forms of MMP-3, MMP-7, and MMP-9 were not altered. Homocysteine at 500 μM did not significantly increase MMP-2 secretion, which is in accordance with previous reports in which such levels of homocysteine were shown to have cytotoxic effect on cultured SMC [22]. In conditioned medium, the increased secretion of latent MMP-2 was associated with an increased secretion of TIMP-2, while specific gelatinase activity revealed by gelatinase activity assay was not altered. Actually, the gelatinase activity assay can only reveal the activity of active MMP-2 (68 kDa) free of TIMPs whereas gelatin zymography reveals also the activity of latent MMP-2 due to the SDS-induced dissociation of latent MMP-2/TIMP-2 complexes and the further activation of latent MMP-2 by SDS-induced conformational change [23]. Together, these results clearly evidence that pathological levels of homocysteine increased the MMP-2 dependant proteolytic potential of human arterial SMC in culture.
We have previously reported that homocysteine was able to activate purified latent MMP-2 through a non-proteolytic process which led to an increased proteolytic activity of the enzyme [24]. Although it has not yet been clearly demonstrated, the absence of increased gelatinase activity in our conditioned medium suggest that homocysteine may not be able to process such a non-proteolytic activation when latent MMP-2 is complexed with TIMP-2, which is the predominant form of MMP-2 secreted by cells [25]. In previous studies, Doronzo et al. found that constitutive secretion of only active form of MMP-2 was increased by homocysteine in cultured human aortic SMC [26], whereas Guo et al. reported that homocysteine increased only latent MMP-2 constitutively secreted by rat aortic SMC [27]. One of the main activating processes of MMP-2 is a cell surface MT1-MMP dependant proteolytic process that also involves TIMP-2. Indeed, it is well known that TIMP-2 is unique as a member of the TIMP family in that, in addition to inhibiting active forms of MMPs, it can selectively interacts with MT1-MMP to trigger or inhibit the cell-surface activation of MMP-2 according to the molecular ratio of TIMP-2/MMP-2 [28]. In our study, the increased secretion of latent MMP-2 by pathological levels of homocysteine was associated with an increase of TIMP-2 secretion, while MT1-MMP expression was not altered in cell lysates. Together, our results are in accordance with the absence of active MMP-2 in the conditioned medium from SMC cultured with pathological levels of homocysteine. Therefore, although it is difficult to reconcile these studies, many factors such as SMC heterogeneity in molecular control of SMC gene expression and in response to numerous stimuli, phenotypic state, and grade of confluence may explain this discrepancy [29].
We suggest that SMC could participate, through the secretion of latent elastolytic MMP-2, to the metalloproteinase-dependant degradation of arterial elastic structures induced by homocysteine. MMP-2 is well known to degrade elastin in vitro [11] and the elastic fibers network ex vivo in human skin [30]. We previously observed an increased immunoreactivity of MMP-2 (latent and active forms) with medial SMC that colocalized with the degradation of elastic structures in arteries cultured with pathological levels of homocysteine (unpublished data). Moreover, in the arterial wall, once secreted by SMC, latent MMP-2 could exert its elastolytic activity against elastic structures since: (i) elastin was previously shown to activate latent MMP-2 by contact autoactivation which results in its own elastolysis [31]; (ii) latent MMP-2 can be activated in vivo by endothelial serine-proteases such as human tissue kallikrein [32] which expression was shown to be increased by homocysteine [10].
An important finding in this study is that ROS are involved in the increased secretion of MMP-2 induced by homocysteine in SMC. Indeed, the increased secretion of latent MMP-2 induced by homocysteine was associated with an increased production of ROS. Furthermore, inhibition of ROS production by SOD alone or in combination with catalase inhibited the increased secretion of latent MMP-2 induced by homocysteine. This finding is consistent with previous observations showing that increased expression of MMP-2 in SMC in response to proatherogenic stimuli is dependant on ROS [33]. Moreover, since we used SOD and catalase that are large molecules and only act as extracellular free oxygen radical inhibitors, the increased expression of MMP-2 induced by homocysteine involved extracellular ROS. This is in agreement with previous in vitro study that clearly demonstrated that extracellular generation of ROS increased MMP-2 expression in human dermal fibroblasts [34]. Recently, Doronzo et al. showed that phosphatidyl inositol 3-kinase (PI3K) and Mitogen-Activated Protein Kinase (MAPK) are involved in increased expression of MMP-2 induced by homocysteine [26]. Since MAPK and PI3K pathway are ROS-sensitive downstream pathways [35], these previous data together with the complete inhibition by antioxidant SOD and catalase of the increased expression of MMP-2 induced by homocysteine suggest that ROS may be upstream activators of MAPK and PI3K pathway leading to increased expression of MMP-2 in SMC stimulated by pathological levels of homocysteine.
