Abstract
Multiple mucosal immune factors, such as TNF-α and IL-1β, are thought to be key mediators involved in inflammatory bowel disease. We evaluated the role of the pro-inflammatory cytokine TNF-α on nitric oxide synthase (NOS) expression in indomethacin-induced jejunoileitis in rats. Jejunoileitis was induced in rats with subcutaneous injections of indomethacin (7.5 mg/kg) 24 h apart for two consecutive days, and animals were randomized into four groups. Group 1 received only indomethacin. Group 2 was treated with a daily dose of phosphodiesterase (PDE) inhibitor (theophylline or pentoxifylline) by oral gavage for 2 days before and 4 days after indomethacin. Group 3 received a single dose of anti-TNF-α monoclonal antibody (TNF-Ab, IP) 30 min before indomethacin. Group 4 was treated with 1 h hyperbaric oxygenation (HBO2) for 5 days after indomethacin. Rats were sacrificed at 12 h or 4 days after final indomethacin injection. PDE inhibitor, TNF-Ab, or HBO2 treatment significantly decreased indomethacin-induced ulceration, myeloperoxidase activity, and disease activity index. Although indomethacin significantly increased serum TNF-α and nitrate/nitrite (NOx) concentrations above control values at 12 h, inducible NOS (iNOS) expression was detected only at day 4. Serum IL-1β levels did not change at 12 h but increased 4-fold after 4 days. Indomethacin had no effect on constitutive NOS. Treatment with PDE inhibitor, TNF-Ab, or HBO2 significantly reduced serum/tissue TNF-α, IL-1β, NOx, and iNOS expression. Our data show TNF-α plays an early pro-inflammatory role in indomethacin-induced jejunoileitis. Additionally, down-regulation of NOx by PDE inhibitors, TNF-Ab, or HBO2 suggests that TNF-α modulates iNOS expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crohn’s disease and ulcerative colitis are chronic inflammatory gastrointestinal disorders that are collectively referred to as inflammatory bowel disease (IBD). The etiology of IBD most likely involves a complex interaction of genetic, environmental, and immunoregulatory factors. Current hypotheses propose that an inappropriate mucosal immune response to normal intestinal constituents is a key factor, leading to an imbalance in local pro- and anti-inflammatory cytokines, including tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1) [1, 2]. Recent in vitro studies suggest that TNF-α and other cytokines [3], including IL-1β, activate nuclear transcription factor-κB (NF-κB), which leads to activation of transcription of various inflammatory genes [4, 5], including inducible nitric oxide synthase (iNOS). Moreover, iNOS is expressed only in inflamed mucosa from patients with ulcerative colitis, but not in healthy tissue [6].
Neutrophil and monocyte influx occurs with subsequent enhanced expression of iNOS and overproduction of nitric oxide (NO), including release of reactive oxygen intermediates leading to the development of chronic gut inflammation in experimental animal [7, 8] and human IBD [9, 10]. It has been shown that NO derived from iNOS promotes gut inflammation [11, 12], and iNOS inhibition reduces indomethacin (INDO)-induced jejunoileitis [13, 14] and trinitrobenzene sulfonic acid (TNBS)-induced colitis [15]. Although the mechanisms of INDO-induced rat intestinal ulceration and human IBD may differ, the fundamental inflammatory processes are similar. Therefore, INDO-induced intestinal ulceration may provide a useful tool to better understand the pathogenesis of IBD and to search for effective therapies. Pharmacologic agents such as aminosalicylates, azathioprine/6-mercaptopurine, and steroids have been mainstays of therapy. However, newer agents including anti-TNF-α monoclonal antibodies are showing great clinical benefit [16, 17].
We hypothesized that TNF-α production could up-regulate NO production by overexpression of iNOS in INDO-induced jejunoileitis. The present study was designed in order to investigate the role of the pro-inflammatory cytokine TNF-α alone, or in concert with other cytokines, on iNOS and constitutive NOS (cNOS) expression in INDO-induced jejunoileitis in rats. Therefore, we utilized the phosphodiesterase inhibitors, theophylline (TF), and pentoxifylline (PF) to inhibit TNF-α release [18], anti-TNF-α monoclonal antibody (TNF-Ab) to neutralize circulating TNF-α, and non-invasive hyperbaric oxygenation (HBO2) treatment to reduce TNF-α and/or IL-1β production [19].