In summary, we have shown that pathological levels of homocysteine increased the MMP-2 dependant proteolytic potential of SMC through a mechanism involving ROS. These results are in favor of an active participation of SMC to the metalloproteinase-dependant arterial elastolysis induced by homocysteine. Further experiments in vivo are required to confirm the active participation of MMP-2-dependant elastolytic potential of SMC in the degradation of arterial elastic structures associated with hyperhomocysteinemia. This study reinforces the key role of oxidative stress in the deleterious effect of homocysteine on arterial wall, suggesting antioxidant therapy may have a beneficial effect on cardiovascular complications of hyperhomocysteinemia.
References
McCully KS (1983) Homocysteine theory of arteriosclerosis: development and current status. Atheroscler Rev 11:157–246
Eikelboom JW, Lonn E, Genest J Jr, Hankey G, Yusuf S (1999) Homocyst(e)ine and cardiovascular disease: a critical review of the epidemiologic evidenc. Ann Intern Med 131:363–375
Wang H, Jiang X, Yang F, Gaubatz JW, Ma L, Magera MJ, Yang X, Berger PB, Durante W, Pownall HJ, Schafer AI (2003) Hyperhomocysteinemia accelerates atherosclerosis in cystathionine beta-synthase and apolipoprotein E double knock-out mice with and without dietary perturbation. Blood 101:3901–3907
Hofmann MA, Lalla E, Lu Y, Gleason MR, Wolf BM, Tanji N, Ferran LJ Jr, Kohl B, Rao V, Kisiel V, Stern DM, Schmidt AM (2001) Hyperhomocysteinemia enhances vascular inflammation and accelerates atherosclerosis in a murine model. J Clin Invest 107:675–683
Austin RC, Lentz SR, Werstuck GH (2004) Role of hyperhomocysteinemia in endothelial dysfunction and atherothrombotic disease. Cell Death Differ 11(Suppl 1):S56–S64
Tsai MY, Arnett DK, Eckfeldt JH, Williams RR, Ellison RC (2000) Plasma homocysteine and its association with carotid intimal-medial wall thickness and prevalent coronary heart disease: NHLBI Family Heart Study. Atherosclerosis 151:519–524
Rolland PH, Friggi A, Barlatier A, Piquet P, Latrille V, Faye MM, Guillou J, Charpiot P, Bodard H, Ghiringhelli O et al (1995) Hyperhomocysteinemia-induced vascular damage in the minipig. Captopril-hydrochlorothiazide combination prevents elastic alterations. Circulation 91:1161–1174
Charpiot P, Bescond A, Augier T, Chareyre C, Fraterno M, Rolland PH, Garcon D (1998) Hyperhomocysteinemia induces elastolysis in minipig arteries: structural consequences, arterial site specificity and effect of captopril-hydrochlorothiazide. Matrix Biol 17:559–574
Augier T, Charpiot P, Chareyre C, Remusat M, Rolland PH, Garcon D (1997) Medial elastic structure alterations in atherosclerotic arteries in minipigs: plaque proximity and arterial site specificity. Matrix Biol 15:455–467
Chaussalet M, Lamy E, Foucault-Bertaud A, Genovesio C, Sabatier F, Dignat-George F, Charpiot P (2004) Homocysteine modulates the proteolytic potential of human vascular endothelial cells. Biochem Biophys Res Commun 316:170–176
Senior RM, Griffin GL, Fliszar CJ, Shapiro SD, Goldberg GI, Welgus HG (1991) Human 92- and 72-kilodalton type IV collagenases are elastases. J Biol Chem 266:7870–7875
Quantin B, Murphy G, Breathnach R (1989) Pump-1 cDNA codes for a protein with characteristics similar to those of classical collagenase family members. Biochemistry 28:5327–5334
Nelson KK, Melendez JA (2004) Mitochondrial redox control of matrix metalloproteinases. Free Radic Biol Med 37:768–784
Papatheodorou L, Weiss N (2007) Vascular oxidant stress and inflammation in hyperhomocysteinemia. Antioxid Redox Signal 9:1941–1958
Grenier G, Remy-Zolghadri M, Guignard R, Bergeron F, Labbe R, Auger FA, Germain L (2003) Isolation and culture of the three vascular cell types from a small vein biopsy sample. In Vitro Cell Dev Biol Anim 39:131–139
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Wang H, Joseph JA (1999) Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med 27:612–616
Mountain DJ, Singh M, Menon B, Singh K (2007) Interleukin-1beta increases expression and activity of matrix metalloproteinase-2 in cardiac microvascular endothelial cells: role of PKCalpha/beta1 and MAPKs. Am J Physiol Cell Physiol 292:C867–C875
Su B, Mitra S, Gregg H, Flavahan S, Chotani MA, Clark KR, Goldschmidt-Clermont PJ, Flavahan NA (2001) Redox regulation of vascular smooth muscle cell differentiation. Circ Res 89:39–46
Knox JB, Sukhova GK, Whittemore AD, Libby P (1997) Evidence for altered balance between matrix metalloproteinases and their inhibitors in human aortic diseases. Circulation 95:205–212
Sasaguri Y, Murahashi N, Sugama K, Kato S, Hiraoka K, Satoh T, Isomoto H, Morimatsu M (1994) Development-related changes in matrix metalloproteinase expression in human aortic smooth muscle cells. Lab Invest 71:261–269
Lee HY, Chae IH, Kim HS, Park YB, Choi YS, Lee YW, Park SJ, Cha YJ (2002) Differential effects of homocysteine on porcine endothelial and vascular smooth muscle cells. J Cardiovasc Pharmacol 39:643–651
Murphy G, Crabbe T (1995) Gelatinases A and B. Methods Enzymol 248:470–484
Bescond A, Augier T, Chareyre C, Garcon D, Hornebeck W, Charpiot P (1999) Influence of homocysteine on matrix metalloproteinase-2: activation and activity. Biochem Biophys Res Commun 263:498–503
Goldberg GI, Marmer BL, Grant GA, Eisen AZ, Wilhelm S, He CS (1989) Human 72-kilodalton type IV collagenase forms a complex with a tissue inhibitor of metalloproteases designated TIMP-2. Proc Natl Acad Sci USA 86:8207–8211
Doronzo G, Russo I, Mattiello L, Trovati M, Anfossi G (2005) Homocysteine rapidly increases matrix metalloproteinase-2 expression and activity in cultured human vascular smooth muscle cells. Role of phosphatidyl inositol 3-kinase and mitogen activated protein kinase pathways. Thromb Haemost 94:1285–1293
Guo H, Wang P, You B, Xing Y, Lee JD (2007) Chinese yellow wine inhibits production of homocysteine-induced extracellular matrix metalloproteinase-2 in cultured rat vascular smooth muscle cells. Clin Nutr 26:348–354
Nagase H (1997) Activation mechanisms of matrix metalloproteinases. Biol Chem 378:151–160
Owens GK, Kumar MS, Wamhoff BR (2004) Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84:767–801
Berton A, Godeau G, Emonard H, Baba K, Bellon P, Hornebeck W, Bellon G (2000) Analysis of the ex vivo specificity of human gelatinases A and B towards skin collagen and elastic fibers by computerized morphometry. Matrix Biol 19:139–148
Emonard H, Hornebeck W (1997) Binding of 92 kDa and 72 kDa progelatinases to insoluble elastin modulates their proteolytic activation. Biol Chem 378:265–271
Schanstra JP, Neau E, Drogoz P, Arevalo Gomez MA, Lopez Novoa JM, Calise D, Pecher C, Bader M, Girolami JP, Bascands JL (2002) In vivo bradykinin B2 receptor activation reduces renal fibrosis. J Clin Invest 110:371–379
Luchtefeld M, Grote K, Grothusen C, Bley S, Bandlow N, Selle T, Struber M, Haverich A, Bavendiek U, Drexler H, Schieffer B (2005) Angiotensin II induces MMP-2 in a p47phox-dependent manner. Biochem Biophys Res Commun 328:183–188
Kawaguchi Y, Tanaka H, Okada T, Konishi H, Takahashi M, Ito M, Asai J (1996) The effects of ultraviolet A and reactive oxygen species on the mRNA expression of 72-kDa type IV collagenase and its tissue inhibitor in cultured human dermal fibroblasts. Arch Dermatol Res 288:39–44
Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Lassegue B, Griendling KK (2007) Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol 27:42–48
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ke, X.D., Foucault-Bertaud, A., Genovesio, C. et al. Homocysteine modulates the proteolytic potential of human arterial smooth muscle cells through a reactive oxygen species dependant mechanism. Mol Cell Biochem 335, 203–210 (2010). https://doi.org/10.1007/s11010-009-0270-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0270-7