Materials and methods
Chemicals
Indomethacin, theophylline, and pentoxifylline were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Anti-TNF-α monoclonal antibody and immunoassay kits for TNF-α and IL-1β were acquired from R&D Systems, Inc. (Minneapolis, MN, USA). Nitrate/nitrite concentration assay kit was from Alexis Bioch Corp. (San Diego, CA, USA), and polyclonal anti-iNOS and anti-cNOS antibodies were from Transduction Lab (Lexington, Kentucky, USA). Goat anti-rabbit IgG conjugated with horseradish peroxidase and ECL detection system were obtained from Amersham (Piscataway, NJ, USA).
Animals
Male pathogen-free Sprague–Dawley rats weighing 150–180 gm were obtained from Taconic Sprague–Dawley, Inc. (Indianapolis, IN, USA). Rats were acclimatized for 1 week before being included in experiments, with unrestricted access to water and standard pelleted rat chow (Diet 5008, Purina Mills Inc., Richmond, IN, USA). All protocols were reviewed and approved by the Committee for the Humane Use of Animals at the SUNY Upstate Medical University at Syracuse. The experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US Public Health Service.
Experimental design
The study was divided into two experiments, each consisting of control and experimental rats. Jejunoileitis was induced in study animals by subcutaneous injections of INDO (7.5 mg/kg in 5% sodium bicarbonate) 24 h apart for two consecutive days. In experiment 1, rats were pretreated with a daily dose of TF (50 or 100 mg/kg) or PF (100 or 200 mg/kg) by oral gavage for 2 days, and then continued for 4 days after final INDO administration. Other rats received a single intraperitoneal dose (25 or 50 μg in 1 ml saline) of rat anti-TNF-α antibody (TNF-Ab) 30 min before the first INDO injection. For hyperbaric oxygenation (HBO2) treatment, rats received 100% oxygen at 2.3 atm absolute for 1 h for 5 days 30 min after the first INDO injection. In experiment 2, rats received TF (100 mg/kg) or PF (200 mg/kg), TNF-Ab (50 μg/rat), as in experiment 1, or HBO2 for 2 days. Control rats received equal volumes of vehicle by corresponding route, with or without HBO2, in the presence or absence of INDO. Animals were weighed and their activity level assessed daily. Consistency and color of stool, and occult blood by benzidine test were recorded daily. All of these parameters were used for measurement of disease activity index (DAI). Rats were killed at 4 days or 12 h after the final INDO injection in experiment 1 and experiment 2, respectively.
Preparation of animals
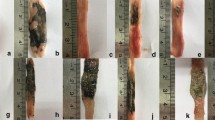
Rats were euthanized using intraperitoneal injections of Nembutal (150 mg/kg). Blood and small bowel were obtained for preparation of serum and three equal parts of tissue samples, respectively, as previously described [13], and stored at −78°C for measurement of TNF-α, nitrate/nitrite (NOx), myeloperoxidase (MPO), and iNOS and cNOS expression. In a duplicate series of experiments, the small bowel was flushed with phosphate buffered saline (PBS) and fixed in a 9:1 mixture of methanol and acetic acid for 24 h for macroscopic evaluation of ulceration. Characteristically, INDO-induced intestinal inflammation was manifested as mucosal ulcerations that extended up to several centimeters in length along the mesenteric border of the jejunum to the distal ileum, with transmural inflammation, fibrosis, adhesions, partial obstruction, and occasional perforation. Total ulcer length (TUL) was obtained by adding the longitudinal length (millimeters) of individual ulcers. A representative intestinal ulceration is shown in Fig. 1. Two independent blinded observers (BS and JMZ) recorded the macroscopic data.
Calculation of DAI
The DAI was determined by scoring changes in body weight, hemoccult positivity, gross bleeding, and stool consistency. After the final INDO injection, daily scores were added for 4 days, and the total values divided by 3, as previously described [14]. These clinical parameters correspond to symptoms observed in human IBD patients.
Measurement of MPO activity
Tissue samples were obtained from different segments and used for assay of MPO activity, as an indicator of granulocyte (mainly neutrophil) infiltration into the tissue using the method described by Grisham et al. [20]. One unit of activity was defined as the amount of enzyme present that produced an absorbance change of 1.0 per minute at 37°C in a final reaction volume of 2.5 ml containing 0.2 M sodium acetate (pH 3.0). MPO activity was expressed as units/minute/gram of tissue.
Serum and tissue TNF-α and IL-1β concentrations
Serum and tissue TNF-α and IL-1β levels were measured using an immunoassay kit specific for each as described by the manufacturer’s manual. Serum was used directly, and tissue TNF-α and IL-1β, frozen samples were homogenized in PBS (1 μl/mg wet tissue weight). The suspensions were centrifuged at 20,000×g for 20 min at 4°C. Supernatant was collected for the measurement of tissue TNF-α and IL-1β levels.
Serum NOx concentrations
Serum NOx concentration was measured using a colorimetric assay kit as previously described by Nandi et al. [14]. Serum NOx concentrations were calculated as μmol/l using a nitrate standard curve.
Tissue iNOS and cNOS expression
Intestinal mucosal extracts were analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) using mouse macrophage as a positive control. Partially purified intestinal mucosal extract (50 μg of protein) for iNOS and cNOS [21] was separated by 10% SDS-PAGE and electrophoretically transferred to nitrocellulose membranes and western blot was performed as previously described by Nandi et al. [14]. The iNOS and cNOS band densities were calculated using ratios to their respective positive control (10 μg of mouse macrophage protein) and expressed as arbitrary units. Data were normalized to 100 for the positive control.
Statistical analysis
The data are expressed as mean ± SEM. Statistical analysis for changes within each group was performed using two-way analysis of variance (ANOVA) for repeated measures and the Bonferroni test was used as a post hoc test. P < 0.05 was considered significant.
Results
At 4 days, the DAI was zero in vehicle-treated control rats with and without HBO2 treatment (data not shown). These rats were used as controls in all experimental measurements of various parameters. The DAI was 2.58 ± 0.2 (n = 8) in INDO-treated rats and was significantly decreased after all drug and HBO2 treatment except in low-dose TF-treated rats, which showed only an 8% decrease in DAI (Fig. 2). The change in DAI in each treatment group was dose-dependent.
Disease activity index (DAI) during acute-phase of indomethacin (INDO)-induced jejunoileitis in rats after treatment with various drugs and hyperbaric oxygenation (HBO2). TF theophylline, PF pentoxifylline, TNF-Ab rat anti-TNF-α antibody, DAI (combined score of weight loss, stool consistency, and bleeding)/3 as described under “Methods”. DAI of INDO treatment alone was 2.58 ± 0.02, and used as 100%. Values on the ordinate are percentage of INDO and expressed as mean ± SEM (n = 8 rats in each treatment group; * P > 0.05 compared to INDO alone, † P > 0.05 compared to TF 50 mg/kg)
There was no intestinal ulceration or inflammation in control rats at 4 days. With INDO treatment, there were no ulcerations observed after 12 h, but there was significant ulceration after 4 days. Like DAI, a significant dose-dependent reduction in INDO-induced ulceration, as measured by TUL, was seen in the drug and HBO2 treatment groups (Fig. 3). Moreover, a linear correlation (r = 0.82, P < 0.025) was found between the percent decrease in DAI and the percent reduction of TUL in various treatment groups.
Total ulcer length (TUL) in indomethacin (INDO)-induced jejunoileitis in rats at 4 days after treatment with various drugs and hyperbaric oxygenation (HBO2). TF theophylline, PF pentoxifylline, TNF-Ab rat anti-TNF-α antibody. TUL of INDO treatment alone was 233 ± 31 mm and used as 100%. Values on the ordinate are percentage of INDO and expressed as mean ± SEM (n = 8 rats in each treatment group; * P > 0.05 compared to INDO alone, † P > 0.05 compared between low and high doses)
INDO significantly increased mucosal MPO activity by 60 and 135% after 12 h and 4 days, respectively, compared to control mucosa (252 ± 30 units/min/g; n = 4). Treatment with HBO2, or a higher dose of TF, PF, or TNF-Ab, significantly decreased MPO activity after 12 h and 4 days, compared to respective INDO (Fig. 4).
Myeloperoxidase (MPO) activity in indomethacin (INDO)-induced jejunoileitis in rats at 12 h and 4 days after treatment with various drugs and hyperbaric oxygenation (HBO2). TF theophylline (100 mg/kg), PF pentoxiphylline (200 mg/kg), TNF-Ab rat anti-TNF-α antibody (50 μg/rat). Control MPO activity was 252 ± 30 (units/min/g), and used as 100%. Values on the ordinate are percentage control and expressed as mean ± SEM (n = 4 rats in each treatment group; * P > 0.05 compared to control, † P > 0.05 compared to INDO alone)
The control tissue TNF-α level was three times higher than control serum level. INDO increased tissue and serum TNF-α levels by 5-fold and 10-fold, respectively, compared to respective control values at 12 h, and remained approximately 2-fold above the control values at 4 days (Table 1). Treatment with HBO2, and higher doses of TF and PF, significantly decreased the tissue and serum TNF-α at 12 h, yet TNF-α was undetectable at 4 days. Animals treated with high-dose TNF-Ab showed no detectable tissue or serum TNF-α at 12 h or 4 days.
In contrast to serum TNF-α, serum IL-1β levels had not increased by 12 h with INDO, but increased 3-fold above the control values at 4 days. Serum IL-1β level remained near control values in all treatment groups either at 12 h or 4 days (Table 2). Unlike serum IL-1β levels, tissue IL-1β levels were much higher with INDO at 12 h and 4 days (Table 2). HBO2 or high-dose TNF-Ab treatment reduced tissue IL-1β levels by 50% at 12 h, compared to INDO, while high-dose TF or PF had no effect. However, tissue IL-1β levels decreased significantly in all treatment groups at 4 days.
Serum NOx levels and mucosal iNOS expression are shown in Fig. 5. Serum NOx levels were significantly increased by INDO (33.1 ± 0.1 μmol/l) at 12 h compared to control value (26.4 ± 1.9 μmol/l), and remained at a higher level (2.5-fold above control) at 4 days. In contrast to NOx, mucosal iNOS protein expression was not significantly increased by INDO at 12 h (Control and INDO were 11 ± 4 and 16 ± 4 arbitrary units, respectively, n = 4), but was increased 6-fold compared to control at 4 days. At 4 days, treatment with TF, PF, TNF-Ab, or HBO2 decreased serum NOx levels to control values with a significant concomitant reduction of iNOS relative density, compared to INDO. There was no change in cNOS expression with or without INDO treatment (Control and INDO were 86 ± 27 and 72 ± 25 arbitrary units, respectively, n = 4).
Serum nitrate/nitrite (NOx) concentrations and tissue iNOS expression in indomethacin (INDO)-induced jejunoileitis in rats at 4 days after treatment with various drugs and hyperbaric oxygenation (HBO2). TF theophylline (100 mg/kg), PF pentoxiphylline (200 mg/kg), TNF-Ab rat anti-TNF-α antibody (50 μg/rat). Values on the ordinate are serum NOx (μmol/l) or iNOS density ratios (arbitrary units), and are expressed as mean ± SEM (n = 4 rats in each treatment group; * P > 0.05 compared to control, † P > 0.05 compared to INDO alone)
Discussion
INDO-induced jejunoileitis in rats shares similar clinical and histological characteristics with human IBD, yet the precise pathophysiological mechanisms may be different [22]. Depending on the dose and route of INDO administration, as well as the strain of rat, INDO-induced jejunoileitis and intestinal inflammation usually resolves spontaneously and completely within 1–2 weeks [23]. The present study was designed in order to avoid the spontaneous remission phase of ulceration [14], and therefore was ended within 5 days after subcutaneous injections of INDO 24 h apart for two consecutive days.
We have shown that INDO-induced jejunoileitis, as evidenced by increases in TUL, DAI, and mucosal MPO activity, was associated with significantly increased production of serum and tissue TNF-α and IL-1β levels. TNF-α levels were higher at 12 h, during the early phase of inflammation (see “Results”). However, levels of TNF-α in both serum and tissue were significantly reduced 4 days after final injection of INDO despite progression of inflammation. Similarly, Videla et al. [24] reported that TNF-α content in colonic homogenates was significantly increased on day 1, reached peak levels on day 4, and returned to baseline levels on day 18 in TNBS-induced chronic colitis in rats. Although the mechanisms of TNBS-induced chronic colitis and INDO-induced acute jejunoileitis may differ, the similarities of clinical manifestations between these two types of animal models suggest that TNF-α plays a key role in the early phase of inflammatory process.
In contrast to tissue TNF-α, levels of the cytokine IL-1β were increased in intestinal mucosa as early as 12 h, and were further elevated by 2-fold at 4 days, after final injection of INDO. Unlike serum TNF-α, elevation of serum IL-1β levels was observed 48 h after INDO injection (data not shown) and remained 4-fold above control at day 4. Similarly, in a dextran sulfate sodium-induced rat colitis model, IL-1β levels were significantly increased only after 7 days in rectal dialysate [25]. This prolonged elevation of mucosal and serum levels shows that IL-1β is involved in the sustained inflammatory process.
We found that PDE inhibitors, TNF-Ab, or HBO2 treatment significantly decreased INDO-induced serum and tissue TNF-α and IL-1β levels, with a concomitant reduction of jejunoileitis. There was a linear relationship between decrease in TUL and DAI, using various concentrations of PDE inhibitors, TNF-Ab, and HBO2 treatment (see “Results”). TNF-α is known to have anorexic effects [26], and its decrease in serum by TNF-Ab may explain the reduction in DAI. Likewise, HBO2 treatment has been shown to suppress immune response [27], and may also account for the reduced DAI. Although reported in vivo and in vitro data on the action of PDE inhibitors on TNF-α and IL-1β levels reach variable conclusions [18, 28–31], our results show that PDE inhibitors decrease DAI less compared to TNF-Ab and HBO2 treatment. Moreover, the 50% reduction in tissue IL-1β level with HBO2 or TNF-Ab treatment, but not PDE inhibitors, at 12 h (Table 2) indicates a modulation of IL-1β by TNF-α.
Measurement of serum NOx level is an indirect indicator of cNOS or iNOS expression in tissue. Generally, a low level of NO produced by cNOS is important for housekeeping function in the gastrointestinal mucosa [32], and this amount was considered the basal serum NOx level. There was a significant increase in serum NOx level with INDO and a concomitant over expression of tissue iNOS at 4 days, but not at 12 h (Fig. 5). Our earlier study showed that the peak serum NOx level and tissue iNOS expression occurred on day 4 [14]. Likewise, iNOS activity was increased in animal models of intestinal inflammation [15, 33] and in patients with Crohn’s disease or ulcerative colitis [10]. We also found that there was no change in cNOS expression with INDO treatment. It also appears that after INDO induction, an initial rise in TNF-α and IL-1β increases iNOS expression and thereby increases NO level, resulting in intestinal injury. The mechanism by which TNF-α modulates iNOS is unclear, but NF-κB has been implicated in in vitro studies [5, 21]. Figure 6 illustrates an immunologic sequence leading to epithelial damage, indicating the central role of TNF-α, and regions where PDE inhibitors, anti-TNF-α antibody, and HBO2 treatment significantly reduced tissue iNOS expression and serum NOx levels.
An immunologic sequence leading to epithelial damage, indicating the central role of TNF-α, and regions where PDE inhibitors, anti-TNF-α antibody, and HBO2 treatment significantly reduced tissue iNOS expression and serum NO levels. APC antigen presenting cell, T T helper cell, MAC macrophage, N neutrophil, ROS reactive oxygen species, NO nitric oxide, INF-γ interferon-γ, HBO 2 hyperbaric oxygenation, TF theophylline, PF pentoxiphylline, TNF-Ab anti-TNF-α antibody
Other factors may be important in the pathogenesis of INDO-induced intestinal lesions. For instance, enterobacteria and their products contribute to the inflammatory response, as evident by attenuated acute INDO-induced small intestine ulceration in germfree rats [34]. Additionally, others have shown that HBO2 treatment reduced translocated mesenteric bacteria in burned mice [35], and inhibited cultured bacterial growth [36, 37]. It is possible that HBO2 treatment reduced growth of intestinal bacteria in our INDO-induced intestinal ulceration model, with concomitant reduction of pro-inflammatory cytokine levels.
The present study has certain limitations. Measurement of serum TNF-α may not accurately reflect INDO-induced intestinal tissue injury, as TNF-α may also be released into serum from other affected tissues. We only measured tissue TNF-α and IL-1β protein levels, and not tissue mRNA expression, which may be a more accurate marker to compare with DAI. Furthermore, we did not perform enterobacteria count after INDO induction and subsequent drugs and HBO2 treatment, and a decreased bacterial level may affect the DAI.
In conclusion, the early pro-inflammatory role of TNF-α has been shown in indomethacin-induced jejunoileitis. The gradual rise in serum IL-1β and iNOS-derived NO levels suggests that TNF-α may up-regulate other cytokines and pro-inflammatory mediators, and thereby cause tissue damage. Finally, the down-regulation of NO by phosphodiesterase inhibitors, anti-TNF-α antibody, or HBO2 treatment suggests that TNF-α modulates iNOS expression, and thereby ameliorates INDO-induced jejunoileitis.
References
Podolsky DK (2000) Medical progress: inflammatory bowel disease. N Engl J Med 347:417–429
Akazawa A, Sakaida I, Higaki S, Kubo Y, Uchida K, Okita K (2002) Increased expression of tumor necrosis factor-alpha messenger RNA in the intestinal mucosal of inflammatory bowel disease, particularly in patients with disease in the inactive phase. J Gastroenterol 37:345–353
Bertrand V, Guimbund R, Tulliez M, Mauprivez C, Sogni P, Couturier D, Giroud JP, Chaussade S, Chauvelot-Moachon L (1998) Increase in tumor necrosis factor-α production linked to toxicity of indomethacin in the rat small intestine. Br J Pharmacol 124:1385–1394
Grisham MB, Pavlic KP, Laroux FS, Hoffnan J, Bharwani S, Wolf RE (2002) Nitric oxide and chronic gut inflammation: controversies in inflammatory bowel disease. J Invest Med 50:272–283
Baeverle PA, Baltimore D (1996) NF-KB: ten years after. Cell 87:13–20
Colon AL, Menchen L, Lizasoain I, Leza JC, Menchen P, Gonzalez-Lara V, Moro MA, Lorenzo P (2000) Inducible nitric oxide synthase activity is expressed not only in inflamed but also in normal colonic mucosa in patients with ulcerative colitis: a potential prognostic marker. Am J Gastroenterol 95:1371–1372
Konaka A, Nishijima M, Tanaka A, Kunikata T, Kato S, Tekeuchi K (1999) Nitric oxide, superoxide radicals and mast cells in pathogenesis of indomethacin-induced small intestinal lesions in rats. J Physiol Pharmacol 50:25–38
Whittle BJR, Laszlo F, Evans SM, Moncada S (1995) Induction of nitric oxide synthase and microvascular injury in the rat jejunum provoked by indomethacin. Br J Pharmacol 116:2286–2290
Singer II, Kawka DW, Scott S, Weidner JR, Mumford RA, Riehl TE, Stenson WF (1996) Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology 111:871–885
Kimura H, Miura S, Shigematsu T, Ohkubo N, Tsuzuki Y, Kurose I, Higuchi H, Akiba Y, Hokari R, Hirokawa M, Serizawa H, Ishii H (1997) Increased nitric oxide production and inducible nitric oxide synthase activity in colonic mucosa of patients with active ulcerative colitis and Crohn’s disease. Dig Dis Sci 42:1047–1054
Grisham MB, Jourd’heuil D, Wink DA (1999) Nitric oxide I physiological chemistry of nitric oxide and its metabolites: implication in inflammation. Am J Physiol 276:G315–G321
Miller MJS, Sandoval M (1999) Nitric oxide III A molecular prelude to inflammation. Am J Physiol 276:G795–G799
Parasher G, Frenklakh L, Siddiqui T, Nandi J, Levine RA (2001) Nitric oxide inhibitors ameliorate indomethacin-induced enteropathy in rats. Dig Dis Sci 46:2536–2541
Nandi J, Saud B, Zinkievich JM, Palma DT, Levine RA (2008) 5-aminosalicylic acid improves indomethacin-induced enteropathy by inhibiting iNOS transcription in rats. Dig Dis Sci 53:123–132
Rachmilewitz D, Karmeli F, Okon E, Bursztyn M (1995) Experimental colitis is ameliorated by inhibition of nitric oxide synthase activity. Gut 37:247–255
Shanahan F (2001) Inflammatory bowel disease: immunodiagnostics, immunotherapeutics, and ecotherapeutics. Gastroenterology 120:622–635
Lugering A, Schmidit M, Lugering N, Pauels HG, Domschke W, Kucharzik T (2001) Infliximab induces apoptosis in monocytes from patients with chronic active Crohn’s disease by using a caspase-dependent pathway. Gastroenterology 121:1145–1157
Santucci L, Fiorucci S, Giansanti M, Buonori PM, DiMatteo FM, Morelli A (1994) Pentoxifylline prevents indomethacin induced acute mucosal damage in rats: role of tumour necrosis factor alpha. Gut 35:909–915
Weisz G, Lavy A, Adir Y, Melamed Y, Rubin D, Eidelman S, Pollack S (1997) Modification of in vitro and in vivo TNF-alpha, IL-1, and IL-6 secretion by circulating monocytes during hyperbaric oxygen treatment in patients with perianal Crohn’s disease. J Clin Immunol 17:154–159
Grisham MB, Benoit JN, Granger DN (1996) Assessment of leukocyte involvement during ischemia and re-perfusion of intestine. Methods Enzymol 186:729–742
Kennedy M, Wilson L, Szabo C, Salzman AL (1999) 5-aminosalicylic acid inhibits iNOS transcription in human intestinal epithelial cells. Int J Mol Med 4:437–443
Anthony A, Pounder RE, Dhillon AP, Wakefield AJ (2001) The inflammatory bowel disease study group. Similarities between ileal Crohn’s disease and indomethacin experimental jejunal ulcers in the rat. Aliment Pharmacol Ther 14:241–245
Yamada T, Deitch E, Specian RD, Perry MA, Sartor RB, Grisham MB (1993) Mechanisms of acute and chronic intestinal inflammation induced by indomethacin. Inflammation 17:641–662
Videla S, Garcia-Lafuente A, Antolin M, Vilaseca J, Guarner F, Crespo E, Lez GG, Salas A, Malagelada JR (1998) Antitumor necrosis factor therapy in rat chronic granulomatous colitis: critical dose-timing effects on outcome. J Pharmacol Exp Ther 287:854–859
Sukumar P, Loo A, Adolphe R, Nandi J, Oler A, Levine RA (1999) Dietary nucleotides augment dextran sulfate sodium-induced distal colitis in rats. J Nutr 129:1377–1381
Nandi J, Meguid MM, Inui A, Xu Y, Makarenko IG, Tada T, Chung C (2002) Central mechanisms involved with catabolism. Curr Opin Clin Nutr Metab Care 5:407–418
Saito K, Tanaka Y, Ota T, Eto S, Yamashita U (1991) Suppressive effect of hyperbaric oxygenation on immune responses of normal and autoimmune mice. Clin Exp Immunol 86:322–327
Doherty GM, Christian J, Alexander HR, Buresh CM, Norton JA (1991) Pentoxifylline suppression of tumor necrosis factor gene transcription. Surgery 110:192–198
Reuter BK, Wallace JL (1999) Phosphodiesterase inhibitors prevent NSAID enteropathy independently of effects on TNF-α release. Am J Physiol 277:G847–G854
Katz J, Willis J, Cooper G, Geraci K, Fiocchi C (1999) Treatment of Crohn’s disease with pentoxifylline: lack of correlation between clinical improvement and mucosal cytokine levels. Am J Gastroenterol 94:A270
Reimund JM, Dumont S, Muller CD, Kenney JS, Kedinger M, Baumann R, Poindron P, Duclos B (1997) In vitro effects of oxpentifylline on inflammatory cytokine release in patients with inflammatory bowel disease. Gut 40:475–480
Lopez-Belmonte J, Whittle BJR, Moncada S (1993) The actions of nitric oxide donors in the prevention of induction of injury to the rat gastric mucosa. Br J Pharmacol 108:73–78
Takeuchi K, Yokota A, Tanaka A, Takahira Y (2006) Factors involved in upregulation of inducible nitric oxide synthase in rat small intestine following administration of nonsteroidal anti-inflammatory drugs. Dig Dis Sci 51(7):1250–1259
Robert A, Asano T (1977) Resistance of germ free rats to indomethacin-induced intestinal lesions. Prostaglandins 14:333–341
Tenenhaus M, Hansbrough JF, Zapata-Sirvent R, Neumann T (1994) Treatment of burned mice with hyperbaric oxygen reduces mesenteric bacteria but not pulmonary neutrophil deposition. Arch Surg 129:1338–1342
Gottlieb SF (1971) Effect of hyperbaric oxygen on microorganisms. Annu Rev Microbiol 25:111–152
Sebesteny M, Balogh A, Nemes A, Besznyak I (1976) Effect of hyperbaric oxygen on aerobic bacteria. Z Exp Chir 9:84–88
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nandi, J., Saud, B., Zinkievich, J.M. et al. TNF-α modulates iNOS expression in an experimental rat model of indomethacin-induced jejunoileitis. Mol Cell Biochem 336, 17–24 (2010). https://doi.org/10.1007/s11010-009-0259-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0259-2